1. Introduction
Candida auris was first isolated from the external ear canal of a Japanese patient in 2009 [
1]. Since then, it was responsible for several infections worldwide with a serious nosocomial health risk [
2]. In fact, this yeast merged pivotal pathogenetic features to pose a threat for public healthcare systems such as antifungal multidrug resistance, biofilm formation, ability to spread through contact transmission and opportunistic behaviour in critically ill and immunocompromised patients [
3]. Furthermore, reliable identification by traditional phenotypic and biochemical methods is challenging:
C. auris could be misidentified with other yeast (
e.g.,
Candida haemulonii,
Candida lusitaniae,
Candida guilliermondii) and its correct identification at species level requires more advanced techniques such as DNA sequencing, Nucleic Acid Amplification Tests or, most frequently, Matrix Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS)[
4,
5].
After its identification as a novel species,
C. auris emerged in Africa, India (2009), China (2011), South (2012) and North America (2013) [
6]. In the same year
C. auris reached the Europe with sporadic cases in the United Kingdom [
7]. In the period 2013-2021, 1812 new cases were reported by 15 countries belonging to the European Union and European Economic Area (EU/EEA). For most cases, carriage status was reported (1146; 63.2%), while a bloodstream or another type of infection was accounted for 277 (15.3%) and 186 (10.3%) patients respectively [
8]. Focusing on Italy,
C. auris was first identified as responsible of invasive infection in a hospital of the Liguria region in 2019 [
9]. Thence, other 360 cases were registered until December 2022 in four Northern Italy regions (Liguria, n=297; Piedmont, n=48; Emilia-Romagna, n=15; Veneto, n=1) [
10]. During this time span,
C. auris epidemic raised two peaks: the first occurred in December 2020 and was related to Liguria local epidemiology, while the second was observed in September 2022, mainly due to the emergence of an outbreak in a referral Intensive Care Unit (ICU) in Turin (Piedmont)[
10,
11].
With regard to
C. auris infection-control strategies, international healthcare agencies and expert consensus recommended disinfection of high touch areas and reusable equipment twice or three time daily in rooms housing patients infected by
C. auris. Chlorine-based agents or other sporicidal disinfectants belonging on List P of Environmental Protection Agency (EPA) - Registered Disinfectants are highly effective in controlling
C. auris cross-transmission and its spreading in healthcare settings[
12,
13]. Moreover, contact tracing of
C. auris positive patients is critical to prevent the yeast diffusion and to determine potential outbreaks. To achieve this purpose, the IR-Biotyper (IR-BT) (Bruker Daltonics GmbH & Co. KG, Bremen, Germany), a novel typing instrument based on Fourier-transform infrared (FTIR) spectroscopy, represents a promising tool for rapid and reliable microbial outbreak analysis in nosocomial settings [
14].
A preliminary analysis on the
C. auris outbreak occurred between July 2021 and March 2022 in the AOU Città della Salute e della Scienza, Turin, Italy, was performed by Corcione et al. in 2022, considering the epidemiological and clinical aspects [
11].
Aim of this work was to broaden and deep-in the previous outbreak analysis by the application of the IR-BT in order to investigate phylogenetic relationship among
C. auris strains isolated in a referral ICU of AOU Città della Salute e della Scienza of Turin, Tertiary University hospital in Piedmont, Italy [
11]. For this purpose, for the first time, data collected from IR-BT analysis were used to experimentally define a clustering cut-off to classify new
C. auris strains as belonging or not to the same cluster.
2. Materials and Methods
The study was performed in the Microbiology and Virology Unit of the University Hospital A.O.U. Città della Salute e della Scienza of Turin, Italy, between December 2021 and February 2023. Positive clinical or surveillance samples for C. auris belonging to patients admitted to the university ICU, were accounted in the present work. Only the first strain per patient was considered. Subsequently to diagnostic routine, the isolated C. auris strains were rendered irreversibly anonymous. The anonymization process was carried out in accordance with the procedure concerning the use of clinical data for scientific purposes and of biological samples stored in the laboratories for retrospective studies; successively the strains were stored at -80 °C.
2.1. C. auris Surveillance Protocol
All patients admitted to the ICU were screened weekly by standard surveillance cultures (urine culture, endotracheal aspirate, rectal swab) for multidrug-resistant bacteria detection. Following the first evidence of a
C. auris positive urine culture during surveillance standard screening and according to international available guidelines, a specific surveillance protocol has been developed and applied for all patients hospitalized in the ICU ward[
15,
16,
17,
18,
19].
C. auris screening was performed weekly through a bilateral axillae and groins eSwab (Copan, Brescia, Italy) before patient hygiene nursing care. After samples collection, they were seeded on BD Sabouraud Agar with gentamicin and chloramphenicol (Becton Dickinson, Franklin Lakes, New Jersey, USA) and on chromogenic agar (Brilliance Candida Agar, Thermo Scientific, Waltham, Massachusetts, USA). Incubation was performed at 37 °C for 48 hours. Isolates yeasts were identified at species level using Microflex LT MALDI-TOF (Bruker Daltonics GmbH&Co.KG, Bremen, Germany) according to manufacturer’s instructions.
2.2. IR-Biotyper Protocol for C. auris Typing
Stored C. auris strains were thawed and grown at 37 °C on BD Sabouraud Agar for 24 hours, and then subcultivated on the same medium for 24 +1 h for IR-BT measurements. Sample preparation was performed accordingly to manufacturer’s instruction. Briefly, two highly filled 1 µl inoculation loop of yeast colonies were suspended in 50 μl of 70% ethanol solution in the suspension vials containing metal beads included in the IR-BT kit (Bruker Daltonics). The suspension was homogenized by vortexing for two minutes, and 50 μl of deionized water were added to reach the final volume (100 μl). After two minutes vortexing, 10 μl of the yeast suspension were spotted in five technical replicates on a silicon sample plate (Bruker Daltonics GmbH&Co.KG) and dried at room temperature. Two IR Tests Standards (Bruker Daltonics GmbH&Co.KG) were included in duplicate in each run. All samples were tested in triplicate (biological replicates) in three different days to overcome the biological variation carried out to cultural and IR-BT tests.
Spectra were acquired and analysed in transmission mode (spectral range: 4000-500 cm-1) through OPUS v.8.2 and IR Biotyper Client v.4.0 software (Bruker Daltonics GmbH & Co. KG). The second-order derivative was calculated using the Savitzky-Golay algorithm on nine datapoints and spectra were vector normalized. The similarity analysis between spectra was carried out with a hierarchical clustering algorithm based on Euclidean single linkage. The above-mentioned software allowed constructing a distance matrix and a dendrogram, which gives a tree-like overview of the spectral relationship. Moreover, the unsupervised dimensionality reduction method Principal Component Analysis (PCA) and the supervised Linear Discriminant Analysis (LDA), which maximizes the inter-group variance and minimizes the intra-group variance, were performed to represent spectra as dots in the two-dimensional (2D) and three-dimensional (3D) scatter plots. Each strain was represented with an average spectrum derived from fifteen IR-BT measurements (five spectra, technical replicates, for three biological replicate).
In order to correctly interpret the local C. auris outbreak, a highly characterized strain, DSM 21092, belonging to DSMZ-German Collection of Microorganisms and Cell Cultures GmbH (Leibniz Institute, Germany) was included as external clustering control.
2.3. Statistical Approach to Define a Clustering Cut-Off for IR-Biotyper and C. auris
Starting from the distance matrix, intra-strain variation (span among technical and biological replicates of the same isolate) and inter-strain variation (span among technical and biological replicates of different isolates) were evaluated for each C. auris strain.
In order to define a C. auris clustering cut-off, intra- and inter-strain values, average, median, standard deviation, 5th percentile and 95th percentile were calculated and graphed. A 5% error was considered acceptable for clustering discrimination.
If the inter-isolate distance between two strains is included within the 95th percentile of the intra-isolate distances, the first strain is considered similar to the technical and biological replicates of other and therefore it could be defined clonal. Assuming this, the 95th percentile of intra-isolate distances was considered the lower cut-off to define a strain as part of the same cluster (minimum clustering cut-off). On the other hand, if the inter-isolate distance between two strains is under the 5th percentile of the inter-isolates distance between two distinct groups, the strains could be considered part of the same cluster. Therefore, the 5th percentile of inter-isolates distances between two clusters will be considered as the higher cut-off to consider a strain as belonging to a different cluster (maximum clustering cut-off). The area between the lower and higher cut-off values will be defined as a grey zone of uncertain clustering result.
3. Results
During the considered period of 14 months, 56 positive cases of
C. auris were identified in patients admitted in the ICU. The majority of
C. auris positive samples (51/56; 91.1%) resulted from surveillance cultures from patients colonized at skin level (45; 80.4%), at the urinary (3; 5.4%) and respiratory tract (3; 5.4%). In 5 cases (8.9%) an invasive infection occurred, with
C. auris isolation from blood cultures and intravascular catheter tip samples (
Table 1).
A total of 855 spectra was obtained by typing the 56 clinical strains of C. auris involved in the outbreak and the external control strain. Intra- and inter-isolate distances of the acquired spectra were analysed in order to obtain a reference clustering cut-off.
3.1. Analysis of Intra-Isolate and inter-Isolates Distances Values and Clustering Cut-Off Definition
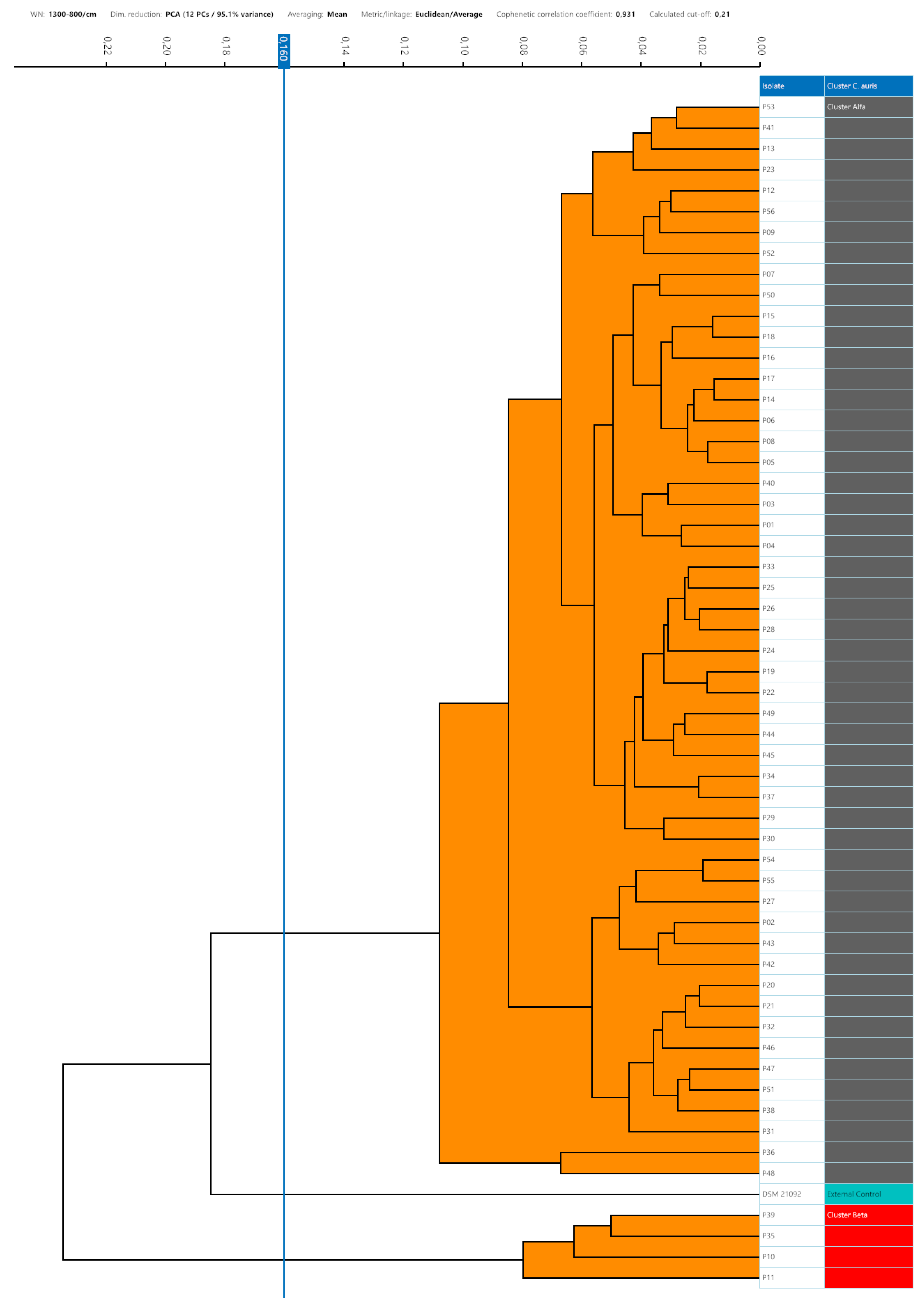
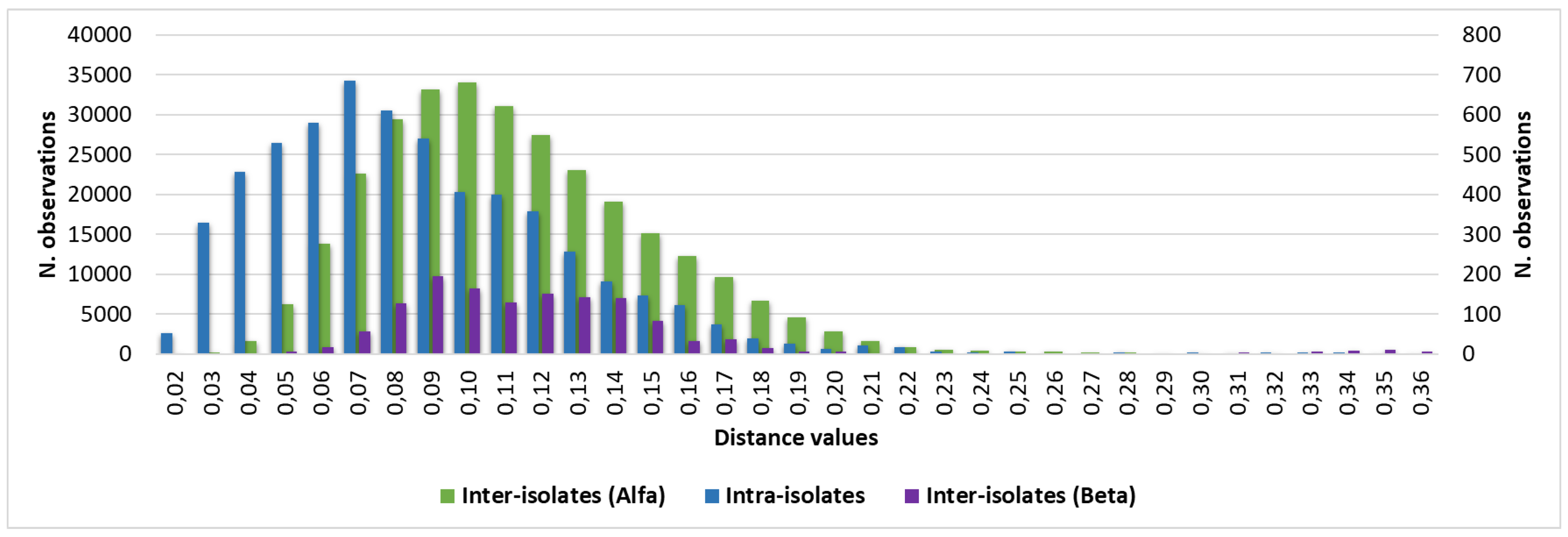
Considering intra-isolate distances of each strain of C. auris under study, mean, median, minimum and maximum, standard deviation, 5th percentile and 95th percentile were calculated. The histogram of the frequencies of all intra-isolate distance values showed a Gaussian distribution with unimodal trend (Figure 3). The median, the 5th and 95th percentile were 0.080, 0.030 and 0.160, respectively. Intra-isolate distance values analysis is reported in detail in the Supplementary material.
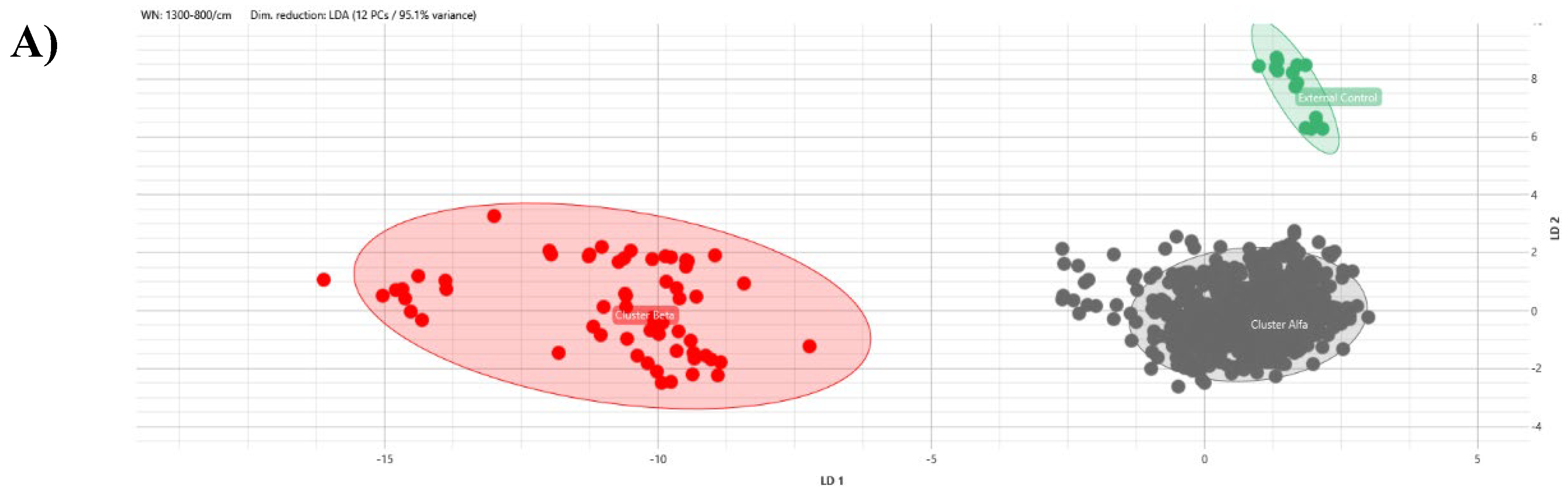
Setting the 95th percentile value, 0.160, as the minimum clustering cut-off, two distinct clusters could be demonstrated by dendrogram and distance matrix analysis: the larger cluster, named Alfa, consisting of 52 (92.9%) strains strictly related to each other and the smaller one, Beta, formed by four (7.1%) similar strains. The third arm of the dendrogram was characterized by the external control spectra of
C. auris interspersed between Alfa and Beta clusters (
Figure 1).
Considering the inter-isolates distances within the major cluster Alfa, the median, the 5th and 95th percentile were 0.107, 0.061 and 0.180, respectively. The inter-isolates distance distributions between strains belonging to the same cluster were consistently overlapping with each other and with the intra-strain distribution (
Figure 3). However, as regards cluster Beta, due to the small number of isolates, the graphic distribution of inter-isolates distances was more irregular. Additional calculated variables and the intra-strains distribution are reported in Supplementary material.
Through the inter-isolates distances analysis between cluster Beta and Alfa, a Gaussian distribution of the distance values could be observed with median, 5th and 95th percentile values of 0.244, 0.160 and 0.354, respectively. Inter-isolates distance analysis and graphic representation are described in detail in the Supplementary material. A summary of the principal calculated distance values is reported in
Table 2.
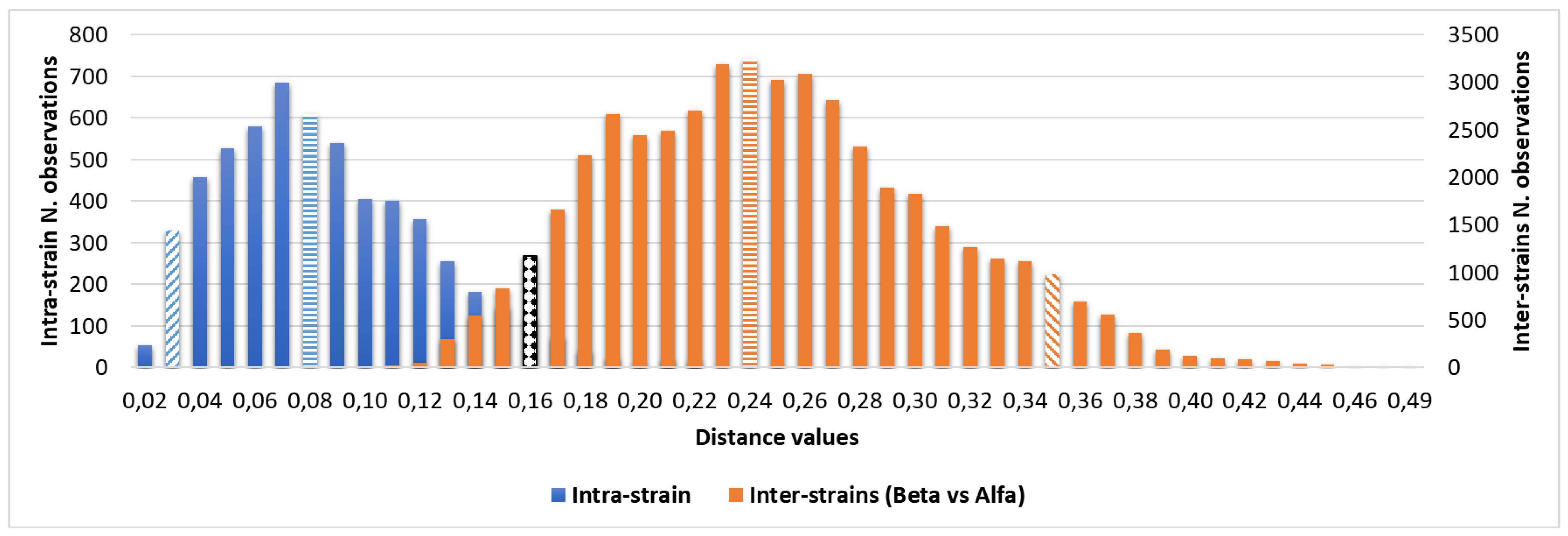
The overlap between the 95th percentile of intra-isolate and the 5th percentile of the inter-cluster distribution could be observed in
Table 2 and
Figure 4.
No grey zone could be observed between minimum and maximum clustering cut-off. Considering the external control position in the dendrogram representation, the cut-off values within which the external control could be considered independent from Alfa or Alfa and Beta clusters were <0.185 and <0.235, respectively. According to 0.160 clustering cut-off, it was separated from both Alfa and Beta clusters.
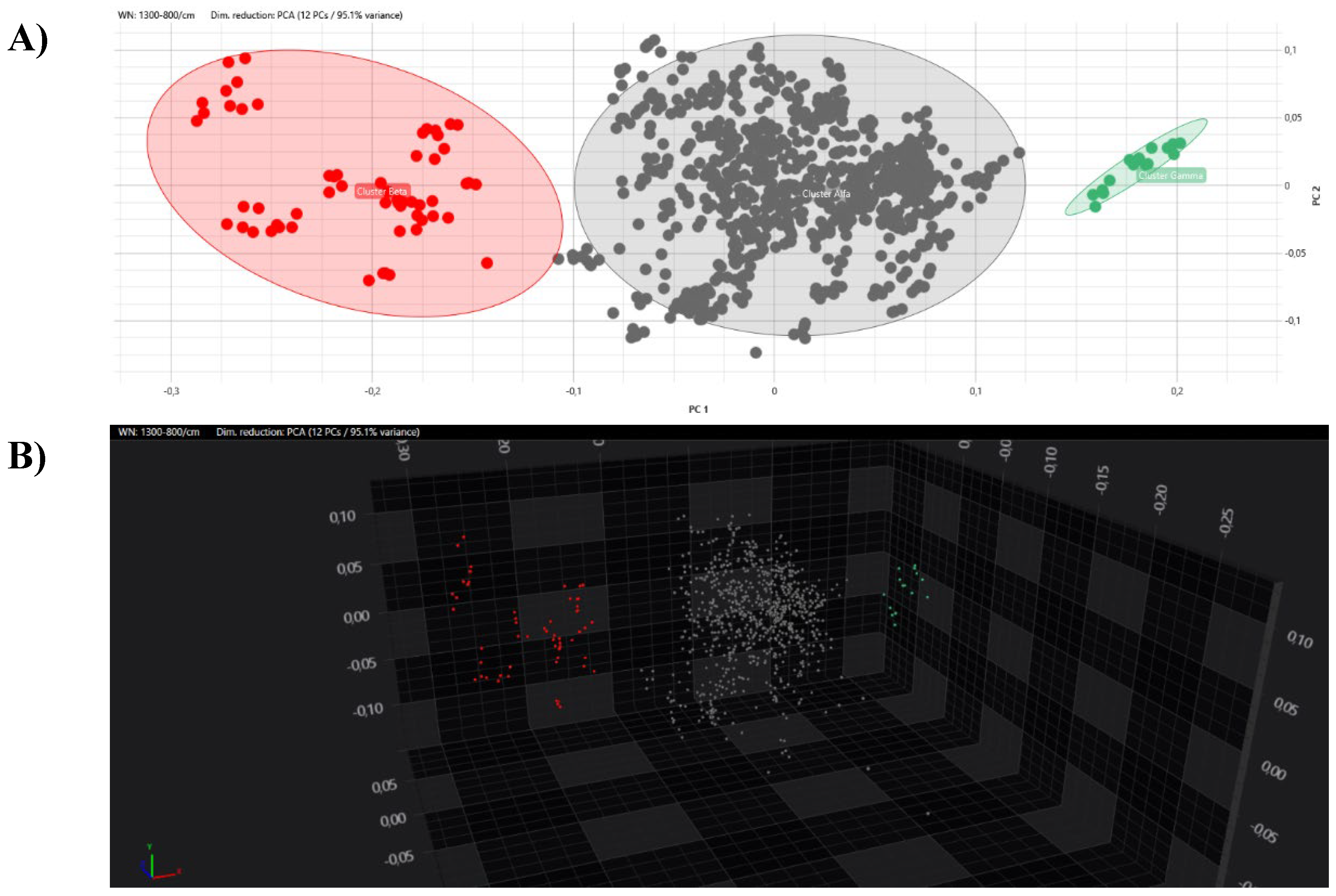
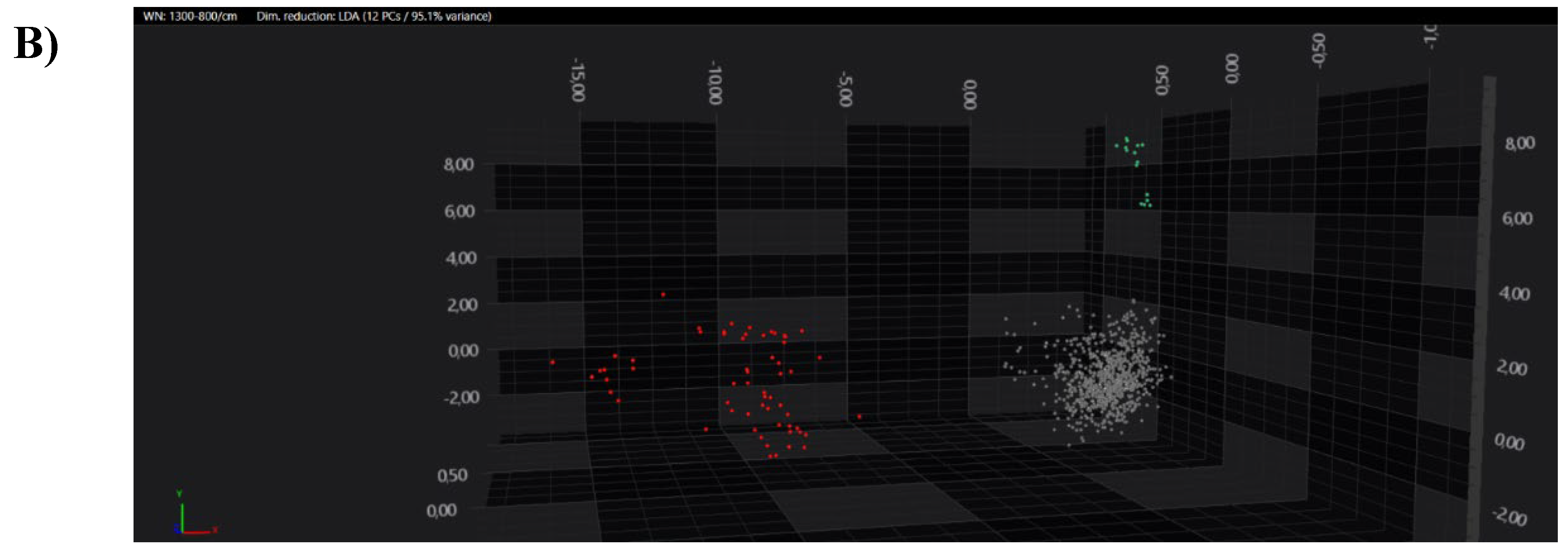
All the obtained spectra were successively analysed by PCA and LDA analysis in 2D and 3D scatter plots. Acquired spectra clearly grouped separated from each other within the belonging cluster in both PCA and LDA analysis (
Figure 5 and
Figure 6).
A subset analysis was conducted to investigate strains features in relationship with samples, antifungal profile, and time of isolation. No differences in clustering analysis were observed between invasive and colonizing strains, antifungal profile, or temporal series of admitted patients and related samples.
4. Discussion
In recent years,
C. auris has emerged as a multi-resistant fungal pathogen, with a significant clinical and health impact and it has been recently reported as a critical issue in the WHO fungal priority list. Unique characteristic of this species is its ability to persist for a long time on the human skin and in hospital environments, resulting in the spread of this microorganism inside and through health networks [
20]. It is therefore of strategic importance to apply correct disinfection guidelines and protocols for the eradication and containment of this microorganism [
21]. Also its rapid and specific diagnosis, as well as the development of genotypical and phenotypic methods for typing, is pivotal in order to investigate with accuracy and speed the relationships between
C. auris isolates involved in nosocomial outbreaks[
22,
23].
The main purpose of this study was the application of FT-IR spectroscopy to investigate the phylogenetic relationships between isolated strains within the local C. auris outbreak.
The first positive case for this emergent microorganism in the ICU ward led to the establishment of an extraordinary surveillance program aimed at identifying patients colonized/infected by
C. auris. During the study period, an outbreak involving overall 56 patients was observed in the aforementioned ward. The phenomenon could be related to the high number of patients transferred from surrounding hospitals during the COVID-19 pandemic, as well as considerable access to transplant assessments and procedures of patients from other regions where
C. auris had already been previously found [
11]. Furthermore, the high clinical severity at admission, the notable invasiveness of treatment (major surgery, extracorporeal support, renal replacement treatment), the need for prolonged ICU length of stay and broad-spectrum antimicrobial therapies characterized the colonized population [
11].
The innovative point of this study consisted in the definition of C. auris clustering cut-offs to discriminate between strains belonging or not to the same cluster.
Assuming that a strain could be considered as part of the same cluster if its inter-strain distance is within the intra-strain distance, the distance between each replicate spectra, the 95th percentile of intra-strain distance was used to define the minimum clustering cut-off with a 5% of accepted classification error. The strains analysed by IR-BT, with the stated 0.160 cut-off, were found to belong to a maximum of two distinct clusters: the major cluster, Alfa, consisting of 52 strains and comprising the index case, and the smaller Beta, consisting of the remaining 4 strains. Adopting this clustering cut-off, the external control was correctly positioned out from the Alfa and Beta clusters. Our minimum clustering cut-off is the result of the 56 C. auris strains’ spectra evaluation, and it is independent from local epidemiology and clustering definition. For these reasons, the 0.160 cut-off value could be proposed as a robust and universal C. auris clustering cut-off whether the incubation and analysis protocol are respected.
The comparison between the intra- and inter-isolate distances of Alfa and Beta clusters shows a clear degree of distributions’ overlapping, supporting the common clonal origin of all the strains belonging to the clusters. The slight shift to the right of the inter-isolate distribution can be explained by the phenomenon of genetic drift due to the selective pressure to which C. auris is subjected in a hospital environment (e.g., use of antifungals, disinfectants, interactions with different hosts’ microbiota and immune systems), which is reflected in phenotypic changes in fungal macromolecules production.
Having ascertained the legitimacy of the two clusters, we focused on the maximum clustering cut-off definition. The inter-strains distance values analysis between Beta and Alfa clusters was performed, assuming the 5th percentile of inter-isolates distance as the maximum clustering cut-off, with a 5% acceptance error. The inter-isolate distances distribution clearly shifted to the right in comparison to intra-strain distribution with only a small and partial overlap. The higher clustering cut-off was 0.160, coincident with the previous one, the minimum clustering cut-off. As validation of the proposed clustering cut-off, the value within which the external control could be considered independent from Alfa or Alfa and Beta clusters, in both dendrogram and distance matrix analysis, was correctly over the defined 0.160 cut-off (<0.185). Moreover, both PCA and LDA analysis clearly confirmed the analysis and the definition of Alfa and Beta clusters.
The application of IR-BT as typing tool for
Candida spp. analysis was investigated in 3 studies up to now. Contreras et al. (2022), by applying a surveillance protocol to over 700 patients admitted to a third-level hospital in Los Angeles, identified 28 positive samples for
C. auris within a year. IR-BT typing allowed to identify two clusters, the first consisting of 27 strains and the second consisting of one isolate. The WGS analysis confirmed this subdivision and allowed to classify the first cluster as belonging to the South African clade and the second as belonging to the South Asian one [
24]. In the Vatanshenassan et al. (2020) study, 96
C. auris strains isolated from 14 geographical regions were analysed using 5 typing protocols (microsatellite analysis, sequencing of ITS regions, AFLP, MALDI-TOF, IR-BT). The results indicated microsatellite analysis as the preferred method for evaluating
C. auris outbreaks. The greater consistency with this method was the sequencing of ITS regions (45% similarity), followed by typing by IR-BT (33% similarity). The analysis of microsatellites allowed to group the isolates in four clusters, corresponding to the four geographical clades (East Asia, South Asia, South Africa and South America), while typing by IR-BT has grouped the strains into two main clusters, independently by their geographical origin [
2].
The study of De Carolis et al. (2023) conducted at the University Hospital A. Gemelli IRCCS in Rome analysed 59 strains of
Candida parapsilosis using IR-BT and microsatellite analysis, observing a concordance between the two methods varying from 47% to 74%, in relation to the cut-off value used in IR-BT analysis (respectively 0.967 - 0.995) [
25].
The agreement discrepancies between microsatellite and IR-BT analysis observed in the two previous studies are presumably due to two reasons: the first lies in the analysis of genetic regions responsible for fluconazole resistance, which contain different mutations depending on C. auris clade and which can be demonstrated exclusively by genotypic analysis, the second reason is the different clonal origin of C. auris strains tested in the study of Vatanshenassan et al. In light of these characteristics, the greater concordance between genotypic (microsatellites) and phenotypic (IR-BT) analysis found by De Carolis et al. appears to be linked to the common geographical origin of Candida parapsilosis strains analysed, presumably genetically related. This is also reflected in a lower rate of mutations responsible for resistance to fluconazole.
The common element of the three studies described above concerns the comparison between genetic analyses (WGS or microsatellites analysis) and IR-BT, in order to evaluate their discriminatory capacity for clustering analysis. On the contrary, in our study, for the first time, a new analytical approach was developed to define a clustering cut-off useful to classify new
C. auris strains without the aid of genetic information. This data could help to overcome traditional typing techniques (WGS, pulsed-field gel electrophoresis - PGFE, MLST) weaknesses such as the access to these technologies, their costs and turn-around time, and the need for expert personnel, features not commonly available in Microbiology laboratories. IR-BT could be a viable alternative for clustering analysis because it is cheaper, faster and has high daily throughput. In addition, its ability to use the cell surface as a sort of “barcode” that can provide more information than PGFE and MLST confirms the robustness of the method for industrial and clinical-epidemiological applications[
23,
26,
27,
28].
5. Conclusions
In this study IR-BT was used to investigate the onset and spread of an outbreak of C. auris at the University Hospital Città della Salute e della Scienza of Turin, Italy. The application of IR-BT based on FT-IR technology has allowed to obtain important information about the phylogenetic relationships between the analysed strains and to define for the first time a clustering cut-off with a “not WGS-based” statistical-mathematical approach, in order to correctly classify C. auris strains as belonging or not to the same cluster. The potential impact of this technology in the management of patients is pivotal not only for infection control policies but also for clinical application. The availability of information about C. auris circulating strains combined with their antifungal profile and virulence, such as their colonizing vs. invasive behaviour, could bring to important changes in patients’ therapeutic management.
Future perspectives will include the results confirmation by WGS genotyping and the typing of new strains of C. auris, in order to test and validate the robustness of the calculated cut-off. A high agreement between the two methods will allows the use of IR-BT as a valid alternative to molecular methods due to its easy to use, its fast turnaround time, its user-friendly interface and its low costs.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org
Funding
This research was supported by EU funding within the MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT).
Author contributions
AC and LP: Conceptualization, Data Curation, Validation, Methodology and Writing - Original Draft; AC, CC, MC: Resources; AB, GP, MG, CP, LC: Investigation; LP, AC, PB and MC: Formal analysis; AC and PB: Visualization; AB, NM, SC, GM, GP and CP: Writing - review & editing; GM, LB and CC: Supervision. All authors contributed to the final version of the manuscript and approved it for publication.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Conflicts of interest
MC is employed at the Bruker Daltonics GmbH & Co. KG. All other authors report no potential conflicts of interest regarding this publication.
References
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida Auris Sp. Nov., a Novel Ascomycetous Yeast Isolated from the External Ear Canal of an Inpatient in a Japanese Hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Vatanshenassan, M.; Boekhout, T.; Mauder, N.; Robert, V.; Maier, T.; Meis, J.F.; Berman, J.; Then, E.; Kostrzewa, M.; Hagen, F. Evaluation of Microsatellite Typing, ITS Sequencing, AFLP Fingerprinting, MALDI-TOF MS, and Fourier-Transform Infrared Spectroscopy Analysis of Candida Auris. J. Fungi Basel Switz. 2020, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida Auris: Epidemiology, Biology, Antifungal Resistance, and Virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef] [PubMed]
- Fasciana, T.; Cortegiani, A.; Ippolito, M.; Giarratano, A.; Di Quattro, O.; Lipari, D.; Graceffa, D.; Giammanco, A. Candida Auris: An Overview of How to Screen, Detect, Test and Control This Emerging Pathogen. Antibiot. Basel Switz. 2020, 9, 778. [Google Scholar] [CrossRef] [PubMed]
- Keighley, C.; Garnham, K.; Harch, S. a. J.; Robertson, M.; Chaw, K.; Teng, J.C.; Chen, S.C.-A. Candida Auris: Diagnostic Challenges and Emerging Opportunities for the Clinical Microbiology Laboratory. Curr. Fungal Infect. Rep. 2021, 15, 116–126. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Sood, P. On the Emergence, Spread and Resistance of Candida Auris: Host, Pathogen and Environmental Tipping Points. J. Med. Microbiol. 2021, 70, 001318. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Isolates of the Emerging Pathogen Candida Auris Present in the UK Have Several Geographic Origins. Med. Mycol. 2017, 55, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Kohlenberg, A.; Monnet, D.L.; Plachouras, D. ; Candida auris survey collaborative group; Candida auris survey collaborative group includes the following national experts Increasing Number of Cases and Outbreaks Caused by Candida Auris in the EU/EEA, 2020 to 2021. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2022, 27, 2200846. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Codda, G.; Orsi, A.; Battaglini, A.; Giacobbe, D.R.; Delfino, E.; Ungaro, R.; Marchese, A. Isolation of Candida Auris from Invasive and Non-Invasive Samples of a Patient Suffering from Vascular Disease, Italy, July 2019. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2019, 24, 1900549. [Google Scholar] [CrossRef]
- Sticchi, C.; Raso, R.; Ferrara, L.; Vecchi, E.; Ferrero, L.; Filippi, D.; Finotto, G.; Frassinelli, E.; Silvestre, C.; Zozzoli, S.; et al. Increasing Number of Cases Due to Candida Auris in North Italy, July 2019-December 2022. J. Clin. Med. 2023, 12, 1912. [Google Scholar] [CrossRef]
- Corcione, S.; Montrucchio, G.; Shbaklo, N.; De Benedetto, I.; Sales, G.; Cedrone, M.; Vita, D.; Costa, C.; Zozzoli, S.; Zaccaria, T.; et al. First Cases of Candida Auris in a Referral Intensive Care Unit in Piedmont Region, Italy. Microorganisms 2022, 10, 1521. [Google Scholar] [CrossRef]
- Ahmad, S.; Asadzadeh, M. Strategies to Prevent Transmission of Candida Auris in Healthcare Settings. Curr. Fungal Infect. Rep. 2023, 17, 36–48. [Google Scholar] [CrossRef] [PubMed]
- USEPA List P: Antimicrobial Products Registered with EPA for Claims against C. Auris. 04 October 2023. [(Accessed on 18 November 2023)]; Available Online: Https://Www.Epa.Gov/Pesticide-Registration/List-p-Antimicrobial-Products-Registered-Epa-Claims-against-Candida-Auris.
- Teng, A.S.J.; Habermehl, P.E.; van Houdt, R.; de Jong, M.D.; van Mansfeld, R.; Matamoros, S.P.F.; Spijkerman, I.J.B.; van Meer, M.P.A.; Visser, C.E. Comparison of Fast Fourier Transform Infrared Spectroscopy Biotyping with Whole Genome Sequencing-Based Genotyping in Common Nosocomial Pathogens. Anal. Bioanal. Chem. 2022, 414, 7179–7189. [Google Scholar] [CrossRef]
- CDC Infection Prevention and Control for C. Auris. 2023. Fungal Diseases, CDC. Https://Www.Cdc.Gov/Fungal/Candida-Auris/c-Auris-Infection-Control.Html#print.
- European Centre for Disease Prevention and Control. C. Auris in Healthcare Settings – Europe – First Update, 23 April 2018. Stockholm: ECDC; 2018.
- Bishop L., Cummins M., Guy R., Hoffman P., Jeffery K., Jefferey-Smith A., Johnson E., Lamagni T., Manuel R., Mohamed-Klein R., Muller-Pebody B., Neely F., Patel B., Sedgwick J., Shetty N., Taori S., Brawn C. (2017). Guidance for the Laboratory Investigation, Management and Infection Prevention and Control for Cases of C. Auris. Public Health England.
- Vuichard-Gysin, D.; Sommerstein, R.; Martischang, R.; Harbarth, S.; Kuster, S.P.; Senn, L.; Widmer, A. Candida Auris - Recommendations on Infection Prevention and Control Measures in Switzerland. Swiss Med. Wkly. 2020, 150, w20297. [Google Scholar] [CrossRef]
- Ong, C.W.; Chen, S.C.-A.; Clark, J.E.; Halliday, C.L.; Kidd, S.E.; Marriott, D.J.; Marshall, C.L.; Morris, A.J.; Morrissey, C.O.; Roy, R.; et al. Diagnosis, Management and Prevention of Candida Auris in Hospitals: Position Statement of the Australasian Society for Infectious Diseases. Intern. Med. J. 2019, 49, 1229–1243. [Google Scholar] [CrossRef]
- Kordalewska, M.; Perlin, D.S. Molecular Diagnostics in the Times of Surveillance for Candida Auris. J. Fungi 2019, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Samaranayake, L.P. Emerging Strategies for Environmental Decontamination of the Nosocomial Fungal Pathogen Candida Auris. J. Med. Microbiol. 2022, 71. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Lyman, M.M.; Sexton, D.J. Tools for Detecting a “Superbug”: Updates on Candida Auris Testing. J. Clin. Microbiol. 2022, 60, e0080821. [Google Scholar] [CrossRef]
- Rakovitsky, N.; Frenk, S.; Kon, H.; Schwartz, D.; Temkin, E.; Solter, E.; Paikin, S.; Cohen, R.; Schwaber, M.J.; Carmeli, Y.; et al. Fourier Transform Infrared Spectroscopy Is a New Option for Outbreak Investigation: A Retrospective Analysis of an Extended-Spectrum-Beta-Lactamase-Producing Klebsiella Pneumoniae Outbreak in a Neonatal Intensive Care Unit. J. Clin. Microbiol. 2020, 58, e00098-20. [Google Scholar] [CrossRef]
- Contreras, D.A.; Morgan, M.A. Surveillance Diagnostic Algorithm Using Real-Time PCR Assay and Strain Typing Method Development to Assist with the Control of C. Auris amid COVID-19 Pandemic. Front. Cell. Infect. Microbiol. 2022, 12, 887754. [Google Scholar] [CrossRef]
- De Carolis, E.; Posteraro, B.; Falasca, B.; Spruijtenburg, B.; Meis, J.F.; Sanguinetti, M. The Fourier-Transform Infrared Spectroscopy-Based Method as a New Typing Tool for Candida Parapsilosis Clinical Isolates. Microbiol. Spectr. 2023, 11, e0238823. [Google Scholar] [CrossRef] [PubMed]
- Deidda, F.; Bozzi Cionci, N.; Cordovana, M.; Campedelli, I.; Fracchetti, F.; Di Gioia, D.; Ambretti, S.; Pane, M. Bifidobacteria Strain Typing by Fourier Transform Infrared Spectroscopy. Front. Microbiol. 2021, 12, 692975. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Cordovana, M.; Deidda, F.; Pane, M.; Ambretti, S. Application of Fourier Transform Infrared Spectroscopy for Real-Time Typing of Acinetobacter Baumannii Outbreak in Intensive Care Unit. Future Microbiol. 2021, 16, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Lozano, I.; Galán-Sánchez, F.; Rodríguez-Iglesias, M. Fourier Transform Infrared Spectroscopy as a New Tool for Surveillance in Local Stewardship Antimicrobial Program: A Retrospective Study in a Nosocomial Acinetobacter Baumannii Outbreak. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2022, 53, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).