Submitted:
27 May 2024
Posted:
28 May 2024
You are already at the latest version
Abstract
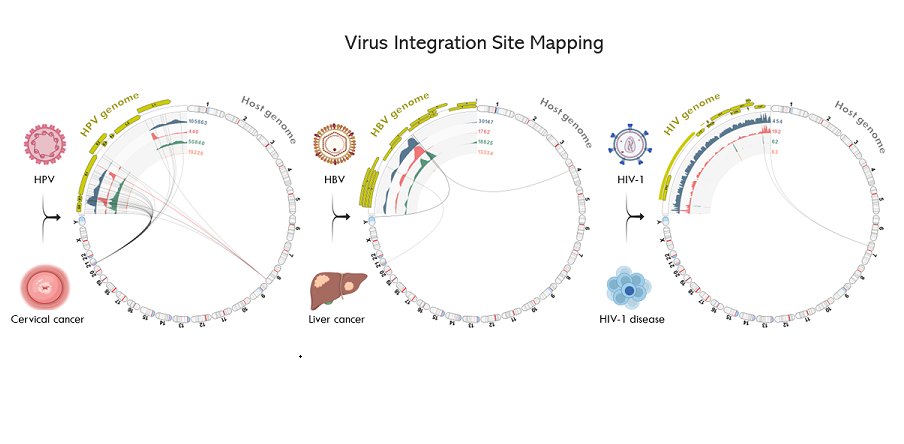
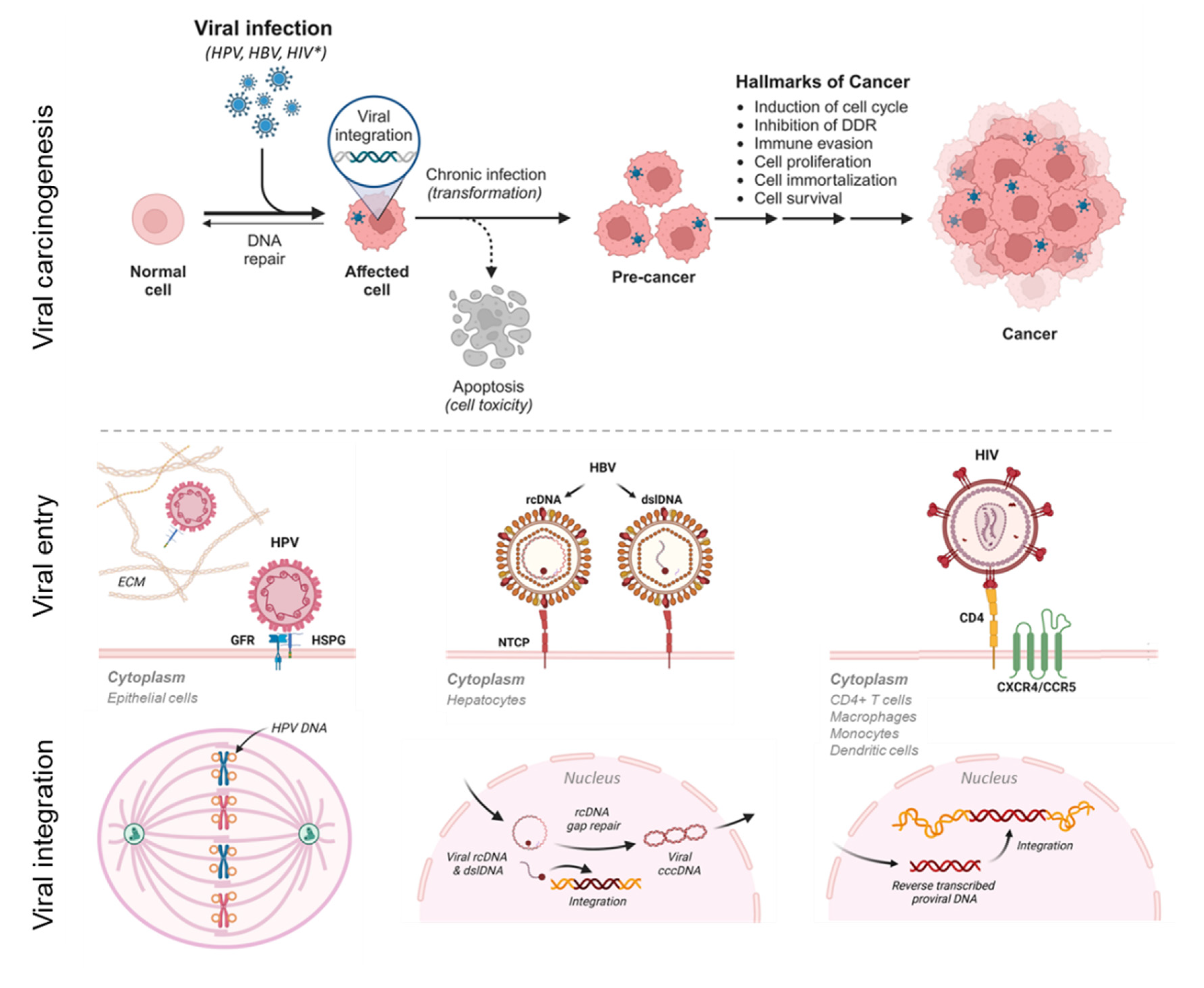
Keywords:
1. Introduction
2. Materials and Methods
2.1. NGS Dataset of HPV, HBV, and HIV-Positive Cell Lines
2.2. Customized HPV, HBV, and HIV-1 Reference Databases for CLC Workflows and Tools
2.3. Whole Genome Alignment and Phylogenetic Analysis of Database Sequences
2.4. Viral Hybrid Capture (VHC) Analysis and Workflow
2.5. Viral Integration Site (VIS) Analysis and Workflow
3. Results
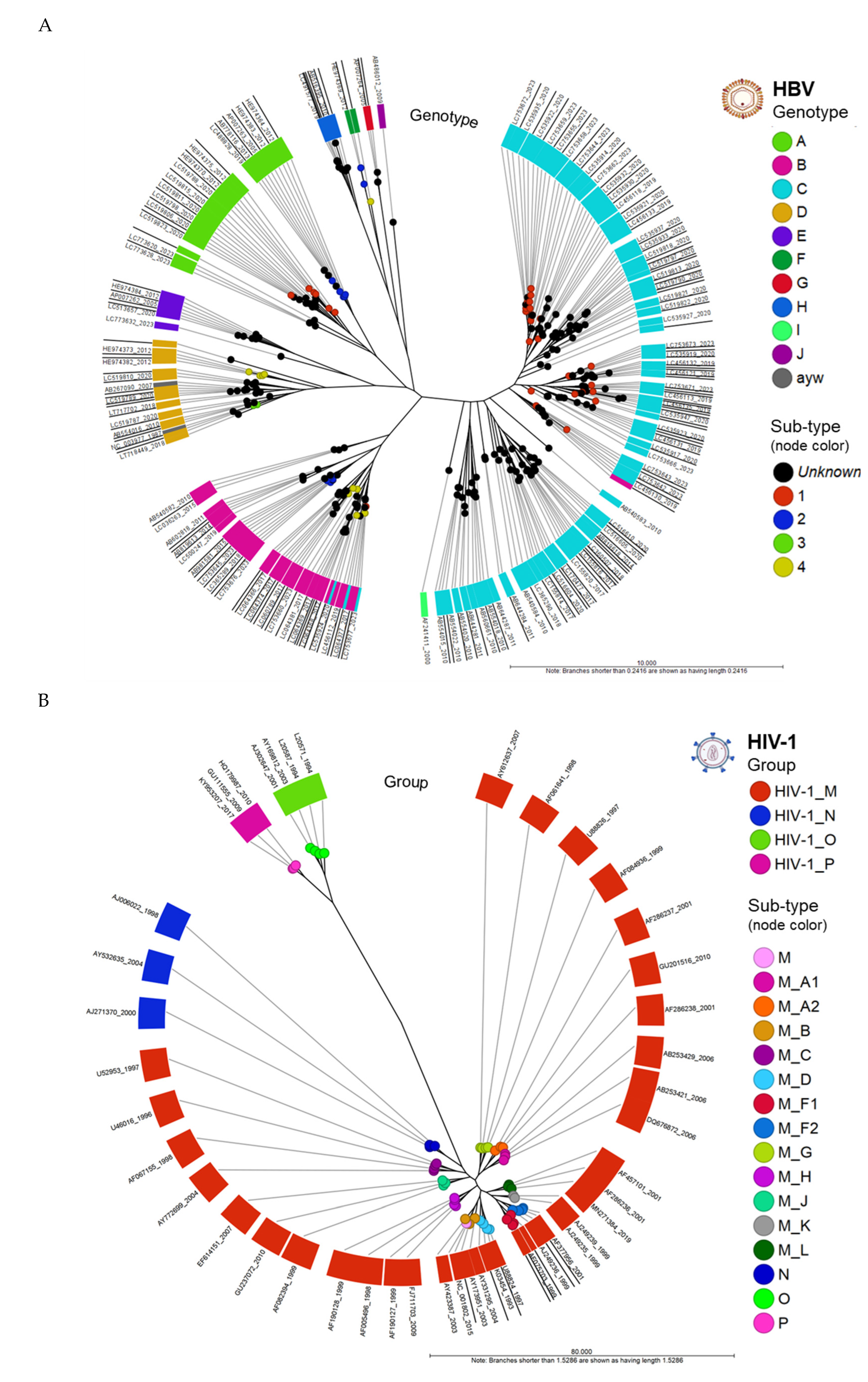
3.1. Whole Genome Alignment (WGA) and Comparison of HBV and HIV-1 genomes
3.1.1. Phylogenetic Trees of HBV and HIV-1 Genomes
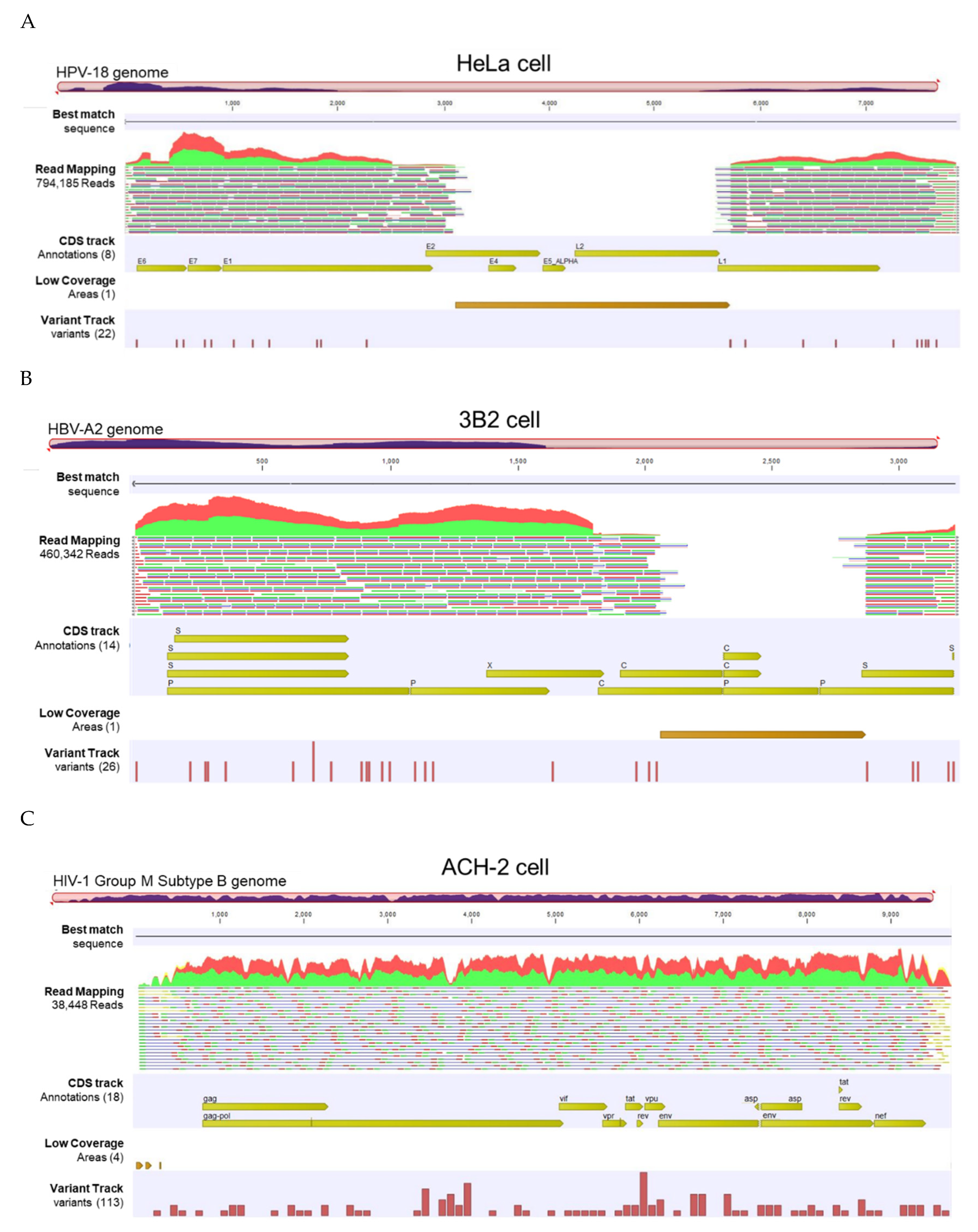
3.2. Viral Hybrid Capture (VHC) Analysis and Visualization
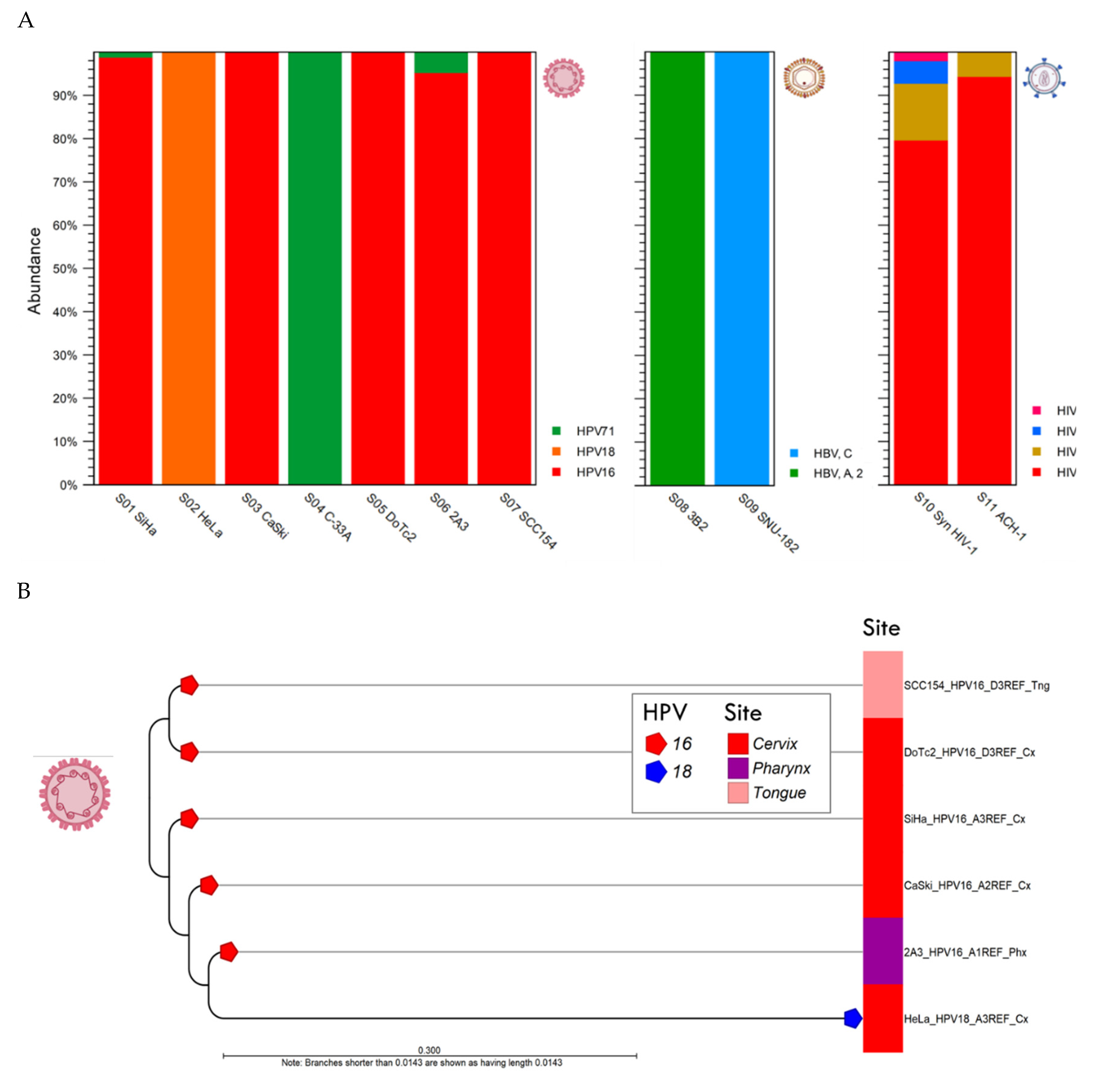
3.2.1. HPV Taxonomic Profiling
3.2.2. Viral Hybrid Capture (VHC) Tracklists
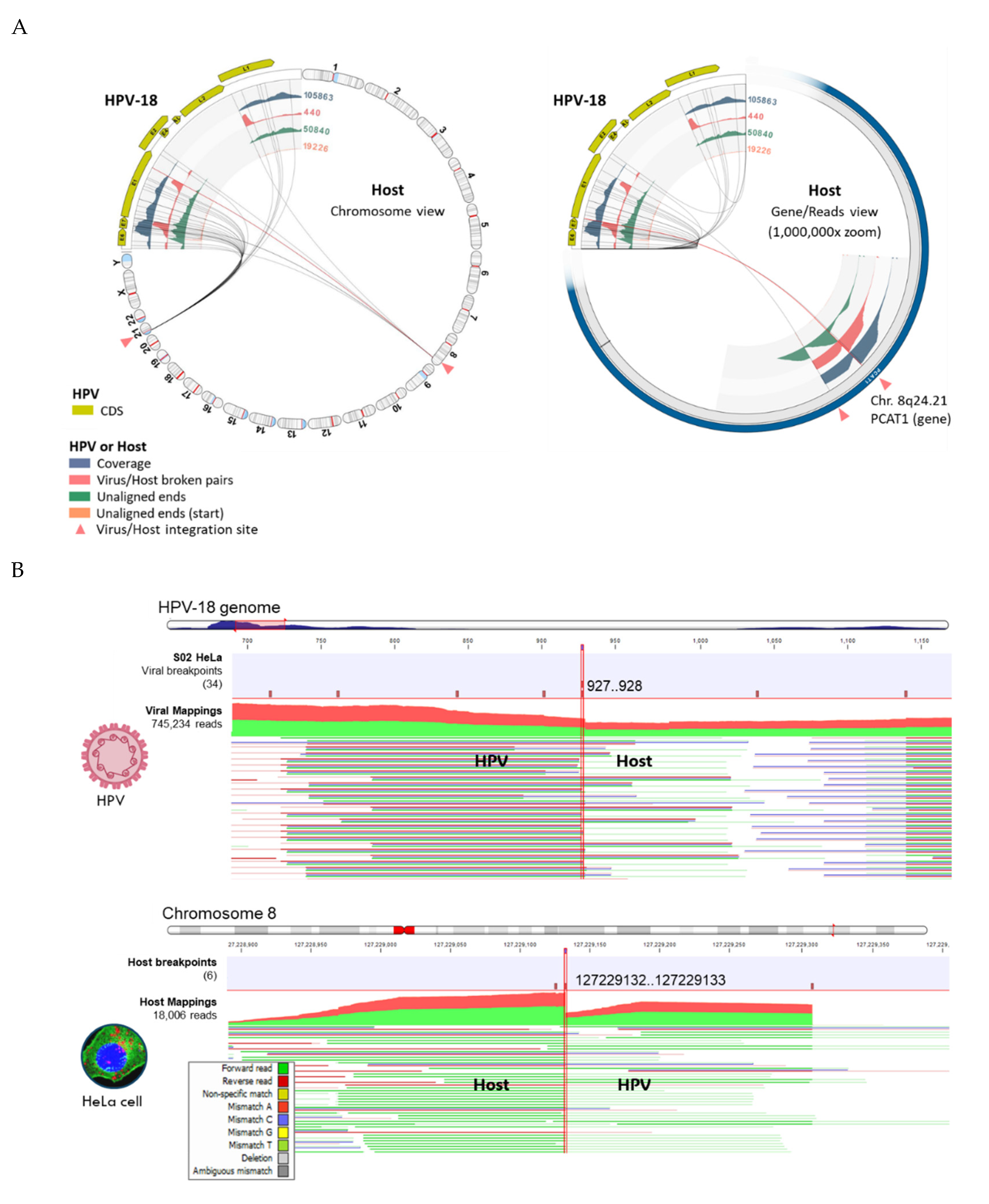
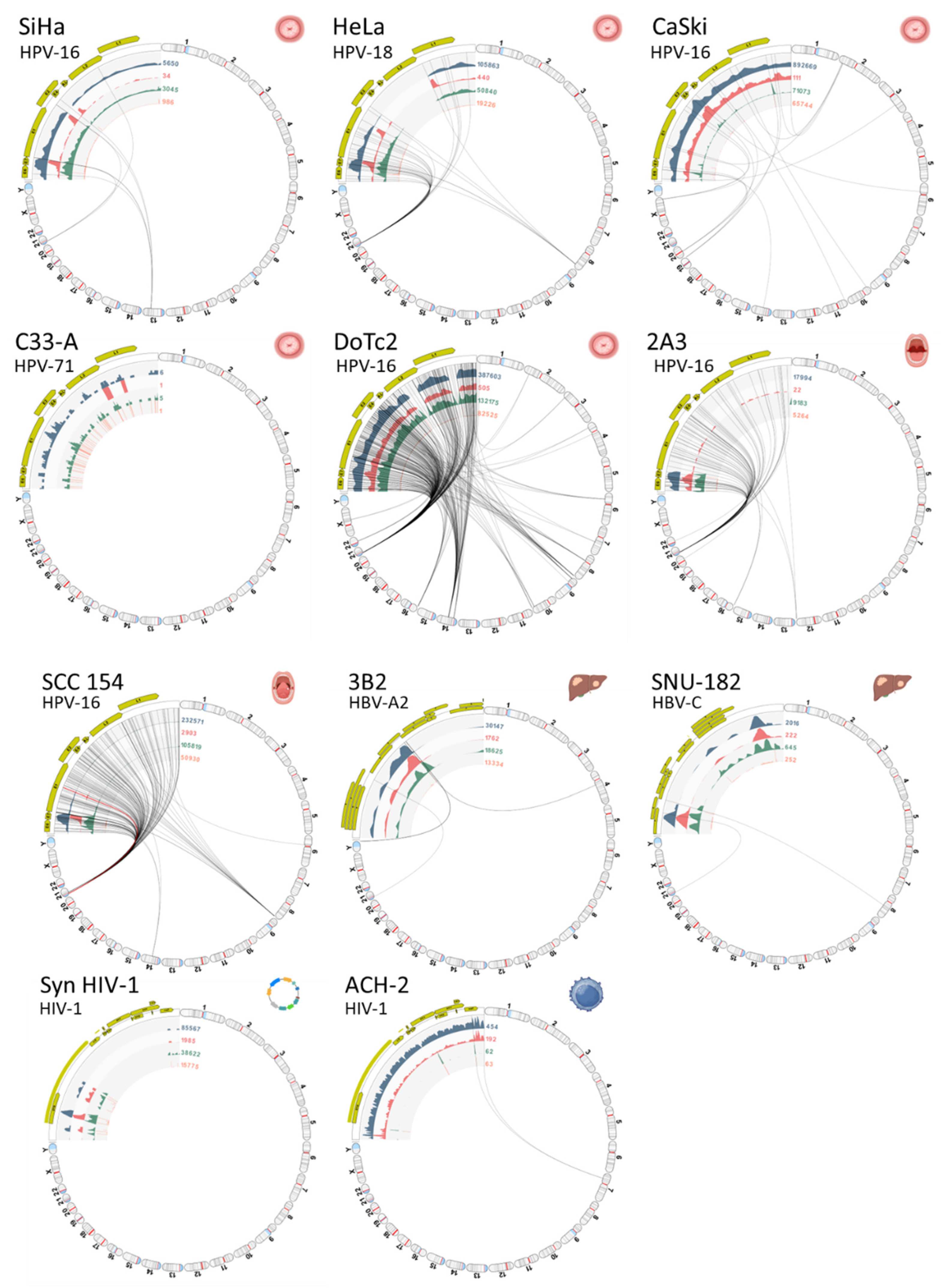
3.3. Viral Integration Site (VIS) Analysis and Visualization
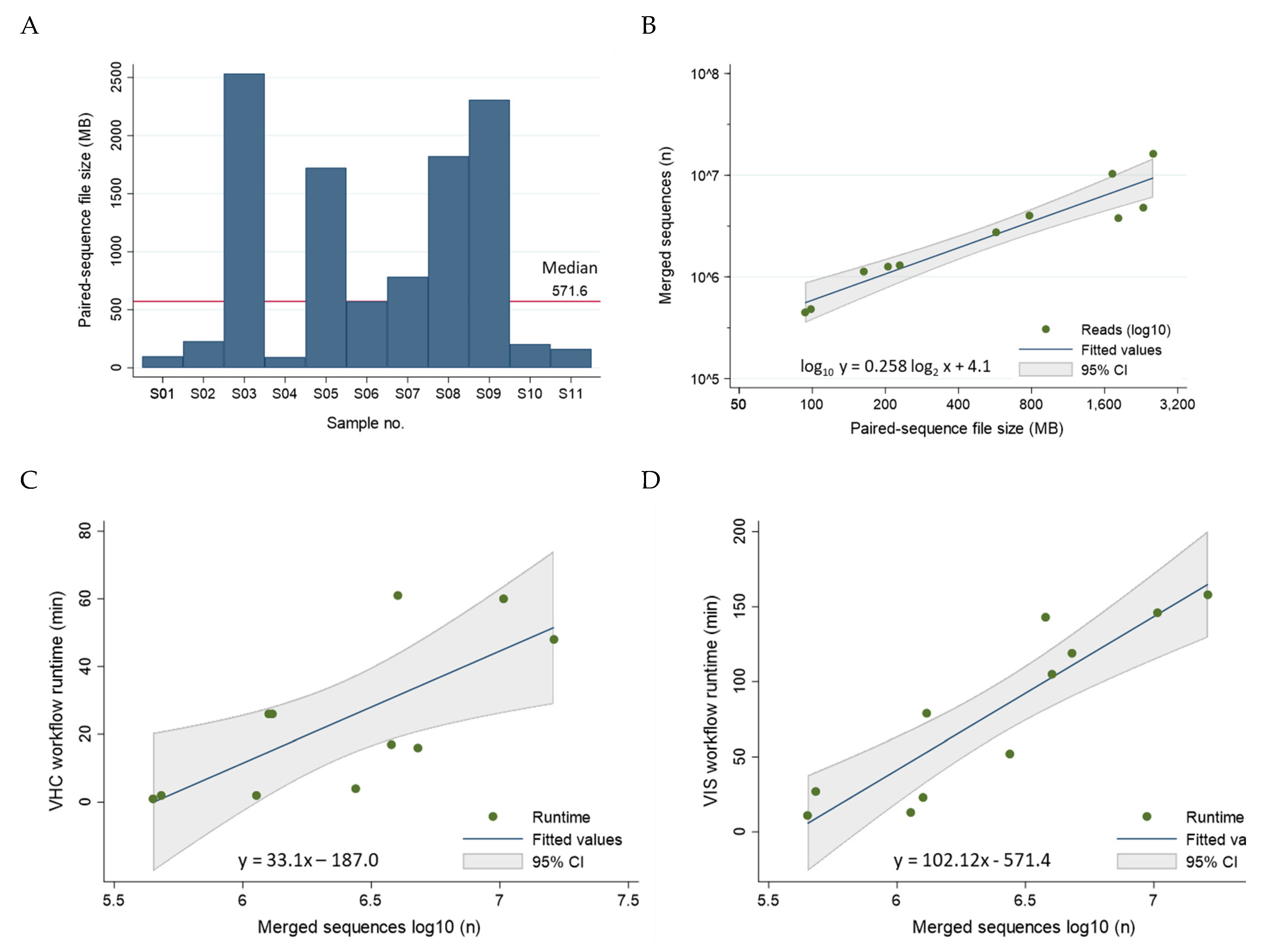
3.4. Workflow Runtimes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Weiss RA, Vogt PK. 100 years of Rous sarcoma virus. J Exp Med. 2011, 208, 2351–2355. [Google Scholar] [CrossRef] [PubMed]
- Lipsick, J. A History of Cancer Research: Tumor Viruses. Cold Spring Harb Perspect Biol. 2021, 13, a035774. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Monographs on the Identification of Carcinogenic Hazards to Humans, volumes 1-135. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed on 2 February2024).
- Proulx J, Ghaly M, Park IW, Borgmann K. HIV-1-Mediated Acceleration of Oncovirus-Related Non-AIDS-Defining Cancers. Biomedicines. 2022, 10, 768. [Google Scholar]
- World Health Organization (WHO). Global cancer burden growing, amidst mounting need for services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 2 February2024).
- International Agency for Research on Cancer (IARC). Cancers Attributable to Infections. Geneva: World Health Organization. Available online: https://gco.iarc.fr/causes/infections/tools-bars?mode=2&sex=0&population=who&country=4&continent=0&agent=0&cancer=0&key=attr_cases&lock_scale=0&nb_results=10 (accessed on 2 February2024).
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention. Lyon: International Agency for Research on Cancer; International Agency for Research on Cancer: Lyon, France, 2020; Available online: http://publications.iarc.fr/586 (accessed on 6 June 2022).
- de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018, 142, 2471–2477. [Google Scholar] [CrossRef]
- World Health Organization (WHO). HIV data and statistics. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 2 February2024).
- Aksoy P, Gottschalk EY, Meneses PI. HPV entry into cells. Mutat Res Rev Mutat Res. 2017, 772, 13–22. [Google Scholar] [CrossRef]
- Park JH, Iwamoto M, Yun JH, Uchikubo-Kamo T, Son D, Jin Z, Yoshida H, Ohki M, Ishimoto N, Mizutani K, Oshima M, Muramatsu M, Wakita T, Shirouzu M, Liu K, Uemura T, Nomura N, Iwata S, Watashi K, Tame JRH, Nishizawa T, Lee W, Park SY. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature. 2022, 606, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- McBride AA, Sakakibara N, Stepp WH, Jang MK. Hitchhiking on host chromatin: how papillomaviruses persist. Biochim Biophys Acta. 2012, 1819, 820–825. [Google Scholar] [CrossRef]
- Coursey TL, McBride AA. Hitchhiking of Viral Genomes on Cellular Chromosomes. Annu Rev Virol. 2019, 6, 275–296. [Google Scholar] [CrossRef]
- Warburton A, Della Fera AN, McBride AA. Dangerous Liaisons: Long-Term Replication with an Extrachromosomal HPV Genome. Viruses. 2021, 13, 1846. [Google Scholar] [CrossRef]
- McBride AA, Warburton A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017, 13, e1006211. [Google Scholar]
- Tu T, Budzinska MA, Shackel NA, Urban S. HBV DNA Integration: Molecular Mechanisms and Clinical Implications. Viruses. 2017, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Tu T, Zhang H, Urban S. Hepatitis B Virus DNA Integration: In Vitro Models for Investigating Viral Pathogenesis and Persistence. Viruses. 2021, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Grandgenett DP, Engelman AN. Brief Histories of Retroviral Integration Research and Associated International Conferences. Viruses. 2024, 16, 604. [Google Scholar] [CrossRef]
- Kumar A, Murthy S, Kapoor A. Evolution of selective-sequencing approaches for virus discovery and virome analysis. Virus Res. 2017, 239, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Shen-Gunther J, Cai H, Wang Y. HPV Integration Site Mapping: A Rapid Method of Viral Integration Site (VIS) Analysis and Visualization Using Automated Workflows in CLC Microbial Genomics. Int J Mol Sci. 2022, 23, 8132. [Google Scholar] [CrossRef] [PubMed]
- Qiagen: QIAseq xHYB Viral STI Panel. Available online: https://www.qiagen.com/us/products/next-generation-sequencing/ metagenomics/targeted-metagenomics/qiaseq-xhyb-viral-and-bacterial-panels/ (accessed on 1 August 2022).
- American Type Culture Collection (ATCC). Available online: https://www.atcc.org/ (accessed on 1 August 2022).
- Cellosaurus. Available online: https://www.cellosaurus.org (accessed on 1 December 2023).
- Iwase SC, Miyazato P, Katsuya H, Islam S, Yang BTJ, Ito J, Matsuo M, Takeuchi H, Ishida T, Matsuda K, Maeda K, Satou Y. HIV-1 DNA-capture-seq is a useful tool for the comprehensive characterization of HIV-1 provirus. Sci Rep. 2019, 9, 12326. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Sequence Read Archive. Available online: https://www.ncbi.nlm.nih.gov/sra (accessed on 5 January 2024).
- Qiagen Digital Insights. Available online: https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-clc-microbial-genomics-module/ (accessed on 12 July 2023).
- Richardson, C. Hepadnaviruses. In Fundamentals of Molecular Virology, 2nd Edition; Editor Acheson, NJ., Ed.; John Wiley & Sons: NJ, USA, 2011; pp. 365–376. [Google Scholar]
- NCBI Virus. Available online: https://www. ncbi.nlm.nih.gov/labs/virus/vssi/#/ (accessed on 25 December 2023).
- NCBI Nucleotide. Available online: https://www.ncbi.nlm.nih.gov/nuccore (accessed on 25 December 2023).
- International Committee on Taxonomy of Viruses (ICTV): Master Species Lists. Available online: https://ictv.global/msl (accessed on 25 December 2023).
- Koonin, E.V.; Krupovic, M.; Agol, V.I. The Baltimore Classification of Viruses 50 Years Later: How Does It Stand in the Light of Virus Evolution? Microbiol. Mol. Biol. Rev. 2021, 85, e0005321. [Google Scholar] [CrossRef]
- Locarnini SA, Littlejohn M, Yuen LKW. Origins and Evolution of the Primate Hepatitis B Virus. Front Microbiol. 2021, 12, 653684. [Google Scholar]
- United Nations Statistical Division-Geographic Regions. Available online: https://unstats.un.org/unsd/methodology/m49/ (accessed on 12 July 2023).
- Cochrane, A. Human Immunodeficiency Virus. In Fundamentals of Molecular Virology, 2nd ed.; Editor Acheson, NJ., Ed.; John Wiley & Sons: NJ, USA, 2011; pp. 354–364. [Google Scholar]
- Los Alamos National Laboratory (LANL). HIV Databases. Available online: https://www.hiv.lanl.gov/content/index (accessed on 22 January 2024).
- Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011, 1, a006841. [Google Scholar]
- Clifford GM, Tenet V, Georges D, Alemany L, Pavón MA, Chen Z, Yeager M, Cullen M, Boland JF, Bass S, Steinberg M, Raine-Bennett T, Lorey T, Wentzensen N, Walker J, Zuna R, Schiffman M, Mirabello L. Human papillomavirus 16 sub-lineage dispersal and cervical cancer risk worldwide: Whole viral genome sequences from 7116 HPV16-positive women. Papillomavirus Res. 2019, 7, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Brothman AR, Persons DL, Shaffer LG. Nomenclature evolution: Changes in the ISCN from the 2005 to the 2009 edition. Cytogenet Genome Res. 2009, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Simons A, Shaffer LG, Hastings RJ. Cytogenetic Nomenclature: Changes in the ISCN 2013 Compared to the 2009 Edition. Cytogenet Genome Res. 2013, 141, 1–6. [Google Scholar] [CrossRef] [PubMed]
- HumCFS: A Database of Human Chromosomal Fragile Sites. Available online: https://webs.iiitd.edu.in/raghava/humcfs/index.html (accessed on 1 January 2024).
- Bodelon C, Untereiner ME, Machiela MJ, Vinokurova S, Wentzensen N. Genomic characterization of viral integration sites in HPV-related cancers. Int J Cancer. 2016, 139, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- NCBI Gene. Available online: https://www.ncbi.nlm.nih.gov/gene (accessed on 1 January 2024).
- Xu X, Han Z, Ruan Y, Liu M, Cao G, Li C, Li F. HPV16-LINC00393 Integration Alters Local 3D Genome Architecture in Cervical Cancer Cells. Front Cell Infect Microbiol. 2021, 11, 785169. [Google Scholar]
- Shen C, Liu Y, Shi S, Zhang R, Zhang T, Xu Q, Zhu P, Chen X, Lu F. Long-distance interaction of the integrated HPV fragment with MYC gene and 8q24.22 region upregulating the allele-specific MYC expression in HeLa cells. Int J Cancer. 2017, 141, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Wilson C, Kanhere A. 8q24.21 Locus: A Paradigm to Link Non-Coding RNAs, Genome Polymorphisms and Cancer. Int J Mol Sci. 2021, 22, 1094. [Google Scholar] [CrossRef]
- Larios-Serrato V, Valdez-Salazar HA, Ruiz-Tachiquín ME. The landscape of 8q24 cytoband in gastric cancer (Review). Oncol Lett. 2024, 27, 179. [Google Scholar] [CrossRef] [PubMed]
- Bi Y, Hu J, Zeng L, Chen G, Cai H, Cao H, Ma Q, Wu X. Characteristics of HPV integration in cervical adenocarcinoma and squamous carcinoma. J Cancer Res Clin Oncol. 2023, 149, 17973–17986. [Google Scholar] [CrossRef]
- Diosdado B, Buffart TE, Watkins R, Carvalho B, Ylstra B, Tijssen M, Bolijn AS, Lewis F, Maude K, Verbeke C, Nagtegaal ID, Grabsch H, Mulder CJ, Quirke P, Howdle P, Meijer GA. High-resolution array comparative genomic hybridization in sporadic and celiac disease-related small bowel adenocarcinomas. Clin Cancer Res. 2010, 16, 1391–1401. [Google Scholar] [CrossRef]
- Tang W, Pei M, Li J, Xu N, Xiao W, Yu Z, Zhang J, Hong L, Guo Z, Lin J, Dai W, Xiao Y, Wu X, Liu G, Zhi F, Li G, Xiong J, Chen Y, Zhang H, Xiang L, Li A, Liu S, Wang J. The miR-3648/FRAT1-FRAT2/c-Myc negative feedback loop modulates the metastasis and invasion of gastric cancer cells. Oncogene. 2022, 41, 4823–4838. [Google Scholar] [CrossRef] [PubMed]
- Xiong T, Li J, Chen F, Zhang F. PCAT-1: A Novel Oncogenic Long Non-Coding RNA in Human Cancers. Int J Biol Sci. 2019, 15, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Wu Y, Mou J, Zhou G, Yuan C. CASC19: An Oncogenic Long Non-coding RNA in Different Cancers. Curr Pharm Des. 2024 Mar 27.
- Wang K, Liang Q, Li X, Tsoi H, Zhang J, Wang H, Go MY, Chiu PW, Ng EK, Sung JJ, Yu J. MDGA2 is a novel tumour suppressor cooperating with DMAP1 in gastric cancer and is associated with disease outcome. Gut. 2016, 65, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Choi EJ, Yoo NJ, Kim MS, An CH, Lee SH. Putative Tumor Suppressor Genes EGR1 and BRSK1 Are Mutated in Gastric and Colorectal Cancers. Oncology. 2016, 91, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Wang WF, Zhong HJ, Cheng S, Fu D, Zhao Y, Cai HM, Xiong J, Zhao WL. A nuclear NKRF interacting long noncoding RNA controls EBV eradication and suppresses tumor progression in natural killer/T-cell lymphoma. Biochim Biophys Acta Mol Basis Dis. 2023, 1869, 166722. [Google Scholar]
- Tachibana M, Kiyokawa E, Hara S, Iemura S, Natsume T, Manabe T, Matsuda M. Ankyrin repeat domain 28 (ANKRD28), a novel binding partner of DOCK180, promotes cell migration by regulating focal adhesion formation. Exp Cell Res. 2009, 315, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Jafari S, Ravan M, Karimi-Sani I, Aria H, Hasan-Abad AM, Banasaz B, Atapour A, Sarab GA. Screening and identification of potential biomarkers for pancreatic cancer: An integrated bioinformatics analysis. Pathol Res Pract. 2023, 249, 154726. [Google Scholar] [CrossRef] [PubMed]
- Xu HW, Wang MQ, Zhu SL. Analysis of IGFBP7 expression characteristics in pan-cancer and its clinical relevance to stomach adenocarcinoma. Transl Cancer Res. 2023, 12, 2596–2612. [Google Scholar] [CrossRef]
- Zhang JJ, Hong J, Ma YS, Shi Y, Zhang DD, Yang XL, Jia CY, Yin YZ, Jiang GX, Fu D, Yu F. Identified GNGT1 and NMU as Combined Diagnosis Biomarker of Non-Small-Cell Lung Cancer Utilizing Bioinformatics and Logistic Regression. Dis Markers. 2021, 2021, 6696198. [Google Scholar]
- Ni Z, Cong S, Li H, Liu J, Zhang Q, Wei C, Pan G, He H, Liu W, Mao A. Integration of scRNA and bulk RNA-sequence to construct the 5-gene molecular prognostic model based on the heterogeneity of thyroid carcinoma endothelial cell. Acta Biochim Biophys Sin (Shanghai). 2024, 56, 255–269. [Google Scholar]
- Wu G, Dong Y, Hu Q, Ma H, Xu Q, Xu K, Chen H, Yang Z, He M. HGH1 and the immune landscape: a novel prognostic marker for immune-desert tumor microenvironment identification and immunotherapy outcome prediction in human cancers. Cell Cycle. 2023, 22, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Matsui Y, Imai A, Izumi H, Yasumura M, Makino T, Shimizu T, Sato M, Mori H, Yoshida T. Cancer-associated point mutations within the extracellular domain of PTPRD affect protein stability and HSPG interaction. FASEB J. 2024, 38, e23609. [Google Scholar] [CrossRef] [PubMed]
- Lyu Y, Wang Y, Ding H, Li P. Hypoxia-induced m6A demethylase ALKBH5 promotes ovarian cancer tumorigenicity by decreasing methylation of the lncRNA RMRP. Am J Cancer Res. 2023, 13, 4179–4191. [Google Scholar] [PubMed] [PubMed Central]
- Das, L. Epigenetic alterations impede epithelial-mesenchymal transition by modulating centrosome amplification and Myc/RAS axis in triple negative breast cancer cells. Sci Rep. 2023, 13, 2458. [Google Scholar] [CrossRef] [PubMed]
- Yang C, Zhang X, Yang X, Lian F, Sun Z, Huang Y, Shen W. Function and regulation of RGS family members in solid tumours: a comprehensive review. Cell Commun Signal. 2023, 21, 316. [Google Scholar] [CrossRef] [PubMed]
- Lin YH, Chen CW, Cheng HC, Liu CJ, Chung ST, Hsieh MC, Tseng PL, Tsai WH, Wu TS, Lai MD, Shih CL, Yen MC, Fang WK, Chang WT. Inhibition of lncRNA RPPH1 activity decreases tumor proliferation and metastasis through down-regulation of inflammation-related oncogenes. Am J Transl Res. 2023, 15, 6701–6717. [Google Scholar] [PubMed] [PubMed Central]
- Yan W, Li SX, Gao H, Yang W. Identification of B-cell translocation gene 1-controlled gene networks in diffuse large B-cell lymphoma: A study based on bioinformatics analysis. Oncol Lett. 2019, 17, 2825–2835. [Google Scholar]
- Jia Z, Wang M, Li S, Li X, Bai XY, Xu Z, Yang Y, Li B, Li Y, Wu H. U-box ubiquitin ligase PPIL2 suppresses breast cancer invasion and metastasis by altering cell morphology and promoting SNAI1 ubiquitination and degradation. Cell Death Dis. 2018, 9, 63. [Google Scholar] [CrossRef]
- Han Z, Wang Y, Han L, Yang C. RPN2 in cancer: An overview. Gene. 2023, 857, 147168. [Google Scholar] [CrossRef]
- Keramati F, Seyedjafari E, Fallah P, Soleimani M, Ghanbarian H. 7SK small nuclear RNA inhibits cancer cell proliferation through apoptosis induction. Tumour Biol. 2015, 36, 2809–2814. [Google Scholar] [CrossRef]
- Chiovaro F, Chiquet-Ehrismann R, Chiquet M. Transcriptional regulation of tenascin genes. Cell Adh Migr. 2015, 9, 34–47. [Google Scholar] [CrossRef]
- Heczko L, Hlaváč V, Holý P, Dvořák P, Liška V, Vyčítal O, Fiala O, Souček P. Prognostic potential of whole exome sequencing in the clinical management of metachronous colorectal cancer liver metastases. Cancer Cell Int. 2023, 23, 295. [Google Scholar] [CrossRef] [PubMed]
- Bao X, Ran J, Kong C, Wan Z, Wang J, Yu T, Ruan S, Ding W, Xia L, Zhang D. Pan-cancer analysis reveals the potential of hyaluronate synthase as therapeutic targets in human tumors. Heliyon. 2023, 9, e19112. [Google Scholar] [CrossRef]
- Vigetti D, Deleonibus S, Moretto P, Bowen T, Fischer JW, Grandoch M, Oberhuber A, Love DC, Hanover JA, Cinquetti R, Karousou E, Viola M, D’Angelo ML, Hascall VC, De Luca G, Passi A. Natural antisense transcript for hyaluronan synthase 2 (HAS2-AS1) induces transcription of HAS2 via protein O-GlcNAcylation. J Biol Chem. 2014, 289, 28816–28826. [Google Scholar] [CrossRef]
- Bogusławska DM, Skulski M, Bartoszewski R, Machnicka B, Heger E, Kuliczkowski K, Sikorski AF. A rare mutation (p.F149del) of the NT5C3A gene is associated with pyrimidine 5′-nucleotidase deficiency. Cell Mol Biol Lett. 2022, 27, 104. [Google Scholar]
- Xu H, Liu P, Yan Y, Fang K, Liang D, Hou X, Zhang X, Wu S, Ma J, Wang R, Li T, Piao H, Meng S. FKBP9 promotes the malignant behavior of glioblastoma cells and confers resistance to endoplasmic reticulum stress inducers. J Exp Clin Cancer Res. 2020, 39, 44. [Google Scholar] [CrossRef]
- Jin Z, Liu B, Lin B, Yang R, Wu C, Xue W, Zou X, Qian J. The Novel lncRNA RP9P Promotes Colorectal Cancer Progression by Modulating miR-133a-3p/FOXQ1 Axis. Front Oncol. 2022, 12, 843064. [Google Scholar] [CrossRef] [PubMed]
- Nodin B, Fridberg M, Uhlén M, Jirström K. Discovery of dachshund 2 protein as a novel biomarker of poor prognosis in epithelial ovarian cancer. J Ovarian Res. 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Shen-Gunther J, Xia Q, Cai H, Wang Y. HPV DeepSeq: An Ultra-Fast Method of NGS Data Analysis and Visualization Using Automated Workflows and a Customized Papillomavirus Database in CLC Genomics Workbench. Pathogens. 2021, 10, 1026. [Google Scholar] [CrossRef]
- HBVdb: The Hepatitis B Virus database. Available online: https://hbvdb.lyon.inserm.fr/HBVdb/HBVdbIndex (accessed on 25 December 2023).
- BV-BRC Viral Sub-species Classification Workshop. Available online: https://www.bv-brc.org/docs/news/2024/2024-04-08-bv-brc-workshop-subspecies.html (accessed on 10 April 2024).
- Hu Z, Zhu D, Wang W, Li W, Jia W, Zeng X, Ding W, Yu L, Wang X, Wang L, Shen H, Zhang C, Liu H, Liu X, Zhao Y, Fang X, Li S, Chen W, Tang T, Fu A, Wang Z, Chen G, Gao Q, Li S, Xi L, Wang C, Liao S, Ma X, Wu P, Li K, Wang S, Zhou J, Wang J, Xu X, Wang H, Ma D. Genome-wide profiling of HPV integration in cervical cancer identifies clustered genomic hot spots and a potential microhomology-mediated integration mechanism. Nat Genet. 2015, 47, 158–163. [Google Scholar] [CrossRef]
- Akagi K, Li J, Broutian TR, Padilla-Nash H, Xiao W, Jiang B, Rocco JW, Teknos TN, Kumar B, Wangsa D, He D, Ried T, Symer DE, Gillison ML. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014, 24, 185–199. [Google Scholar] [CrossRef]
- Shi Z, Lopez J, Kalliney W, Sutton B, Simpson J, Maggert K, Liu S, Wan J, Stack MS. Development and evaluation of ActSeq: A targeted next-generation sequencing panel for clinical oncology use. PLoS One. 2022, 17, e0266914. [Google Scholar]
- Olthof NC, Huebbers CU, Kolligs J, Henfling M, Ramaekers FC, Cornet I, van Lent-Albrechts JA, Stegmann AP, Silling S, Wieland U, Carey TE, Walline HM, Gollin SM, Hoffmann TK, de Winter J, Kremer B, Klussmann JP, Speel EJ. Viral load, gene expression and mapping of viral integration sites in HPV16-associated HNSCC cell lines. Int J Cancer. 2015, 136, E207–E218. [Google Scholar]
- Ku JL, Park JG. Biology of SNU cell lines. Cancer Res Treat. 2005, 37, 1–19. [Google Scholar] [CrossRef]
- Sunshine S, Kirchner R, Amr SS, Mansur L, Shakhbatyan R, Kim M, Bosque A, Siliciano RF, Planelles V, Hofmann O, Ho Sui S, Li JZ. HIV Integration Site Analysis of Cellular Models of HIV Latency with a Probe-Enriched Next-Generation Sequencing Assay. J Virol. 2016, 90, 4511–4519. [Google Scholar] [CrossRef]
- VIS Atlas: A Database of Virus Integration Sites in Human Genome from NGS Data to Explore Integration Patterns. Genomics Proteomics Bioinformatics. 2023, 21, 300–310. [CrossRef]
- VIS Atlas. Available online: http://www.vis-atlas.tech/ (accessed on 1 April 2024).
- Tang D, Li B, Xu T, Hu R, Tan D, Song X, Jia P, Zhao Z. VISDB: a manually curated database of viral integration sites in the human genome. Nucleic Acids Res. 2020, 48, D633–D641. [Google Scholar] [CrossRef] [PubMed]
- VISDB Viral Integration Site DataBase. Available online: https://bioinfo.uth.edu/VISDB/index.php/homepage (accessed on 1 April 2024).
- Vučković N, Hoppe-Seyler K, Riemer AB. Characterization of DoTc2 4510-Identifying HPV16 Presence in a Cervical Carcinoma Cell Line Previously Considered to Be HPV-Negative. Cancers (Basel). 2023, 15, 3810.
- Vogt, PK. Retroviral oncogenes: a historical primer. Nat Rev Cancer. 2012, 12, 639–648. [Google Scholar] [CrossRef]
- Law EW, Settell ML, Kurani SS, Eckert EC, Liu MC, Greenberg-Worisek AJ. Liquid Biopsy: Emergence of an Alternative Cancer Detection Method. Clin Transl Sci. 2020, 13, 845–847. [Google Scholar] [CrossRef]
- Sastre-Garau X, Diop M, Martin F, Dolivet G, Marchal F, Charra-Brunaud C, Peiffert D, Leufflen L, Dembélé B, Demange J, Tosti P, Thomas J, Leroux A, Merlin JL, Diop-Ndiaye H, Costa JM, Salleron J, Harlé A. A NGS-based Blood Test For the Diagnosis of Invasive HPV-associated Carcinomas with Extensive Viral Genomic Characterization. Clin Cancer Res. 2021, 27, 5307–5316. [Google Scholar] [CrossRef] [PubMed]
- Li CL, Ho MC, Lin YY, Tzeng ST, Chen YJ, Pai HY, Wang YC, Chen CL, Lee YH, Chen DS, Yeh SH, Chen PJ. Cell-Free Virus-Host Chimera DNA From Hepatitis B Virus Integration Sites as a Circulating Biomarker of Hepatocellular Cancer. Hepatology. 2020, 72, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi J, Olivo A, Laeyendecker O, Forberg K, Ndembi N, Mbanya D, Kaptue L, Quinn TC, Cloherty GA, Rodgers MA, Berg MG. Universal Target Capture of HIV Sequences From NGS Libraries. Front Microbiol. 2018, 9, 2150. [Google Scholar]
- How to BLAST Guide National Center for Biotechnology Information. Available online: https://ftp.ncbi.nlm.nih.gov/pub/factsheets/HowTo_BLASTGuide.pdf (accessed on 6 June 2022).
 ); (B) WGA workflow steps (1-4) with user-defined parameter settings for WGA and annotation e.g., HBV RefSeq (*) genome; (C) Create Average Nucleotide Identity Comparison workflow inputs the WGA file for quantification of the similarity between genomes, and outputs a pairwise comparison matrix; (D) VHC workflow steps (1-11) with user-defined parameter settings for Taxonomic Profiling (*) e.g., HBV reference index and Host genome index; (E) VIS workflow steps (1-4) with selected HBV(*) and Host reference genome databases and user-defined search parameters entered for this study.
); (B) WGA workflow steps (1-4) with user-defined parameter settings for WGA and annotation e.g., HBV RefSeq (*) genome; (C) Create Average Nucleotide Identity Comparison workflow inputs the WGA file for quantification of the similarity between genomes, and outputs a pairwise comparison matrix; (D) VHC workflow steps (1-11) with user-defined parameter settings for Taxonomic Profiling (*) e.g., HBV reference index and Host genome index; (E) VIS workflow steps (1-4) with selected HBV(*) and Host reference genome databases and user-defined search parameters entered for this study.
 ); (B) WGA workflow steps (1-4) with user-defined parameter settings for WGA and annotation e.g., HBV RefSeq (*) genome; (C) Create Average Nucleotide Identity Comparison workflow inputs the WGA file for quantification of the similarity between genomes, and outputs a pairwise comparison matrix; (D) VHC workflow steps (1-11) with user-defined parameter settings for Taxonomic Profiling (*) e.g., HBV reference index and Host genome index; (E) VIS workflow steps (1-4) with selected HBV(*) and Host reference genome databases and user-defined search parameters entered for this study.
); (B) WGA workflow steps (1-4) with user-defined parameter settings for WGA and annotation e.g., HBV RefSeq (*) genome; (C) Create Average Nucleotide Identity Comparison workflow inputs the WGA file for quantification of the similarity between genomes, and outputs a pairwise comparison matrix; (D) VHC workflow steps (1-11) with user-defined parameter settings for Taxonomic Profiling (*) e.g., HBV reference index and Host genome index; (E) VIS workflow steps (1-4) with selected HBV(*) and Host reference genome databases and user-defined search parameters entered for this study.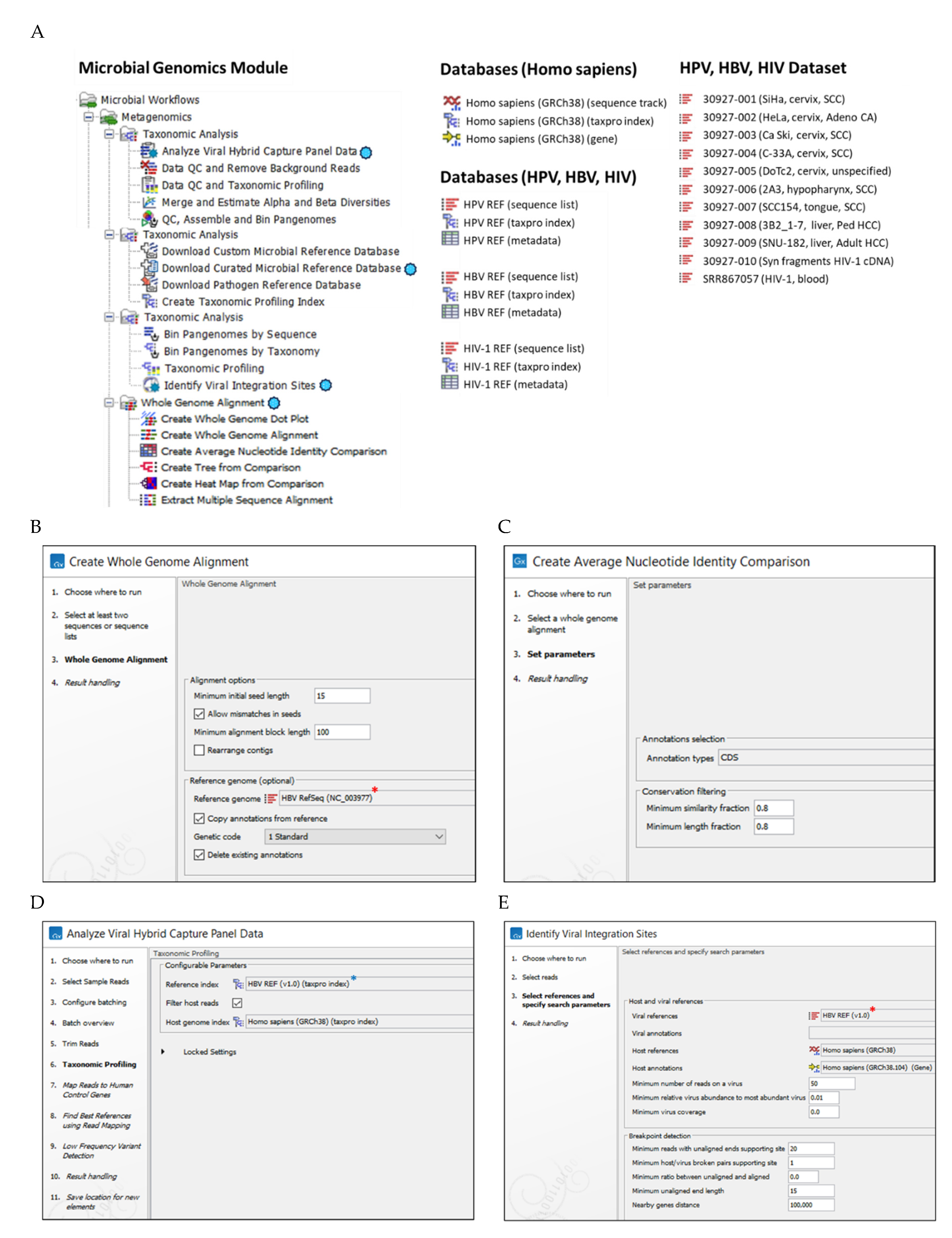
| Sample No. | Cell line or construct1 | Age | Genome ancestry | Virus-genotype | Tumor site | Histology |
|---|---|---|---|---|---|---|
| S01 | SiHa | 55 | NE. Asian (Japanese) | HPV-16 | Cervix | SCCA |
| S02 | HeLa | 30 | African (American) | HPV-18 | Cervix | AdenoCA |
| S03 | CaSki | 40 | N European | HPV-16 | Cervix (met)5 | SCCA |
| S04 | C-33A | 66 | N European | P53+, pRB+ | Cervix | SCCA |
| S05 | DoTc2 | NS | N European | HPV-16 | Cervix | NS |
| S06 | 2A3 | 56 | SE Asian (Indian) | HPV-164 | Hypopharynx | SCCA |
| S07 | SCC154 | 54 | Caucasian | HPV-164 | Tongue | SCCA |
| S08 | 3B2.1-7 | 8 | African (American) | HBV-A24 | Liver | HCCA |
| S09 | SNU-182 | 24 | NE Asian (Korean) | HBV-C4 | Liver | HCCA |
| S10 | Syn HIV-12 | NA | NA | HIV-1 M4 | NA | NA |
| S11 | ACH-23 | 3 | Caucasian | HIV-1 M4 | Blood | T-cell |
| VIS | Cell line | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cervix | Oropharynx | Liver | T-cell | ||||||
| SiHa | HeLa | CaSki | DoTc2 | 2A3 | SCC154 | 3B2 | SNU-182 | ACH-2 | |
| 1 | 13q22.11,2,3 LINC003934 KLF125 |
8q24.212,3 PCAT14 CASC194 MYC5 |
2p11.2 LINC01830 PRR30 |
2p22.3 LINC004864 |
12q24.331 FBRSL1 | 6p12.2 7SK4 |
4q13.36 | 1q25.11 TNR |
7p14.3 NT5C3A FKBP95 RP95 |
| 2 | 21p11.22 MIR3648-15 RNA5-8SNx |
21p11.22 MIR3648-15 RNA5-8SNx |
3q236 |
3p25.11 ANKRD284 RN7SL4P4 |
16p13.3 METTL26 |
8q24.31,2 HGH14 |
13q31.35 | 2q34 UNC804 |
|
| 3 | 6p21.16 |
4p15.311 IGFBP75 |
20q11.231 RPN24 |
14q21.3 RN7SL24 |
16q11.26 | 4q31.35 | |||
| 4 | 10p146 | 6p21.32 ZBTB22 |
21p11.22 MIR3648-15 RNA5-8SNx |
21p11.22 MIR3648-15 RNA5-8SNx |
21p11.22 MIR3648-15 RNA5-8SNx |
8q24.131,2 HAS2-AS14 |
|||
| 5 | 11p15.46 | 7q21.3 GNGT14 |
22q11.22 PPIL24 |
21q21.17 | Yq126 | 17p11.16 | |||
| 6 | 14q21.3 MDGA24 |
8p11.21 PLAT4 HGH14 |
21p11.22 MIR3648-15 RNA5-8SNx |
||||||
| 7 | 19q13.421 BRSK14 |
9p23 PTPRD4 RN7SL5P RMRP4 |
|||||||
| 8 | 20p11.16 | 10q26.111 TUBGCP24 RGS104 |
|||||||
| 9 | Xq27.31, 6 | 14q21.3 RPPH14 RN7SL24 RPS294 RN7SL14 |
|||||||
| 10 | 16p13.3 METTL26 |
||||||||
| 11 | 21p11.22 MIR3648-15 RNA5-8SNx |
||||||||
| 12 | 22q11.22 PPIL24 |
||||||||
| 13 | Xq21.2 DACH24 |
||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





