1. Introduction
Recessive Dystrophic Epidermolysis Bullosa (RDEB) is an orphan genetic disease caused by mutations in
COL7A1 [
1] leading to skin and mucosal blisters and erosions upon minor trauma. Skin fragility results in chronic inflammatory wounds or dystrophic scars in RDEB patients, which promote the development of cutaneous squamous cell carcinomas (SCCs). SCCs represent the main cause of death in RDEB patients due to their recurrent, sometimes multifocal, and early metastasizing characteristics [
2,
3].
The pathogenesis of SCCs in RDEB patients is not completely elucidated. Previous studies have suggested the implication of a permissive microenvironment in chronic wounds or lesional skin as a driving mechanism in SCC development [
4,
5]. Investigations of the tumor microenvironment (TME) have revealed reduced infiltration of CD3+, CD4+, and CD68+ immune cells in RDEB-SCCs compared to sporadic SCCs [
6,
7,
8], indicating an immunosuppressive TME in RDEB-SCCs. It is now well established that cell-TME interactions play a key role in cancer development and metastasis [
9,
10]. Neutrophils, in particular, have emerged as important components of the TME. Although they can have dual functions [
11], several studies have reported that neutrophil infiltration can favor tumor progression and metastasis in many cancer types by promoting tumor cell growth and immunosuppression [
12,
13]. Upon activation, neutrophils can release decondensed nuclear DNA-histone complexes associated with proteases and inflammatory mediators, known as neutrophil extracellular traps (NETs) [
14]. NETs have been shown to contribute to cancer cell progression [
15,
16] and metastasis spread in mouse models [
17,
18,
19] and patients [
20,
21]. Recently, a marker of NETs, citrullinated histone H3, was found to be elevated in the blood and associated with a poor prognosis in patients with advanced cancer [
22,
23].
In the present study, we investigated the immune profiles of 38 SCCs in a cohort of 20 RDEB patients (RDEB-SCC) and compared them with clinical-histopathological and prognostic features. We report a higher proportion of neutrophils to lymphocytes and NET formation in the TME of high-risk primary RDEB-SCCs. Furthermore, RDEB patients with high-risk primary SCC displayed increased levels of citrullinated histone H3 in the serum, suggesting this NET marker as a potential biomarker of poor prognosis in RDEB cancer progression.
2. Materials and Methods
2.1. Study Design and Ethics
The SIMOCEB study was approved by the Paris Nord Ethics Review Committee for Biomedical Research Projects (CEERB: n°2020-010 / ClinicalTrials.gov: NCT04285294). Consecutive patients were followed in the MAGEC reference center, (Centre de référence des maladies rares de la peau et des muqueuses d’origine génétique Nord) at Saint-Louis Hospital, and were included from March 2015 to December 2022 after written informed consent was obtained.
The diagnosis of RDEB subtypes: severe RDEB (RDEB-Sev), intermediary RDEB (RDEB-Int), or inversed RDEB (RDEB-Inv), relied on clinical findings, and was supported by type VII collagen expression and COL7A1 mutation analysis.
The diagnosis of SCCs was established by histopathological analysis of biopsies from suspicious skin lesions before surgery.
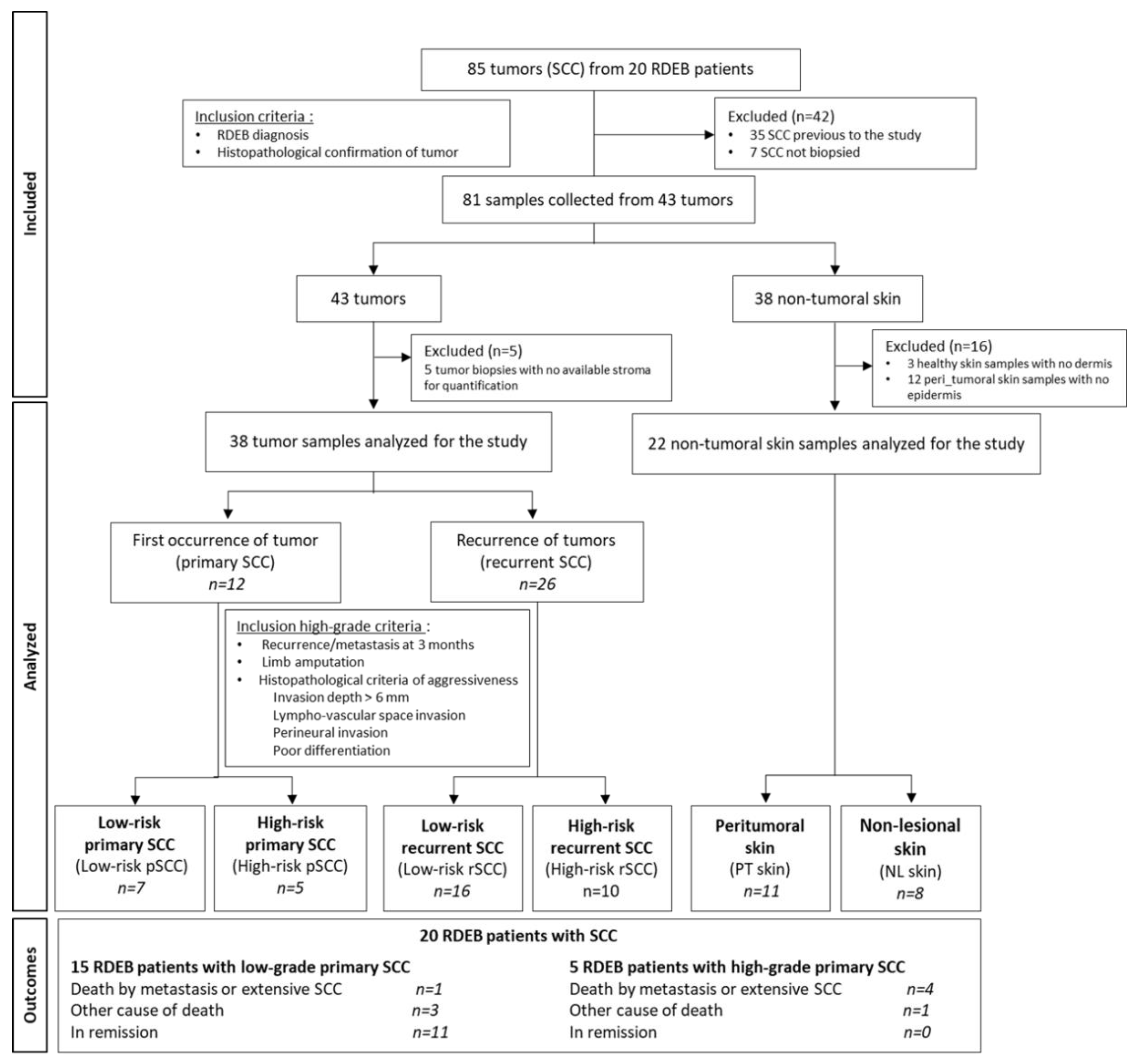
At the time of the surgery, 3 different types of samples were collected from surgical resections and residues using punch biopsy, when possible, from RDEB patients: the tumor, the lesional peri-tumoral skin, and the non-lesional skin for immunohistological and/or molecular analyses. Indeed, depending on the size of the surgical excision, not all biological samples could be collected in the 3 locations for the different experiments. In consequence, the collection of samples were prioritized based on the type of analysis required. The flow chart of the analyzed samples was described in the
Figure 1. Biological material was collected from 43 RDEB-SCCs. The morphological parameters of the 43 SCCs were defined by pathologists and 5 of these were discarded due to an unsatisfactory stroma quality. The biological material available for the study is listed in the
Supplementary table S1.
The diagnostic stage was based on the American Joint Committee on Cancer staging Manual, 8
th Edition classification system
[24], and adapted for the size criteria for the classification of the RDEB-SCCs. High-risk RDEB-SCC conditions were validated in consultation both by dermatologist, pathologist, and surgeon, according to the following features: local recurrence or metastasis at 3 months, massive local extension with recommendation of limb amputation, and histopathological criteria of aggressiveness (invasion depth > 6mm, lympho-vascular space invasion, perineural invasion, poor differentiation). Four SCC groups were analyzed: low-risk primary SCCs (low-risk-pSCC), high-risk primary SCCs (high-risk-pSCC), low-risk recurrent SCCs (low-risk-rSCC), and high-risk recurrent SCCs (high-risk-rSCC). The proportion of aggressiveness parameters in high-risk SCC groups is listed in
Supplementary Table S2.
2.2. Histology and Immunolabeling
Tissue samples were fixed in 4% formaldehyde in PBS pH 7.4 for 24 h, then embedded in paraffin. Hematoxylin-eosin (HE) staining was performed on 5µm paraffin-embedded sections using standard histological techniques. Representative images were taken using a slide scanner system Nanozoomer 2.0 HT (Hamamatsu) at the SFR Necker’s cell imaging platform.
Immunostaining analyses were performed on 5μm-paraffin sections of samples on the entire width of the punch-biopsy, including 32 of the 38 analyzed RDEB-SCCs (n=6/7 for low-risk primary SCCs, n=5/5 for high-risk primary SCCs, n=13/16 for low-risk recurrent SCCs, and n=8/10 for high-risk recurrent SCCs). Sections were deparaffinized, rehydrated, and followed by antigen retrieval using sodium citrate buffer (pH6). The primary antibodies listed in Supplementary table S3 were incubated at 4°C overnight. The following day, the appropriate secondary antibodies were incubated for 45 minutes at room temperature. Nuclei were stained using DAPI (1μg/ml). Positive cells were quantified at 200x magnification under a Zeiss Axiovert 200 microscope. Two non-consecutive sections per sample were analyzed for each staining and at least 5 fields were captured along the invasive margin of the tumors or the papillary dermis of the non-tumoral skin sample. Positive cells per field were quantified using the ImageJ software (NIH, Bethesda, USA). Representative images were taken using a Leica TCS SP8 SMD confocal microscope at 400x or 630x magnification.
2.3. Citrullinated Histone H3 ELISA
Histone H3 citrullination was measured in the serum of RDEB patients or healthy controls using the EpiQuik circulating Histone H3 Citrullination ELISA kit, according to the manufacturer's instructions (#P-3097, EpiGentek, NY). RDEB samples were collected during routine medical follow-up with or without SCC occurrence (n=11 and n=13 respectively). Briefly, samples and diluted standards were added to the plate coated with an anti-histone H3 (Citrulline R2+R8+R17) antibody and incubated for 60 min at 37 °C. After three washes, the detection antibody solution was added to each well for 60 min at RT. The plates were then washed four times and incubated with the detection substrate solution for 10 min at RT in the dark. Finally, a stop solution was added and the signal was measured on an automated analyzer Tecan (Research Triangle Park, NC, U.S.A.). The optical density was measured at 450 nm. Histone H3 citrullination concentration in the serum was calculated based on the histone H3R2R8R17cit3 standard curve, according to manufacturer's instructions.
2.4. Statistical Analysis
Analyses were conducted using The GraphPad Prism v10 software (La Jolla, CA). Values are given as means ± standard error of the mean. Each dot represents the mean of the quantifications for one sample. A Shapiro-Wilk normality test was used to assess whether the data followed the Gauss law. Depending on the normality of the dataset, statistical differences between the studied groups were assessed by Kruskal-Wallis or ANOVA tests followed by Dunn’s or Tukey’s multiple comparison test. Differences were considered significant for p<0.05.
3. Results
3.1. Two Distinct Clinical Outcomes of RDEB Patients with SCCs
Twenty consecutive RDEB patients were included in the study, representing a total of 85 clinically reported cutaneous SCCs. The disease characteristics of the patients are described in
Table 1 and
Supplementary table S1.
RDEB subtypes included 16 RDEB-Sev (80%), 2 RDEB-Int (10%), and 2 RDEB-Inv (10%). The median age of onset of the first SCC was 29 years (range: 18-48 years). At least one recurrent SCC was developed for 18 out the 20 RDEB patients, with a median of 5.1 SCCs per patient (range: 1-17) and a median frequency of recurrence of 14 months (range: 1-85 months). The median survival after the first SCC occurrence was 4.6 years (range: 0.4-19.1 years) regardless RDEB subtypes. The median survival was 2.3 years for RDEB patients with RDEB-Sev subtype (range: 0.4-14.6 years) in line with previous study [
3]. None of the RDEB patients received preoperative treatments including chemiotherapy, radiotherapy, or immunotherapy.
High-risk primary SCC (high-risk pSCC) was described in 5 RDEB-sev patients. Four out the 5 developed a high-risk recurrence locally or metastasis within 3 months, leading to death by extensive SCCs or metastasis of the patients. The fifth patient died from sepsis not related to his SCC. The median survival of RBEB patients with high-risk pSCC was 1.2 years (range: 1.1-2.2 years).
In contrast, 15 RDEB patients presented a low-risk primary SCC (low-risk pSCC) without aggressive criteria (11/15 RDEB-Sev (68.8%); 2/2 RDEB-Int (100.0%); 2/2 RDEB-Inv (100.0%)). At least one high-risk recurrent SCC (high-risk rSCC) has been developped in 4 of these 15 patients, leading to death from metastasis in 1 patient 8 months after the high-risk rSCC diagnosis. Three additional RDEB patients died from complications unrelated to their SCC, including 1 patient by sepsis 5 months after their low-risk pSCC. To date, 11 patients are in remission. The median overall survival of the 15 patients with low-risk pSCC was significantly higher than that of the 5 patients with high-risk pSCC (9.3 years ; range: 0.4-19.1 years) (p=0.003 by Mann-Whitney test).
3.2. Clinical-Histopathological Features of RDEB-SCCs
Biological tissues were collected from 43 out of the 85 SCCs (
Table 1). RDEB-SCCs were predominantly located on the extremities (31/43; 72%). Based on the histological reports mentioning the size of the tumor, 27 SCCs had a size greater than 2 cm (27/36; 67.5%), a factor of T2 stage
[25]. An invasion deeper than 6 mm was measured in 7 of 39 SCCs (16.3%), whose extension into the dermis was mentionned. Good differentiation was observed in 28 SCCs (28/43; 65.1%), while 15 SCCs exhibited moderate to poor differentiation (34.9%). Most patients were initially diagnosed with stage II disease (39/43; 90.7%)
RDEB-SCCs were divided into 4 groups according to their grading determined by clinical-histopathological parameters and recurrence (
Figure 1 and
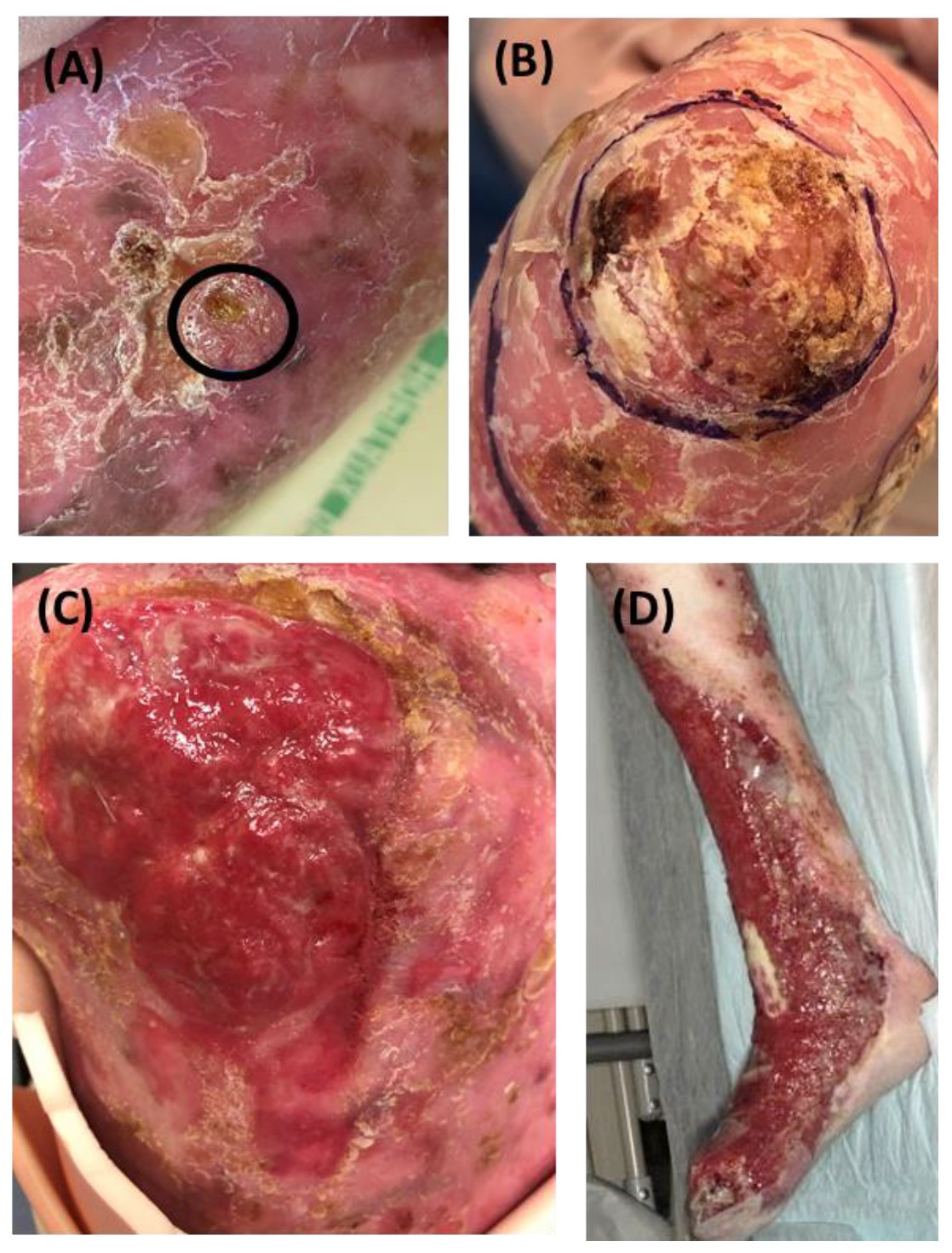
Supplementary table S2). Representative clinical pictures of RDEB-SCCs from each subgroup are depicted in
Figure 2.
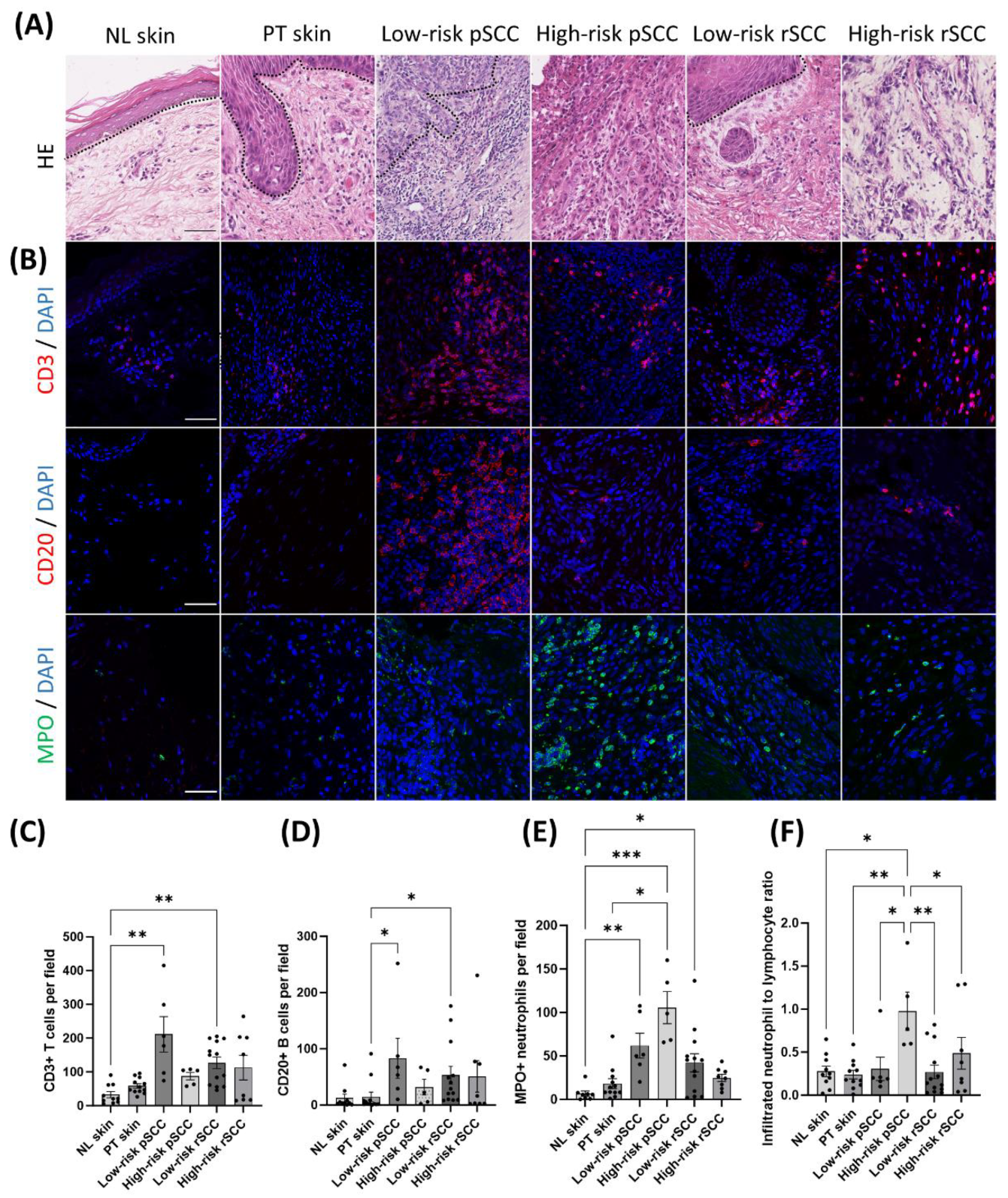
Representative HE stainings of the different groups are shown in
Figure 3A. Most of the low-risk pSCCs (5/6) and all low-risk recurrent SCCs (low-risk rSCC) (16/16) were well differentiated at the initial diagnosis. Conversely, 4 of the 5 high-risk pSCCs showed poor differentiaton, while 1 high-risk pSCC was well differentiated. One RDEB patient developed 2 synchronous primary SCCs in the same location, one moderately-differentiated low-risk pSCC and one poorly-differentiated high-risk pSCC. The majority of high-risk rSCCs (7 out of 10) originated from local recurrences or metastases of previously resected high-risk SCCs. One high-risk rSCC arose from a prior tumor not included in our cohort, for which histological parameters were not available. Two high-risk rSCCs were classified as new tumors. Three out of the 10 high-risk rSCCs were poorly differentiated, 4 showed moderate differentiation and 3 were well-differentiated. Ki67-positive nuclei were detected mainly in the outer layers of the tumor nests in low-risk pSCCs and low-risk rSCCs (
Supplementary figure S1A). The number of Ki67-positive tumor cells was significantly higher in both high-risk pSCCs and high-risk rSCCs compared to non-lesional skin (
Supplementary Figure S1B). Finally, the transcript expression of epithelial differentation markers, CDH1, OCLN, and KRT1
[26,27,28], was decreased in high-risk pSCCs compared with non-lesional skin or peri-tumoral skin (
Supplementary figure S1C).
3.3. Infiltrating Neutrophil-to-Lymphocyte Ratio Is Increased in High-Risk Primary SCCs in RDEB Patients
The TME of SCCs, peri-tumoral skin, and non-lesional skin of RDEB patients with different clinical-histopathological features was investigated by assessing immune cell infiltration (
Figure 3 and
Supplementary Figure 2).
Infiltrating lymphocytes were exclusively detected in the invasive margin (
Figure 3A,B). Very few immune cells were infiltrating the intra-tumoral region in the SCC groups. The density of immune cells was quantified in the invasive margin of the tumor or in the stromal region of non-tumor samples. The density of CD20+ B cells and CD3+ T cells was increased in low-risk pSCCs and low-risk rSCCs compared with peri-tumoral skin and non-lesional skin respectively (
Figure 3C,D). No significant difference was noted in the proportion of CD4+ helper T cells and CD8+ cytotoxic T cells relative to CD3+ T cells in the different groups (
Supplementary figures S2B-2C).
Innate immune cells were also detected in the invasive margin of the tumors (
Figure 3B and
Supplementary figure S3). Specifically, the number of infiltrated CD163+ macrophages was increased in low-risk rSCCs compared with peri-tumoral skin and non-lesional skin (
Supplementary Figure 3B). The proportion of tryptase+ mast cells was not different among the different groups (
Supplementary Figure 3C). Conversely, the proportion of myeloperoxidase (MPO) positive neutrophils was significantly increased in low-risk pSCCs, high-risk pSCCs, and low-risk rSCCs compared with non-lesional skin (
Figure 3E). Finally, the ratio of tumor-infiltrated neutrophils to lymphocytes (defined by CD20+ and CD3+ cells), which has previously been associated with poor prognosis in several cancers
[29], was specifically increased in high-risk pSCCs (
Figure 3F).
3.4. Increased Pro-Inflammatory Mediators and Neutrophil Extracellular Traps in the TME and the Serum of RBEB Patients with High-Risk Primary SCC
Neutrophils have been shown to promote cancer dissemination through the formation of NETs in the TME [
30]. To assess whether neutrophil infiltration in the TME of RDEB-SCCs was associated with NET formation, we performed immunostaining for MPO and citrullinated histone H3 (citH3), which are specific markers for neutrophils and NETosis [
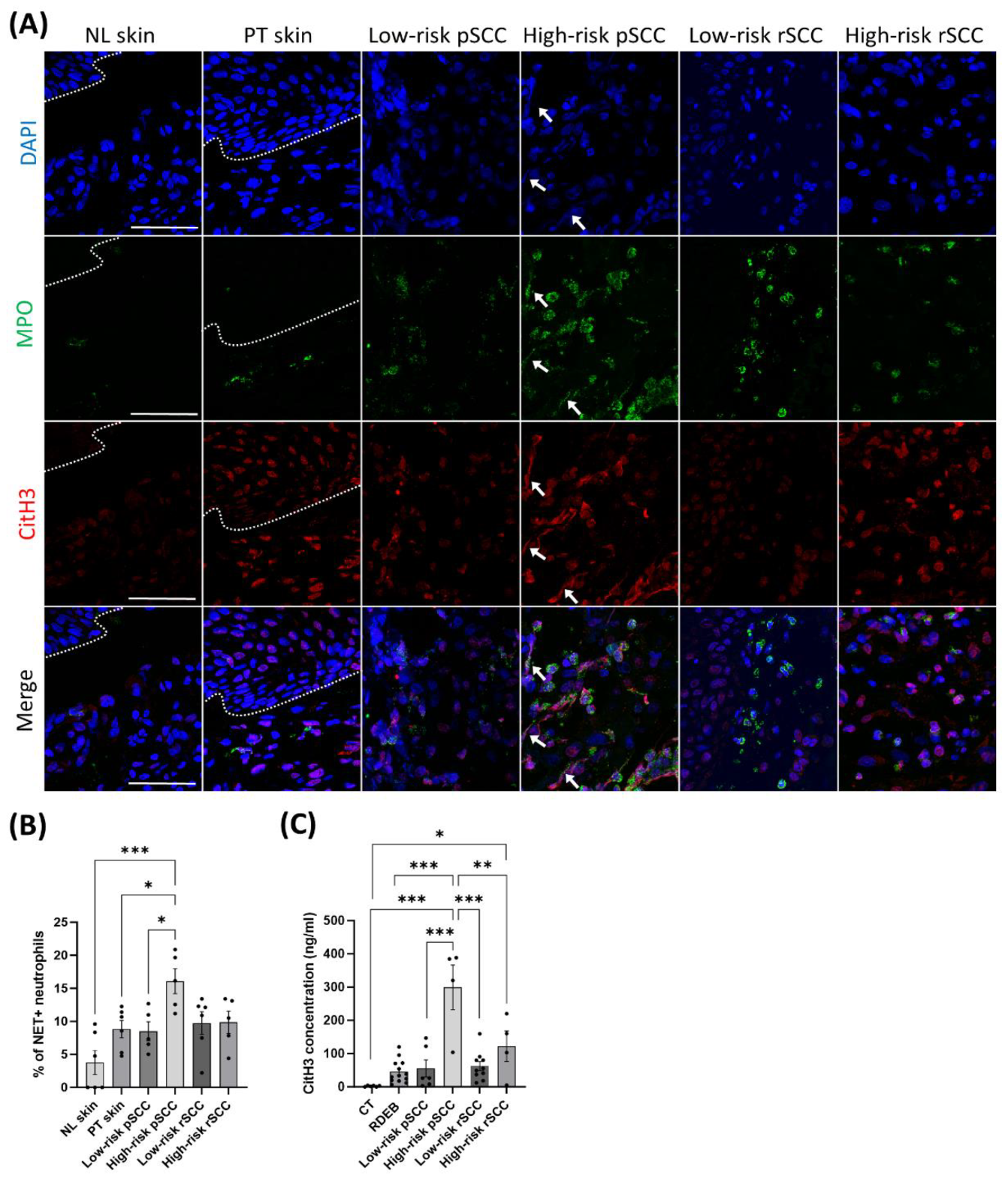
31], respectively, and with DAPI for DNA (
Figure 4A). Immunostaining revealed increased NET formation in high-risk pSCCs compared with low-risk pSCCs, peri-tumoral skin, and non-lesional skin, as measured by co-staining for MPO, citH3, and DAPI. (
Figure 4B).
Several studies have reported that NET formation can be mediated by various stimuli including Interleukin-1β (IL-1β/IL1B)
[32,33], High-mobility group box 1 (HMGB1/HMGB1)
[34], as well as by regulators of neutrophil recruitment including Interleukin-8 (IL-8/CXCL8)
[32,35] and Granulocyte-(macrophage) colony-stimulating factor (GM-CSF/CSF2 or G-CSF/CSF3)
[36,37]. Quantitative RT-PCR analysis of the 3 high-risk pSCCs which could be studied (
Supplementary figure S4) revealed higher levels of CSF2 or CSF3 transcripts (2/3) or both (1/3) (
Supplementary figures S4A and 4B). IL1B expression was also increased in high-risk pSCC and high-risk rSCC samples, with the expression of CXCL8 and PADI4 being specifically increased in the high-risk pSCC group (
Supplementary figures S4C-S4D). Conversely, HMGB1 expression was decreased in low-risk pSCCs and low-risk rSCCs compared with non-lesional skin (
Supplementary figure S4E).
Finally, the serum concentration of the NET marker citH3 was enhanced in RDEB patients with high-risk pSCC compared with RDEB patients with low-risk pSCC or without SCC (
Figure 4C).
4. Discussion
Our study aimed at better characterizing the immune TME of SCCs in a cohort of 20 RDEB patients and comparing these results with clinical-histopathological features.
Consistent with the literature, our data support the notion that SCCs develop in RDEB patients from an early age with a median age 29 years at the initial diagnosis, and display a high risk of recurrence observed in 90% of RDEB patients in our cohort
[3,38]. We found a median survival of 4.6 years after the diagnosis of the first SCC, considering all RDEB subtypes, and 2.3 years for the RDEB-Sev subtype. Our observations are in line with previous studies based on American, English, Australian, and Dutch EB registries (i.e. 2.4 years, 4 years, and 3.5 years, respectively) [
3,
38,
39,
40]. The severe RDEB subtype has previously been shown to have the highest cumulative risk of death after the first SCC occurrence
[3]. Previous studies have shown that most RDEB patients developed invasive, recurrent or metastatic life-threatening SCC, with fewer RDEB patients having a prolonged phase of localized tumor growth [
2,
38,
41]. These two distinct clinical outcomes were observed among patients with similar clinical severity in the cohort we studied. However, RDEB patients with a more favorable disease course were the most numerous. Specifically, 15 out of 20 patients developed several recurrent SCCs with an overall median survival of 9.3 years. This relatively long period is in accordance with a previous study of the Spanish EB registry [
42]. Conversely, 5 RDEB-Sev patients developed several aggressive recurrences or metastases after their primary SCC occurrence, leading to a median survival of 1.2 years. Four out of these RDEB patients with severe prognosis, developed a poorly differentiated primary SCC, suggesting an early aggressive tumor. Therefore, new biomarkers need to be identified to predict a potential poor prognosis after the occurrence of the first SCC. Some potential tissue biomarkers have been associated with poor prognosis in RDEB-SCCs, including miR-10b or Periostin
[43,44]. A recent study reported miRNA signatures of RDEB-SCC derived exosomes as potential circulating diagnostic biomarkers [
45]. By contrast, no circulating prognostic biomarkers have been identified to predict the two distinct clinical outcomes of SCCs in RDEB patients.
To determine whether a specific immune TME could be associated with distinct clinical-histopathological features, we explored the immune cell infiltration in SCCs occurring in our RDEB cohort. The analyses highlighted a TME enriched in neutrophil infiltrates compared with lymphocytes in the invasive margin of high-risk pSCCs. Neutrophils are the first cells to respond to inflammatory or infectious conditions. The accumulation of neutrophils in the TME has previously been reported as being a factor of poor prognosis in different cancer types [
46,
47]. Likewise, an increased concentration of circulating neutrophils or an elevated ration of infiltrating neutrophil-to-lymphocyte have been considered as poor prognostic factors in cancers, including SCCs [
46,
48,
49,
50].
Neutrophil infiltration may result from a response to increased bacterial invasion in RDEB-SCCs. Consistent with this possibility, skin wounds of RDEB patients commonly show increased colonization and infections with different bacteria
[51,
52]. Furthermore, signatures of enhanced antibacterial immunity were identified in RDEB-SCCs, suggesting that the response to bacteria may be associated with an aggressive progression in RDEB-SCCs
[53]. Previous studies have shown that infiltrated neutrophils in the TME could favor cancer cell growth and metastasis through the secretion of tumor growth-promoting factors
[54,
55] or the inhibition of the immune response
[56]. In our study, we found no significant B and T cell infiltration in the TME of high-risk pSCCs and rSCCs. These observations are in line with recent studies that described a low immune cell infiltration in RDEB-SCCs in comparison with sporadic SCCs, with lower CD4+ and CD8+ cell infiltration
[6,
7,
8].
Neutrophils may also participate in cancer progression with NET formation through systemic inflammation and thrombosis
[16,
17,
18,
31]. Increased blood levels of citH3, the marker of NETs, have been found to be a prognostic marker for endotoxic choc, suggesting citH3 is a potential therapeutic target in this severe condition
[57,
58]. By contrast, several studies identified NETs as drivers of epithelial-mesenchymal transition in human colon and breast cancers [
19,
30,
59]. The presence of NETs was previously described in lesional RDEB skin and RDEB-SCCs
[60]. Herein, NETs tended to increase in the peri-tumoral skin and were significantly increased in high-risk pSCCs. Interestingly, we found that NET formation in TME of high-risk pSCCs was associated with increased serum levels of the NET marker citH3. These results are consistent with previous studies reporting an association of biomarkers of NETs with adverse clinical outcomes in two cohorts of patients with aggressive cancers
[22,
23]. In contrast, NET formation was not significantly increased in the TME and the serum of RDEB patients with high-risk rSCC in our study. This difference could be explained by the fact that 7 out of 10 high-risk rSCCs were local recurrences or metastases from previously resected high-risk SCCs. These data suggest that NET formation may be transient in the early stage of aggressive tumor progression and that additional mechanisms associated with aggressiveness may be involved in the reactivation of tumor cells in later stages.
To investigate the underlying molecular mechanisms involved in high-risk pSCCs, we measured transcript levels of pro-inflammatory markers in the tumors. Surprisingly, the expression of
HMGB1, a gene encoding a danger-associated molecular pattern, was not increased in RDEB-SCCs. This result is in agreement with published RNAseq data from other investigators
[61], but contrasts with other studies showing increased levels of HMGB1 in blister fluid, lesional RDEB skin and RDEB-SCCs
[7,
62]. Conversely, we found increased transcript levels of IL1β, IL8, and PAD4 associated with enhanced expression of GM-CSF and/or G-CSF in high-risk pSCCs. IL-1β has been shown to drive NET formation through IL-8 or G-CSF production in inflammatory diseases including cancers
[32,
33,
35], but also to directly target epithelial-mesenchymal transition
[63]. Therefore, additional investigations are needed to identify the signaling pathways underlying tumor progression and aggressiveness in RDEB-SCCs, i.e., tumor growth, EMT, or cell dissemination through NETs. The main limitation of our study is the small number of patients with high-risk pSCC due to the orphan nature of the disease. Nevertheless, the present study, based on national recruitment, represents one of the most important cohort of this ultra-rare condition. The relevance of circulating citH3 as a prognostic biomarker for RDEB patients with SCCs has to be confirmed by a prospective study of RDEB patients with primary SCCs. Furthermore, the value of increased circulating citH3 as a reliable marker of local and/or systemic inflammation needs to be confirmed. Due to the dominant role of NETs in adaptive anti-tumor immune escape, pharmacological inhibition of PADI4 could sensitize cancer cells to combined immunotherapy with anti-PD-1 and anti-CTLA-4 checkpoint inhibitors
[64]. Thus, it may be relevant to evaluate the potential of a combinatorial therapy targeting neutrophil activation and immune checkpoint inhibitors for the treatment of aggressive RDEB-SCCs with high systemic citH3 levels.
5. Conclusion
In conclusion, the present study describes two distinct clinical outcomes in RDEB patients with cutaneous SCCs. RDEB-SCCs with the poorest prognosis were associated with a TME enriched in neutrophil to lymphocyte infiltration and an Il1β related signature. We showed for the first time, increased levels of systemic citH3, a marker of NETs, in the serum of RDEB patients with the most aggressive SCCs, suggesting that citH3 could be a potential marker of unfavorable prognosis in RDEB-SCCs.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary figure S1: Detection of Ki67 and epithelial gene markers in RDEB-SCCs with different severity. Supplementary figure S2: Detection of adaptive immune cells in RDEB-SCCs with different severity. Supplementary figure S3: Detection of innate immune cells in RDEB-SCCs with different severity. Supplementary figure S4: Gene expression of inflammatory mediators involved in neutrophil activation by quantitative RT-PCR. Supplementary table S1: Supplementary RDEB patient and SCC tumor sample details. Supplementary table S2: Aggressiveness parameters in high-risk SCC groups. Supplementary table S3: Listing of antibodies. S4. Listing of primers used for RT-quantitative PCR.
Author Contributions
Conceptualization, H.R., E.B., A.H. S.G. and M.T.; methodology, H.R., A.H. and M.T. ; software, H.R., J.B. and M.D.C; validation, H.R., S.G., E.B., and M.B. formal analysis, H.R.; investigation, H.R., J.B. and M.D.C.; resources, R.B., E.B. and M.B.; data curation, H.R. and M.D.C.; writing—original draft preparation, H.R., A.H., and M.T. ; writing—review and editing, all authors; supervision, E.B, A.H. and M.T. ; project administration, HR., S.G. and E.B. ; funding acquisition, H.R, E.B and A.H. All authors have read and agreed to the published version of the manuscript.”.
Funding
This study was supported by grants from the Dystrophic Epidermolysis Bullosa Research Association (DEBRA) France and from the Société Française de Dermatologie.
Institutional Review Board Statement
The SIMOCEB study was approved by the Paris Nord Ethics Review Committee for Biomedical Research Projects (CEERB: n°2020-010/ClinicalTrials.gov: NCT04285294).
Informed Consent Statement
Written informed consent for publication must be obtained from participating patients who can be identified (including by the patients themselves). Please state “Written informed consent has been obtained from the patient(s) to publish this paper” if applicable.
Data Availability Statement
Acknowledgments
We thank the SFR Necker Cell Imaging platform for technical support. We acknowledge Lucile Marchal, PhD student (Imagine Institute) for the proof-reading support.
Conflicts of Interest
Pr. Hovnanian has served on the scientific advisory board for EB Research Partnership. Pr. Battistella has consulting role from Takeda, Innate Pharma, and Kyowa Kirin.
References
- Hilal L, Rochat A, Duquesnoy P, Blanchet-Bardon C, et al. A homozygous insertion-deletion in the type VII collagen gene (COL7A1) in Hallopeau-Siemens dystrophic epidermolysis bullosa. Nat Genet. 1993, 5, 287-93.
- Montaudie H, Chiaverini C, Sbidian E, Charlesworth A, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016, 11, 117. [CrossRef] [PubMed]
- Fine JD, Johnson LB, Weiner M, Li KP, et al. Epidermolysis bullosa and the risk of life-threatening cancers: the National EB Registry experience, 1986-2006. J Am Acad Dermatol. 2009, 60, 203-11.
- Guerra L, Odorisio T, Zambruno G, Castiglia D. Stromal microenvironment in type VII collagen-deficient skin: The ground for squamous cell carcinoma development. Matrix Biol. 2017, 63, 1–10. [CrossRef] [PubMed]
- Nystrom A, Bornert O, Kuhl T, Gretzmeier C, et al. Impaired lymphoid extracellular matrix impedes antibacterial immunity in epidermolysis bullosa. Proc Natl Acad Sci U S A. 2018, 115, E705-E14.
- Filoni A, Cicco G, Lospalluti L, Maglietta A, et al. Morphological and morphometric analysis of cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa: a retrospective study. J Eur Acad Dermatol Venereol. 2020, 34, 1707-14.
- Filoni A, Cicco G, Cazzato G, Bosco A, et al. Immune Disregulation in Cutaneous Squamous Cell Carcinoma of Patients with Recessive Dystrophic Epidermolysis Bullosa: A Single Pilot Study. Life (Basel). 2022, 12(2).
- Rafei-Shamsabadi D, Scholten L, Lu S, Castiglia D, et al. Epidermolysis-Bullosa-Associated Squamous Cell Carcinomas Support an Immunosuppressive Tumor Microenvironment: Prospects for Immunotherapy. Cancers (Basel). 2024, 16(2).
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010, 140, 883-99.
- Galdiero MR, Garlanda C, Jaillon S, Marone G, et al. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013, 228, 1404-12.
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016, 16, 431-46.
- Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 2015, 528, 413-7.
- Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019, 16, 601-20.
- Brinkmann V, Reichard U, Goosmann C, Fauler B, et al. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532-5.
- Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, et al. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016, 76, 1367-80.
- Jung HS, Gu J, Kim JE, Nam Y, et al. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS One 2019, 14, e0216055.
- Albrengues J, Shields MA, Ng D, Park CG, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409).
- Cools-Lartigue J, Spicer J, McDonald B, Gowing S, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013.
- Xiao Y, Cong M, Li J, He D, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021, 39, 423-37 e7.
- Yang L, Liu Q, Zhang X, Liu X, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020, 583, 133-8.
- Zhang Y, Hu Y, Ma C, Sun H, et al. Diagnostic, Therapeutic Predictive, and Prognostic Value of Neutrophil Extracellular Traps in Patients With Gastric Adenocarcinoma. Front Oncol. 2020, 10, 1036. [CrossRef] [PubMed]
- Thalin C, Lundstrom S, Seignez C, Daleskog M, et al. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One 2018, 13, e0191231.
- Grilz E, Mauracher LM, Posch F, Konigsbrugge O, et al. Citrullinated histone H3, a biomarker for neutrophil extracellular trap formation, predicts the risk of mortality in patients with cancer. Br J Haematol. 2019, 186, 311-20.
- Ruiz ES, Karia PS, Besaw R, Schmults CD. Performance of the American Joint Committee on Cancer Staging Manual, 8th Edition vs the Brigham and Women's Hospital Tumor Classification System for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2019, 155, 819-25.
- Morgan FC, Ruiz ES, Karia PS, Besaw RJ, et al. Brigham and Women's Hospital tumor classification system for basal cell carcinoma identifies patients with risk of metastasis and death. J Am Acad Dermatol. 2021, 85, 582-7.
- Onder TT, Gupta PB, Mani SA, Yang J, et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008, 68, 3645-54.
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003, 116(Pt 10), 1959-67.
- Joosse SA, Hannemann J, Spotter J, Bauche A, et al. Changes in keratin expression during metastatic progression of breast cancer: impact on the detection of circulating tumor cells. Clin Cancer Res. 2012, 18, 993–1003. [CrossRef] [PubMed]
- Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013, 88, 218-30.
- Martins-Cardoso K, Almeida VH, Bagri KM, Rossi MID, et al. Neutrophil Extracellular Traps (NETs) Promote Pro-Metastatic Phenotype in Human Breast Cancer Cells through Epithelial-Mesenchymal Transition. Cancers (Basel). 2020, 12(6).
- Park J, Wysocki RW, Amoozgar Z, Maiorino L, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016, 8, 361ra138.
- Keshari RS, Jyoti A, Dubey M, Kothari N, et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS One 2012, 7, e48111.
- Gomes T, Varady CBS, Lourenco AL, Mizurini DM, et al. IL-1beta Blockade Attenuates Thrombosis in a Neutrophil Extracellular Trap-Dependent Breast Cancer Model. Front Immunol. 2019, 10, 2088. [CrossRef]
- Tadie JM, Bae HB, Jiang S, Park DW, et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiol Lung Cell Mol Physiol. 2013, 304, L342-9.
- Alfaro C, Teijeira A, Onate C, Perez G, et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin Cancer Res. 2016, 22, 3924-36.
- Wang TT, Zhao YL, Peng LS, Chen N, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut 2017, 66, 1900-11.
- Demers M, Wagner DD. Neutrophil extracellular traps: A new link to cancer-associated thrombosis and potential implications for tumor progression. Oncoimmunology 2013, 2, e22946. [CrossRef]
- Robertson SJ, Orrin E, Lakhan MK, O'Sullivan G, et al. Cutaneous Squamous Cell Carcinoma in Epidermolysis Bullosa: a 28-year Retrospective Study. Acta Derm Venereol. 2021, 101, adv00523. [CrossRef]
- Kim M, Li M, Intong-Wheeler LRA, Tran K, et al. Epidemiology and Outcome of Squamous Cell Carcinoma in Epidermolysis Bullosa in Australia and New Zealand. Acta Derm Venereol. 2018, 98, 70-6.
- Harrs C, van den Akker PC, Baardman R, Duipmans JC, et al. The aggressive behaviour of squamous cell carcinoma in epidermolysis bullosa: analysis of clinical outcomes and tumour characteristics in the Dutch EB Registry. Br J Dermatol. 2022, 187, 824-6.
- Bonamonte D, Filoni A, De Marco A, Lospalluti L, et al. Squamous Cell Carcinoma in Patients with Inherited Epidermolysis Bullosa: Review of Current Literature. Cells. 2022, 11(8).
- Castelo B, Vinal D, Maseda R, Ostios L, et al. Epidemiology and natural history of cutaneous squamous cell carcinoma in recessive dystrophic epidermolysis bullosa patients: 20 years' experience of a reference centre in Spain. Clin Transl Oncol. 2019, 21, 1573-7.
- Lincoln V, Chao L, Woodley DT, Murrell D, et al. Over-expression of stromal periostin correlates with poor prognosis of cutaneous squamous cell carcinomas. Exp Dermatol. 2021, 30, 698–704. [CrossRef]
- Wimmer M, Zauner R, Ablinger M, Pinon-Hofbauer J, et al. A cancer stem cell-like phenotype is associated with miR-10b expression in aggressive squamous cell carcinomas. Cell Commun Signal. 2020, 18, 61. [CrossRef]
- Zauner R, Wimmer M, Atzmueller S, Proell J, et al. Biomarker Discovery in Rare Malignancies: Development of a miRNA Signature for RDEB-cSCC. Cancers (Basel). 2023,15(13).
- Wu M, Ma M, Tan Z, Zheng H, et al. Neutrophil: A New Player in Metastatic Cancers. Front Immunol. 2020, 11, 565165. [CrossRef] [PubMed]
- Xiao WK, Chen D, Li SQ, Fu SJ, et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer 2014, 14, 117.
- Hu K, Lou L, Ye J, Zhang S. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open 2015, 5, e006404. [CrossRef] [PubMed]
- Guo Q, Shao Z, Xu D, Fan L, et al. Prognostic value of neutrophil-to-lymphocyte ratio in peripheral blood and pathological tissue in patients with esophageal squamous cell carcinoma. Medicine (Baltimore) 2020, 99, e21306. [CrossRef] [PubMed]
- Tessier-Cloutier B, Twa DD, Marzban M, Kalina J, et al. The presence of tumour-infiltrating neutrophils is an independent adverse prognostic feature in clear cell renal cell carcinoma. J Pathol Clin Res. 2021, 7, 385-96.
- van der Kooi-Pol MM, Duipmans JC, Jonkman MF, van Dijl JM. Host-pathogen interactions in epidermolysis bullosa patients colonized with Staphylococcus aureus. Int J Med Microbiol. 2014, 304, 195–203. [CrossRef] [PubMed]
- Mellerio JE. Infection and colonization in epidermolysis bullosa. Dermatol Clin. 2010, 28, 267-9, ix.
- Foll MC, Fahrner M, Gretzmeier C, Thoma K, et al. Identification of tissue damage, extracellular matrix remodeling and bacterial challenge as common mechanisms associated with high-risk cutaneous squamous cell carcinomas. Matrix Biol. 2018, 66, 1–21. [CrossRef]
- Schaider H, Oka M, Bogenrieder T, Nesbit M, et al. Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. Int J Cancer 2003, 103, 335-43.
- Jablonska E, Kiluk M, Markiewicz W, Piotrowski L, et al. TNF-alpha, IL-6 and their soluble receptor serum levels and secretion by neutrophils in cancer patients. Arch Immunol Ther Exp (Warsz). 2001, 49, 63-9.
- el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987, 139, 2406-13.
- Pan B, Alam HB, Chong W, Mobley J, et al. CitH3: a reliable blood biomarker for diagnosis and treatment of endotoxic shock. Sci Rep. 2017, 7, 8972.
- Deng Q, Pan B, Alam HB, Liang Y, et al. Citrullinated Histone H3 as a Therapeutic Target for Endotoxic Shock in Mice. Front Immunol. 2019, 10, 2957.
- Stehr AM, Wang G, Demmler R, Stemmler MP, et al. Neutrophil extracellular traps drive epithelial-mesenchymal transition of human colon cancer. J Pathol. 2022, 256, 455-67.
- Hoste E, Maueroder C, van Hove L, Catrysse L, et al. Epithelial HMGB1 Delays Skin Wound Healing and Drives Tumor Initiation by Priming Neutrophils for NET Formation. Cell Rep. 2019, 29, 2689-701 e4.
- Cho RJ, Alexandrov LB, den Breems NY, Atanasova VS, et al. APOBEC mutation drives early-onset squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2018, 10(455).
- Hoste E, Arwert EN, Lal R, South AP, et al. Innate sensing of microbial products promotes wound-induced skin cancer. Nat Commun. 2015, 6, 5932. [CrossRef] [PubMed]
- Zhou J, Zheng S, Liu T, Liu Q, et al. IL-1beta from M2 macrophages promotes migration and invasion of ESCC cells enhancing epithelial-mesenchymal transition and activating NF-kappaB signaling pathway. J Cell Biochem. 2018, 119, 7040-52.
- Teijeira A, Garasa S, Gato M, Alfaro C, et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856-71 e8.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).