1. Introduction
Nitrogen fertilizer is applied to increase crop yield but may pollute the nearby environment when it is not managed well [
1,
2]. A high accumulation of soil mineral N after N fertilization, especially at the early stage of crop growth, has an increased risk of loss via leaching or gas emissions, eventually leading to water eutrophication, climate change, and losses of biodiversity [
3,
4]. Therefore, designing appropriate measures to enhance fertilizer-N conservation in cropland is crucial. In recent decades, split applications have been created to meet the N requirements of crops in different growth periods while avoiding excessive mineral N accumulation in the early stages [
5,
6]. However, because of the interference of field crops, topdressing is hard to apply on large scales by machinery and must be spread by hand, which increases labor inputs. Based on technical and economic considerations, most of the small household farms in China still prefer using a single fertilization regime instead of split fertilization [
6,
7].
In addition to split fertilization, other strategies such as reduced N application, nitrification inhibitor (NI) application, and straw incorporation have also been developed to regulate N transformations, avoid mineral N accumulation in the soil at the early stage, and reduce fertilizer-N losses [
8,
9,
10]. Based on data from several field, lysimeter, and monitoring studies, Ju et al. found that knowledge-based N reduction in intensive double-cropping systems in China can potentially maintain crop yields and decrease N loss to the environment [
11]. Nitrification inhibitor application can also reduce nitrate accumulation and its leaching and denitrification losses. Several meta-analyses have suggested that NI amendment enhanced fertilizer-N use efficiency (NUE) by 11%–27% in various cropping systems [
5,
12,
13,
14] while reducing N
2O emission by 34%–48% [
15,
16,
17]. Straw incorporation can enhance the transformation from fertilizer-N to soil organic N through microbial-mediated immobilization in the early stage of crop growth, then release it to benefit crop growth at later stages [
18]. The vital role of the exogenous organic matter input on soil N cycling has been widely confirmed [
19,
20,
21]. Through modeling, Wang et al. estimated that corn yield in Northeast China can increase by 5.8% under the optimal straw return rate (48% of the straw at harvesting) [
22].
Northeast China is a vital food production base due to its high soil fertility [
23]. However, intensive agrarian management with high synthetic fertilizer input and low crop residue return has led to the degradation of soil quality [
24]. Since the 2010s, the Ministry of Agriculture and Rural Affairs of China has implemented the “double reduction” (reducing fertilizer and pesticide applications) and “black land protection and utilization” strategies to improve fertilizer-NUE and enhance the sustainability of soil nutrient supply. These strategies include reduced N application, NI, and straw incorporation. However, how these strategies change the fertilizer-N fates during the crop growing season is still unclear, and their effectiveness on grain yield, crop N uptake, NUE, fertilizer-N retention by soil, and losses remain to be evaluated in this region.
Here, we conducted a field 15N tracer study in Northeast China to track the fates of fertilizer-N in soil and plant pools and the effects of reduced N application, NI, and straw incorporation during the first growing season. We hypothesized that these practices would improve the fertilizer-N transformation to more favorable pathways, eventually benefiting fertilizer-N use and minimizing fertilizer-N loss.
2. Materials and Methods
2.1. Study Site
The trial was conducted on a maize field in Gongzhuling, Jilin Province (43°30’N, 124°48’E), which lies in the Songnen Plain, a critical corn production base in China. The site has a semi-humid continental monsoon climate and has been cultivated for decades. The average annual precipitation and temperature for the study site during 2011–2020 were 666 mm and 6.8 °C, respectively (
http://tjj.jl.gov.cn/tjsj/tjnj/). The soil is named black soil and is classified as a Mollisol according to the US soil taxonomy. Two days before this trial, we collected soil samples (0–20 cm) and measured soil characteristics: organic C, 19.1 g kg
−1; total N, 1.52 g kg
−1; NH
4+-N, 14.5 mg kg
−1; NO
3−-N, 29.6 mg kg
−1; pH, 6.19.
2.2. Experimental Design
Three blocks were arranged on the field in 2016, each containing six plots with an area of 25 m2 each, which were assigned six treatments: 1) Control, no N fertilization; 2) 100%N, applying 200 kg urea-N ha−1; 3) 100%N+S, applying 200 kg urea-N ha−1 and 2400 kg dry straw ha−1; 4) 80%N, applying 160 kg urea-N ha−1; 5) 80%N+NI, applying 160 kg urea-N ha−1 and nitrification inhibitor (Nitrapyrin, C6H3Cl4N, 1.6 kg ha−1); 6) 80%N+NI+S, applying urea 160 kg N ha−1, nitrification inhibitor (Nitrapyrin, C6H3Cl4N, 1.6 kg ha−1), and straw. The amount of straw added was 2400 kg dry straw ha−1, approximately 25% of the aboveground biomass (excluding grain) at the physiological maturity stage of maize.
We arranged the trial as ridge-furrow cultivation and strip fertilization, similar to the management practice used by local farmers. In detail, after plowing and ridging, all fertilizers, nitrification inhibitors, and maize straw were placed in each ridge and then covered with soils from the furrows. The abundance of applied 15N-urea was 1.19%, corresponding to δ15N (‰) of 2276. In addition, all plots received 90 kg P2O5 ha−1 and 90 kg K2O ha−1. The maize straw was applied after being crushed into pieces smaller than 3 cm. After fertilization, a hand-powered hole-drilling machine was used for sowing on the ridge.
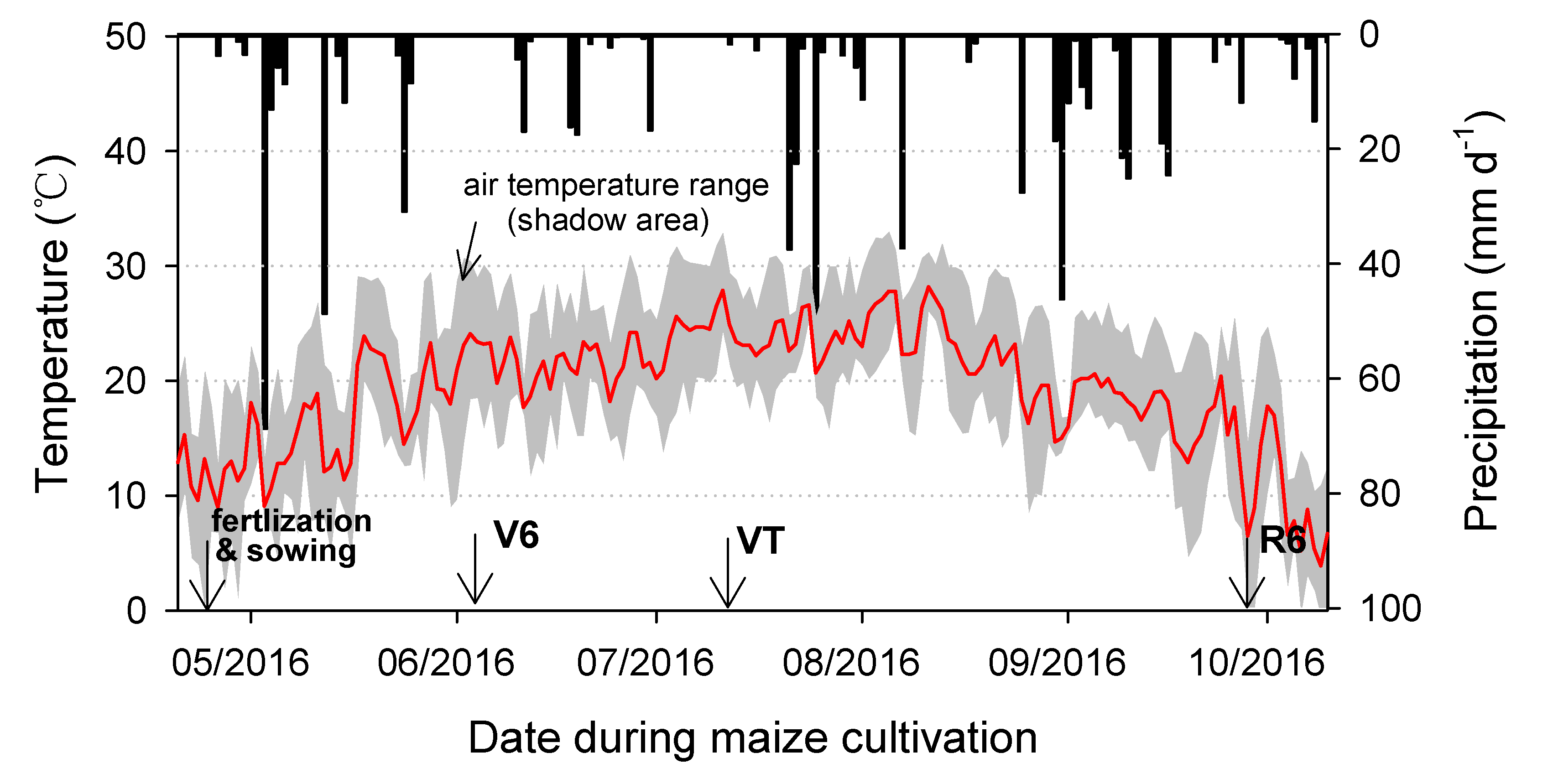
The maize variety in this study was Xianyu 335, and the planting density was 70000 plants per hectare. The row and plant spacings were 60 and 20 cm, respectively. Other agronomic practices during the maize growth period followed local farmers’ procedures. Irrigation and fertilization were no longer carried out during the growth period. A nearby meteorological station recorded daily mean air temperature and precipitation during the trial period (
Figure 1).
2.3. Sampling
Soil and plant samples were collected three times during the growth period (
Figure 1), on days 42 (V6, six-leaf stage), 82 (VT, tasseling stage), and 152 (R6, physiological maturity). Details of the sampling process for soil and plant samples were similar to those of Quan et al. [
25]. In detail, 0–40 cm soils were sampled in 10 cm intervals. Because fertilizer was unevenly distributed in the soil, a stainless-steel sampling frame was used to sample soils, with a length, width, and height of 60 cm, 25 cm, and 20 cm, respectively. When sampling, the sampling frame was first pressed into the field using a hammer (covering both ridge and furrow parts with a plant in the center), then all 0–10 cm and 10–20 cm soils were dug out separately. After mixing the soil thoroughly by hand and taking a portion as soil samples, the 20–30 and 30–40 cm layers were directly sampled by a soil auger within the frame. The soil bulk density was determined simultaneously with each sampling.
Three plants in the central area of each plot were sampled. Plant samples at different growth stages were divided into one to five organs (cob, root, stem, leaf, and grain) and weighed separately. Fresh samples of various organs were cut into small pieces of <3 cm with a chopper and mixed well for later processes. Parts of the samples were bagged and brought to the laboratory and then dried in an oven (70 °C) to determine their water content and calculate the dry weight of the plants. Only the visible parts in the 0–20 cm layer were collected for maize roots. Dried plant samples were stored in individually sealed bags to determine N concentration and 15N abundance.
2.4. Chemical and Isotope Analysis
Fresh soil samples were taken from the field to the laboratory and passed through a 2-mm sieve. Sub-samples of fresh soil were extracted with 2M potassium chloride (soil:water=1:4) and shaken for one hour, then filtered through filter papers. The mineral nitrogen (NH4+-N, NO3−-N) concentrations in the extracts were measured by a continuous chemical analyzer (Smart Chem 200, Italy).
Soil and plant samples were further pulverized, finely ground, and then analyzed for total N (TN) concentration and 15N abundance using an elemental analyzer (Elementar Vario MICRO cube, Germany) coupled with a stable isotope ratio mass spectrometer (Isoprime 100, UK). During the measurement, four standard samples were used (acetanilide, L-histidine, glycine, and D-glutamic acid) to correct the drift and blank.
2.5. Calculation
15N atomic abundance (A
x,
15N as a percentage of the total atomic N) and
15N enrichment relative to the standard (
) in soil and plant samples were calculated as follows:
where R
x is the “
15N and
14N atomic ratio” in the tested sample, which can be measured directly with the stable isotope mass spectrometer. The isotope values of dinitrogen in the atmosphere were assumed to be constant and considered the standard (
= 0.003676,
= 0.3663%,
= 0‰).
The fate of fertilizer-N in the soil and plant maize pools was calculated as follows [
25]:
where
is the proportion of crop N derived from in-season applied fertilizer and
is N concentration (%).
,
, and
represent
15N atomic abundance of the related N pools, background (in the control), and tracer (
15N-labeled urea), respectively.
and
represent recovery (%) of
15N-labeled urea in the related soil and plant N pools.
is the rate of
15N-urea applied (200 kg N ha
−1).
V is the volume of the soil (1000 m
3 ha
−1 in every 10-cm layer), and
DW is the dry weight of related organs (kg ha
−1).
Crop NUE at the R6 stage was calculated as the total
15N recovery by the maize plant:
where NUE
15N is the sum of all crop
.
2.6. Statistical Analysis
Statistical analysis in this study was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance was used to test differences among the six treatments (n=3 for each treatment), and multiple comparisons were performed using the least significant difference (LSD) test with a 95% confidence interval. In addition, the linear mixed effect model was used to test the effects of fertilization treatment, sampling time, soil layer or plant organs, and their interactions on soil and plant N index. Figures were made with SigmaPlot 14.0 software.
3. Results
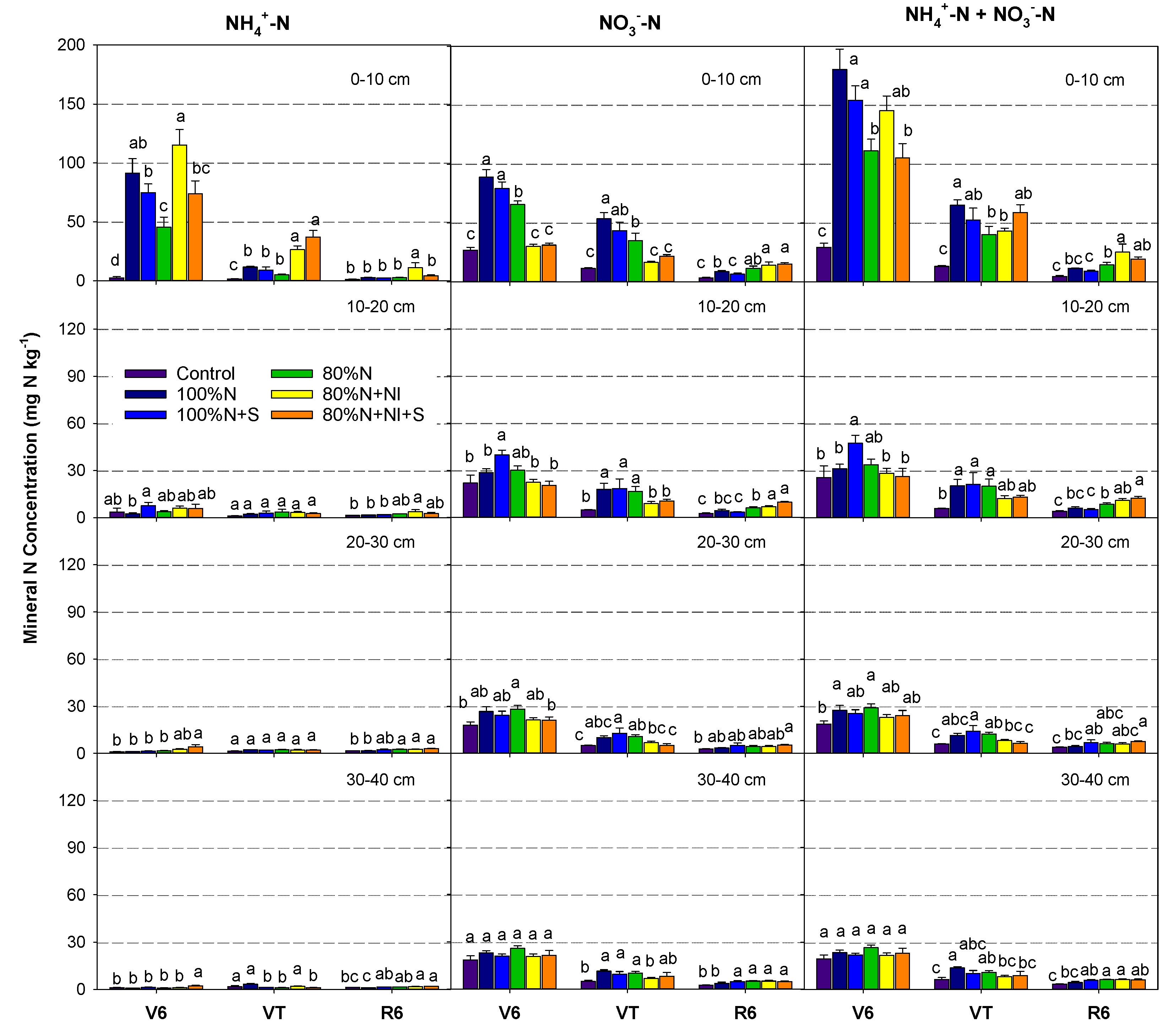
3.1. Soil Mineral N Dynamics
Soil NH
4+ and NO
3- concentrations decreased along with the increase of soil depth and growth stage, and the differences among treatments were mainly displayed in the 0–10 cm soil layer (
Figure 2). Soil mineral N (NH
4+ + NO
3-) concentrations in the 0–10 cm were < 30 mg N kg
-1 during the whole growth period in the Control and N fertilization significantly enhanced their concentrations. Soil mineral N concentrations in the 100%N treatment were 180, 65, and 12 mg N kg
-1 at the V6, VT, and R6 stages, respectively. Compared with the 100%N treatment, mineral N concentrations in the 100%N+S, 80%N, 80%N+NI and 80%N+NI+S treatments were reduced by 15%–42% in 0–10 cm soil layer at the V6 stage.
Compared with the 80%N treatment, soil NH
4+ concentration in 0–10 cm in 80%N+NI treatment was significantly enhanced by 150%, 410% and 290% at V6, VT and R6 stages, respectively (P<0.05), while soil NO
3- concentration was significantly reduced by 54% and 52% at the V6 and VT stages (P<0.05) (
Figure 2). Compared with the 80%N+NI treatment, soil NH
4+-N concentration in 0–10 cm in 80%N+NI+S treatment was significantly reduced by 36% and 61% at the V6 and R6 stages (P<0.05).
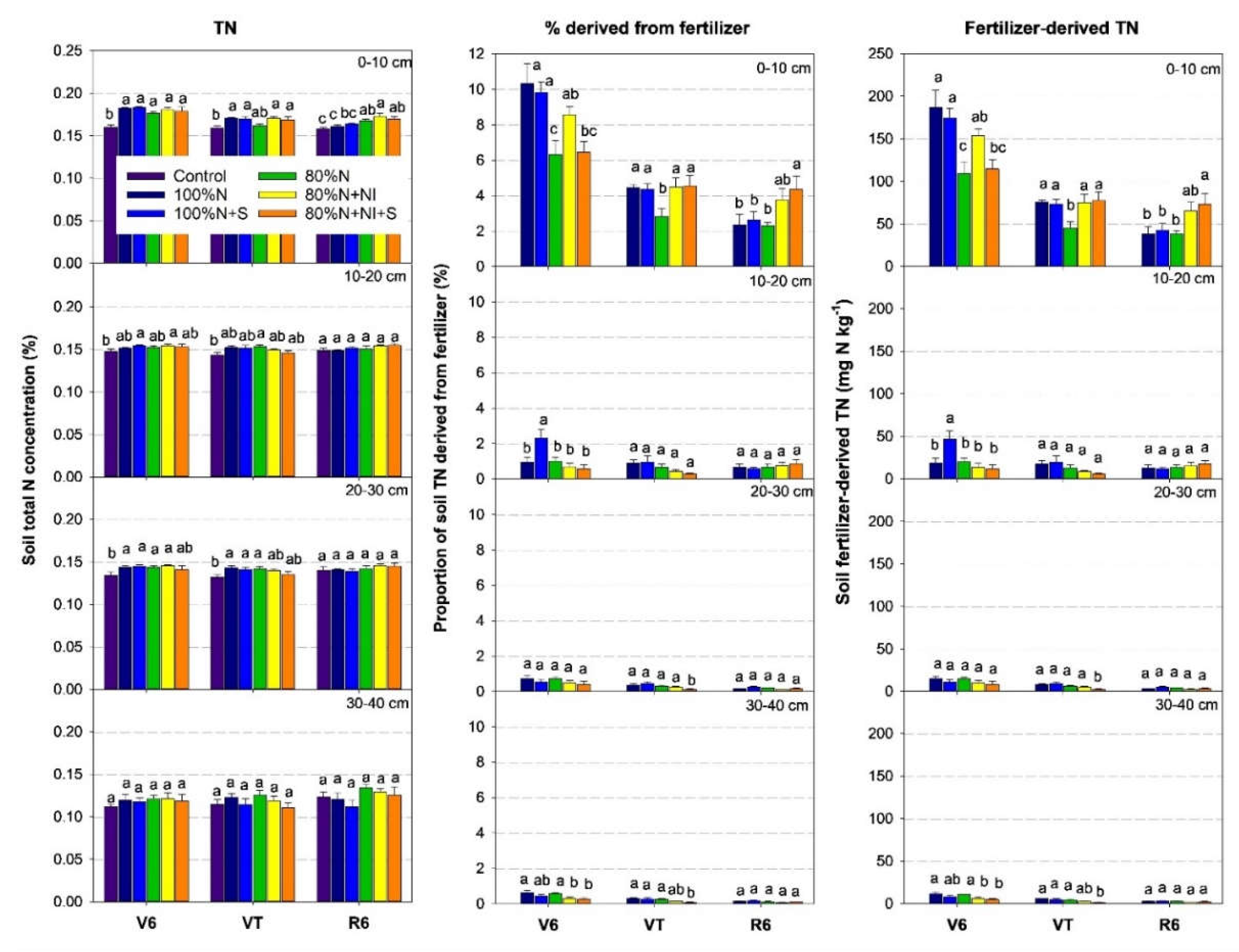
3.2. Soil Total and Fertilizer-Derived N Concentrations
Fertilizer-N mainly remained in the 0–10 cm soil and was less distributed in the 10–40 cm soil. Compared with the control, soil TN concentrations in the 0–10 cm layer were enhanced by 2%–18% in the five N-fertilized treatments (
Figure 3). As the growing season progressed, the proportion of TN derived from fertilizer in the 0–10 cm soil layer decreased significantly from 10% (V6) to 2% (R6) for the 100%N treatment and from 6% (V6) to 2% (R6) for the 80%N treatment. Compared with the 80%N treatment, the 80%N+NI+S treatment increased fertilizer-derived TN by 71% and 90% in the 0–10 cm soil at the VT and R6 stages (P < 0.05), and reduced fertilizer-derived TN by 63% and 68% in 20–30 cm and 30–40 cm soil, respectively, at the VT stage (P < 0.05).
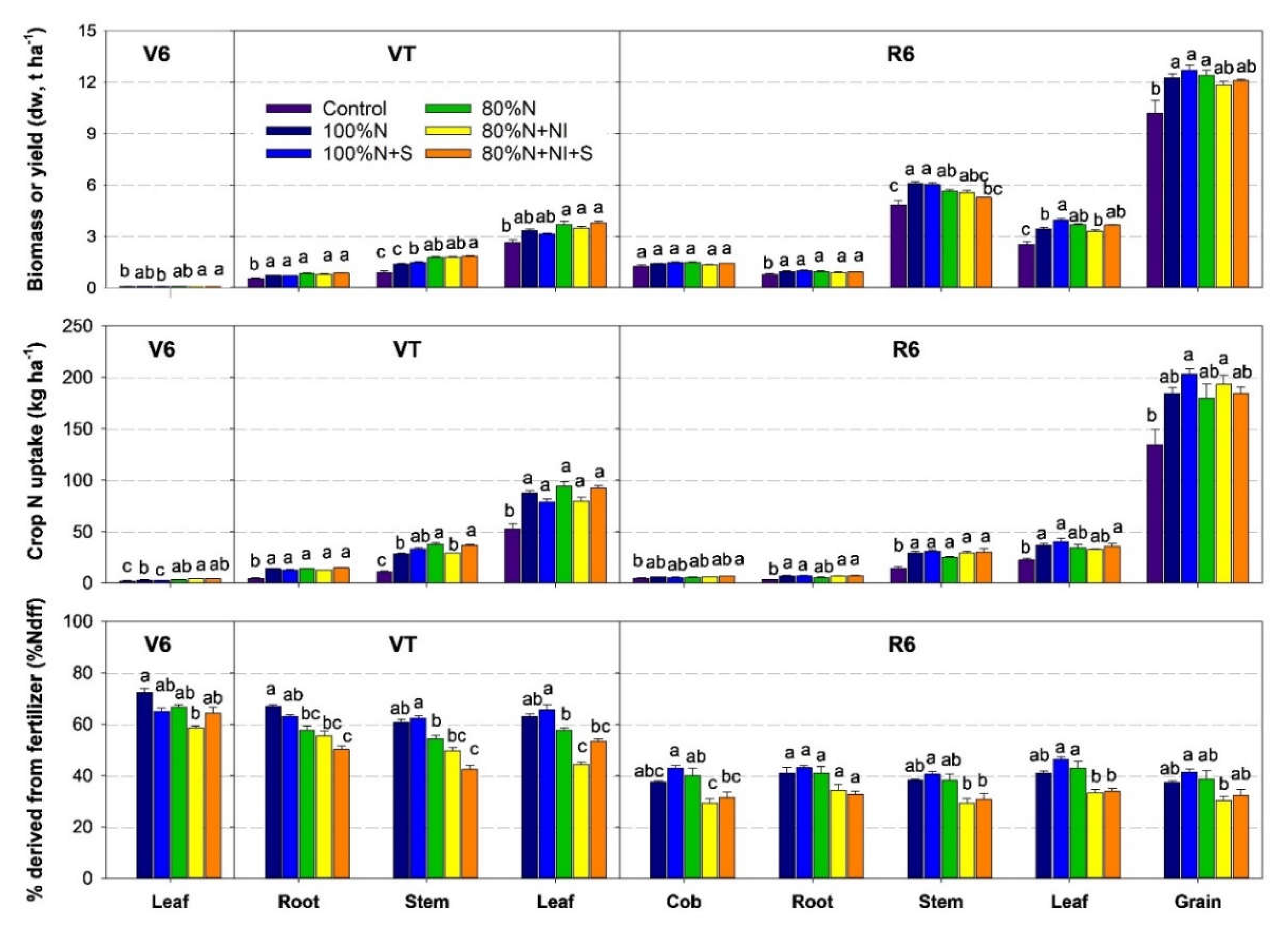
3.3. Maize Biomass, Yield, and Fertilizer-Derived N Uptake
Grain yield and crop N uptake in the control were 10.2 t ha
−1 and 180 kg N ha
−1 at harvesting (R6 stage), respectively. N-fertilized treatments had significantly higher grain yields (16–25%), plant N concentration (25%–48%) and crop N uptake (39%–60%) (
Figure 4,
Figure S1). However, the differences among the five N-fertilized treatments were not statistically significant. Across the five N-fertilized treatments, 72% of the
15N taken up by crops was distributed to grain, with the remaining 28% distributed to other organs (2%, 3%, 10%, and 13% for cob, root, stem, and leaf, respectively).
The proportion of crop N derived from fertilizer (%Ndff), calculated by plant
15Nδ, varied widely among growth stages and treatments, but little among organs (
Table S1,
Figure S2). The %Ndff for the whole maize plant decreased from 59%–72% at the V6 stage to 47%–64% at the VT stage, and finally to 31%–42% at the R6 stage. Nitrification inhibitor application (80%N+NI and 80%NI+NI+S) significantly reduced the %Ndff by 4%–12%, 12%–15%, and 13%–18% at the V6, VT, and R6 stages, respectively, compared with the 80%N treatment. The effect of straw incorporation was not significant.
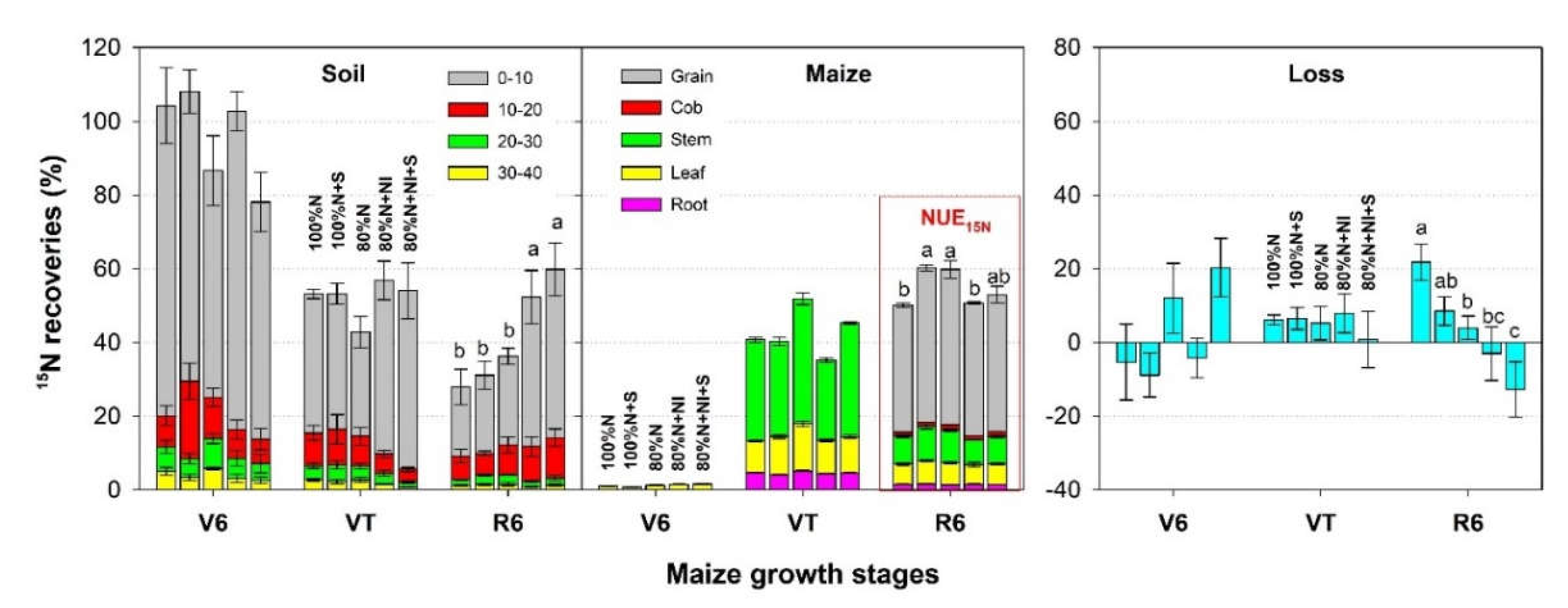
3.4. 15N Recoveries in the Soil-Maize System
As maize growth progressed, soil residual
15N concentration gradually decreased and maize uptake of
15N gradually increased (
Figure 5). At harvesting (R6 stage), 28%–59% was retained in the soil, and 50%–60% was taken up by maize. Most soil
15N was recovered from the 0–10 cm layer (19%–46%). Compared with the 100%N treatment, the 100%N+S treatment increased
15N retention in the 0–10 cm soil layer by 12% (P > 0.05) and significantly promoted plant
15N recovery (NUE
15N) by 20% at the R6 stage (P < 0.05). NI co-application enhanced
15N retention in the 0–10 cm soil layer by 98% (80%N+NI, P > 0.05) and 108% (80%N+NI+S, P < 0.05) at the R6 stage (
Figure 3), and reduced NUE
15N by 15% (P < 0.05) and 12% (P > 0.05), compared with the 80%N treatment.
Because of error propagation, the proportion of fertilizer-N loss calculated by the difference method varied largely and had negative values (
Figure 5). Reduced N application, nitrification inhibitor co-application and straw incorporation were all beneficial to mitigate the proportion of fertilizer-N loss (
15N-loss%). Compared with the 100%N treatment, the 80%N treatment significantly reduced
15N-loss% from 22% to 4% (P < 0.05), while the 80%N+NI+S treatment further reduced fertilizer-N loss to –13% at the R6 stage (P < 0.05).
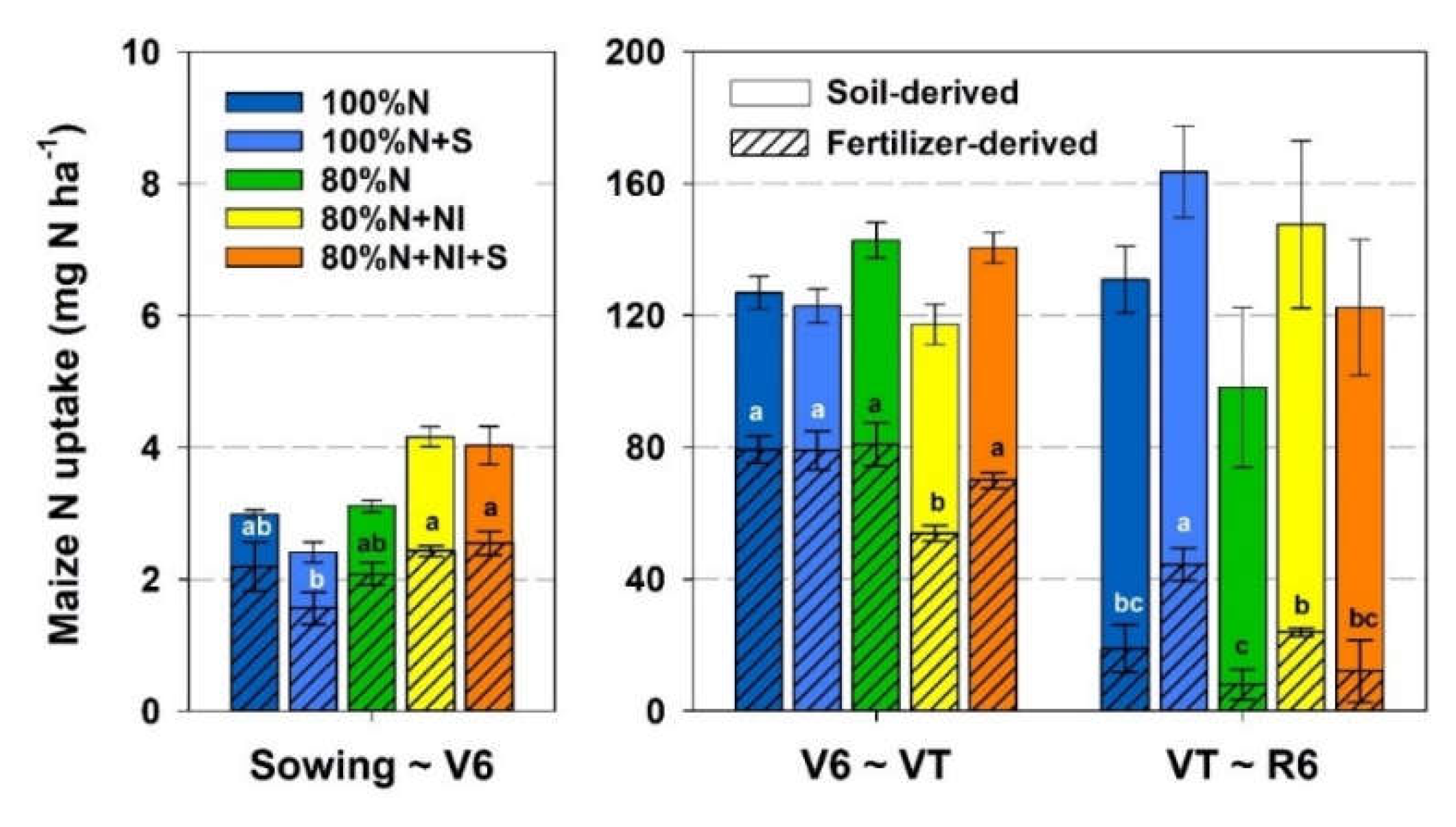
The N absorbed by crops was mainly derived from the applied fertilizer at the V6 and VT stages, and then shifted to being derived mainly from the soil at the R6 stage (
Figure 6). At the V6 stage, NI application treatments had much higher fertilizer-derived N uptake than the 100%N+S treatment (2.4–2.5 kg N ha
−1 vs. 1.5 kg N ha
−1, P < 0.05). However, from the V6 to VT stage, the 80%N+NI treatment had much lower fertilizer-derived N uptake than other treatments. From the VT to R6 stage, compared with the 100%N and 80%N treatments, the 100%N+S and 80%N+NI treatments significantly promoted fertilizer-derived N uptake (P < 0.05).
4. Discussion
This study compared the effect of reduced N application, NI, and straw incorporation on fertilizer-N fates. Reducing N application by 20% improved NUE15N and reduced 15N-loss% remarkably, while maintaining crop N uptake. In contrast, NI co-application significantly increased fertilizer-N turnover and retention in soil, resulting in decreases in NUE15N and 15N-loss%. Compared with NI, straw incorporation decreased the magnitude of fertilizer-N retention and promoted NUE15N.
4.1. Effects of Reduced N Application on Fertilizer-N Fates
Compared with the 100%N treatment, the control still achieved 83% of the grain yield and 73% of the crop N uptake (
Figure 4), implying that the soil had a high nutrient-supply capacity. Generally, high-fertility soils with high nutrient-supply capacity can satisfy most of the crop demand for nutrients in one or several growing seasons, even with no exogenous nutrient input. Consequently, in this study, the differences in grain yield and N uptake between the 100%N and 80%N treatments were small and insignificant owing to the buffering effect of soil fertility (including the legacy fertility of previously applied fertilizer). In a meta-analysis based on
15N tracer studies in maize cropping systems worldwide, Quan et al. found that reducing N rates from approximately 200 kg N ha
−1 to 140 kg N ha
−1 maintained maize N uptake in the North China Plain (NCP) [
26]; however, this practice failed in the American Corn Belt. Cropland soils in the NCP were reported to have accumulated a large amount of mineral N due to long-term over-fertilization and high atmospheric N deposition, which may be related to the insensitivity of fertilizer-N reduction on crop N uptake and grain yield [
26,
27]. Although the treatment of reducing the fertilizer-N rate improved NUE
15N remarkably because of the “law of diminishing return” (the efficiency of crop N uptake instinctively decreases with the increase of N input), its potential effects on grain yield and crop N uptake must be further assessed using long-term trials along with the soil fertility [
28,
29].
In this study, we found soil N imbalance, even in the 100%N treatment. Crop N uptake was approximately 250 kg N ha
−1 for the N fertilization treatments, much higher than the application rate of urea N (200 kg N ha
−1) by local farmers. Even if the gap can be partly replenished by atmospheric N deposition and non-symbiotic N fixation, it is unlikely to balance the input and output of soil N stock. Straw incorporation may relieve the imbalance because 100% straw returning can provide almost one-third of crop N uptake (approximately 85 kg N ha
−1) (
Figure 4). However, in the studied region, most of the straw is removed from the field to feed livestock or for burning [
30]. Another critical evidence supporting soil N imbalance is the Ndfs (crop N derived from the soil), which ranged from 154 to 186 kg N ha
−1 among the five N fertilization treatments, much higher than the corresponding fertilizer-N retention in 0–40 cm soil (only 56–96 kg N ha
−1) (
Figure 2 and
Figure 5). Soil N supply capacity is primarily sustained by human-controllable external N input, and long-term soil N deficit weakens soil N fertility [
29,
31]. Therefore, reducing fertilizer-N application can only be sustainable when soil N balance is considered and accompanied by other N-replenishing measures to prevent soil N deficit.
4.2. Effects of Nitrification Inhibitor Co-Application on Fertilizer-N Fates
Our study showed that blending fertilizers with NI suppressed fertilizer-derived N uptake (
Figure 6), which might be related to the changes in soil N availability, as NI application changed the relative proportions of NH
4+-N and NO
3−-N [
32,
33]. The inhibitory effect of NI application on ammonium oxidation increased the residence time of ammonium in the soil [
14,
34]. Consequently, the fertilizer-N supply was stabilized by biotic NH
4+-N immobilization (microbial assimilation) and abiotic NH
4+-N fixation (2:1 clay minerals), which could be responsible for the increased
15N retention and decreased
15N-nitrate. Therefore, maize roots absorbed less fertilizer-N because maize is a nitrate-preferring crop [
26,
33,
35]. This finding was roughly in line with other
15N studies using straw-biochar as the exogenous additive to modify soil in wheat and cotton-barley cropping systems, where increased fertilizer-N retention in the soil and decreased fertilizer-N uptake by crops were both observed [
36,
37]. The effect of NI on crop N uptake varied among different soil conditions [
38]. In a meta-analysis by Quan et al., enhanced-efficiency N fertilizers (including urease inhibitor or NI application and slow-release fertilizers) were also found effective in promoting fertilizer-N retention and reducing
15N-loss%, %Ndff, and NUE
15N, but these effects were not significant [
26]. As upland crops prefer to absorb nitrate as N nutrition, we speculate that the major role of NI application in upland agroecosystems may be the preservation of fertilizer-N to facilitate long-term N supply, rather than the in-season utilization by crops.
Although NI decreased NUE
15N in some studies, it may maintain crop N uptake and grain yield by changing native soil N conversion and availability, especially for alkaline soils where nitrification is reported to be much stronger than in acidic soils [
16]. In this study, we found that crop N uptake was not affected because maize absorbed more N from the native soil N pool attributed to the so-called “added N interaction” (ANI, increased N uptake derived from native soil organic N induced by NI addition) (
Figure 6). Given that part of the increased crop N uptake under N fertilization cannot be traced by
15N-tracer owing to the influence of ANI, the estimated NUE
15N might be apparent and usually lower than the actual value [
29,
36]. In non-labeling trials, the effect of NI on NUE enhancement tended to be positive [
5]. The integrated effects of NI application on soil N supply and crop N uptake still need to be tested in long-term studies by synthesizing results from both non-labeling and labeling trials.
4.3. Effects of Straw Incorporation on Fertilizer-N Fates
Straw incorporation can increase soil N availability because it accelerates the process of soil N mineralization and immobilization turnover (MIT). However, it may also decrease soil N availability because it fuels denitrifiers and increases N gaseous losses [
39,
40,
41]. In this study, straw incorporation increased fertilizer-N retention in soil, promoted fertilizer-N uptake, and reduced fertilizer-N loss, although the difference was mostly insignificant. Similar findings were also obtained by Yuan et al. and Cao et al., where straw incorporation enhanced fertilizer-derived N uptake by 15%–23% [
9,
40]. The newly immobilized N after C source addition, like a biological controlled-release fertilizer, can enhance native soil N availability, benefiting long-term crop N uptake [
41]. The effect of organic additives on the fate of fertilizer-N depends on the activity of the organic material [
42,
43]. In the presence of an activated C source such as glucose, negative ANI was usually observed because of the intense microbial immobilization, which reduced the crop N uptake derived from either fertilizer or indigenous soil [
44,
45,
46]. In contrast, in the presence of an inert C source such as maize straw, its effect on fertilizer-N transformation would be much lower, mainly because of the slow degradation of straw materials [
41,
47]
In addition to promoting the MIT and denitrification processes, straw returning also provides other essential nutrients (e.g., phosphorus, potassium) for crop growth [
25,
48]. Thus, straw returning is also considered an effective nutrient-conserving practice to increase nutrient supply capacity in the long term [
49]. Based on
15N tracer studies, Ding et al. reported that straw N is also a critical source of maize growth, contributing approximately 10% to crop N uptake in the current season [
50]. Straw incorporation has a slower effect on the soil N cycle than NI, but its overall impact on the soil environment is more prominent and needs to be tested in long-term trials. Based on published results, soil microbial activity and buffering ability under the practice of long-term straw returning can be continuously increased, resulting in improved soil fertility, weakened fertilizer dependency, and strengthened crop yield [
35,
51,
52,
53].
5. Conclusions
Understanding the dynamic of fertilizer-N fate in soil-plant systems is vital for designing appropriate N management strategies for high NUE in cultivated croplands. Through a field 15N tracer study, we quantified the fate of urea-N in a black soil-spring maize cropping system in Northeast China and the effects of reduced N application, applying NI, and straw incorporation. Our results show that reducing N application by 20% increased NUE15N, and simultaneously maintained grain yield and crop N uptake, but might face a risk of soil N imbalance. NI addition and straw incorporation are efficient strategies to stabilize fertilizer-N and maintain the short-term N supply of soil in the maize cropping system, but the effect of NI application was more significant than straw incorporation. This study was limited by the short study period, and whether those practices can eventually improve soil fertility and long-term NUE should be examined on a prolonged temporal scale.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1 Results (F values) of linear mixed effect model testing of the effects of fertilization treatment (F), sampling time (T), soil layer or plant organs (D), and their interactions on soil and plant N index. Figure S1 Carbon concentration (top), N concentration (middle) and C/N (bottom) in different organs of maize at three sampling periods during cultivation. Figure S2 13Cδ (top), and 15Nδ (bottom) in different organs of maize at three sampling periods during cultivation.
Author Contributions
Conceptualization, Z.Q. and Y.F.; methodology, Z.Q.; software, Z.Q.; validation, Z.Q., S.L. and Y.F.; formal analysis, Z.Q.; investigation, Z.Q. and S.L.; resources, Z.Q., S.L., Z.X., C.L., D.L., and Y.F.; data curation, Z.Q. and S.L.; writing—original draft preparation, Z.Q.; writing—review and editing, Z.Q., S.L., Y.W., X.Z., M.Y., Y.F.; visualization, Z.Q.; supervision, C.L., X.C., Y.F.; project administration, Z.Q. and Y.F.; funding acquisition, Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28020302), the National Key Research and Development Program of China (2023YFD1501400; 2023YFD1500802), the National Natural Science Foundation of China (42177214), the Shandong Provincial Natural Science Foundation (ZR2023YQ030), the Liaoning Vitalization Talents Program (XLYC2203058), Shenyang Science and Technology Talent Program (RC230102), and the Taishan Scholars (202211306). We also acknowledge support from the K.C. Wong Education Foundation (Y.F.) and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (Z.Q.).
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Xiaoming Fang, Meixia Gao, Chenxia Su and Linlin Song for their help with sample processing and laboratory analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reis S, Bekunda M, Howard CM, Karanja N, Winiwarter W, Yan X, Bleeker A, Sutton MA (2016) Synthesis and review: Tackling the nitrogen management challenge: from global to local scales. Environmental Research Letters 11, 120205.
- Battye W, Aneja VP, Schlesinger WH (2017) Is nitrogen the next carbon? Earth’s Future 5: 894-904.
- Erisman JW, Galloway JN, Dice NB, Sutton MA, Bleeker A, Grizzetti B, Leach AM, de Vries W (2015) Nitrogen too much of a vital resource. Science Brief WWF Netherlands, Zeist, The Netherlands.
- Yu C, Huang X, Chen H, Godfray HCJ, Wright JS, Hall JW, Gong P, Ni S, Qiao S, Huang G, Xiao Y, Zhang J, Feng Z, Ju X, Ciais P, Stenseth NC, Hessen DO, Sun Z, Yu L, Cai W, Fu H, Huang X, Zhang C, Liu H, Taylor J (2019) Managing nitrogen to restore water quality in China. Nature 567: 516-520.
- Xia L, Lam SK, Chen D, Wang J, Tang Q, Yan X (2017) Can knowledge-based N management produce more staple grain with lower greenhouse gas emission and reactive nitrogen pollution? A meta-analysis. Global Change Biology 23: 1917-1925.
- Hu C, Sadras VO, Lu G, Zhang P, Han Y, Liu L, Xie J, Yang X, Zhang S (2021) A global meta-analysis of split nitrogen application for improved wheat yield and grain protein content. Soil and Tillage Research 213: 105111.
- Shen J, Li Y, Wang Y, Zhu X, Jiang W, Li Y, Wu J (2022) Soil nitrogen cycling and environmental impacts in the subtropical hilly region of China: Evidence from measurements and modeling. Frontiers of Agricultural Science and Engineering 9: 407-424.
- Liu C, Lu M, Cui J, Li B, Fang C (2014) Effects of straw carbon input on carbon dynamics in agricultural soils: a meta-analysis. Global Change Biology 20: 1366-1381.
- Yuan L, Chen X, Jia J, Chen H, Shi Y, Ma J, Liang C, Liu Y, Xie H, He H, Zhang X, Peng X, Lu C (2021) Stover mulching and inhibitor application maintain crop yield and decrease fertilizer N input and losses in no-till cropping systems in Northeast China. Agriculture, Ecosystems and Environment 312: 107360.
- Liu J, Jiang B, Shen J, Zhu X, Yi W, Li Y, Wu J (2021) Contrasting effects of straw and straw-derived biochar applications on soil carbon accumulation and nitrogen use efficiency in double-rice cropping systems. Agriculture, Ecosystems and Environment 311: 107286.
- Ju X, Xing G, Chen X, Zhang S, Zhang L, Liu X, Cui Z, Yin B, Christie P, Zhu Z, Zhang F (2009) Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proceedings of the National Academy of Sciences of the United States of America 106: 3041-3046.
- Abalos D, Jeffery S, Sanz-Cobena A, Guardia G, Vallejo A (2014) Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agriculture, Ecosystems and Environment 189: 136-144.
- Li T, Zhang W, Yin J, Chadwick D, Norse D, Lu Y, Liu X, Chen X, Zhang F, Powlson D, Dou Z (2018) Enhanced-efficiency fertilizers are not a panacea for resolving the nitrogen problem. Global Change Biology 24: e511-e521.
- Sha Z, Ma X, Wang J, Lv T, Li Q, Misselbrook T, Liu X (2020) Effect of N stabilizers on fertilizer-N fate in the soil-crop system: A meta-analysis. Agriculture, Ecosystems and Environment 290: 106763.
- Qiao C, Liu L, Hu S, Compton JE, Greaver TL, Li Q (2015) How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Global Change Biology 21: 1249-1257.
- Yang M, Fang,Y, Sun D, Shi Y (2016) Efficiency of two nitrification inhibitors (dicyandiamide and 3, 4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: a meta-analysis. Scientific Reports 6: 22075.
- Gao J, Luo J, Lindsey S, Shi Y, Sun Z, Wei Z, Wang L (2020) Benefits and risks for the environment and crop production with application of nitrification inhibitors in China. Journal of Soil Science and Plant Nutrition 21: 497-512.
- Luo G, Li L, Friman VP, Guo J, Guo S, Shen Q, Ling N (2018) Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biology and Biochemistry 124: 105-115.
- Lu C, Chen H, Teng Z, Yuan L, Ma J, He H, Chen X, Zhang X, Shi Y (2018a) Effects of N fertilization and maize straw on the dynamics of soil organic N and amino acid N derived from fertilizer N as indicated by 15N labeling. Geoderma 321: 118-126.
- Lu C, Wang H, Chen H, Yuan L, Ma J, Shi Y, Zhang X, He H, Chen X (2018b). Effects of N fertilization and maize straw on the transformation and fate of labeled (15NH4)2SO4 among three continuous crop cultivations. Agricultural Water Management 208: 275-283.
- Elrys AS, Uwiragiye Y, Zhang Y, Abdel-Fattah MK, Chen Z, Zhang L, Meng L, Wang J, Zhu T, Cheng Y, Zhang J, Cai Z, Chang SX, Müller C (2023) Expanding agroforestry can increase nitrate retention and mitigate the global impact of a leaky nitrogen cycle in croplands. Nature Food 4: 109-121.
- Wang S, Huang X, Zhang Y, Yin C, Richel A (2021) The effect of corn straw return on corn production in Northeast China: An integrated regional evaluation with meta-analysis and system dynamics. Resources, Conservation and Recycling 167: 105402.
- Niu X, Xie R, Liu X, Zhang F, Li S, Gao S (2013) Maize yield gains in Northeast China in the last six decades. Journal of Integrative Agriculture 12: 630-637.
- Liu N, He H, Xie H, Bai Z, Zhang X, Peng C, Zhu P, Ren J, Wang L (2010) Impacts of long-term inorganic and organic fertilization on lignin in a Mollisol. Journal of Soils and Sediments 10: 1466-1474.
- Quan Z, Li S, Zhu F, Zhang L, He J, Wei W, Fang Y (2018) Fates of 15N-labeled fertilizer in a black soil-maize system and the response to straw incorporation in Northeast China. Journal of Soils and Sediments 18: 1441-1452.
- Quan Z, Zhang X, Davidson EA, Zhu F, Li S, Zhao X, Chen X, Zhang LM, He JZ, Wei W, Fang Y (2021a) Fates and use efficiency of nitrogen fertilizer in maize cropping systems and their responses to technologies and management practices: A global analysis on field 15N tracer studies. Earth’s Future 9: e2020EF001514.
- Ju X, Liu X, Zhang F, Roelcke M (2004) Nitrogen fertilization, soil nitrate accumulation, and policy recommendations in several agricultural regions of China. AMBIO: A Journal of the Human Environment 33: 300-305.
- Wallace AJ, Armstrong RD, Grace PR, Scheer C, Partington DL (2020) Nitrogen use efficiency of 15N urea applied to wheat based on fertiliser timing and use of inhibitors. Nutrient Cycling in Agroecosystems 116: 41-56.
- Quan Z, Zhang X, Fang Y, Davidson EA (2021b) Different quantification approaches for nitrogen use efficiency lead to divergent estimates with varying advantages. Nature Food 2: 241-245.
- Gao L, Ma L, Zhang W, Wang F, Ma W, Zhang F (2009) Estimation of nutrient resource quantity of crop straw and its utilization situation in China. Trans CSAE 25: 173-179. (in Chinese with English abstract).
- Powlson DS, Jenkinson DS, Johnston AE, Poulton PR, Glendining MJ, Goulding KWT (2010) Comments on “Synthetic nitrogen fertilizers deplete soil nitrogen: a global dilemma for sustainable cereal production”, by R. L. Mulvaney, S. A. Khan, and T. R. Ellsworth in the Journal of Environmental Quality, 2009 38: 2295-2314. Journal of Environmental Quality 39, 749-752.
- Dawar K, Khan A, Sardar K, Fahad S, Saud S, Datta R, Danish S (2021) Effects of the nitrification inhibitor nitrapyrin and mulch on N2O emission and fertilizer use efficiency using 15N tracing techniques. Science of The Total Environment 757: 143739.
- Ma Q, Wu Z, Shen S, Zhou H, Jiang C, Xu Y, Liu R, Yu W (2015) Responses of biotic and abiotic effects on conservation and supply of fertilizer N to inhibitors and glucose inputs. Soil Biology and Biochemistry 89: 72-81.
- Alonso-Ayuso M, Gabriel JL, Quemada M (2016) Nitrogen use efficiency and residual effect of fertilizers with nitrification inhibitors. European Journal of Agronomy 80: 1-8.
- Ma Q, Jiang C, Li S, Yu W (2021a) Maize yield and nitrogen-use characteristics were promoted as consistently improved soil fertility: 6-year straw incorporation in Northeast China. Plant, Soil and Environment 67: 383-389.
- Wang Z, Wang Z, Luo Y, Zhan Y, Meng Y, Zhou Z (2020) Biochar increases 15N fertilizer retention and indigenous soil N uptake in a cotton-barley rotation system. Geoderma 357: 113944.
- Shi W, Bian R, Li L, Lian W, Liu X, Zheng J, Cheng K, Zhang X, Drosos M, Joseph S, Pan G (2021) Assessing the impacts of biochar-blended urea on nitrogen use efficiency and soil retention in wheat production. GCB-Bioenergy 14: 65-83.
- Linquist BA, Liu L, van Kessel C, van Groenigen KJ (2013) Enhanced efficiency nitrogen fertilizers for rice systems: Meta-analysis of yield and nitrogen uptake. Field Crops Research 154: 246-254.
- Said-Pullicino D, Cucu MA, Sodano M, Birk JJ, Glaser B, Celi L (2014) Nitrogen immobilization in paddy soils as affected by redox conditions and rice straw incorporation. Geoderma 228-229: 44-53.
- Cao Y, Sun H, Zhang J, Chen G, Zhu H, Zhou S, Xiao H (2018) Effects of wheat straw addition on dynamics and fate of nitrogen applied to paddy soils. Soil and Tillage Research 178: 92-98.
- Yu C, Xie X, Yang H, Yang L, Li W, Wu K, Zhang W, Feng C, Li D, Wu Z, Zhang L (2020) Effect of straw and inhibitors on the fate of nitrogen applied to paddy soil. Scientific Reports 10: 21582.
- Chen B, Liu E, Tian Q, Yan C, Zhang Y (2014) Soil nitrogen dynamics and crop residues. A review. Agronomy for Sustainable Development 34: 429-442.
- Pan F, Yu W, Ma Q, Zhou H, Jiang C, Xu Y, Ren J (2017) Influence of 15N-labeled ammonium sulfate and straw on nitrogen retention and supply in different fertility soils. Biology and Fertility of Soils 53: 303-313.
- Cheng Y, Wang J, Wang J, Chang SX, Wang S (2017) The quality and quantity of exogenous organic carbon input control microbial NO3- immobilization: A meta-analysis. Soil Biology and Biochemistry 115: 357-363.
- Liu S, Wang J, Pu S, Blagodatskaya E, Kuzyakov Y, Razavi BS (2020) Impact of manure on soil biochemical properties: A global synthesis. Science of The Total Environment 745: 141003.
- Ma Q, Zhang X, Wu Z, Li S, Xu Z, Zhou C, Xia Z, Zhu M, Gao Y, Yu W. (2021b) Occurrence of added nitrogen interaction affected by nitrogen stabilizer and glucose additions in an Alfisol. European Journal of Soil Biology 103: 103285.
- Ding X, Han X, Zhang X (2013) Long-term impacts of manure, straw, and fertilizer on amino sugars in a silty clay loam soil under temperate conditions. Biology and Fertility of Soils 49: 949-954.
- Hu G, Liu X, He H, Zhang W, Xie H, Wu Y, Cui J, Sun C, Zhang X (2015) Multi-seasonal nitrogen recoveries from crop residue in soil and crop in a temperate agro-ecosystem. PLoS One 10: e0133437.
- Wang M, Pendall E, Fang C, Li B, Nie M (2018) A global perspective on agroecosystem nitrogen cycles after returning crop residue. Agriculture, Ecosystems and Environment 266: 49-54.
- Ding W, Li S, He P, Huang S (2019) Contribution and fate of maize residue-15N and urea-15N as affected by N fertilization regime. PLoS One 14: e0210176.
- Li F, Wang Z, Dai J, Li Q, Wang X, Xue C, Liu H, He G (2015) Fate of nitrogen from green manure, straw, and fertilizer applied to wheat under different summer fallow management strategies in dryland. Biology and Fertility of Soils 51, 769-780.
- Bird JA, Horwath WR, Eagle AJ, van Kessel C (2001) Immobilization of fertilizer nitrogen in rice: Effects of straw management practice. Soil Science Society of America Journal 65: 1143-1152.
- Liu X, Zhou F, Hu G, Shao S, He H, Zhang W, Zhang X, Li L (2019) Dynamic contribution of microbial residues to soil organic matter accumulation influenced by maize straw mulching. Geoderma 333: 35-42.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).