1. Introduction
Corneal ulcer is a common and potentially serious condition in horses. Damage to the cornea, resulting in loss of epithelial cells with exposed corneal stroma, creates an ulcer on the corneal surface [
1]. Squinting (blepharospasm) or keeping an eye shut and tearing (epiphora) are often the first signs owners will notice in pets with corneal ulcers. Fluorescein stain uptake in the ulcerated region confirms the diagnosis of an ulcer. Categorization of the ulcer is based on a combination of clinical signs and visual inspection of the eye [
1,
2].
Indolent corneal ulcers, also known as refractory ulcers, "boxer" ulcers, spontaneous chronic corneal epithelial defects (SCCEDs), and recurrent erosions, are a special type of superficial ulcer that fails to resolve through the normal wound-healing process [
3]. Hallmark clinical and histologic features of SCCEDs include a superficial corneal ulcer that (1) does not extend into the stroma; (2) is associated with redundant, non-adherent corneal epithelial borders that may be associated with an acellular hyaline zone in the anterior stroma; (3) persists for weeks or months if not adequately addressed; and (4) may or may not include neovascularization and edema [
4,
5,
6].
The underlying pathophysiology of SCCEDs is not completely understood, and there is some thought that these ulcers may be the shared end result of a variety of pathways. Ultimately, indolent corneal ulcers result from dysmaturation of corneal epithelia that do not properly attach to the underlying stroma of the eye, creating a lesion composed of loose, poorly adhered epithelia overlying corneal stroma (lipping) [
5,
7].
Traditional treatments for corneal ulcer in horses include antibiotics, anti-inflammatory medications, and surgery [
1,
7]. However, these treatments can be costly, time-consuming, and sometimes ineffective [
8]. In recent years, regenerative medicine has emerged as a promising approach for treating various conditions, including corneal wounds [
9]. One of the most promising alternatives developments is the use of mesenchymal stem cells (MSCs) [
10,
11,
12]. In MSCs therapy, stem cells are harvested from the patient or from a donor and then cultured in a laboratory. The MSCs are then administered to the patient to promote healing and tissue regeneration. However, recent studies have shown that the beneficial effects of MSCs therapy may not be solely due to the stem cells themselves, but rather to the secretion of various factors, known as the secretome also called conditioned medium (CM) by the MSCs [
13,
14]. MSCs secretome refers to the collection of bioactive molecules, including growth factors, cytokines, and extracellular vesicles (EVs), secreted by these cells. The secretome of MSCs has gained significant attention due to its potential to modulate the wound healing process by regulating various cellular processes, such as inflammation, angiogenesis, and extracellular matrix remodeling [
15]. Furthermore, the use of the secretome eliminates the need for direct cell transplantation, reducing the risks associated with cell-based therapies, such as immune rejection and potential tumorigenicity [
16].
This case report describes a novel approach to treating a corneal wound in a Hispano-bretón mare using the secretome derived from adipose tissue mesenchymal stem cells (ASCs). We describe the clinical presentation, treatment protocol, and outcome, highlighting the potential of ASC secretome as a promising therapeutic strategy for corneal wound healing in veterinary ophthalmology.
2. Case Description
A 28-year-old mare of the Hispano Breton breed kept in a paddock with two other horses. The general examination of the animal does not show any alteration, the ocular examination shows blepharospasm and moderate blepharitis, moderate/mild epiphora and the tear secretion is serous. The pupil appears miotic, with moderate corneal edema and localized corneal neovascularization in the dorsal region, with conjunctival hyperemia. Fluorescein staining revealed the presence of a corneal ulcer of about 7 mm in diameter, apparently uncomplicated.
Treatment was started with tobramycin in eye drops (Tobrex®) every 4 hours, atropine (Colircusí® Atropina 1%) in eye drops every 12 hours during the first 48 hours and then as needed, autologous serum every 2 hours in the morning and every 3 hours in the afternoon and intravenous flunixin meglumine (Finadyne®) at a dose of 1.1mg/kg every 24 hours for two days.
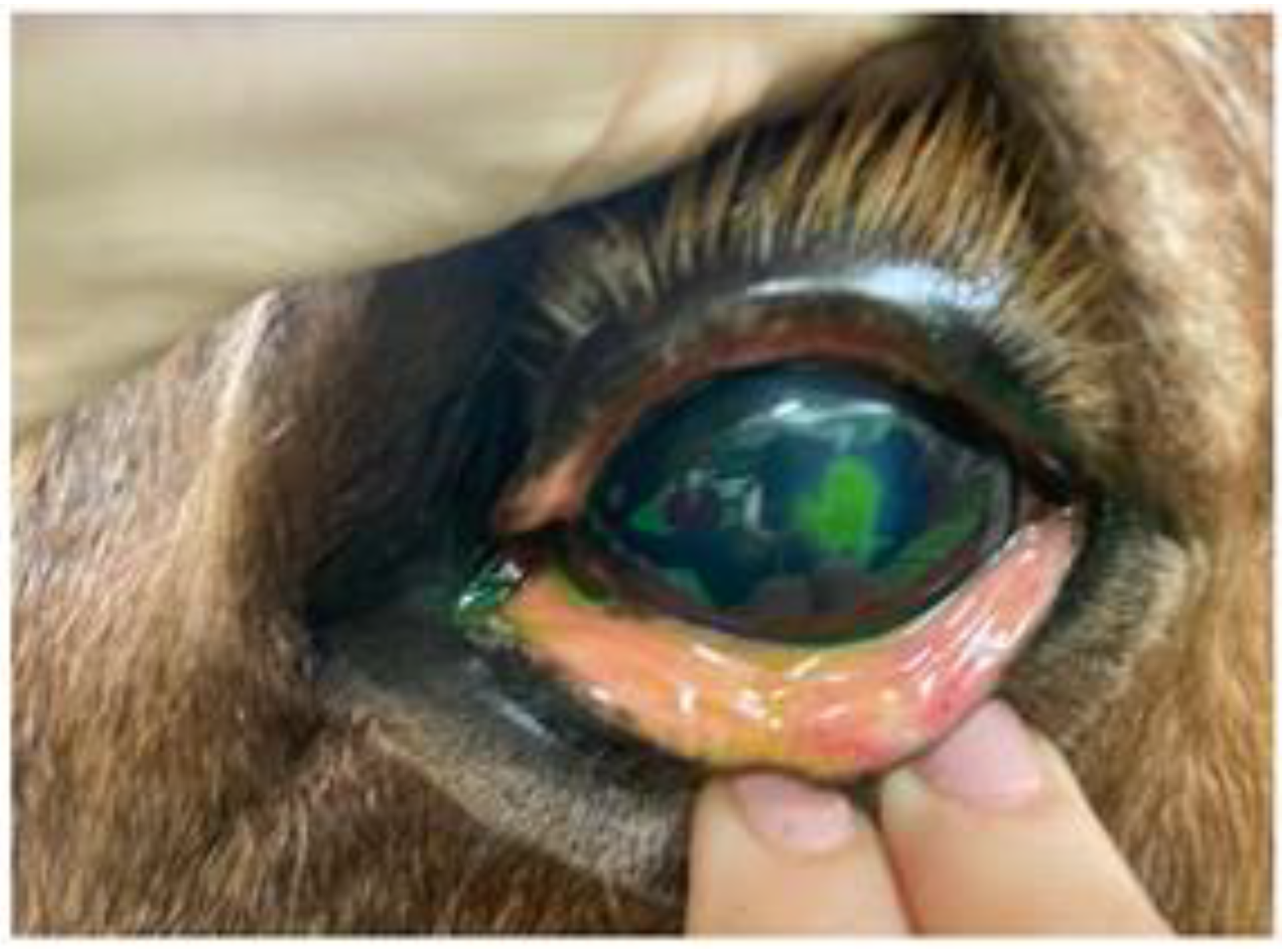
A first review after 4 days showed improvement of blepharospasm and corneal edema, although epiphora persisted. Fluorescein staining revealed dye infiltration under the epithelial borders of the lesion. Slit-lamp biomicroscopic examination and fluorescein staining revealed detached epithelial edges (
Figure 1), confirming the diagnosis of indolent/nonhealing corneal ulcer. The examination was completed, ruling out the existence of a foreign body, and debridement was performed. After sedation with detomidine (Domosedan
®) and butorphanol (Torbugesic Vet
®), in addition to topical anesthesia, mechanical debridement of the lesion was performed with a Algerbrush 3.5 mm
® diamond bur. The non-adherent epithelium was removed along the entire contour of the lesion and the lesion bed was also reamed.
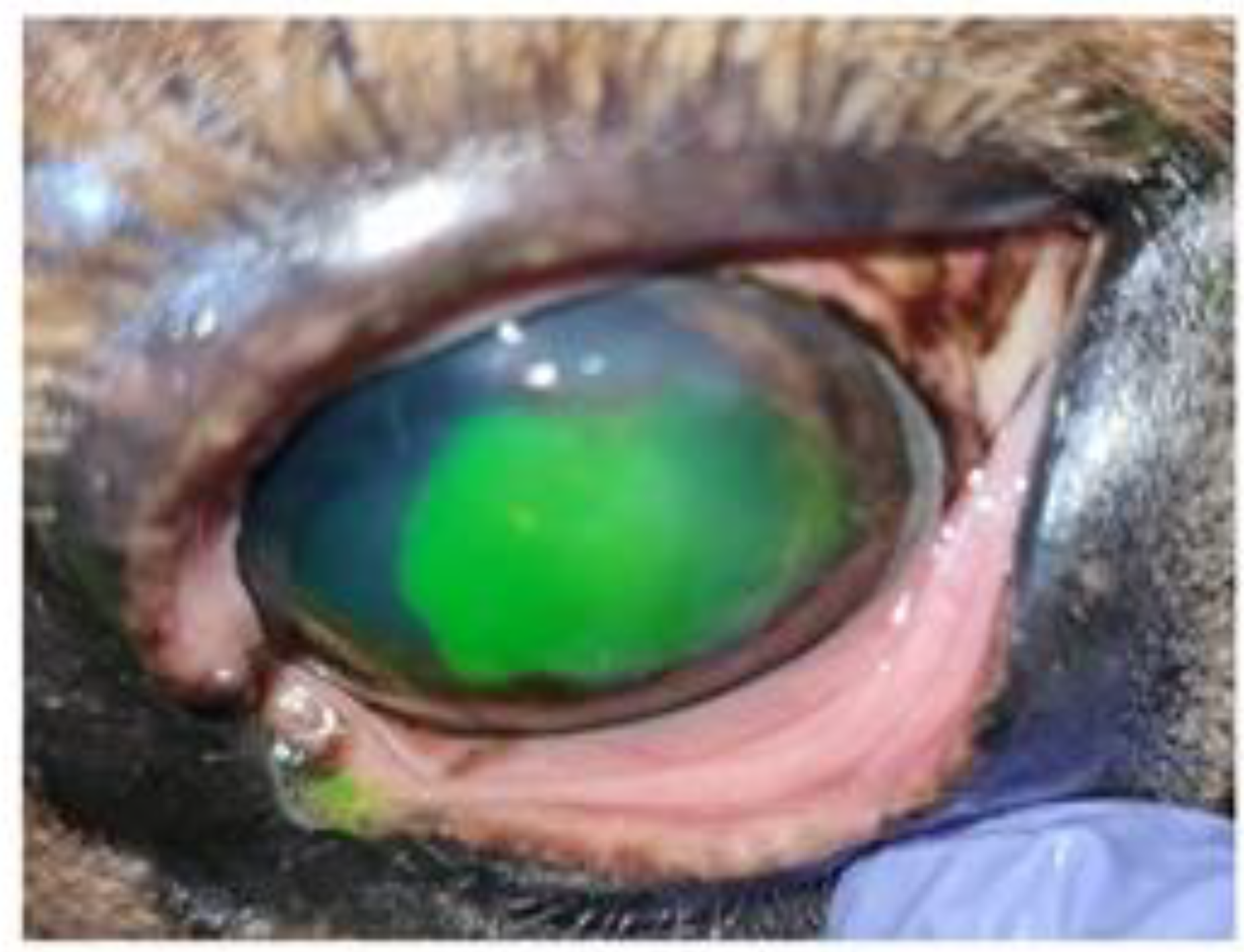
The result was a significant increase in the extent of the corneal ulcer. It occupied 2/3 of the corneal surface (
Figure 2). The previously prescribed medical treatment with systemic flunixin meglumine was maintained for two days.
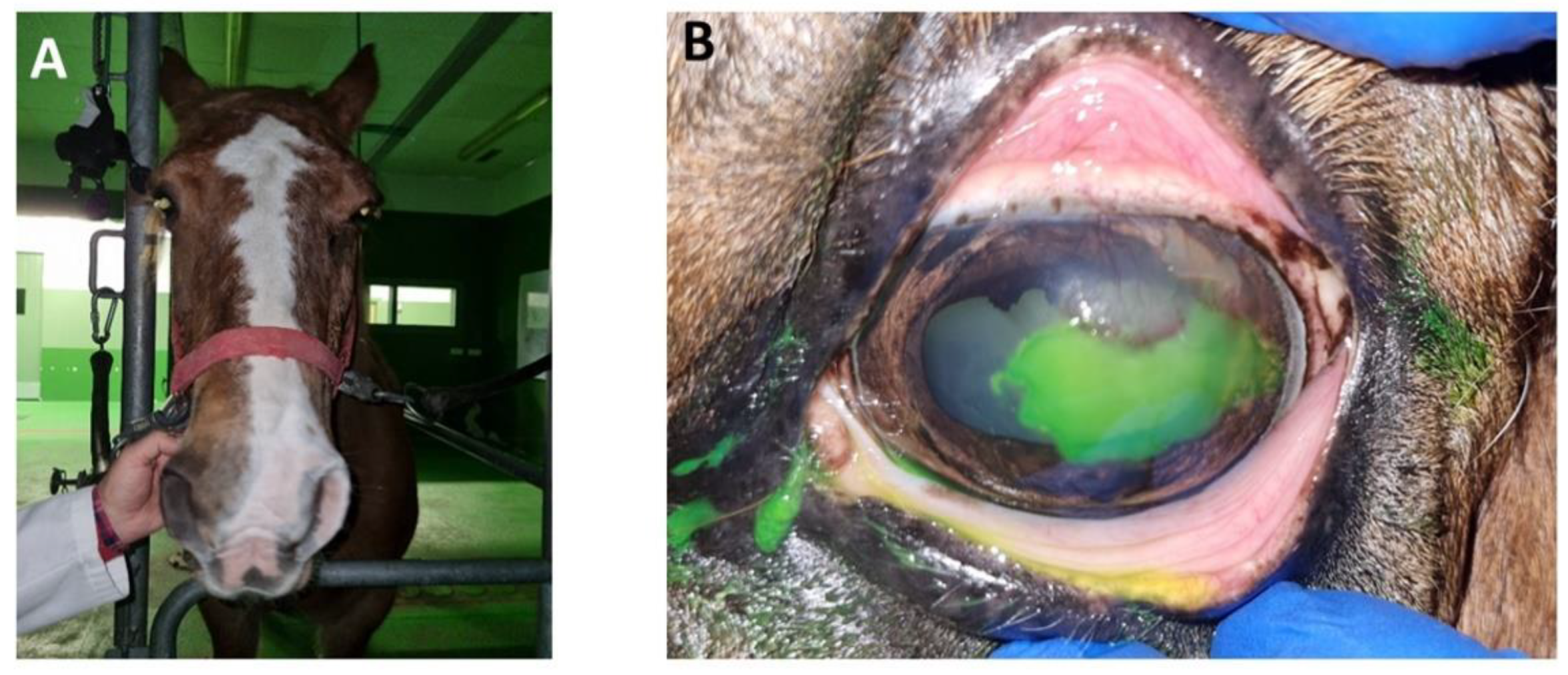
At the next examination 1 week later, moderate blepharospasm (
Figure 3.A) was observed. The lesion was fluorescein positive (
Figure 3.B) and was accompanied by significant cellular and vascular infiltration in the superotemporal quadrant of the cornea. He underwent another debridement with diamond burr. The result was a reduction of the ulcerated surface, with respect to the previous debridement. Medical treatment was maintained, in this case with flunixin meglumine for 3 days.
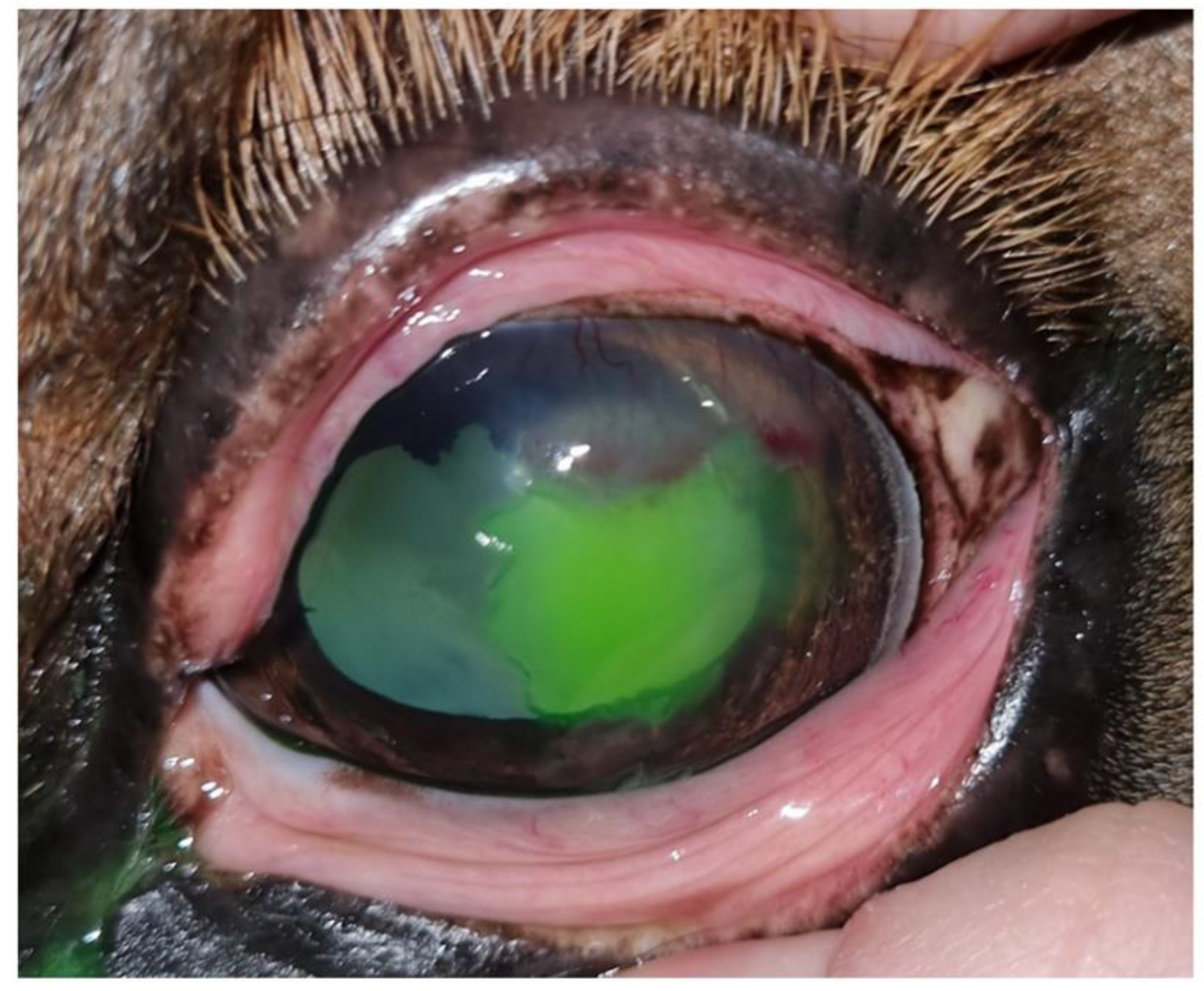
One week later, at the second examination, the clinical signs of blepharospasm and epiphora were still present, with significant cellular and vascular infiltration of the cornea. Fluorescein staining was negative but the epithelium did not appear to be completely adherent to the stroma on biomicroscopic examination, a touch with the Kimura spatula revealed sliding of the epithelium over the corneal stroma. Debrided, the final result is a lesion of greater extent than after the previous debridement (
Figure 4). There is no improvement from the previous consultation.
After this conventional treatment without significant evolutions we consider the need to modify the therapeutic strategy in order to promote proper re-epithelialization, the patient underwent therapeutic intervention with ASCs secretome. Adipose tissue was collected from the subcutaneous fat deposits of healthy horses during routine surgical procedures. The ASCs were isolated from the adipose tissue through enzymatic digestion with collagenase at 37ºC for 2 hours. The purified ASCs were cultured until confluence. When 80% confluence was reached, ASCs were maintained in serum-free DMEM medium for 48 hours to produce CM. The CM was collected and centrifuged to remove cellular debris. The cell-free CM, containing the secretome of proteins, cytokines, and other factors secreted by the equine ASCs, was concentrated using ultrafiltration membranes.
After informing the owners of the experimental treatment condition and obtaining their consent we administered in drops topically the secretome obtained from ASCs every 4 hours.
One week later the clinical signs of blepharospasm and epiphora had disappeared, the lesion was fluorescein negative (
Figure 5), on biomicroscopic examination the lesion appeared well re-epithelialized, yet swabbing was performed on the surface of the lesion, which revealed adherence of the epithelium to the corneal stroma.
The re-epithelialized area had regained transparency, cellular infiltration had disappeared, and improved corneal neovascularization was observed. The administration of secretoma was maintained for 3 more days and the patient was discharged with no recurrence in a year and a half after treatment.
4. Discussion
The findings from this case report highlight the potential therapeutic benefits of using mesenchymal stem cell (MSC) secretome, specifically from adipose-derived MSCs (ASCs) [
17], for treating non-healing corneal ulcers in horses [
10,
18]. Despite initial conventional treatment with antibiotics, anti-inflammatory drugs, and debridement, the corneal ulcer in this Hispano-Bretón mare failed to heal properly, exhibiting signs of persistent epithelial defects.
The lack of significant improvement with standard therapy prompted the exploration of an alternative regenerative approach using the secretome derived from cultured equine ASCs [
8]. The ASCs secretome, a rich milieu of trophic factors, cytokines, and extracellular vesicles, has been shown to modulate various cellular processes crucial for wound healing, including inflammation, angiogenesis, and extracellular matrix remodeling [
19,
20] .
By harnessing the paracrine effects of the ASC secretome, topical administration facilitated the re-epithelialization of the corneal ulcer and resolution of associated clinical signs within a week of treatment initiation.
These findings corroborate previous studies that have demonstrated the therapeutic potential of MSC secretomes in promoting corneal wound healing [
21]. For instance, a study by Saccu et al. reported significantly faster healing times and reduced complications in corneal ulcers treated with bone marrow-derived MSC secretome compared to a placebo group [
22]. Similarly, Cunha et al. [
23] observed enhanced corneal re-epithelialization and reduced inflammation in rabbits treated with ASC secretome after corneal alkali burns. However, there is a paucity of research investigating the use of stem cell therapies for the treatment of corneal ulcers in veterinary medicine. This gap in knowledge is even more pronounced when considering the equine species. To date, no studies have evaluated the therapeutic effects of stem cell secretomes, specifically those derived from ASCs, on corneal ulcers in horses.
The regenerative capacity of MSC secretomes is attributed to their ability to modulate the wound microenvironment through various mechanisms [
20]. The secreted factors can exert anti-inflammatory effects by regulating the activity of immune cells and reducing oxidative stress. Additionally, the secretome contains growth factors and cytokines that stimulate the proliferation and migration of corneal epithelial cells, facilitating re-epithelialization [
24,
25]. Furthermore, the extracellular vesicles present in the secretome can transfer bioactive molecules, such as proteins, lipids, and nucleic acids, to target cells, influencing their behavior and promoting tissue repair [
26].
While the results from this case report are promising, it is important to acknowledge the limitations of a single case study. Further controlled studies with larger sample sizes are necessary to validate the efficacy and safety of ASCs secretome therapy for treating corneal ulcers in horses. Additionally, optimizing the dosing regimen, administration route, and formulation of the secretome could potentially enhance its therapeutic effects.
5. Conclusions
Overall, these findings suggest that ASCs secretome therapy may be a safe and effective novel regenerative therapy for treating non-healing corneal ulcers in horses. The topical application of the ASCs secretome facilitated corneal re-epithelialization and resolution of clinical signs in a case refractory to conventional treatment. These findings contribute to the growing body of evidence supporting the use of MSC secretomes as a cell-free, minimally invasive approach for promoting tissue repair and regeneration in veterinary ophthalmology.
Author Contributions
Conceptualization, V.S. and G.R.; methodology, C.S., G.C., G.F. and G.R.; validation, V.S., G.R., G.F., G.C., G.R. and C.S.; formal analysis, V.S., G.R., G.F., G.C., G.R. and C.S.; data curation, V.S., G.R. and C.S.; writing—original draft preparation, V.S.; writing—review and editing, V.S., G.R., G.F., G.C., G.R. and C.S.; supervision, V.S., G.R., G.F., G.C., G.R. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The protocol was approved by the Ethical Committee of the University of León (Ref:ULE-063-2023) for compliance with the provisions of Regulation (RD 53/2013).
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank Mª Elisa López González and Cintia Miranda Rodríguez for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hartley, C. Differential Diagnosis and Management of Corneal Ulceration in Horses, Part 2. In Pract. 2015, 37, 23–30. [Google Scholar] [CrossRef]
- Michau, T.M.; Schwabenton, B.; Davidson, M.G.; Gilger, B.C. Superficial, Nonhealing Corneal Ulcers in Horses: 23 Cases (1989–2003). Vet. Ophthalmol. 2003, 6, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, C. Ophthalmology for the Veterinary Practitioner. Can. Vet. J. 2009, 50, 1074. [Google Scholar]
- Williams, L.B.; Pinard, C.L. Corneal Ulcers in Horses. Compend. Contin. Educ. Vet. 2013, 35, E4. [Google Scholar] [PubMed]
- Cooley, P.L.; Wyman, M. Indolent-like Corneal Ulcers in 3 Horses. J. Am. Vet. Med. Assoc. 1986, 188, 295–297. [Google Scholar] [PubMed]
- Hempstead, J.E.; Clode, A.B.; Borst, L.B.; Gilger, B.C. Histopathological Features of Equine Superficial, Nonhealing, Corneal Ulcers. Vet. Ophthalmol. 2014, 17 Suppl 1, 46–52. [Google Scholar] [CrossRef]
- Morgan, R.V.; Abrams, K.L. A Comparison of Six Different Therapies for Persistent Corneal Erosions in Dogs and Cats.; 1994.
- Prucha, V.J.S.; Tichy, A.; Nell, B. Equine Non-Healing Corneal Ulcers: A Retrospective Evaluation of 57 Cases (2001-2017). Tierarztl. Prax. Ausg. G. Grosstiere. Nutztiere. 2020, 48, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Falcão, M.S.A.; Brunel, H.D.S.S.; Peixer, M.A.S.; Dallago, B.S.L.; Costa, F.F.; Queiroz, L.M.; Campbell, P.; Malard, P.F. Effect of Allogeneic Mesenchymal Stem Cells (MSCs) on Corneal Wound Healing in Dogs. J. Tradit. Complement. Med. 2020, 10, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Falcão, M.S.A.; Brunel, H. dos S. S.; Peixer, M.A.S.; Dallago, B.S.L.; Costa, F.F.; Queiroz, L.M.; Campbell, P.; Malard, P.F. Effect of Allogeneic Mesenchymal Stem Cells (MSCs) on Corneal Wound Healing in Dogs. J. Tradit. Complement. Med. 2020, 10, 440–445. [Google Scholar] [CrossRef]
- Singh, V.K.; Sharma, P.; Vaksh, U.K.S.; Chandra, R. Current Approaches for the Regeneration and Reconstruction of Ocular Surface in Dry Eye. Front. Med. 2022, 9, 885780. [Google Scholar] [CrossRef]
- Alió del Barrio, J.L.; De la Mata, A.; De Miguel, M.P.; Arnalich-Montiel, F.; Nieto-Miguel, T.; El Zarif, M.; Cadenas-Martín, M.; López-Paniagua, M.; Galindo, S.; Calonge, M.; et al. Corneal Regeneration Using Adipose-Derived Mesenchymal Stem Cells. Cells 2022, 11. [Google Scholar] [CrossRef]
- Xia, J.; Minamino, S.; Kuwabara, K.; Arai, S. Stem Cell Secretome as a New Booster for Regenerative Medicine. Biosci. Trends 2019, 13, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Makridakis, M.; Roubelakis, M.G.; Vlahou, A. Stem Cells: Insights into the Secretome. Biochim. Biophys. Acta 2013, 1834, 2380–2384. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Novel Frontiers in Regenerative Medicine. Stem Cell Res. Ther. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Pawitan, J.A. Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. Biomed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef]
- Bellei, B.; Migliano, E.; Tedesco, M.; Caputo, S.; Papaccio, F.; Lopez, G.; Picardo, M. Adipose Tissue-Derived Extracellular Fraction Characterization: Biological and Clinical Considerations in Regenerative Medicine. Stem Cell Res. Ther. 2018, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Peyrecave-Capo, X.; Saulnier, N.; Maddens, S.; Gremillet, B.; Desjardins, I. Equine Umbilical Cord Serum Composition and Its Healing Effects in Equine Corneal Ulceration. Front. Vet. Sci. 2022, 9, 843744. [Google Scholar] [CrossRef] [PubMed]
- K. B., G.; D.S., S.; A.F., E.; D.N., S.; A.C., D.A.; R.R., D.S.; M.B.P., S.; Villarreal C.F. AO - Soares Cristiane Flora; ORCID: http://orcid.org/0000-0002-0113-7864, M.B.P.O. http://orcid. org/000.-0001-7549-2992 A.O.-V.; Gama, K.B.; Santos, D.S.; et al. Conditioned Medium of Bone Marrow-Derived Mesenchymal Stromal Cells as a Therapeutic Approach to Neuropathic Pain: A Preclinical Evaluation. Stem Cells Int. 2018, 2018, 1–12. [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- An, S.; Anwar, K.; Ashraf, M.; Lee, H.; Jung, R.; Koganti, R.; Ghassemi, M.; Djalilian, A.R. Wound-Healing Effects of Mesenchymal Stromal Cell Secretome in the Cornea and the Role of Exosomes. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Saccu, G.; Menchise, V.; Gai, C.; Bertolin, M.; Ferrari, S.; Giordano, C.; Manco, M.; Dastrù, W.; Tolosano, E.; Bussolati, B.; et al. Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Promote Corneal Wound Repair by Regulating Inflammation and Angiogenesis. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Na, K.S.; Putra, I.; Lee, H.J.; Hull, S.; Cheng, Y.C.; Blanco, I.J.; Eslani, M.; Djalilian, A.R.; Myung, D. Corneal Wound Healing Effects of Mesenchymal Stem Cell Secretome Delivered Within a Viscoelastic Gel Carrier. Stem Cells Transl. Med. 2019, 8, 478–489. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Helal, M.A.Y.; Tanaka, R. The Pivotal Role of Stem Cells in Veterinary Regenerative Medicine and Tissue Engineering. Vet. Sci. 2022, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal Stem Cell-Derived Exosomes for Clinical Use. Bone Marrow Transplant. 2019, 54, 789–792. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).