1. Introduction
Picophytoplankton, including picoeukaryotes (PE),
Prochlorococcus spp. (Pro), and
Synechococcus spp. (Syn), contribute to phytoplankton biomass and nutrient cycling in aquatic ecosystems, and their importance is expected to increase in the future with global warming [
1,
2]. It is observed that these cells undergo rapid divisions (up to once a day or more) and react very rapidly to environmental fluctuations, such as changes in cloud cover [
3], vertical mixing [
4], and nutrients pulses [
5]. There is little knowledge about the effect of environmental factors on phytoplankton populations over the short term. To better understand the factors limiting and regulating picophytoplankton, it is important to investigate picophytoplankton population variability on short timescales. It is particularly interesting since changes in these short-term processes are likely to influence long-term changes in populations [
4].
There is no doubt that light is a major factor driving the variability of picophytoplankton throughout the day. Previously, it was shown that Syn cell cycles were phased with the daily light cycle [
6,
7], possibly because of a genetic clock [
8]. There is, however, evidence in natural ecosystems and cultures that the division of Syn, Pro, and PE does not occur simultaneously [
4,
9]. There is no clear indication of whether phase differences between groups are related to their differential sensitivity to light [
10], but Jacquet et al. [
11] suggest that the cell cycle of Pro is closely linked to irradiance levels. Furthermore, in oceanic environments, photosynthetic release is believed to be one of the main sources of dissolved organic matter (DOM) for marine bacteria [
12]. As a consequence, intensive research has been conducted to determine if phytoplankton-bacteria interactions influence short-term bacterial variability since a tight coupling between primary production and bacterial activity is expected to result in periodic and significant fluctuations in bacterial heterotrophic activity in marine environments. In addition to ultraviolet radiation, bacterivory, and viral lysis, other factors that affect marine bacteria also vary daily [
13]. In general, diel patterns in abundance are more altered than other parameters, suggesting an imbalance between growth and loss. Due to diel variations in picophytoplankton community structure indicating that loss processes occur differently during the day [
14,
15], nanoflagellate grazing activity may vary with picophytoplankton cell cycle [
7], and viral infection could display diel variability [
14], disruption of the diel periodicity in nanoflagellates or virus abundance could increase prey abundance.
The concept of mixotrophy, the combination of photosynthesis with phagotrophy in one microorganism, is well-established among the vast majority of photosynthetic species. There is a growing recognition that mixotrophs play an important role in biogeochemical cycling in aquatic ecosystems, as well as their wide distribution in aquatic ecosystems. It has previously been demonstrated [
16] that pigmented nanoflagellates (PNF) are the key grazers of Syn populations in subtropical western Pacific coastal waters. In response to daily fluctuations in the size, abundance, biomass, or composition of prey, grazers may respond differently to their diverse nutritional requirements [
17]. According to the following study, diel variations in ingestion rates are affected by non-dividing cells of Syn [
18], implying food size selectivity is responsible for PNF grazing impact on Syn. Moreover, it was estimated that 52% of the total bacteria consumption was accounted for by the 3–6 m PNF, which was a major consumer of nanoflagellates [
19]. In these situations, heterotrophy enables mixotrophic PNF to acquire nutrients from their prey when nutrients are scarce. Further, photosynthetic PE is an important primary producer in oceanic and coastal environments. Aside from being primary producers, PE has been found in several studies to be mixotrophs and major predators of bacteria [
20,
21]. It is important to consider quantitatively the proportion of phagotrophs in microbial food webs, even though it was highly variable in short-term samplings.
In coastal waters of the western Pacific subtropical ocean, we analyzed the relationship between marine viruses, bacteria, and picophytoplankton and their nanoplanktonic protistan consumers during the diel cycle. The aim of this study was to document the diel variations in viral, picoplankton, and nanoflagellate abundance using flow cytometry samples taken at a high frequency (4 h intervals) during two cycles of 48 h in spring 2024. In addition, we examined diel samples taken from the same study site of marine viruses, bacteria, picophytoplankton, and nanoflagelates in a 10L incubation bottle in order to compare the difference in population abundance between field and incubation conditions.
2. Materials and Methods
2.1. Study Site and Samplings
We selected a semi-enclosed port, which is a shallow (3 m depth) oligotrophic coastal station with relatively insignificant wave effects inside the port, located on the north coast of Taiwan as the site of study (
Figure 1). A previous study has found that the temperatures of the water exhibited a clear seasonal cycle and ranged between 17°C (January) and 31℃ (July). The concentration of chlorophyll
a in this study ranged from 0.03 to 6.45 mg m
-3 (average 2.29 mg m
-3), with the highest concentrations occurring during the warm season (May to October) [
22]. During two successive three-day periods (from 9-11 March 2024), two diel cycles were studied in this study. The sampling of surface water was performed at 0.5-m depth with polycarbonate carboys at a frequency of six samplings per day (every 4 h), and then the samples were transported to a laboratory within 20 minutes for analysis. Temperature and salinity of the waters were measured with a Multiparameter (HI98194). In addition, seawater was also transferred directly into 10 L polycarbonate bottles for incubation under natural light outside the lab under an in situ temperature tank when used for incubation experiments. Water samples in incubation were collected every 4 h during the study period.
2.2. Net Increased Abundance of Bacteria and Picophytoplankton
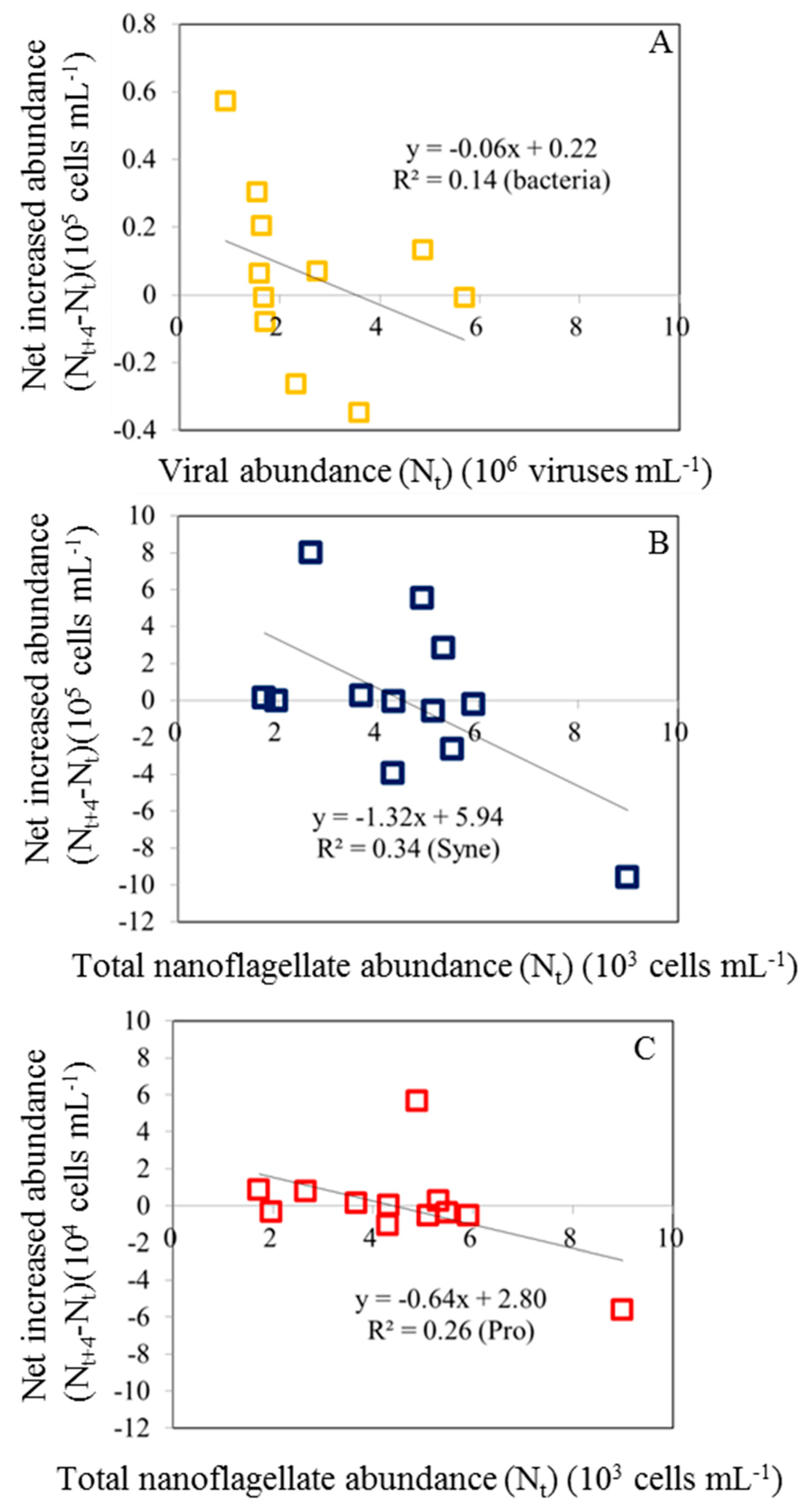
In this study, to determine the effect of nanoflagellate and viral abundance changes on diel variability, we calculate the net increased abundance of bacteria and picophytoplankton at each sampling time. Increased abundance of picoplankton is a result of net growth rate (growth rate - grazing rate). A net increased abundance can be calculated as Nt+4-Nt, where N corresponds to the abundance of bacteria or picophytoplankton, t+4, and t is measured every four hours.
2.3. Flow Cytometric Analyses
We collected 2 ml seawater samples from each treatment, preserved them in 0.5% paraformaldehyde (final concentration), flash-frozen, and stored them in liquid nitrogen for enumerating nanoflagellates, picophytoplankton and heterotrophic bacteria. Samples were frozen at −80°C until analysis in the laboratory a CytoFLEX S flow cytometer (Beckman Coulter, Indianapolis) equipped with a 488 nm air-cooled argon-ion laser, a standard 525 nm filter, and an SYBR signal trigger. To minimize interference from high particle density, viral samples were diluted 1:10 in TE buffer (pH 8.0, EM grade) prior to staining. SYBR Green I (final concentration 1:50,000 commercial stock) was stained onto the diluted samples and incubated in the dark for 10 minutes at 80°C. Following the staining process, samples were cooled to 25 °C in an ice bath and analyzed by FCM according to Brussaard [
23]. To detect and eliminate buffer noise, blank controls of TE buffer stained with SYBR Green I were used. According to Hammes and Egli [
24], bacteria samples were stained with SYBR Green I (final concentration 1:10,000) for 15 minutes in the dark, then processed by FCM. Based on flow cytometric analysis, on the basis of their red fluorescence from chlorophyll (>650 nm) and orange fluorescence from phycoerythrin (578 nm) and light scatter signals (SSC), picophytoplankton from the area were separated into two groups (Syne and Pro) according to Calvo-Díaz and Morán [
25]. Furthermore, in this study, heterotrophic and pigmented nanoflagellates enumeration was also performed using flow cytometer according to Rose et al. [
26].
3. Results
3.1. Temporal Variability in Bacterial and Viral Abundance
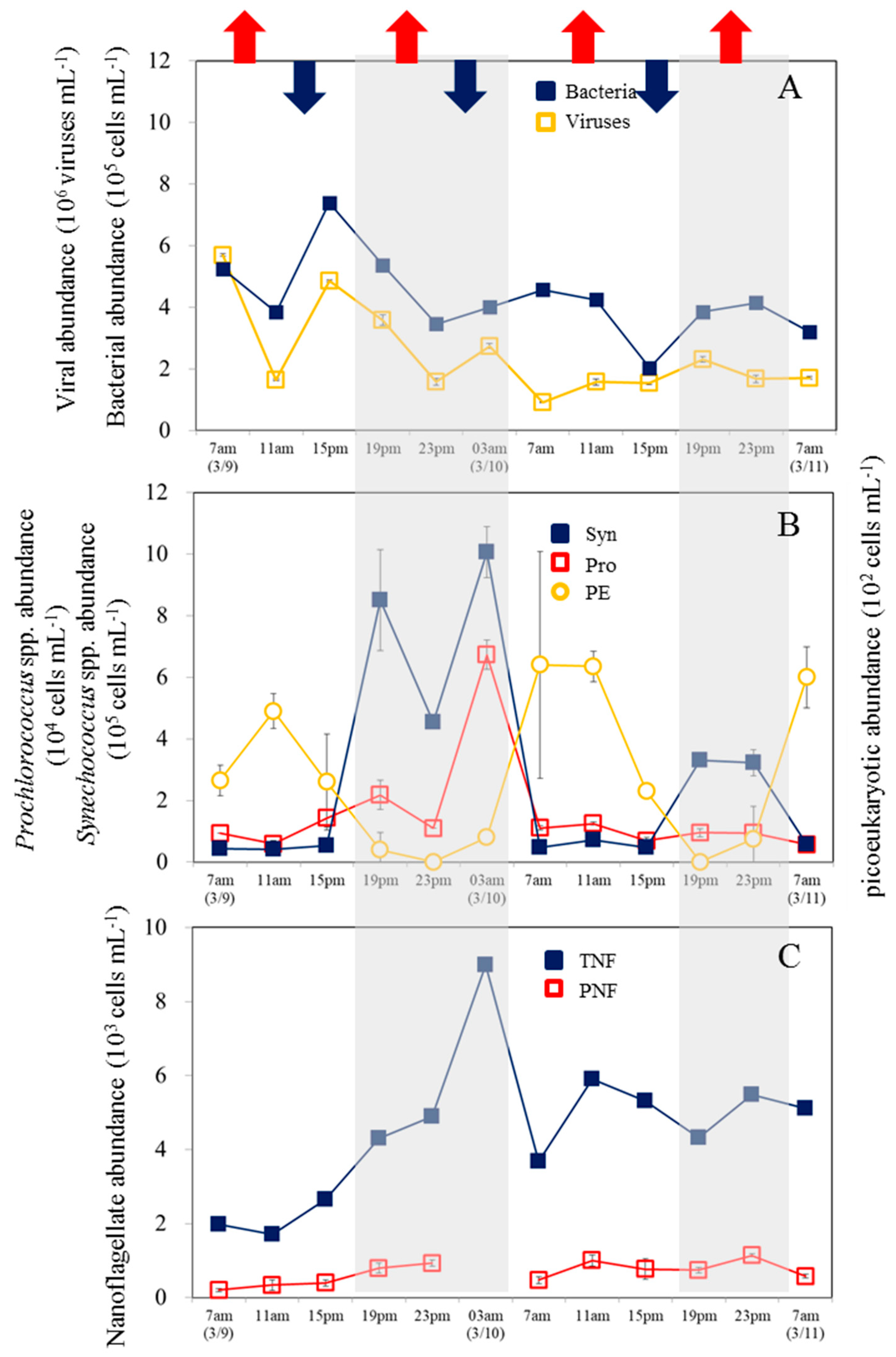
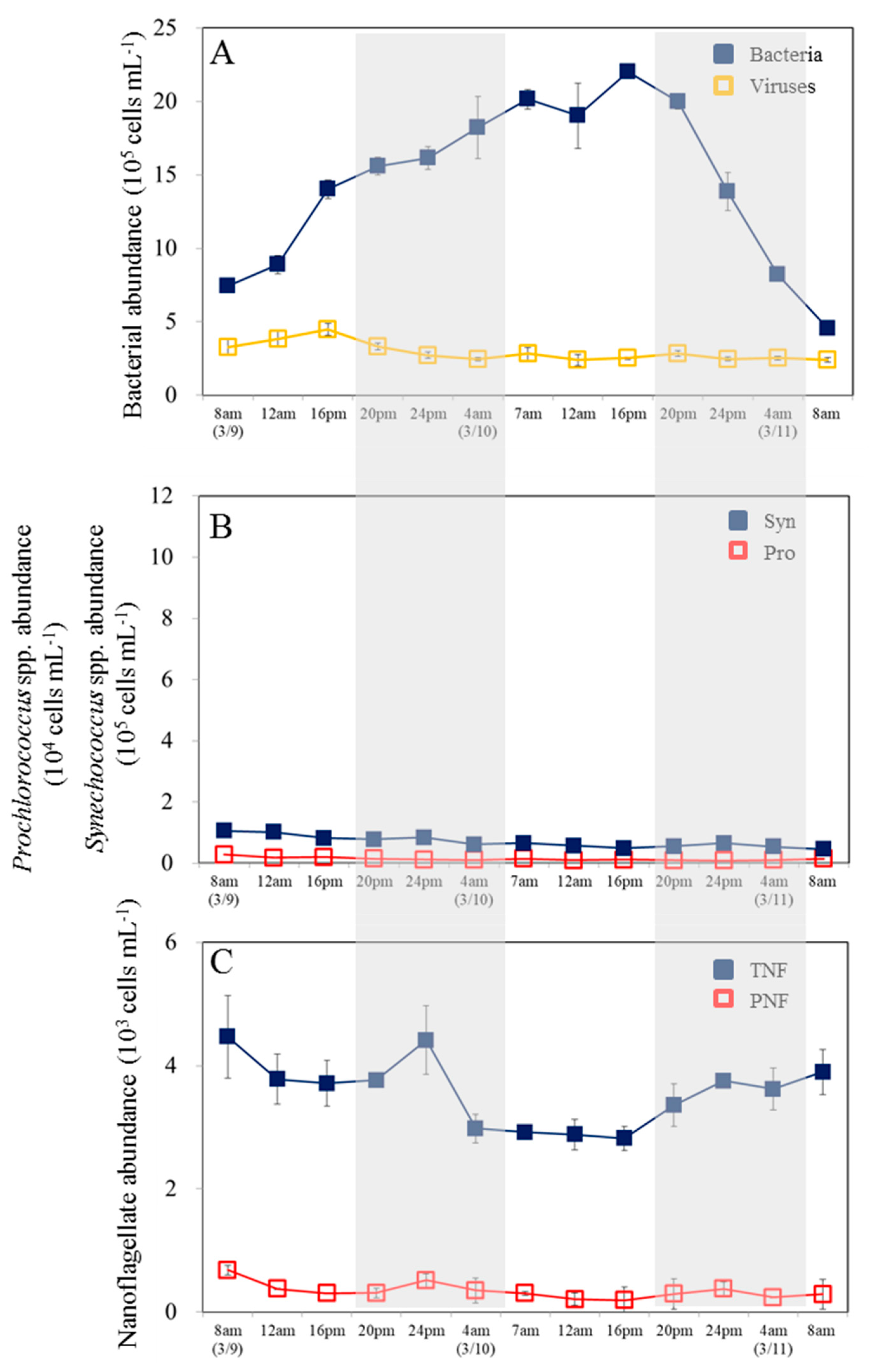
Temperature variations varied from 18.3 to 20.1°C during sampling times over the 48-hour period (data not shown). In our observation period, the semi-diurnal tide exhibited irregular diurnal patterns, with the highest water levels around 10 a.m. and 18:15 p.m. and the lowest levels around 14:40 p.m. and 02:40 a.m. (
Figure 2). Furthermore, bacterial abundance ranged between 0.91 and 5.7 x 10
5 cells ml
−1, with the highest value occurring at 7 am in the first cycle. The abundance of bacteria did not show a consistent diel pattern (
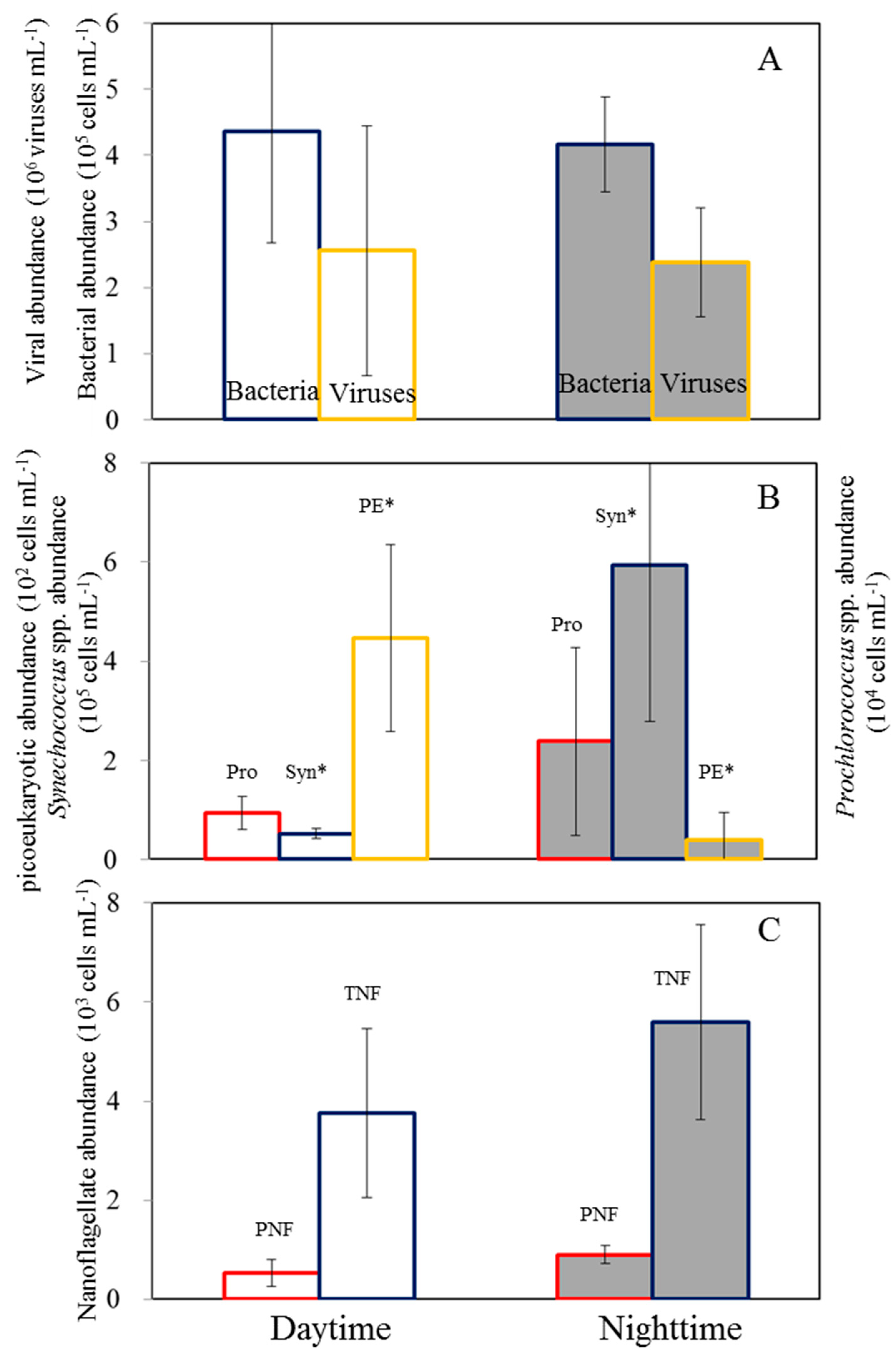
Figure 2A). Furthermore, there was no significant difference in bacterial abundance between the day and night (
p > 0.05,
t-test) (
Figure 3A). The virus density ranged from 0.91 to 5.7 × 10
6 viruses ml
–1, with no significant difference (
p >0.05) between daytime and nighttime (
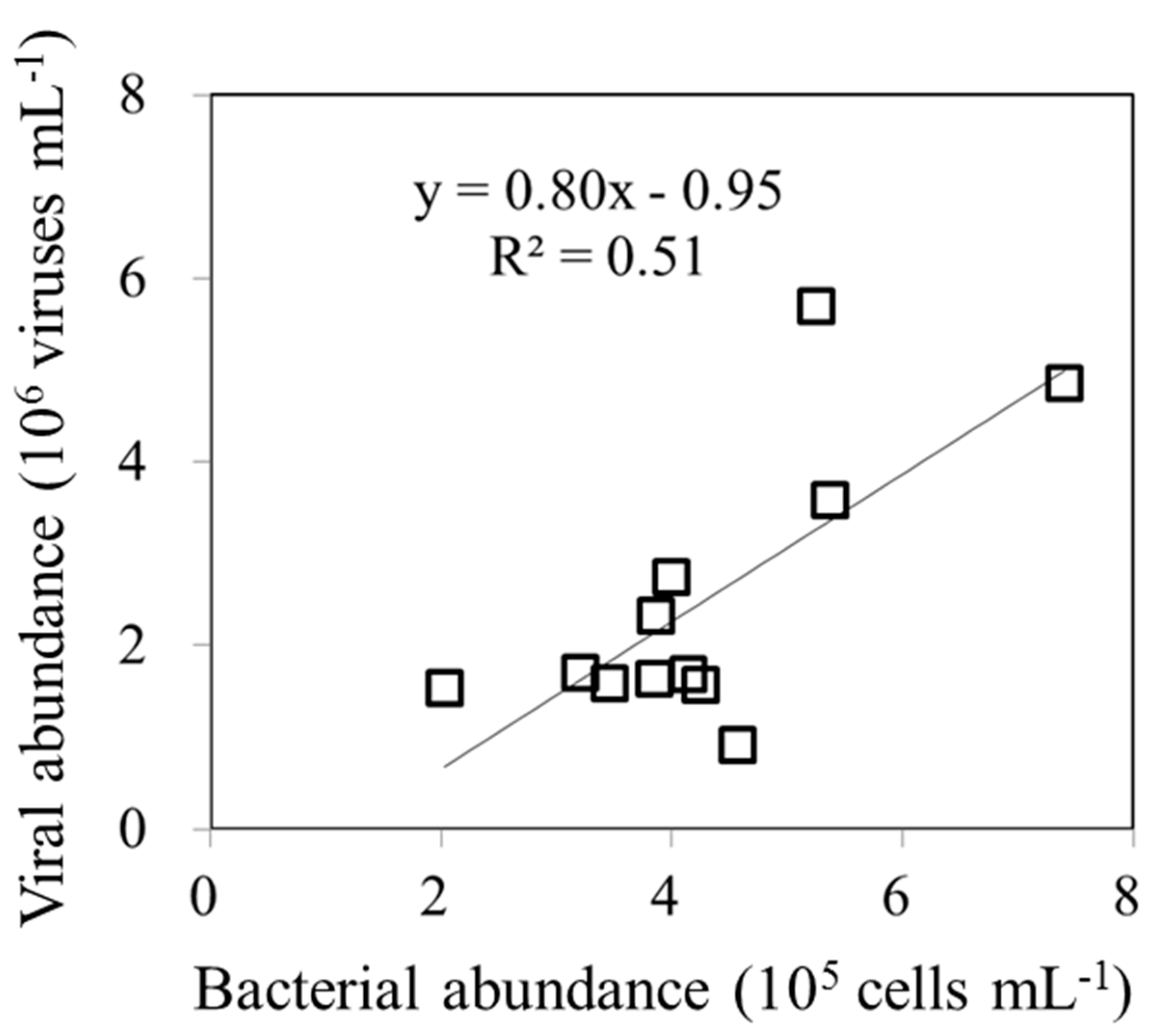
Figures 2A and 3A). Based on the results of our analysis, it was found that bacterial abundance showed a similar pattern as viral abundance and viral abundance was positively correlated with bacterial abundance (
Figure 4).
3.2. Temporal Variability in Picophytoplankton and Nanoflagellate Abundance
There is a general tendency for Syn abundance to increase during the dark period and decrease during the light period. First cycle (March 9-10), Syne abundance followed a clear 24-h cycle (0.41 to 10.1 x 10
5 cells ml
−1) (
Figure 2B). There was a less pronounced diel pattern in Syne abundance in the second diel cycle due to partly cloudy and rainy conditions on 10 March (
Figure 2B). There was a higher average abundance of Syne at night than during the day (
p < 0.05,
t-test) (
Figure 3B). Additionally, Pro showed a significant increase in abundance during the first diel cycle at night, peaking at 3 am (March 10) (
Figure 2B). During the second cycle, no diel pattern of Pro abundance was observed. Moreover, there was no significant difference between average abundance values at night and during the day during the study period (
p > 0.05,
t-test) (
Figure 3B). Surprisingly, the opposite pattern was observed in PE, whose concentration increased during the daytime and decreased during the dark phase (
Figure 2B). Note that the average abundance of PE during the daytime was significantly higher (
t-test,
p < 0.005) than at night (
Figure 3B). Furthermore, the heterotrophic nanoflagellate (HNF) was numerically dominant among the total nanoflagellate (TNF) communities analyzed, ranging from 1.4 × 10
3 cells ml
−1 to 9.0 × 10
3 cells ml
−1 (data not shown). During the first diel cycle, the highest TNF concentrations were observed at 3am, but no apparent trend was noticed during the second diel cycle (
Figure 2C). There was no significant difference in TNF and PNF abundance between night and day in our study (
Figure 3C).
3.3. Net Increased Abundance of Bacteria and Picophytoplankton
An increase in picoplankton abundance is a result of an increase in net growth rate (growth rate minus grazing rate). In our study, we found that the net abundance of bacteria had a negative relationship with the viral abundance (
Figure 5A). In the present study, diel variations in bacterial abundance were clearly associated with viral lysis rates. Furthermore, with all data pooled, the regression analyses also revealed that a significant negative relationship between the net increased abundance of Syn and Pro and total nanoflagellate abundance (
Figure 5B,C).
3.4. Temporal Variability in Microbial Communities in the Experimental Incubations
The bacteria in our study grew rapidly in the incubation experiments, reaching a maximum abundance of 22.0 × 10
5 cells ml
–1 after 32 h (
Figure 6A), corresponding to bacterial growth rate was 0.81 d
-1. Viral abundance varied from 2.4 × 10
6 to 4.5 × 10
6 viruses ml
−1, however, it was not influenced by diel changes of bacteria during incubation (
Figure 6A).
Overall, cell abundance of Pro and Syn in the experimental incubations, varied from 0.10 × 10
4 to 0.29 × 10
4 cells ml
−1 and 0.46 × 10
5 to 1.06 × 10
5 cells ml
−1, respectively (
Figure 6B). As for diel variations, we found no obvious trend for Syn or Pro abundance (
Figure 6B). As for nanoflagellates, the abundance of PNF followed a slightly diel cycle during the incubation period (
Figure 6C). TNF abundance increased slightly in the dark period, peaking at midnight and decreasing until dawn during the first cycle. In the daytime after dawn, only the lower values were observed in TNF concentrations (
Figure 6C).
Surprisingly, PE abundance showed a significant diel periodicity throughout the study period in the incubation (
Figure 7). A significantly higher variance was shown by PE over the diel cycle than by Syne and Pro (
Figure 6B). The second cycle showed a significant increase in PE abundance from noon to nighttime, and a decrease in bacterial abundance during nighttime, to show the prey-predator cycle (
Figure 7).
4. Discussion
Observing short-term patterns of picoplanktonic populations is largely influenced by the natural alternation of day and night. Our study focused on the diel variability of different picophytoplankton populations and observed changes in bacterial and viral abundance during the short-term period. Briefly, our results indicate (i) clear diel variations in picophytoplankton, (ii) differences in the microbial community diel patterns in the field and incubated samplings, and (iii) a potential role for picoeukaryotes. The results of this study are discussed in the following section.
4.1. Diel Patterns in Bacterial and Viral Abundance
Surface waters are driven by a complex infrastructure of biological and physicochemical processes driven by sunlight and the marine biota, resulting in day and night variations in heterotrophic activity. Sunlight and aquatic bacteria interact in a variety of ways, including direct effects on cells through irradiation or indirect effects caused by shifting primary production rates and changes in the nature of dissolved organic matter [
27,
28]. These interactions are a major factor in short-term variation in aquatic bacterial communities. In several marine environments, bacterial activity is significantly influenced by photosynthetic rates [
26,
27]. An earlier study, on the other hand, indicated that bacterial growth in ocean surface water in a diel pattern opposite to phytoplankton. This study also suggested that inorganic nutrients, organic substrate supply rates, and bacterivory all contribute to the bacterial diel cycle [
29]. Besides these reasons, about half of the euphotic layer is exposed to UV radiation, which more adversely affects bacteria than phytoplankton [
30]. Furthermore, until now, diurnal fluctuations in bacterial activity have mostly been studied at the community level, and very few studies have addressed this issue at the single-cell or group level. There may be differences in substrate preferences or phytoplankton species among different phylogenetic groups [
31]. Even though we did not measure bacterial activity and different groups at different times of the day, future experiments should estimate bacterial growth changes over time.
In this study, we found that the abundance of bacteria did not show a consistent diel pattern. Furthermore, there was no significant difference in bacterial abundance between the day and night during the study period (
Figure 4A). The results of this study are similar to those of another, which found that the fluctuations in bacterial abundance over a day seem to be quite stable, where bacterial production and mortality are generally considered to be balanced [
32]. Our study found that bacteria had no diel pattern of abundance (
Figure 3A), but varied with viral abundance (
Figure 5). In this situation, we propose the following hypothetical scenario for diel variations in viral infection and lysis of bacteria in coastal waters. Indeed, two dial incubations were performed at the same study site previously, and viral production was higher during the day than at night [
33]. Thus, diel changes in viral production indicate that estimates of virus-mediated bacterial mortality and regeneration DOM and nutrients release also vary during the day [
34].
Viral diel dynamics were implicated by previous culture experiments and field studies, based on the light-dark cycle and host replication in addition to transcriptional and metabolic processes [
13,
35,
36]. Several studies have demonstrated the ability of viral abundance to fluctuate daily and even hourly [
37], implying that changes in viral production can occur even over short periods of time [
33]. Moreover, in the present study, there was no significant difference in viral abundance between the day and night during the study period (
Figure 4A). For a comprehensive analysis of viral dynamics, measurements of viral production and decay must be made independently. Several studies have shown that UV radiation damages DNA-containing viruses in marine environments in the past decade [
38,
39]. It was shown by Suttle and Chen [
38] that areas with high exposure to sunlight experience higher virus decay rates, ranging from 40 to 80% per hour. In other studies, UV damage was identified as one of the most significant factors that contribute to viral decay [
39]. It was not apparent that daytime and nighttime viral abundance differed significantly in this study. It is certainly possible that virus populations increase in production during daytime, and have higher viral decay under sunlight.
4.2. Diel Patterns in Picophytoplankton Abundance
The abundance of picophytoplankton groups changes daily due to physical (e.g., light and temperature) and biological (e.g., cell division, grazing, and viral lysis) factors [
4,
6,
7,
9,
11]. In this study, abundances of Syn varied dramatically across the 48-hour sampling period, with high values recorded in samples taken in the early evening or at midnight (
Figure 2B). Moreover, partly cloudy and rainy conditions on 10 March resulted in a less pronounced diel pattern in Syn abundance (
Figure 2B). Different sunlight intensities during the study period are partially responsible for the diel variations of Syn mentioned above. Responses of phytoplankton to light variability are crucial for their dynamics because they enable them to adapt to the surrounding environment and optimize their performance. Additionally, picophytoplankton was more sensitive to changes in light during the day than larger phytoplankton [
40]. In other study also suggested that light is clearly one of the most important factors in regulating the cell cycle, with cell division occurring from late afternoon to early evening [
41].
A high-frequency sampling strategy is needed to identify the key biological factors (such as growth and mortality rates) influencing picophytoplankton population abundance on a time scale of hours. Increases in picophytoplankton abundance can only be explained by cell division in the absence of strong advection, while decreases usually result from cell death, grazing, or viral infections [
42,
43]. According to most studies, Syn abundance varies significantly with the day and night, with peak abundance occurring late afternoon or early night, with inverse patterning of cell size [
18,
41], despite considerable differences between depths and locations. Previously, we reported that the ingestion rates of Syn are affected by diel changes in non-dividing Syn cells, implying food selectivity is a factor in the effects of nanoflagellate grazing on Syn [
18]. It is worth noting that the study of Tsai et al. [
18], suggested that day/night net growth rates allow us to speculate two mechanisms for diel variation in the abundance of Syn: 1. dividing cells of Syn are too large to be consumed by nanoflagellates during the day; or 2. the ingestion rates increase in response to higher abundances of non-dividing Syn at night. These results strongly indicated that nanoflagellate grazing was the underlying biological factor regulating diel variations in Syn abundance in our study site.
In the present study, Pro and Syn abundance demonstrated significant higher values at midnight during the first diel cycle (
Figure 2B). Weaker but significant diel periodicity in Pro abundance was detected in our study. Even though Syn and Pro are commonly co-occurring, their adaptation to biogeochemical conditions is different. Light is most likely to play a role in the differential distribution of Sy and Pro among physicochemical factors [
44]. In previous studies, strong light intensities (including UV radiation) were suggested to be detrimental to prokaryotes, especially Pro, leading to fluorescence quenching, growth slowdown, and a reduction in DNA synthesis [
4]. Apart from light, water turbidity, disturbances, competition within groups, and grazing pressure are also important factors controlling Pro and Syn fluctuations [
45,
46].
4.3. Potential Role of Picoeukaryotes
An interesting result of our study is that the PE abundance increased during the daytime and decreased during the dark phase (
Figure 2B). Note that the average abundance of PE during the daytime was significantly higher than at night. In this study, PE shows the opposite diel dynamic to Syn and Pro. As a result of these discoveries, PE may have distinctive ecological characteristics compared to other picophytoplankton (Syn and Pro). According to our understanding, photosynthetic picoeukaryotes contribute significantly to phytoplankton biomass and primary production [
47]. There have been more studies demonstrating that photosynthetic picoeukaryotes can be mixotrophs than previously thought [
20,
21].
As we observed in the culture experiments, PE was also possible for bacterial prey with heterotrophs (
Figure 7). In comparison with similar-sized autotrophs, phagotrophic mixotrophs should have an advantage when dissolved nutrients are scarce as compared to prey nutrients [
48]. Consequently, we found no diel trend for Syn or Pro abundance, and maintained the lower daytime abundance in the culture experiments (
Figure 6B). However, a significantly diel cycle was shown by PE, and show significant increase in PE abundance from noon to nighttime, with a decrease in bacterial abundance during nighttime. The data from our study site suggest that PE may be a significant bacterivore during the springtime. Despite being outnumbered by picocyanobacteria (Syn and Pro, > 10
4 cells mL
-1), the larger cell size of PE makes it impossible for them to compete for nutrients. Small mixotrophic eukaryotes may obtain nutrients through the consumption of prey in this situation. Mixotrophs may be particularly advantageous in oligotrophic ecosystems since nutrients are often limited to phototrophs that utilize mixotrophic (autotrophy and heterotrophy) pathways to acquire nutrients [
20,
21]. There are many similarities between picoeukaryotes and prokaryotes, but they also differ fundamentally in their ecological roles, functionality, and evolutionary paths. Future studies aiming to understand the interactions between prokaryotes and picoeukaryotes within marine microbial communities should take these differences into account.
To summarize, detailed analyses of diel patterns of bacteria and picophytoplankton remain crucial to understanding how populations develop and their physiological status. In this study, we presented a description of diel patterns of variability in bacterial and picophytoplankton abundances and attempted to analyze them. As far as the abundance of bacteria is concerned, it seems that it did not follow a consistent diel pattern. Overall, bacterial mortality and production are considered to be balanced. The diel patterns of picophytoplankton show a great deal of complexity, with each population displaying its behaviors which are partly influenced by the amount of light available. An interesting result of our study is that predation on prokaryotes (heterotrophic bacteria and picocyanobacteria) may be another pathway for picoeukaryotes. Biogeochemical cycles in the ocean could be significantly impacted by shifts in species composition and the relative proportion of mixotrophic and strictly autotrophic PE.
Author Contributions
Conceptualization: A.-Y.T.; methodology: A.-Y.T.; P.W.-Y.C.; M.O.; validation: A.-Y.T.; formal analysis: P.W.-Y.C., A.-Y.T., and M.O.; investigation: A.-Y.T.; P.W.-Y.C.; M.O.; G.S.; U.M.; L.T.; S.E.; S.; I. D. P.; and A.G.; resources: A.-Y.T.; data curation: A.-Y.T.; Writing—Original draft preparation: A.-Y.T.; P.W.-Y.C.; Writing—Review and editing: A.-Y.T. and V.M.; funding acquisition: A.-Y.T.; V.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was conducted in the frame of the Russian state assignments No. 121040600178-6, AAAA-A21-121121700354-9 and supported by RFBR projects 18-44-920026 (works on the GAF phenomenon) and 21-55-52001, and the Ministry of Science and Technology, ROC (Taiwan), grant number MOST 111-2119-M-019-002.
Acknowledgments
We appreciate the language editing and helpful comments related to this manuscript from Choice Language Service.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moran, X.A.G.; Lopez-Urrutia, A.; Calvo-Diaz, A.; et al. Increasing importance of small phytoplankton in a warmer ocean. Global Chang. Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Flombaum, P.; Wang, W.-L.; Primeau, F.W.; et al. Global picophytoplankton niche partitioning predicts overall positive response to ocean warming. Nat Geosci. 2020, 13, 116–120. [Google Scholar] [CrossRef]
- Jacquet, S.; Lennon, J.-F.; Marie, D.; Vaulot, D. Picoplankton population dynamics in coastal waters of the N. W. Mediterranean Sea. Limnol Oceanogr. 1998, 43, 1916–1931. [Google Scholar] [CrossRef]
- Vaulot, D.; Marie, D. Diel variability of photosynthetic picoplankton in the equatorial Pacific. J Geophys Res. 1999, 104, 3297–3310. [Google Scholar] [CrossRef]
- Vaulot, D.; Lebot, N.; Marie, D.; Fukai, E. Effect of phosphorus on the Synechococcus cell cycle in surface in Mediterranean waters during summer. Appl Environ Microbiol. 1996, 132, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.Y.; Chiang, K.P.; Chang, J.; Gong, G.C. Seasonal diel variations of picoplankton and nanoplankton in a subtropical western Pacific coastal ecosystem. Limnol Oceanogr. 2005, 50, 1221–1231. [Google Scholar] [CrossRef]
- Lefort, T.; Gasol, J.M. Short-time scale coupling of picoplankton community structure and single-cell heterotrophic activity in winter in coastal NW Mediterranean Sea waters. J Plank Res. 2014, 36, 243–258. [Google Scholar] [CrossRef]
- Johnson, C.H.; Golden, S.S.; Ishiura, M.; Kondo, T. Circadian clocks in prokaryotes. Mol. Microbiol. 1996, 21, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, S.; Partensky, F.; Lennon, J.F.; et al. Diel patterns of growth and division in marine picoplankton in cultures. J Phycol. 2001, 37, 357–369. [Google Scholar] [CrossRef]
- Sommaruga, R.; Hofer, J.S.; Alonso-Sáez, L.; et al. Differential sunlight sensitivity of picophytoplankton from surface Mediterranean coastal waters. Appl Environ Microbiol. 2005, 71, 2154–2157. [Google Scholar] [CrossRef]
- Jacquet, S.; Partensky, F.; Marie, D.; et al. Cell cycle regulation by light in Prochlorococcus strains. Appl Environ Microbiol. 2001, 67, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, L.R.; leB. Williams, P.J.; Azam, F.; Hobbie, J.E. The microbial loop. Oceanogr. 2007, 20, 28–33. [Google Scholar] [CrossRef]
- Winter, C.; Herndl, G.J.; Weinbauer, M.G. Diel cycles in viral infection of bacterioplankton in the North Sea. Aquat Microb Ecol. 2004, 35, 207–216. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Gong, G.C.; Sanders, R.W.; Chiang, K.P.; Huang, J.K.; Chan, Y.F. Viral lysis and nanoflagellate grazing as factors controlling diel variations of Synechococcus spp. summer abundance in coastal waters of Taiwan. Aquat Microb Ecol. 2012, 66, 159–167. [Google Scholar] [CrossRef]
- Connell, P.E.; Ribalet, F.; Armbrust, E.V.; White, A.; Caron, D.A. Diel oscillations in the feeding activity of heterotrophic and mixotrophic nanoplankton in the North Pacific Subtropical Gyre. Aquat Microb Ecol. 2020, 85, 167–181. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Chiang, K.P.; Chan, Y.F.; Lin, Y.C.; Chang, J. Pigmented nanoflagellates in the coastal western subtropical Pacific are important grazers on Synechococcus populations. J Plank Res. 2007, 29, 71–77. [Google Scholar] [CrossRef]
- Stoecker, D.K.; Hansen, P.J.; Caron, D.A.; Mitra, A. Mixotrophy in the marine plankton. Annu Rev Mar Sci. 2017, 9, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.Y.; Chin, W.M.; Chiang, K.P. Diel patterns of grazing by pigmented nanoflagellates on Synechococcus spp. in the coastal ecosystem of subtropical western Pacific. Hydrobiol. 2009, 636, 249–256. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Gong, G.C.; Sanders, R.W.; Chen, W.H.; Chao, C.F.; Chiang, K.P. Importance of bacterivory by pigmented and heterotrophic nanoflagellates during the warm season in a subtropical western Pacific coastal ecosystem. Aquat Microb Ecol. 2011, 63, 9–18. [Google Scholar] [CrossRef]
- Sanders, R.W.; Gast, R.J. Bacterivory by phototrophic picoplankton and nanoplankton in Arctic waters. FEMS Microbiol Ecol. 2012, 80, 242–253. [Google Scholar] [CrossRef]
- McKie-Krisberg, Z.M.; Sanders, R.W. Phagotrophy by the picoeukaryotic green alga Micromonas: Implications for Arctic Oceans. The ISME J. 2014, 8, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-F.; Tsai, A.-Y.; Ishikawa, A.; Chiang, K.P. Seasonal dynamics of ciliate cysts and the impact of short-term change of salinity in a eutrophic coastal marine ecosystem. Terr Atmo Ocean Sci. 2013, 24, 1051. [Google Scholar] [CrossRef]
- Brussaard, C.P.D. Optimization of procedures for counting viruses by flow cytometry. Appl Environ Microbiol. 2004, 70, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Egli, T. Cytometric methods for measuring bacteria in water: Advantages, pitfalls and applications. Anal Bioanal Chem. 2010, 397, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Díaz, A.; Morán, X.A.G. Seasonal dynamics of picoplankton in shelf waters of the southern Bay of Biscay. Aquat Microb Ecol. 2006, 42, 159–174. [Google Scholar] [CrossRef]
- Rose, J.M.; Caron, D.A.; Sieracki, M.E.; Poulton, N. Counting heterotrophic nanoplanktonic protists in cultures and aquatic communities by flow cytometry. Aquat Microb Ecol. 2004, 34, 263–277. [Google Scholar] [CrossRef]
- Ghiglione, J.F.; Mevel, G.; Pujo-Pay, M.; Mousseau, L.; Lebaron, P.; Goutx, M. Diel and seasonal variations in abundance, activity and community structure of particle-attached and free-living bacteria in NW Mediterranean Sea. Microb Ecol. 2007, 54, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-González, C.; Lefort, T.; Massana, R.; Simó, R.; Gasol, J.M. Diel changes in bulk and single-cell bacterial heterotrophic activity in winter surface waters of the northwestern Mediterranean Sea. Limnol Oceanogr. 2012, 57, 29–42. [Google Scholar] [CrossRef]
- Shiah, F.K. Diel cycles of heterotrophic bacterioplankton abundance and production in the ocean surface waters. Aquat Microb Ecol. 1999, 17, 239–246. [Google Scholar] [CrossRef]
- Jeffrey, W.H.; Pledger, R.J.; Aas, P.; Hager, S.; Coffin, R.B.; Haven, R.V.; Mitchell, D.L. Diel and depth profiles of DNA photodamage in bacterioplankton exposed to ambient solar ultraviolet radiation. Mar Ecol Prog Ser. 1996, 137, 283–291. [Google Scholar] [CrossRef]
- Alonso-Sáez, L.; Gasol, J.M. Seasonal variations in the contributions of different bacterial groups to the uptake of low-molecular-weight compounds in northwestern Mediterranean coastal waters. Appl Environ Microbiol. 2007, 73, 3528–3535. [Google Scholar] [CrossRef] [PubMed]
- Šimek, K.; Pernthaler, J.; Weinbauer, M.G.; Hornák, K.; Dolan, J.R.; Nedoma, J.; et al. Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl Environ Microbiol. 2001, 67, 2723–2733. [Google Scholar] [CrossRef]
- Ho, P.C.; Gong, G.C.; Hsieh, C.H.; Chen, P.W.Y.; Tsai, A.Y. Diel variation of viral production in a coastal subtropical marine system. Diver. 2021, 13, 426. [Google Scholar] [CrossRef]
- Winget, D.M.; Wommack, K.E. Diel and daily fluctuations in virioplankton production in coastal ecosystems. Environ Microbiol. 2009, 11, 2904–2914. [Google Scholar] [CrossRef] [PubMed]
- Aylward, F.O.; Boeuf, D.; Mende, D.R.; Wood-Charlson, E.M.; Vislova, A.; Eppley, J.M.; Romano, A.E.; DeLong, E.F. Diel cycling and long-term persistence of viruses in the ocean’s euphotic zone. Proc Natl Acad Sci USA. 2017, 114, 11446–11451. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nishimura, Y.; Watai, H.; Haruki, N.; Morimoto, D.; Kaneko, H.; Honda, T.; Yamamoto, K.; Hingamp, P.; Sako, Y.; et al. Locality and diel cycling of viral production revealed by a 24 h time course cross-omics analysis in a coastal region of Japan. ISME J. 2018, 12, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, S.; Heldal, M.; Iglesias-Rodriguez, D.; Larsen, A.; Wilson, W.; Bratbak, G. Flow cytometric analysis of an Emiliana huxleyi bloom terminated by viral infection. Aquat Microb Ecol. 2002, 27, 111–124. [Google Scholar] [CrossRef]
- Suttle, C.A.; Chen, F. Mechanisms and rates of decay of marine viruses in seawater. Appl Environ Microbiol. 1992, 58, 3721–3729. [Google Scholar] [CrossRef]
- Noble, R.T.; Fuhrman, J.A. Virus decay and its cause in coastal waters. Appl Environ Microblol. 1997, 63, 77–83. [Google Scholar] [CrossRef]
- Brunet, C.; Casotti, R.; Vantrepotte, V.; Conversano, F. Vertical variability and diel dynamics of picophytoplankton in the Strait of Sicily, Mediterranean Sea, in summer. Mar Ecol Prog Ser. 2007, 346, 15–26. [Google Scholar] [CrossRef]
- Binder, B.J.; DuRand, M.D. Diel cycles in surface waters of the equatorial Pacific. Deep Sea Res II Top Stud Oceanogr. 2002, 49, 2601–2617. [Google Scholar] [CrossRef]
- Christaki, U.; Giannakourou, A.; Van Wambeke, F.; Grégori, G. Nanoflagellate predation on auto-and heterotrophic picoplankton in the oligotrophic Mediterranean Sea. J Plankton Res. 2001, 23, 1297–1310. [Google Scholar] [CrossRef]
- Sullivan, M.B.; Waterbury, J.B.; Chisholm, S.W. Cyanophages infecting the oceanic cyanobacterium Prochlorococcus. Nature 2003, 424, 1047–1051. [Google Scholar] [CrossRef]
- Moore, L.R.; Goericke, R.; Chisholm, S.W. Comparative physiology of Synechococcus and Prochlorococcus: Influence oflight and temperature on growth pigments fluorescence and absorptive properties. Mar Ecol Prog Ser. 1995, 16, 259–275. [Google Scholar] [CrossRef]
- Grébert, T.; Doré, H.; Partensky, F.; Farrant, G.K.; Boss, E.S.; Picheral, M.; Acinas, S.G. Light color acclimation is a key process in the global ocean distribution of Synechococcus cyanobacteria. Proc Natl Acad Sci USA 2018, 115, E2010–E2019. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, J.; Zhang, X.; Wang, J.; Huang, K. Picophytoplankton size and biomass around equatorial eastern Indian Ocean. Microbiol Open. 2019, 8, e00629. [Google Scholar] [CrossRef]
- Jardillier, L.; Zubkov, M.V.; Pearman, J.; Scanlan, D.J. Significant CO2 fixation by small prymnesiophytes in the subtropical and tropical northeast Atlantic Ocean. ISME J. 2010, 4, 1180–1192. [Google Scholar] [CrossRef]
- Rothhaupt, K.O. Laboratorary experiments with a mixotrophic chrysophyte and obligately phagotrophic and photographic competitors. Ecol. 1996, 77, 716–724. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).