1. Introduction
Introducing primary percutaneous coronary intervention (pPCI) in the treatment of patients with ST-elevation myocardial infarction (STEMI) has significantly reduced mortality and the occurrence of complications [
1]. However, despite timely and successful myocardial reperfusion, some patients with STEMI have a high risk of mortality and/or other adverse events.
Diabetes mellitus (DM) is not only a strong risk factor for coronary artery disease, but also has a negative prognostic impact on mortality and other adverse events in all patients with acute myocardial infarction (AMI) [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11]. Data from literature indicate that the negative prognostic impact of DM in patients with AMI differs depending on DM therapy and that patients with insulin-treated DM (ITDM) have a poorer prognosis compared not only to patients without DM but also to patients with non-insulin-treated DM (NITDM) [
2,
4,
6,
11].
Another very strong prognostic marker in patients with AMI is left ventricular systolic function (most frequently defined as the value of the ejection fraction - EF) [
2,
3]. It is well known that patients with AMI and a reduced EF < 50% are at risk of mortality and other adverse events, and the risk increases with further decline of the EF [
1]. At the same time, the presence of DM in patients with a reduced EF can further worsen the prognosis of these patients.
It has been shown that the negative prognostic impact of ITDM is significantly stronger than the negative impact of NITD on mortality in patients with AMI with symptoms and signs of heart failure or an EF <35%. However, it must be noted that only a small percentage of STEMI patients included in these studies were treated with pPCI [
2,
3]. Also, studies involving patients with heart failure of various etiologies with a reduced EF <40% and a mildly reduced EF 40-49% also indicate that patients with ITDM have a higher risk of adverse events compared to patients with NITDM [
9,
10]. To the best of our knowledge, the prognostic significance of ITDM and NITDM in the long-term follow-up of patients with STEMI treated with pPCI, who had a reduced EF, has not been analyzed in literature.
It has been shown that the negative prognostic impact of ITDM is significantly greater than the negative impact of NITD on mortality in patients with AMI who display symptoms and signs of heart failure or have an EF < 35%, however, only a small percentage of STEMI patients included in these studies were treated with pPCI [
2,
3]. Also, studies involving patients with heart failure of various etiologies with a reduced EF (< 40%) and a mildly reduced EF (40 – 49%) indicate that patients with ITDM have a higher risk of adverse events, as compared to patients with NITDM [
9,
10]. To the best of our knowledge, the prognostic significance of ITDM and NITDM in long-term follow-up of patients with STEMI treated with pPCI, who also have a reduced EF, has not as yet been analyzed in literature.
The present study aims to analyze the impact of ITDM and NTIDM on the incidence of all-cause mortality and major adverse cardiovascular events (MACE) in the eight-year follow-up of patients with a reduced EF after STEMI.
2. Materials and Methods
2.1. Study Population, Inclusion and Exclusion Criteria, Data Collection, and Definitions
In the present study, we included 2,230 consecutive STEMI patients with an EF < 50% hospitalized between December 2005 and January 2012, who were also included in the prospective University Clinical Center of Serbia STEMI Register. The purpose of the prospective University Clinical Center of Serbia STEMI Register has already been published elsewhere [
12]. The objective of the Register is to gather data on the management and short- and long-term outcomes of patients with STEMI, treated with pPCI. All consecutive STEMI patients, aged 18 years or older, who were admitted to the Coronary Care Unit after being treated with pPCI in the Catheterization Lab of the Center, were included in the Register. All involved patients received written information of their participation in the Register and the long-term follow-up, and their verbal and written consent was obtained [
12]. Patients with cardiogenic shock at admission were excluded from the Register. For the purpose of this study, patients with an EF ≥ 50% were excluded from the analysis.
The flowchart of patient selection is presented in Figure 1.
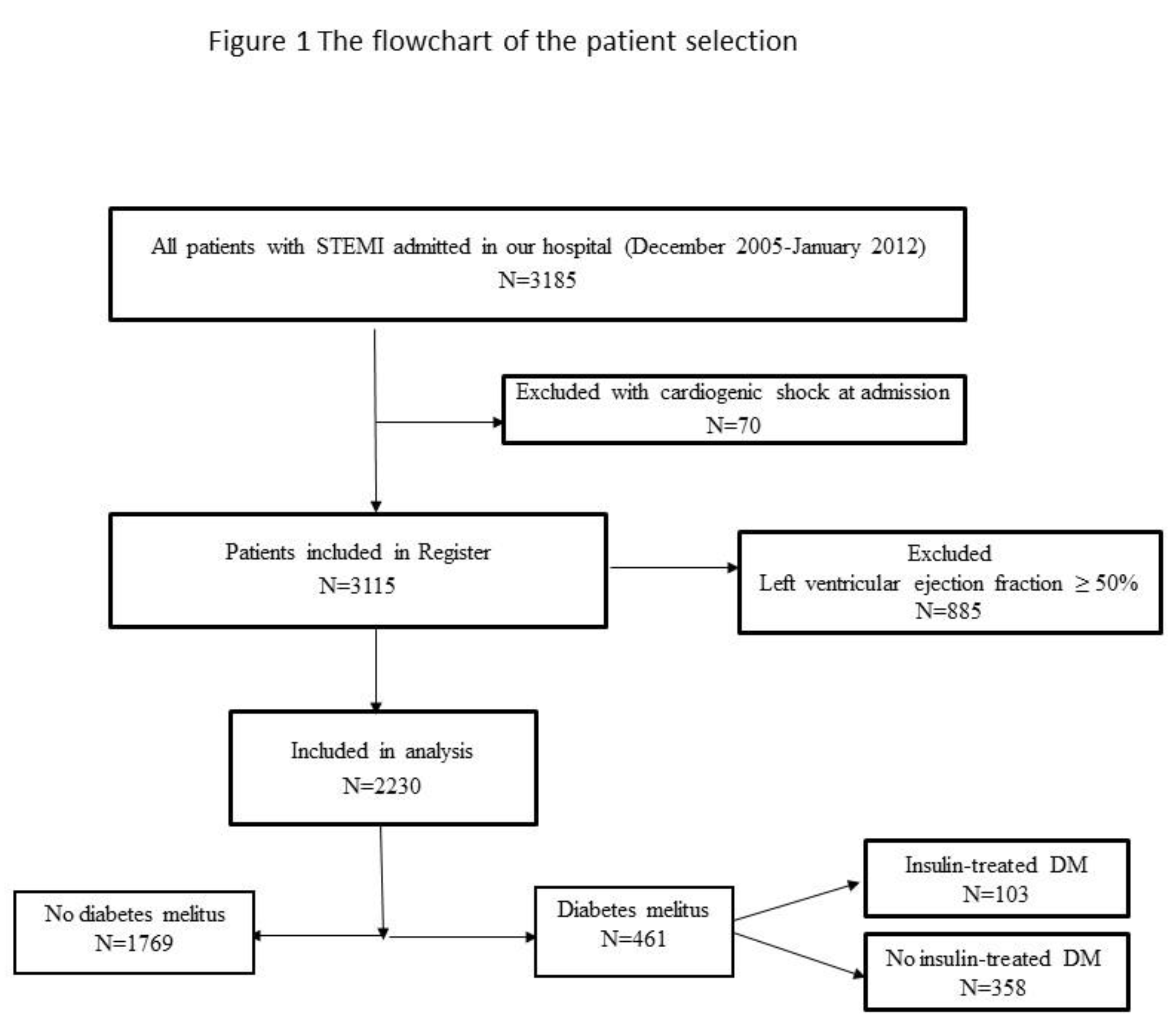
Coronary angiography was performed via the femoral approach. Primary PCI and stenting of the IRA were performed using the standard technique. Loading doses of aspirin (300 mg) and clopidogrel (600 mg) were administered to all patients before pPCI. Selected patients were also given the GP IIb/IIIa receptor inhibitor during the procedure. After pPCI, patients were treated in keeping with the current guidelines.
Demographic, baseline clinical, laboratory, angiographic, and procedural data were collected and analyzed. An echocardiographic examination was performed in the first three days after intervention (pPCI). The left ventricular EF was assessed according to the biplane method. Baseline kidney function (blood for creatinine analysis was drawn before pPCI and before iodine contrast administration) was assessed using the Modification of Diet in Renal Disease equation, and the value of estimated glomerular filtration rate (eGFR) below 60 ml/min/m2 was considered as chronic kidney disease (CKD). Patients were divided into three groups: no DM, ITDM, and NITD. The diabetic status of the patients was determined at hospital admission by the attending physician by careful assessment of past medical history and based on the use of oral antidiabetic drugs or insulin.
Patients were followed up at eight years after enrolment into the Register. Follow-up data were obtained with telephone interviews and during outpatient visits. We analyzed all-cause mortality and composite endpoint major adverse cardiovascular events (MACE), which included cardiovascular death, non-fatal reinfarction, non-fatal ischemic stroke, and target vessel revascularization (TVR). The cause of death was obtained from death certificates or discharge forms (if the patient had been hospitalized). Cardiovascular death included any death due to a proximate cardiac cause (myocardial infarction, low-output heart failure, fatal arrhythmia, sudden death), and death caused by non-coronary vascular causes, such as cerebrovascular disease [
12]. Non-fatal recurrent myocardial infarction was defined according to the Fourth Universal Definition for Myocardial Infarction [
13]. TVR was defined as ischemia-driven percutaneous revascularization of the target vessel performed for restenosis or other complications. Stroke was defined as a new onset of focal or global neurological deficit lasting more than 24 hours. Computed tomography was used to diagnose (ischemic) stroke. The Emergency Hospital neurologist was responsible for the diagnosis and treatment of stroke [
12].
2.2. Ethics
The study protocol was approved by the Ethics Committee of the University of Belgrade, Faculty of Medicine (approval number 470/II-4, February 21, 2008). The study was conducted in keeping with the principles outlined in the Helsinki Declaration. Written informed consent was obtained from all patients for their participation in the Register.
2.3. Statistical Analysis
Categorical variables were expressed as frequency and percentage, and continuous variables were expressed as the median (med), with 25th and 75th quartiles (IQR). Analysis for normality of data was performed using the Kolmogorov-Smirnov test. Baseline differences between groups were analyzed using the Kruskal-Wallis and Man-Whitney tests for continuous variables, and the Pearson χ² test for categorical variables. The Kaplan-Meier method was used for constructing the probability curves for eight-year mortality and the incidence of MACE, while the difference between patients with ITDM, NITDM, and without DM was tested with the Log-Rank test. The Cox proportional hazard model (backward method, with p < 0.10 for entrance into the model) was used to identify univariable and multivariable predictors for the occurrence of all-cause mortality and MACE. Two-tailed p-values of less than < 0.05 were considered statistically significant. We used the SPSS statistical software, Version 19, for statistical analysis (SPSS Inc, Chicago, IL).
3. Results
Of the 2,230 patients analyzed, 461 (20.7%) patients had DM. Among the patients with DM, 103 (22.3%) patients had ITDM, and 358 (77.7%) patients had NITDM. The mean age of all analyzed patients was 61 (53, 70) years. A total of 632 (28.3%) patients were female. Overall, 948 (42.6%) patients had an EF < 40%, while 1,282 (57.4%) patients had an EF ranging from 40% to 49%.
Compared to patients without DM, patients with ITDM and NITDM were older and more likely to be female; they more often presented with HF, complete atrioventricular (AV) block, and atrial fibrillation; they were more likely to have reduced baseline kidney function, multivessel disease on the initial angiogram, a post-procedural TIMI flow < 3 and a lower pre-discharge EF. When we compared patients with ITDM and NITDM, we found that patients with ITDM more often presented with HF, complete AV block, and AF, as well as that they more often had CKD, multivessel coronary artery disease, a postprocedural TIMI flow < 3, and a lower EF. Procedural characteristics and therapy at discharge did not differ among the analyzed groups. In-hospital mortality was significantly higher in patients with DM, when compared with patients without DM. Also, among patients with DM, in-hospital mortality was significantly higher in patients with ITDM, as compared to patients with NITDM. Baseline characteristics, laboratory, angiographic, and procedural characteristics, in-hospital mortality, and therapy at discharge, in patients without DM, with ITDM, and with NITDM, are presented in
Table 1.
At eight-year follow-up, all-cause mortality and MACE were registered in a total of 252 (11.3%) and 379 (16.9%) patients, respectively. Causes of mortality were predominantly cardiovascular in all of the analyzed groups; non-cardiovascular causes of death (such as cancer, ileus, pneumonia, and dementia) were registered in a total of 19 patients (7.5% of all deaths). Eight-year all-cause mortality and MACE were significantly higher in patients with DM, compared with patients without DM (19.8% vs. 9.4%, respectively, p < 0.001 and 25.5% vs. 15.2%, respectively, p < 0.001). The highest mortality and MACE rates in all groups were registered in the first year after the index event.
Eight-year all-cause mortality and MACE in patients with and without DM are presented in
Table 2.
Kaplan Meier curves showing eight-year all-cause mortality (Curve a) and MACE (Curve b) are presented in Figure 2.
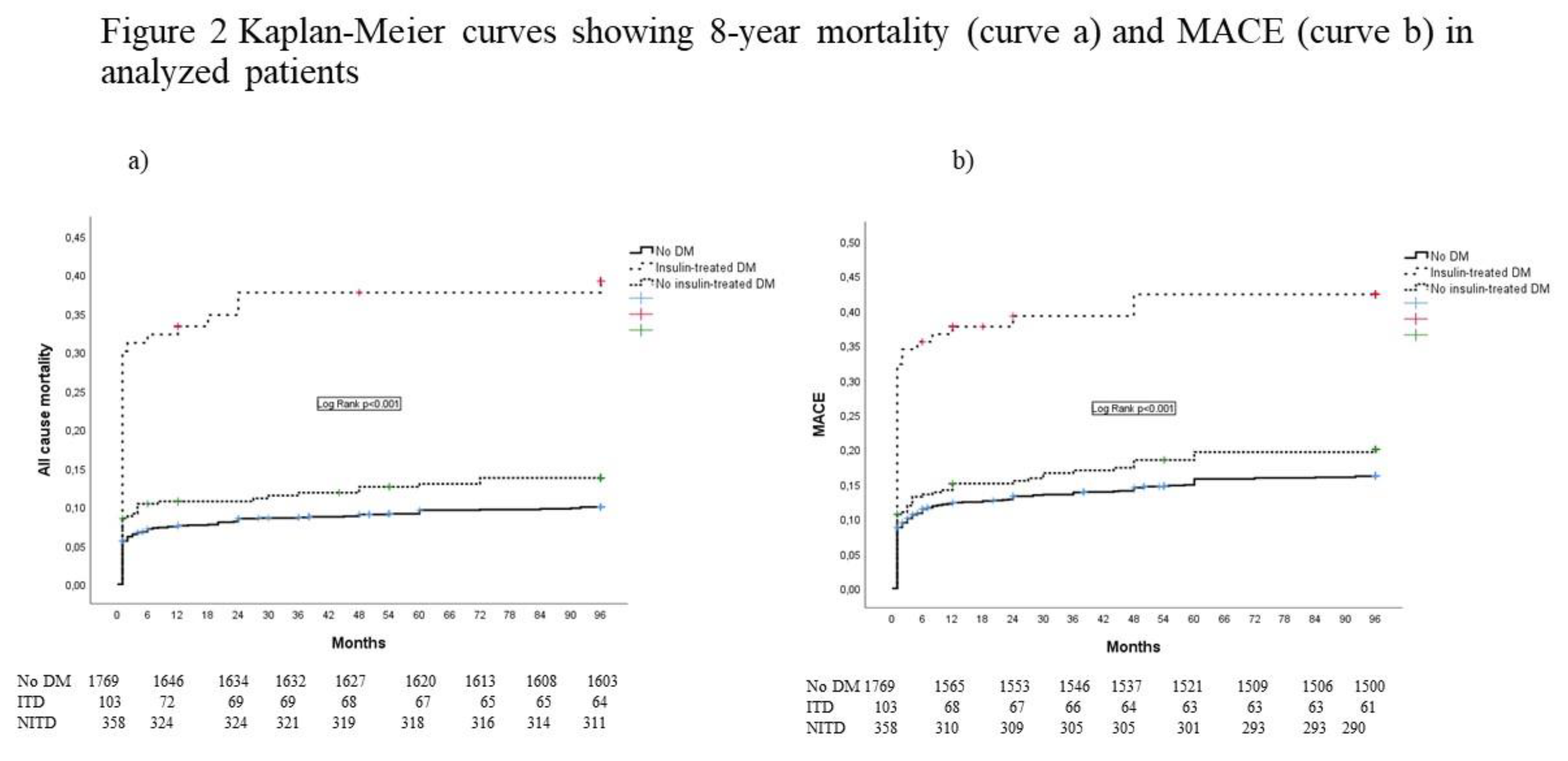
After adjustment for confounders, ITDM, but not NITDM, was an independent predictor of all-cause mortality and MACE in eight-year follow-up. Predictors for the occurrence of eight-year mortality and MACE are presented in
Table 3.
4. Discussion
Our results showed that 20.7% of analyzed patients had DM and that 22.2% of patients with DM had ITDM. Patients with ITDM had a higher burden of cardiovascular diseases and other comorbidities, and a higher incidence of eight-year all-cause mortality and MACE, as compared with patients with NITDM and patients without DM. The difference in the incidence of MACE was predominantly caused by higher cardiovascular mortality; there was no significant difference in the occurrence of non-fatal recurrent ischemic events among these three groups of patients. In the patients analyzed, ITDM, but not NITDM, was an independent predictor of long-term mortality and composite end-point MACE.
4.1. Baseline Characteristics
The clinical characteristics of our patients from all three analyzed groups are in keeping with the data found in literature [
2,
10,
11], and the differences in the percentage of patients with ITDM and NITDM can be explained by the difference in patient selection, i.e., the different study design. According to data from literature, among the patients with DM, around 30% are patients with ITDM [
5,
6,
14,
15,
16], which is also the case with our findings. It is a common finding that patients with DM are older and develop coronary disease earlier, they have more comorbidities, as well as poorer angiographic findings, when compared to patients without DM. This finding suggests that patients with diabetes have a higher atherosclerotic burden [
5,
6]. Patients with ITDM are usually older, as compared to patients with NITD, however, this was not the case in our study, but patients with ITDM did have more comorbidities, poorer angiographic findings, and they more often presented with signs of heart failure and/or heart rhythm disorders, which is also in keeping with the findings found in literature [
2,
3,
11,
16].
4.2. Prognostic Impact of ITDM and NITD in Patients with Myocardial Infarction and/or Heart Failure
The relative lack of studies analyzing the prognostic significance of NITDM and ITDM in patients with reduced EF after STEMI prevents a direct comparison of our results with those of other authors. What is well known is that despite dramatic improvements in survival rates in patients with STEMI undergoing pPCI, the overall risk of adverse events in patients with ITDM has not significantly changed and remains increased, as compared not only with patients without DM, but also with patients with NITD [
2,
6,
7,
11,
17].
In a study by Hoebers et al. wherein over 4,000 STEMI patients treated with pPCI were included, ITD was found to be an independent predictor of long-term mortality (up to 5 years), while long-term mortality was similar in patients with NITDM and patients without DM, which is identical to our findings. However, this study did not present echocardiographic data for the analyzed patients [
11].
Similar are the results of the study by Antonniucci et al. where it was found that six-month mortality was the highest in the ITD group (26%), and similar in patients with NITD and non-diabetic patients (7% vs. 4%, respectively) [
18].
In a study by Reinstadler et al., wherein patients treated with pPCI were analyzed, it was found that the presence of DM was associated with an increased risk of one-year MACE (defined in this study as all-cause mortality, non-fatal reinfarction, and new congestive heart failure), and when MACE events were analyzed individually, DM was not associated with a higher incidence of non-fatal reinfarction. Also, there was a similar risk of MACE in non-diabetic patients and patients with NITDM, but the group with ITD showed a significantly higher incidence of MACE [
6], which is in keeping with our findings. The cited article revealed that the success of reperfusion and the size of myocardial necrosis (analyzed using cardiac magnetic resonance imaging) were similar in patients with DM and patients without DM [
6], indicating that the size of the necrotic myocardium was not the mechanism that could explain the poorer prognosis of the analyzed patients with DM compared to nondiabetic patients.
In a study by Biswas et al., patients with ITDM and NITDM who underwent PCI were compared. In this study, there were about 20% of patients with STEMI in both analyzed groups and about 30% of patients with an EF < 45%. It was found that patients with ITDM had significantly higher twelve-month MACCE (all-cause mortality, myocardial infarction, TVR, and stroke) and mortality as compared with NITDM, while only ITDM (and not NITDM) was an independent predictor of twelve-month MACCE. When the patients were "stratified" in relation to therapy, a gradual increase in the risk of MACCE was found to exist with the escalation of treatment for DM (from a hygienic diet regimen, through oral therapy, all the way to insulin therapy) [
16].
Finally, a meta-analysis by Bundhum et al. included 21 studies wherein patients who underwent PCI were included (without data on what percentage of patients had ACS). The results of this meta-analysis showed that mortality, as well as the occurrence of MACE (mortality, myocardial infarction, TVR, and stent thrombosis) in one-year, but also in long-term follow-up after PCI, were significantly higher in patients with ITDM, as compared to patients with NITD [
19].
Similar results can be found in studies wherein patients with HF and/or a reduced EF were analyzed, especially in cases with ischemic etiology. In a study by Rosello et al, patients who had suffered high-risk myocardial infarction (heart failure or EF ≤ 35%), included in four large, randomized studies (VAALINAT, EPHESUS, OPTIMAAL, CAPRICORN) were analyzed. In this study, the highest total and cardiovascular mortality during an approximately three-year follow-up was registered in patients with ITDM, while patients with NITDM had significantly lower mortality, albeit higher, as compared to patients without DM. The highest percentage of deaths was due to cardiovascular causes in all three analyzed groups, which is in keeping with our findings. ITDM significantly increased the risk of mortality, compared not only to non-diabetic patients but also to patients with NITDM. However, both ITD and NITD were independent predictors of mortality in this study, with the independent and negative prognostic impact of ITDM being stronger, as compared to NITDM [
1]. Also, patients with ITDM had a higher risk of non-fatal events during follow-up, compared with patients with NITDM and patients without DM. The difference in the results between our study and the cited study can be explained by the different populations of analyzed patients, the small number of patients who were treated with pPCI (which can certainly affect the prognosis of the patient), and the lack of angiographic data, which is also stated in the study's limitations [
1].
In a study by Li et al, the prognostic significance of DM type 2 in patients with acute coronary syndrome (ACS) who have HF with mid-range EF (40% – 49%) was analyzed. During three-year follow-up, it was found that the incidence of total mortality, HF rehospitalization, cardiovascular death, and unplanned revascularization was higher in DM compared to non-DM, and DM was an independent predictor of all the analyzed adverse events, except for unplanned revascularization [
5]. The incidence of all adverse events was significantly higher in patients with poorly regulated DM (HgbA1c > 7.5%), as compared to patients with well-regulated DM [
10]. Unlike our study, this article did not analyze the prognostic impact of DM in relation to antidiabetic treatment.
In a post-hoc analysis – Systolic Heart Failure Treatment with the inhibitor ivabradine Trial (SHIFT), patients with sinus rhythm, an EF ≤ 35% and with ITD were shown to have a 33% higher risk of cardiovascular death or hospitalization due to heart failure exacerbation as compared to patients with NITDM. There was no difference in the incidence of cardiovascular death and hospitalization due to heart failure exacerbation between patients with NITDM and patients without DM [
20].
In a study by Schupp et al., the prognostic influence of DM type 2 on the long-term prognosis of patients with heart failure of different etiology and a mid-range EF (40% - 49%) (HFmrEF) was analyzed, with ischemic etiology being present in 20% - 30% of the analyzed patients. Patients with DM had higher mortality, but not a higher risk of rehospitalization, as compared to non-diabetic patients; patients with ITDM had a higher risk of 30-month mortality, as compared to NITDM patients, but the risk for rehospitalization, due to HF, did not differ [
9]. Also, the post-hoc analysis of the SOLVD study showed that the presence of DM generally adversely affected the prognosis of patients with left ventricular systolic dysfunction, if it was of ischemic etiology, whereas DM had no prognostic impact in patients with non-ischemic etiology [
21].
4.3. Possible Mechanisms for Higher Adverse Events Rates in Patients with ITDM
There are numerous and still insufficiently explained mechanisms that could explain the worse prognostic impact of ITD on patients with AMI and/or HF, as compared to the prognostic impact of NITDM. There are data from experimental and non-randomized studies showing that the use of insulin in the treatment of DM led to the progression of cardiovascular diseases, especially in patients with previous macrovascular complications. The introduction of insulin therapy in patients with DM (DM type 2, which also includes our patients) is a surrogate marker for poor glycemic management with oral medication and for longer duration of DM, which explains the finding showing that patients with ITDM are older and have more comorbidities, as compared to patients with NITDM. Patients with ITDM also have an elevated concentration of glycosylation end products, which have an atherogenic and proinflammatory effect [
6,
7,
10,
16], and iatrogenic hyperinsulinemia also itself has a proinflammatory effect [
18]. Long-term DM often leads to an atypical clinical presentation of ischemia, and often to silent ischemia, resulting in a delayed visit to the doctor, i.e., longer chest pain duration before the first contact with a medical professional in patients with STEMI [
11]. In our study, patients with DM had longer pain duration, as compared to non-diabetic patients. On the other hand, there was no difference in the duration of chest pain between patients with ITD and NITD. Some authors believe that reperfusion in ITD is less successful despite an optimal TIMI flow [
11,
22], which indirectly indicates the presence of microvascular disease [
3]. In our study, we found the highest percentage of patients with post-procedural TIMI flow < 3 in the group with ITD. However, even after adjusting for this variable, ITD remained an independent predictor of mortality and MACE. Patients with long-term DM often have an altered myocardium even before the occurrence of infarction (formerly known as "diabetic cardiomyopathy"), which is characterized by preserved systolic and disturbed diastolic function [
9,
10]. This relatively altered myocardium of diabetic patients may be more prone to ischemia-reperfusion injury than the myocardium of patients without diabetes [
8]. Finally, underutilization of evidence-based therapy after MI has been suggested to explain worse outcomes in patients with DM in some older studies [
23], but our results do not support this theory, because in our study there was no difference in the therapy at discharge among analyzed patients.
4.4. Clinical Implications
Our study confirms previous reports on the unfavorable prognostic impact of ITDM in patients with STEMI and a reduced EF and also adds to the knowledge in this area by providing information on long-term outcomes in patients with a mildly reduced EF (40% - 49%). Differentiation between ITDM and NITD should be taken into account in the individual patient with DM. In patients with STEMI and a reduced EF, the need for insulin therapy provides further prognostic value in diabetic patients and should be taken into account to further predict cardiovascular risk. In addition to the usual treatment that is administered to all patients after STEMI (including dual antiplatelet therapy, beta-blockers, ACE inhibitors/sartanas, statins, etc.) introducing sodium glucose transporter inhibitors-2 (SGLT-2 inhibitors) in all patients with DM, especially those with ITDM and a reduced or mildly reduced EF, should be considered [
2,
24].
4.5. Study Limitations
This study should be viewed in the context of its limitations. The study is unicentric, and observational, but it is controlled, prospective, and has included consecutive patients, limiting possible selection bias.
Patients included in the study were hospitalized between 2005 and 2012. Patients with cardiogenic shock at admission were excluded from our Register. All patients were treated with clopidogrel. There were no patients treated with more recently developed antiplatelet drugs (ticagrelor and prasugrel were not available for routine administration to patients at the time of their entry into the Register). Had the patients received more advanced therapy this may have influenced the patients’ prognosis, i.e. reduced the occurrence of cardiovascular mortality or the incidence of non-fatal ischemic events.
We did not use other measures for determining systolic function such as myocardial deformation imaging. However, many clinical trials so far have used EF to stratify patients and have demonstrated its benefit in determining the outcome of therapy [
9,
10,
11].
There are no data on follow-up echocardiographic examinations to show whether there has been a certain degree of recovery or deterioration in the myocardial contractility.
Patients with only insulin resistance or some other prediabetic condition were included in the group of non-diabetic patients. However, they may have developed DM during the eight-year follow-up, which could have affected their long-term prognosis. Diabetic status was assessed at admission based on the known history of DM; we did not routinely measure hemoglobin A1c.
We had no data on the specific criteria for initiating insulin therapy. We also had no data on the duration of DM or insulin treatment. New-generation oral antidiabetic drugs, such as sodium glucose transporter-2 (SGLT-2) inhibitors, were unavailable at the time of patient inclusion. The study was not designed to evaluate whether changing pharmacological treatment during follow-up would have an impact on the long-term outcome in the analyzed patients.
5. Conclusion
Patients with DM had a significantly higher all-cause eight-year mortality and MACE rate, as compared with patients without DM. Patients with insulin-treated DM had a higher burden of comorbidities, a worse finding on the coronary angiogram, and a higher incidence of the analyzed adverse events, as compared with patients with non-insulin-treated DM. Insulin-treated DM significantly and independently increased the incidence of long-term mortality and composite end-point MACE. Non-insulin-treated DM had no significant impact on long-term mortality and MACE rates in the analyzed patients.
Disclosure statement: The authors report that there are no competing interests to declare.
Author Contributions
LS and IM devised the study and participated in its design, acquisition of data, and coordination. LS performed statistical analysis. MA, SS, GK, RL, DM, and DS participated in the study design and helped draft the manuscript. All the authors have read and approved the final manuscript. .
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University of Belgrade, Faculty of Medicine (approval number 470/II-4, February 21, 2008).
Acknowledgments
The authors would like to express their gratitude to the physicians and nurses of the Coronary Unit and the Catheterization Laboratory who participated in the primary PCI program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ng, V.G.; Lansky, A.J.; Meller, S.; Witzenbichler, B.; Guagliumi, G.; Peruga, J.Z.; et al. The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Eur Heart J Acute Cardiovasc Care 2014, 3, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Rossello, X.; Ferreira, J.P.; McMurray, J.J.; Aguilar, D.; Pfeffer, M.A.; Pitt, B.; et al. High-Risk Myocardial Infarction Database Initiative. Editor's Choice- Impact of insulin-treated diabetes on cardiovascular outcomes following high-risk myocardial infarction. Eur Heart J Acute Cardiovasc Care 2019, 8, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Tajik, A.A.; Dobre, D.; Aguilar, D.; Kjekshus, J.; Zannad, F.; Dickstein, K. High-Risk MI Database Scientific Committee. A history of diabetes predicts outcomes following myocardial infarction: an analysis of the 28 771 patients in the High-Risk MI Database. Eur J Heart Fail 2017, 19, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Chen, C.Y.; Lin, H.D.; Wu, H.P. Impact of diabetes and hypertension on cardiovascular outcomes in patients with coronary artery disease receiving percutaneous coronary intervention. BMC Cardiovasc Disord 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tong, Y.; Zhang, Y.; Tang, L.; Lv, Q.; Zhang, F.; et al. Effects on All-cause Mortality and Cardiovascular Outcomes in Patients With Type 2 Diabetes by Comparing Insulin With Oral Hypoglycemic Agent Therapy: A Meta-analysis of Randomized Controlled Trials. Clin Ther 2016, 38, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Reinstadler, S.J.; Stiermaier, T.; Eitel, C.; Metzler, B.; de Waha, S.; Fuernau, G.; et al. Relationship between diabetes and ischaemic injury among patients with revascularized ST-elevation myocardial infarction. Diabetes Obes Metab 2017, 19, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Ritsinger, V.; Lagerqvist, B.; Lundman, P.; Hagström, E.; Norhammar, A. Diabetes, metformin and glucose lowering therapies after myocardial infarction: Insights from the SWEDEHEART registry. Diab Vasc Dis Res 2020, 17, 1479164120973676. [Google Scholar] [CrossRef] [PubMed]
- Kerola, A.M.; Semb, A.G.; Juonala, M.; Palomäki, A.; Rautava, P.; Kytö, V. Long-term cardiovascular prognosis of patients with type 1 diabetes after myocardial infarction. Cardiovasc Diabetol 2022, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Schupp, T.; Abumayyaleh, M.; Weidner, K.; Lau, F.; Reinhardt, M.; Abel, N.; et al. Prognostic Implications of Type 2 Diabetes Mellitus in Heart Failure with Mildly Reduced Ejection Fraction. J Clin Med 2024, 13, 742. [Google Scholar] [CrossRef]
- Li, L.; Li, G.; Chen, H.; Feng, Z.; Zhang, L.; Chen, L.; et al. Role of Diabetes Mellitus in Acute Coronary Syndrome Patients with Heart Failure and Midrange Ejection Fraction Who Have Undergone Percutaneous Coronary Intervention: A 3-Year Case-Series Follow-Up Retrospective Study. Diabetes Metab Syndr Obes 2021, 14, 4931–4944. [Google Scholar] [CrossRef]
- Hoebers, L.P.; Claessen, B.E.; Woudstra, P.; DeVries, J.H.; Wykrzykowska, J.J.; Vis, M.M.; et al. Long-term mortality after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction in patients with insulin-treated versus non-insulin-treated diabetes mellitus. EuroIntervention 2014, 10, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Mrdovic ISavic, L.; Lasica, R.; Krljanac, G.; Asanin, M.; Brdar, N.; et al. Efficacy and safety of tirofiban-supported primary percutaneous coronary intervention in patients pretreated with 600 mg clopidogrel: Results of propensity analysis using the clinical center of serbia STEMI register. Eur. Heart J Acute Cardiovasc Care 2014, 3, 56–66. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Rosselló, X.; Huo, Y.; Pocock, S.; Van de Werf, F.; Chin, C.T.; Danchin, N.; Lee, S.W.; Medina, J.; Vega, A.; Bueno, H. Global geographical variations in ST-segment elevation myocardial infarction management and post-discharge mortality. Int J Cardiol 2017, 245, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Massalha, S.; Luria, L.; Kerner, A.; Roguin, A.; Abergel, E.; Hammerman, H.; et al. Heart failure in patients with diabetes undergoing primary percutaneous coronary intervention. Eur Heart J Acute Cardiovasc Care 2016, 5, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Dinh, D.; Andrianopoulos, N.; Lefkovits, J.; Ajani, A.; Duffy, S.J.; et al. Comparison of Long-Term Outcomes After Percutaneous Coronary Intervention in Patients With Insulin-Treated Versus Non-Insulin Treated Diabetes Mellitus. Am J Cardiol 2021, 148, 36–43. [Google Scholar] [CrossRef] [PubMed]
- van der Schaaf, R.J.; Henriques, J.P.; Wiersma, J.J.; Koch, K.T.; Baan JJr Mulder, K.J.; et al. Primary percutaneous coronary intervention for patients with acute ST elevation myocardial infarction with and without diabetes mellitus. Heart. 2006, 92, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Antoniucci, D.; Valenti, R.; Migliorini, A.; Parodi, G.; Moschi, G.; Memisha, G.; et al. Impact of insulin-requiring diabetes mellitus on effectiveness of reperfusion and outcome of patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol 2004, 93, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Bundhun, P.K.; Li, N.; Chen, M.H. Adverse cardiovascular outcomes between insulin-treated and non-insulin treated diabetic patients after percutaneous coronary intervention: a systematic review and meta-analysis. Cardiovasc Diabetol 2015, 14, 135. [Google Scholar] [CrossRef]
- Komajda, M.; Tavazzi, L.; Francq, B.G.; Böhm, M.; Borer, J.S.; Ford, I.; et al. SHIFT Investigators. Efficacy and safety of ivabradine in patients with chronic systolic heart failure and diabetes: an analysis from the SHIFT trial. Eur J Heart Fail 2015, 17, 1294–1301. [Google Scholar] [CrossRef]
- Dries, D.L.; Sweitzer, N.K.; Drazner, M.H.; Stevenson, L.W.; Gersh, B.J. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol 2001, 38, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Nahser, P.J., Jr.; Brown, R.E.; Oskarsson, H.; Winniford, M.D.; Rossen, J.D. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation 1995, 91, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Norhammar, A.; Malmberg, K.; Rydén, L.; Tornvall, P.; Stenestrand, U.; Wallentin, L. Register of Information and Knowledge about Swedish Heart Intensive Care Admission (RIKS-HIA). Under utilisation of evidence-based treatment partially explains for the unfavourable prognosis in diabetic patients with acute myocardial infarction. Eur Heart J 2003, 24, 838–844. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; et al. ESC Scientific Document Group, 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 2023, 44, 3627–3639. [Google Scholar]
Table 1.
Baseline clinical, laboratory, angiographic, procedural characteristics, therapy at discharge, and intrahospital mortality of the study patients.
Table 1.
Baseline clinical, laboratory, angiographic, procedural characteristics, therapy at discharge, and intrahospital mortality of the study patients.
| Characteristics |
No DM
N= 1769 |
P value |
ITDM
N= 103 |
NITDM
N=358 |
P value
|
| Age, years med(IQR) |
60(52,70) |
<0.001 |
63(57, 72) |
64(58, 73) |
0.971 |
| Female, n(%) |
459(25.9) |
<0.001 |
37(35.9) |
131(36.6) |
0.901 |
| BMI, med (IQR) |
25.9(24.2, 27.8) |
0.493 |
27.3(24.5, 29.7) |
26.8(24.8, 29.4) |
0.644 |
| Previous MI, n(%) |
197(11.1) |
0.009 |
22(21.3) |
49(13.7) |
0.037 |
| Previous angina, n(%) |
126(7.1) |
0.032 |
10(9.7) |
38(10.6) |
0.791 |
| Previous stroke, n(%) |
69(3.9) |
<0.001 |
8(7.7) |
25(7.9) |
0.786 |
| Hypertension, n(%) |
1130(63.9) |
<0.001 |
80(77.7) |
295(82.1) |
0.277 |
| HLP, n(%) |
1030(58.2) |
0.192 |
58(56.3) |
225(62.8) |
0.230 |
| Smoking, n(%) |
965(54.6) |
<0.001 |
29(28.2) |
112(31.3) |
0.544 |
| Family hystory, n(%) |
577(32.6) |
<0.001 |
20(19.4) |
85(23.7) |
0.356 |
| Pain duration, hours med(IQR) |
2.5(1.5, 4.5) |
<0.001 |
3(1.5, 5) |
3(2, 5.5) |
0.861 |
| Atrial fibrillation on initial ECG, n(%) |
147(8.3) |
0.950 |
20(19.4) |
21(5.9) |
<0.001 |
| Complete AV block, n(%) |
86(4.9) |
0.086 |
18(17.4) |
11(3.1) |
<0.001 |
| Killip class >1, n(%) |
262(14.8) |
<0.001 |
49(47.5) |
76(21.1) |
<0.001 |
| Systolic BP at admission, med(IQR) |
130(120, 150) |
<0.001 |
130(110, 150) |
140(130, 160) |
<0.001 |
| Heart rate at admission med(IQR) |
80(70, 90) |
<0.001 |
80(70, 100) |
82(75, 97) |
0.235 |
| Multivessel disease, n(%) |
991(56) |
<0.001 |
89(5.1) |
239(66.7) |
0.002 |
| 3-vessel disease, n(%) |
455(25.7) |
<0.001 |
61(59.2) |
128(35.7) |
<0.001 |
| LM stenosis, n(%) |
113(6.9) |
0.030 |
10(9.8) |
30(8.4) |
0.679 |
| Postprocedural flow TIMI <3, n(%) |
94(5.3) |
0.019 |
16(15.5) |
23(6.4) |
0.003 |
| Stent implanted, n(%) |
1634(92.4) |
0.091 |
92(89.4) |
325(90.8) |
0.958 |
| Acute stent thrombosis, n(%) |
22(1.2) |
0.261 |
4(3.9) |
4(1.1) |
0.058 |
| Glicoprotein IIb/IIIa inhibitor, n(%) |
701(39.6) |
0.007 |
42(40.1) |
112(31.2) |
0.078 |
| CK MB max, med (IQR) |
2389(1598, 4025) |
0.024 |
1788(952, 4003) |
1673(708, 3054) |
0.102 |
| Troponin max, med (IQR) |
37.5(11.9, 103) |
0.045 |
53(12.6, 119) |
27.4(7.8, 96.2) |
0.219 |
| Hemoglobin at admission g/L, med (IQR) |
141(131, 152) |
<0.001 |
141(124, 158) |
150(138, 159) |
0.081 |
| Baseline CKD, n(%) |
282(15.9) |
<0.001 |
38(36.9) |
85(23.7) |
0.008 |
| EF, med(IQR) |
44(40, 49) |
<0.001 |
40(34, 45) |
42(39, 45) |
<0.001 |
| EF<40%, n(%) |
704(39.7) |
|
66(64.1) |
178(49.7) |
<0.001 |
| EF 40-49%, n(%) |
1065(60.2) |
|
37(35.9) |
180(50.3) |
<0.001 |
| Therapy at discharge* |
|
|
|
|
|
| Beta blockers, n(%) |
1432(80.9) |
0.077 |
60(3.4) |
284(79.3) |
0.085 |
| ACE inhibitors, n(%) |
1349(76.2) |
0.015 |
58(3.3) |
275(76.8) |
0.275 |
| Statin, n(%) |
1430(80.8) |
0,779 |
62(3.5) |
271(75.7) |
0.162 |
| Diuretic, n(%) |
320(18.1) |
<0.001 |
25(1.4) |
82(22.9) |
0.080 |
| Calcium antagonist, n(%) |
64(3.6) |
0.589 |
1(0.9) |
13(36.3) |
0.823 |
| Amiodarone, n(%) |
54(3.1) |
0.065 |
1(0.9) |
5(1.4) |
0.934 |
| In-hospital mortality, n(%) |
82(4.6) |
<0.001 |
24(23.3) |
24(6.7) |
<0.001 |
Table 2.
All-cause mortality and composite end-point MACE in study patients.
Table 2.
All-cause mortality and composite end-point MACE in study patients.
| |
No DM
N=1769 |
P value |
ITDM
N=103 |
NITDM
N=358 |
P value |
| All-cause death, n(%) |
166(9.4) |
<0.001 |
39(37.8) |
47(13.1) |
<0.001 |
| MACE, n(%) |
269(15.2) |
<0.001 |
42(40.8) |
68(18.9) |
<0.001 |
| Cardiovascular death, (%) |
154(8.7) |
<0.001 |
36(34.9) |
43(12) |
<0.001 |
| Non-fatal recurrent infarction, n(%) |
96(5.5) |
0.943 |
9(8.7) |
18(5.1) |
0.226 |
| TVR, n(%) |
116(6.5) |
0.070 |
19(18.4) |
39(10.9) |
0.607 |
| Non-fatal stroke, n(%) |
23(1.3) |
0.022 |
3(2.9) |
6(1.7) |
0.250 |
Table 3.
Independent predictors for 8-years all-cause mortality and MACE (Cox regression model) in all analyzed patients.
Table 3.
Independent predictors for 8-years all-cause mortality and MACE (Cox regression model) in all analyzed patients.
| |
Univariable analysis |
Multivariable analysis |
| HR (95%CI) |
p value |
HR (95%CI) |
p value |
| All-cause mortality |
| Age, years |
1.04(1.02-1.06) |
<0.001 |
1.05(1.03-1.06) |
<0.001 |
| Killip >1 at admission |
2.81(2.11-3.73) |
<0.001 |
2.80(2.14-3.62) |
<0.001 |
| Postprocedural TIMI<3 |
2.67(1.91-3.96) |
<0.001 |
2.61(1.87-3.69) |
<0.001 |
| AF at admission |
1.88(1.36-2.59) |
<0.001 |
1.84(1.33-2.54) |
<0.001 |
| Previous infarction |
1.51(1.06-2.12) |
0.020 |
1.50(1.08-2.16) |
0.016 |
| ITDM |
1.78(1.16-2.35) |
0.008 |
1.72(1.12-2.62) |
0.013 |
| 3-vessel disease |
1.32(1.10-1.89) |
0.051 |
|
|
| CKD |
1.29(1.10-1.88) |
0.050 |
|
|
| |
|
MACE |
|
|
| Age, years |
1.04(1.02-1.06) |
<0.001 |
1.02(1.01-1.03) |
<0.001 |
| Postprocedural TIMI<3 |
2.19(1.61-2.98) |
<0.001 |
2.13(1.57-2.91) |
<0.001 |
| Killip >1 at admission |
2.0(1.57-2.54) |
<0.001 |
2.0(1.56-2.48) |
<0.001 |
| ITDM |
1.59(1.09-2.13) |
0.017 |
1.49(1.01-2.19) |
0.041 |
| Complete AV block at admission |
1.41(1.05-1.52) |
0.022 |
1.36(1.01-1.43) |
0.044 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).







