1. Introduction:
The term bacteriocins refers to proteins or peptides of ribosomal production that must display inhibitory or lytic activity against bacteria cells, whether they are of the same genus of the producing bacteria, close genera or even covering a wide spectrum of microorganisms in some cases [
17]. These products might have post-translation modifications or perform their function with their original structure [
18].
These biomolecules correspond to products of the secondary metabolism of several bacterial genera and take part in the elimination of competing microorganisms present in the same ecological niche. However, the production of bacteriocins is an energy and nutrient demanding process, so not all strains carry out this process continuously, in fact the production of these metabolites responds to genetic self-regulation systems known as
quorum sensing mechanisms (QS) [
20].
The fact that these metabolites have antimicrobial activity against either pathogenic microorganisms or microorganisms known as deteriorators, gives them high importance for the pharmaceutical and/or biotechnology industry. The first report of a characterized bacteriocin dates to 1925, being named
colicin, a name given based on the microorganism from which it was isolated:
Escherichia coli [
17,
18].
2. Bacteriocin Classification
Telling on the theme of the classification of bacteriocins, this has been fluctuating and evolving in parallel with the increase in knowledge of these biomolecules. Initial classifications dating back to 1993 and 1995 were based solely on physicochemical properties such as thermostability and molecular weight. Later were classified by their sensitivity to enzymes, their post-translation modifications (if present) or the presence of specific functional groups [
21]. This classification system, although no longer recognized, is still used as a basis for the current system, which was developed by various investigations during 2012 to 2018. From this development, a branching criterion was related to the producing microorganism, for which we have: Gram-positive bacteriocins and gram-negative bacteriocins [
21].
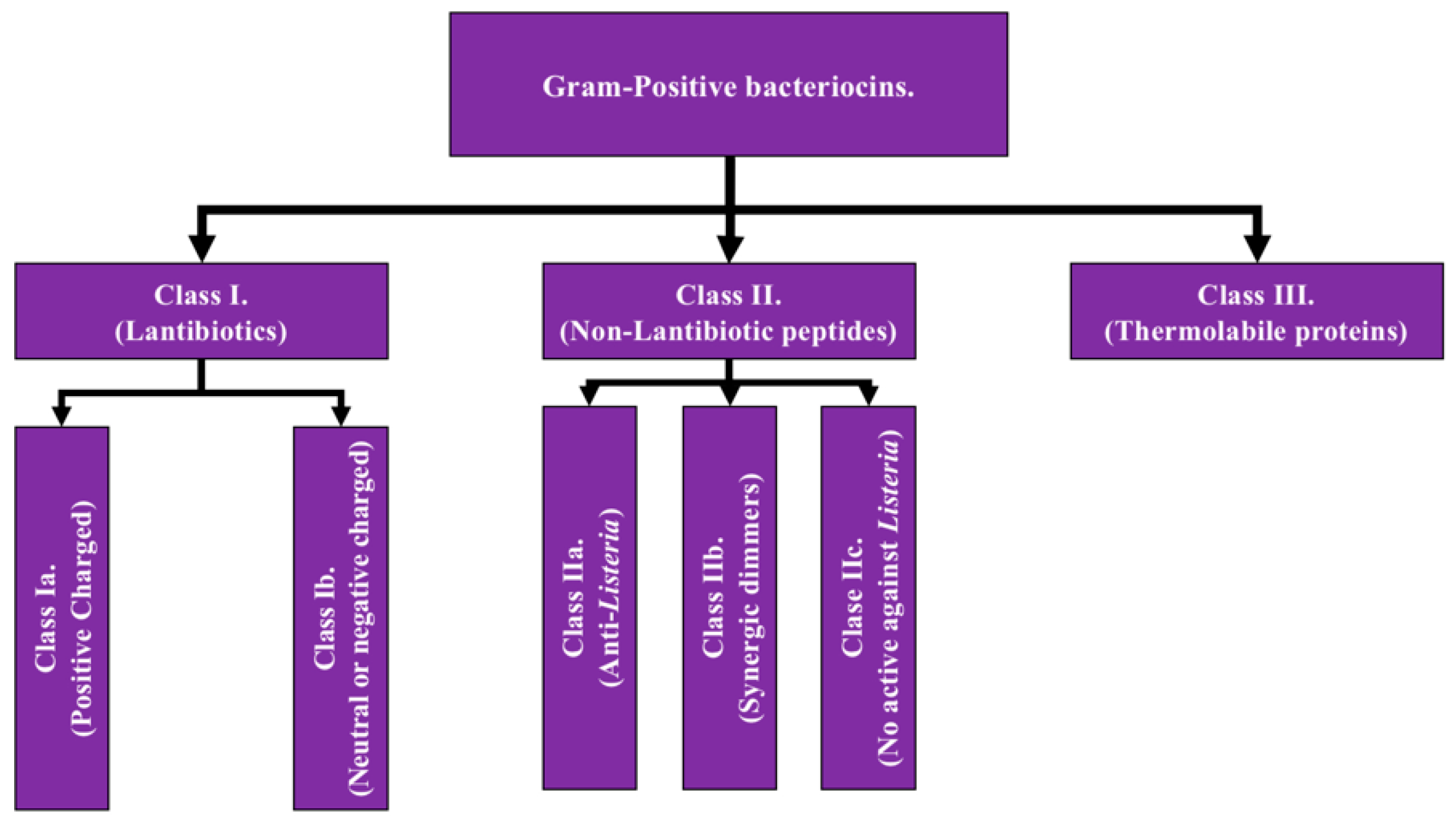
2.1. Gram-Positive Bacteriocins: as the name implies, they are those whose production comes from gram-positive bacterial genera such as Lactobacillus and Staphylococcus. Gram-positive bacteriocins are subclassified into 3 groups based on differences detailed below and
Figure 1 [
18,
21]:
Class I: Also known as lantibiotics, they are molecules with a molecular weight less than 5 kDa, thermostable and presenting a high degree of post-translation modifications. Within its structure there is a high proportion of amino acids such as lanthionine and methyl-lanthionine (amino acids that give its name to this class) as well as unsaturated amino acids. This class has two additional subdivisions:
Class Ia: grouping those structures that are polar having a positive net charge [
18].
Class Ib: which includes those that lack a net charge or have a negative net charge [
18].
Class II: The second class of bacteriocins of gram-positive bacteria covers equally small molecules, however, with a broader range of activity, this being <10 kDa subdivided into four subclasses. All subclasses share the characteristic of having minimal, or even null, post-translation modifications [
21,
31].
Subclass IIa: covers those peptides that have activity against
Listeria (pathogenic bacterial genus that causes food born disease) [
18].
Subclass IIb: corresponds to peptides that act in a dimeric conformation, in which two unaltered peptides act synergistically to achieve the antimicrobial effect [
18,
21].
Subclass IIc: include peptides with a circular structure [
21].
Subclass IId: includes those linear peptides that do not have activity against
Listeria [
21].
Class III: Encompasses proteinaceous bacteriocins with relatively high molecular weight (>30kDa), which have the characteristic of being thermolabile. This group has the particularity of encompassing some bacteriocins that are also produced by gram-negative bacteria under some circumstances, such as the
klebcin [
18].
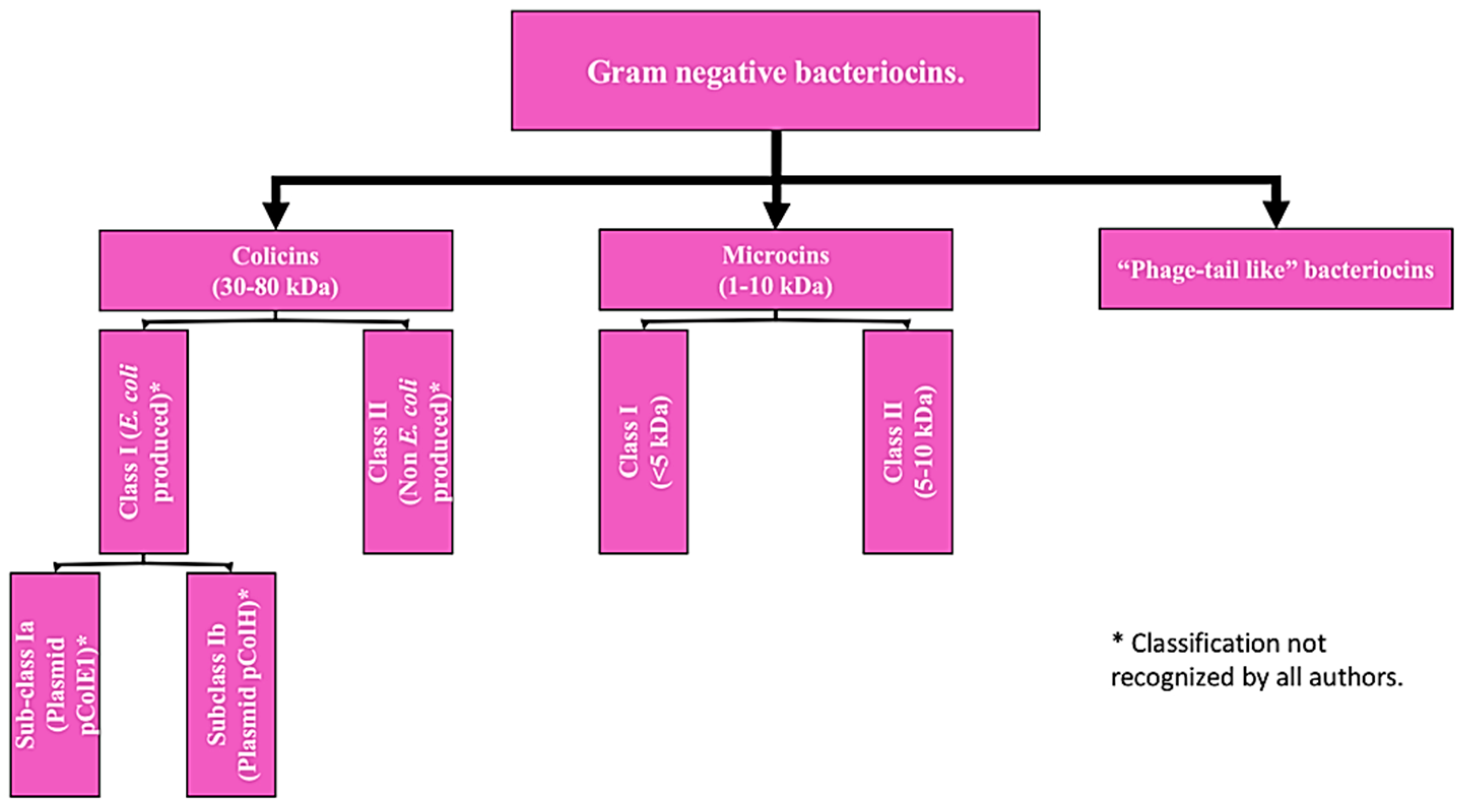
2.2. Gram-Negative Bacteriocins: Regarding the bacteriocins produced by gram-negative microorganisms, the classification is limited to two groups, due to the little information available to date on this class of biomolecules. Gram-negative bacteriocins are, in general, mostly isolated from producing strains of E. coli or from other enterobacteria. The two types of bacteriocins that make up this group are: Colicins (class to which the colicin bacteriocin described above belongs) and microcins, their differentiation is based on their molecular weight [
18,
21]. However, there is a third type not yet fully characterized, which will be addressed as a pseudo-third type (
Figure 2).
Colicins: They are biomolecules with a molecular weight of 30-80 kDa, they are generally produced by strains of
E. coli that harbor a plasmid called colicinogenic, some authors propose the subdivision of this group into two classes:
Colicins produced by
E. coli specifically, which is additionally subdivided according to the type of plasmid from which they originate, and in another group that includes those
colicins produced by other member of Enterobacteriaceae, however, this classification is not yet adopted by all authors [
18,
23].
Microcins: They include low molecular weight bacteriocins, being peptides of 1 to 10 kDa with a highly stable molecular structure, active at a wide pH range, little sensitive to the activity of proteases (a highly desirable characteristic in microbiomes such as the human digestive system) and resistant to temperature changes [
23].
These bacteriocins are encoded in the bacterial genomic DNA, unlike
colicins [
18,
23]. Like previous type, this classification also has a sub-classification that have not yet been fully adopted and is based on their molecular weight: Class I (<5 kDa) and class II (5 to 10 kDa) [
18].
Phage Tail-Like Bacteriocins: They correspond to the third hypothetical type of gram-negative bacteriocin, they are molecules that hypothetically have antimicrobial activity based on their structure, but there is still not much information about them [
23].
However, it is important to highlight that the classification of bacteriocins is still a fluctuating topic, with authors recommending different ways of classifying these biomolecules [
24].
3. Bacteriocins Mechanism of Action
On this context, it is important to highlight that there is no certainty about the mechanism of action of 100% of those described today. An example can be observed in bacteriocins such as
PLNC8, which has inhibitory capacity against
Helicobacter pylori, but its mechanism of action is unknown [
22]. On the other side, there are groups whose mechanism of action is fully described. An example of these groups would correspond to the bacteriocins produced by bacterial genera of lactic acid bacteria, such as
Lactobacillus, also known as LAB-bacteriocins [
21].
3.1. LAB-Bacteriocins: They group together gram-positive bacteriocins, among which the most common and at the same time the best known correspond to lantibiotics (class I) [
21,
30].
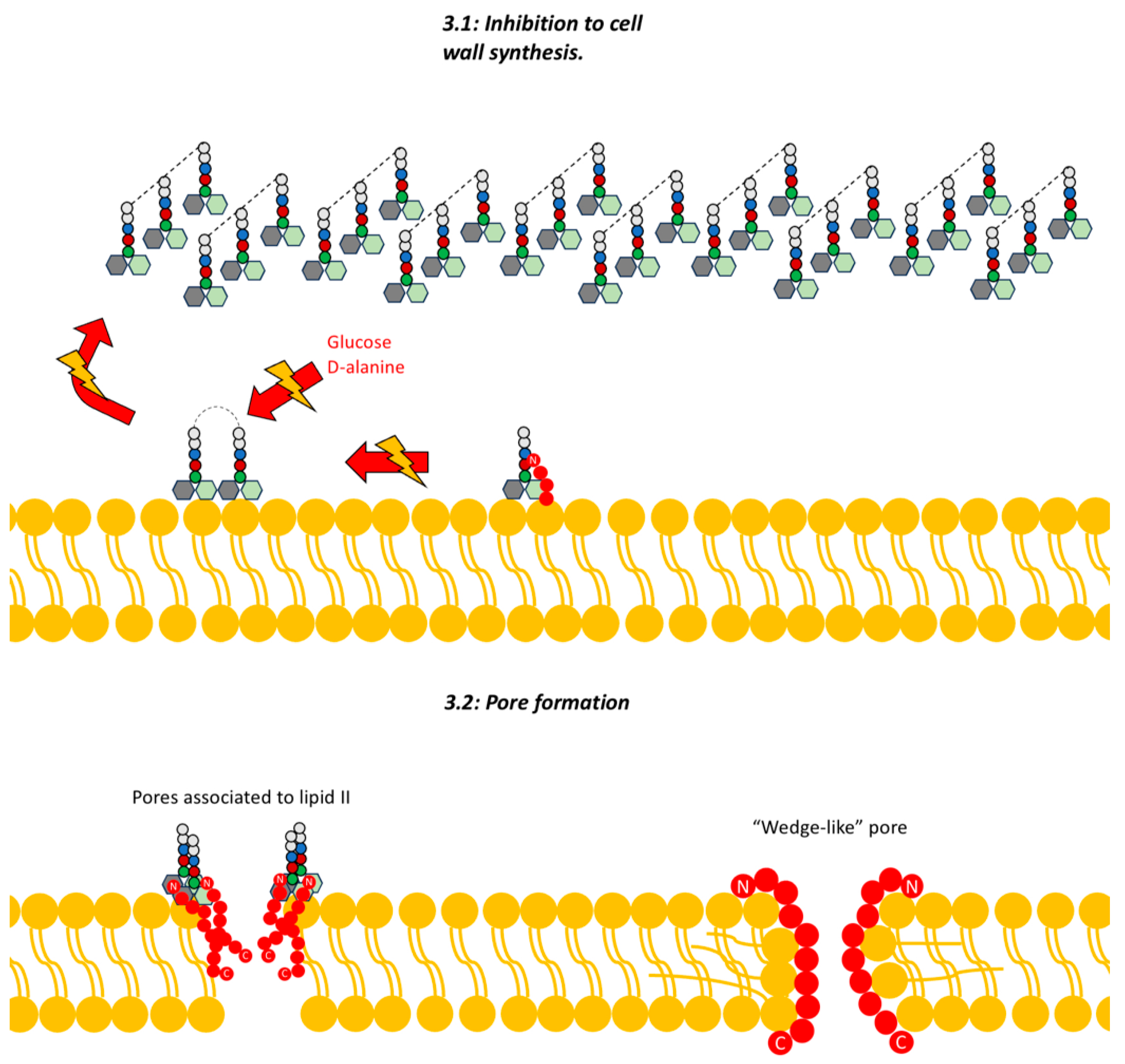
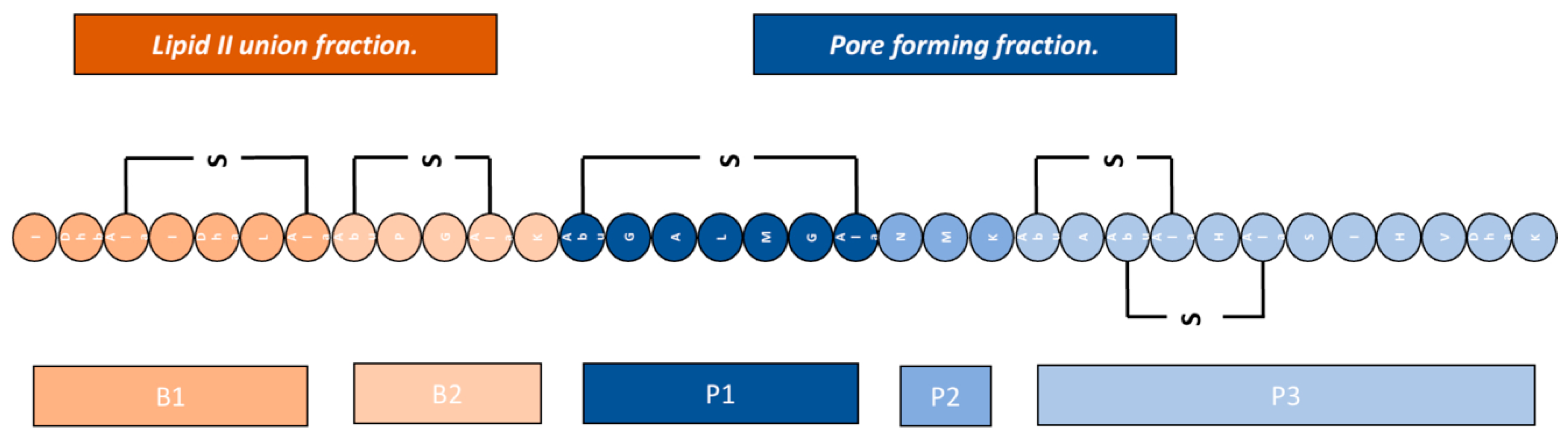
Lantibiotics have demonstrated two different mechanisms to exert their bacterial lysis function: The first corresponds to the disruption of cell wall synthesis, the second corresponds to the formation of pores.
Disruption of Cell Wall Synthesis: In this area, various
lantibiotics show antibiotic activity through two mechanisms of inhibition of cell wall synthesis, the first is the binding to lipid II (an important intermediate in the trans-glycosylation reaction), an example of a bacteriocin that uses this mechanism is
gallidermin, a type of
lantibiotic [
21,
32]. The second mechanism of inhibition of cell wall synthesis corresponds to the blocking of the incorporation of glucose and D-alanine on the precursors of cell wall molecules, thus inhibiting the synthesis of peptidoglycan, however, it was demonstrated in studies by various authors that this mechanism is also dependent on the availability of lipid II (
Figure 3.1) [
21].
Pore Formation: The second way in which gram-positive bacteriocins carry out their bacteriocidal activity corresponds to their ability to attack the integrity of the cell membrane (
Figure 3.2).
Within this mechanism there are two models currently proposed, these being the “barrel-stave”; in which the bacteriocin binds in parallel to the bacterial membrane, which through its difference in charges causes the loss of membrane potential and the formation of accumulations of water and pores, all this leads to the leakage of solutes and biomolecules from the cytoplasm to the external medium [
21,
32].
The second model corresponds to the “wedge” in which the interaction of the bacteriocin occurs in a trans-membrane manner, via the interaction of the charged components of the bacteriocin with the polar head of the lipid bilayer and the interaction of the peptide chain with the non-polar tail of the hydrocarbon. This insertion of the bacteriocin generates deformations in the membrane and fissures [
21]. It has been noted that pore formation can be mediated by binding to lipid II as well [
21,
31].
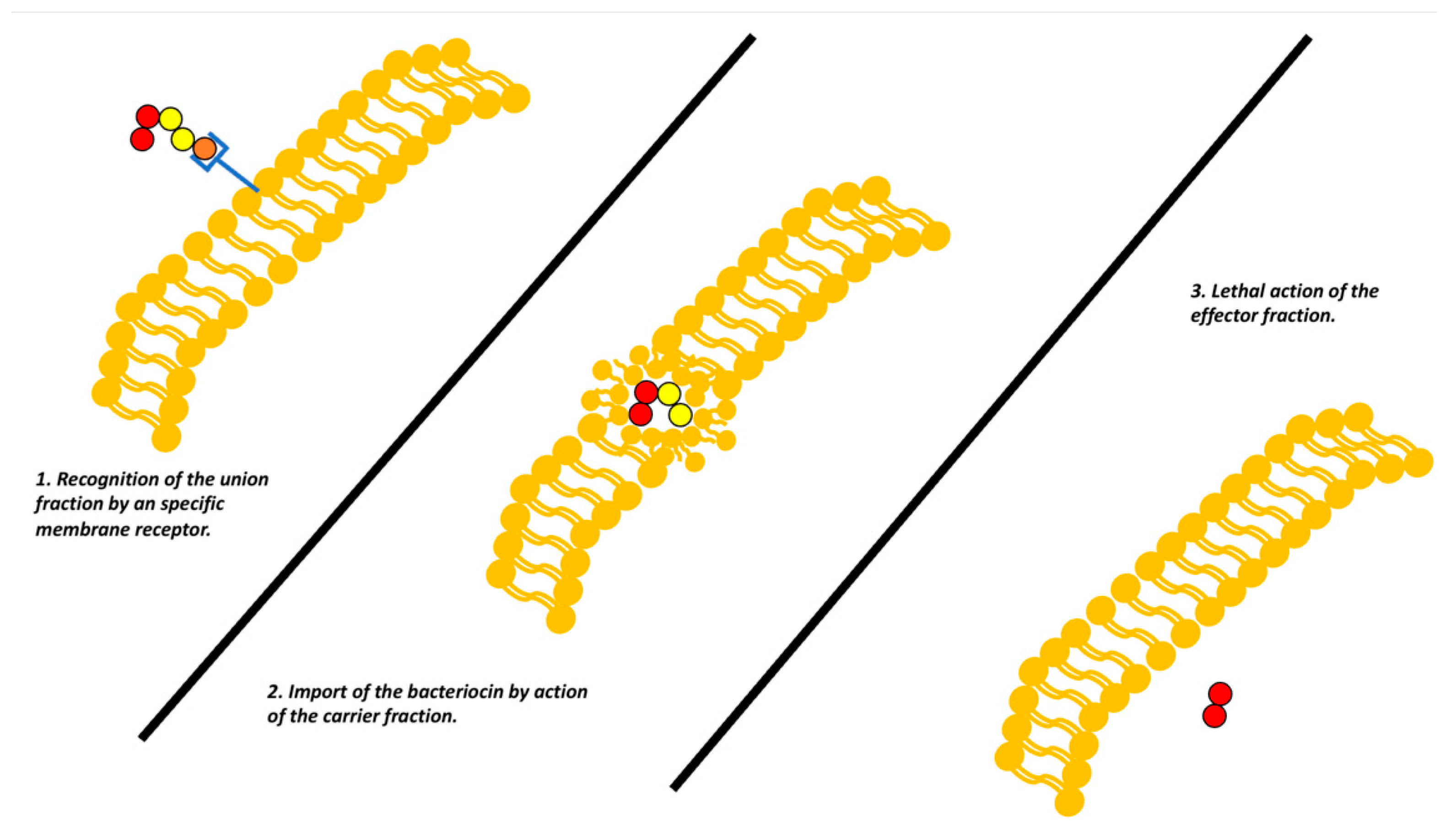
3.2. Colicins: On the other hand, a group of bacteriocins that has also been adequately characterized are the colicins; biomolecules produced by E. coli and other enterobacteria, which are specialized in the elimination of other gram-negative bacteria [
24,
33].
The mechanism of function of these bacteriocins is based on their structure. These colicins, generally present three domains that each have a function, the first will be an antigen-like recognition for anchoring, a mechanism similar for antibodies, a second domain is responsible for the introduction of the bacteriocin to the target bacterial cell, finally the last domain contains the toxic function. Currently there are three mechanisms described for them [
24,
33]:
However, it is key to clarify that the accuracy of how colicins exert these mechanisms may vary (
Figure 4) [
24].
4. Bacteria Genera Producing Bacteriocins
As already mentioned, the production of bacteriocins corresponds to a natural process of various bacterial genera in response to competitors in the microenvironment. This opens the door to assuming that this metabolic activity is common in most bacterial genera discovered today, but this has not been confirmed [
17]. However, research has managed to find bacterial species and/or groups that are certain to produce at least one bacteriocin of any type, thus being a fundamental factor in the formation of the micro-environments where these microorganisms grow [
17].
The first group corresponds to the Enterobacteriaceae family, where we can find species such as
E. coli,
Enterobacter spp.,
Klebsiella spp., among others. These groups are recognized by the production of bacteriocins of the colicin type, especially
E. coli, or
microcins in the case of the rest of the enterobacteria [
24].
On the other hand, a group of bacteria well known for their production of bacteriocins corresponds to lactic acid bacteria, that, in addition to the production of non-protein antimicrobial substances such as lactic acid, are recognized producers of
lantibiotics [
12]. Within this group we find bacteria of the genus
Lactobacillus, species of this genus are used nowadays as oral probiotics, which are commonly recommended to patients after antibiotic therapies or cases of stomach infections due to pathogenic microorganisms, with the aim of recovering the balance of the microbiome by taking advantage of their ability to secrete bacteriocins that attack colonizing foreign microorganisms [
14,
16].
Additionally, recent research has discovered the production of bacteriocins by the genus
Bacillus,
Staphylococcus and
Streptococcus (in particular, beta-hemolytic species), whose bacteriocins have recently been isolated and are in the process of developing a possible biotechnological application [
4,
13,
19].
Finally, it should be noted that the procedure for detecting bacteriocins in bacterial isolates can be cumbersome and repetitive, which is why in recent years “machine learning” mechanisms have been developed to assist in the detection of genes that codify for the synthesis of these bioproducts [
2].
Regarding the genes involved on the production of bacteriocins, two types of them have been reported, the first being chromosomal gene clusters known as “operons”; an example of them, is the “
thermophilin 13 operon” that allows certain strains of
Streptococcus thermophilus to produce the bacteriocin
thermophilin [
25]. The second type of bacteriocin encoding genes are related to the presence of “orphan genes” which are single genes that allow by themselves the production of a certain type of bacteriocin, example of this can be found on certain strains of
Lactobacillus plantarum, which can carry orphan genes like
PlnJ and
PlnNC8, and have been noted to be closely related to other bacteriocin orphan genes from closely related strains, suggesting that the orphan genes probably come from a common ancestor and are transmitted via plasmids or other gene transferring strategy [
26].
4.1. Bacteria Source and Selection: Having discussed the bacteria genera that could produce bacteriocins, the next question to address is the source from which said bacteria could be isolated from. Multiple studies have been successful in isolating potential bacteriocin producing strains from natural sources as river water, grass silage and soil [
34], additionally producing strains can also be found on prepared food items, as example the fermented meals as Korean traditional Kimchi, dairy items as cheese, milk, and buttermilk [
36].
Additionally, another source from which researchers have been able to recover and investigate bacteriocin producing bacteria is samples of healthy microbiomes, like those taken from either the gut or the oral cavity of healthy individuals, from which multiple bacteria species known for producing bacteriocins, generally enterobacteria like
E. coli or
Enterobacter spp. [
35].
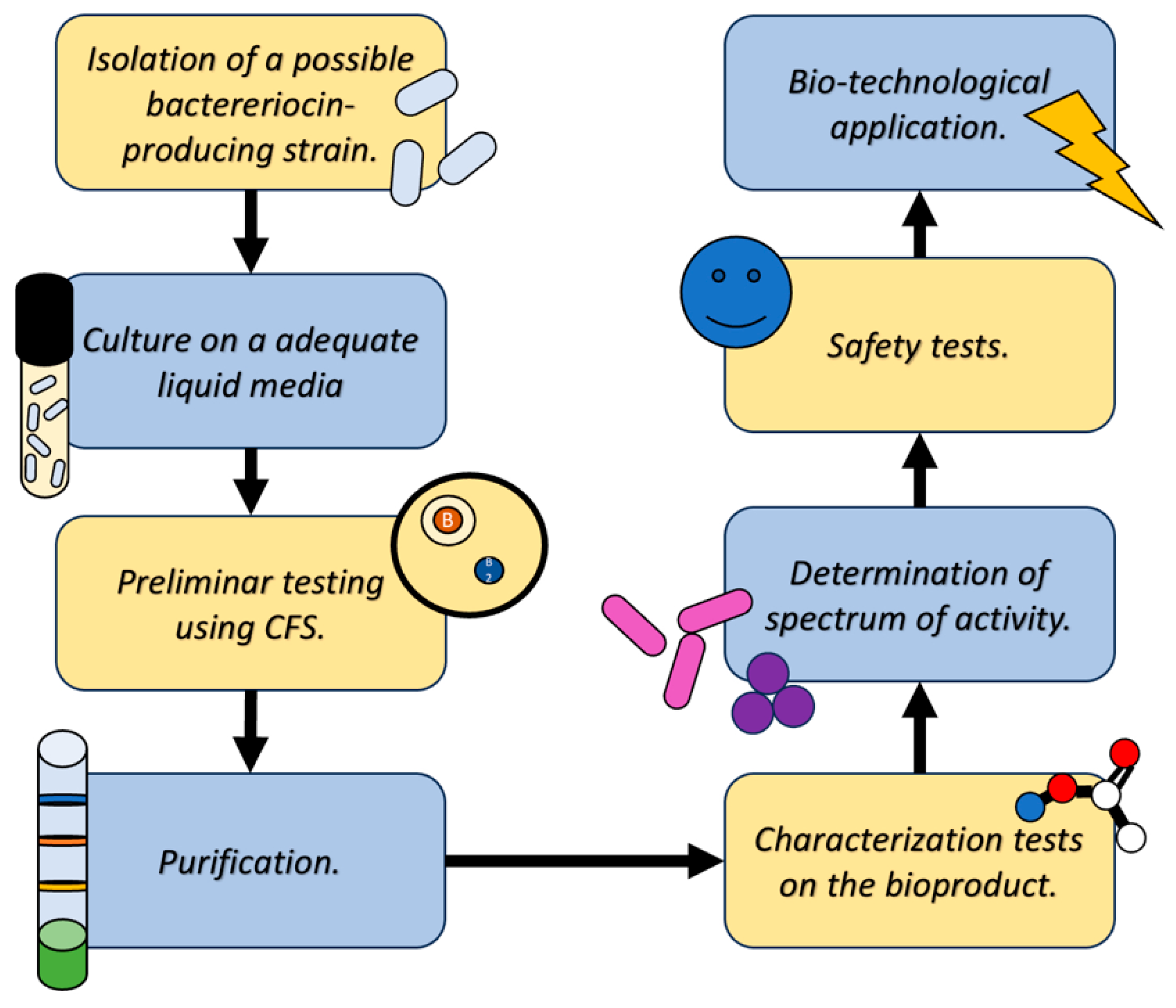
5. Isolation and Uses of Bacteriocins
Regarding the isolation and use of bacteriocins, the methodologies for detection, determination of action spectrum, isolation and subsequent characterization have been evolving in parallel with the knowledge about these molecules.
On first instance, the detection of strains that possibly have the capacity to produce a bacteriocin of possible biotechnological interest is carried out by various methods, among which are: point inoculation method, cross-streak method, radial-streak method, agar insert method, disk diffusion method, Oxford cup method and diffusion-well method [
3].
These methods are based on the inhibition of the growth of “indicator” strains caused by the presence of the strain with possible bacteriocin production or using liquid culture supernatants after centrifugation (known as Cell Free Supernatant or CFS), the which can be placed in contact with the indicator strains using various vehicles [
3].
Aside of the conventional methods described before, more recent research has been able to develop methods of detecting possible bacteriocin producing strains using molecular methods that allow the detection of genes or gene clusters that code to produce these biomolecules [
2].
Once the identification of a bacterial strain that produces a bacteriocin of interest is achieved, the extraction and purification proceed. The first step corresponds to the cultivation of the producing strain in an appropriate liquid medium, from which, the CFS will be obtained, where a part of several metabolic products produced by the bacteria, the bacteriocins are found [
16].
Subsequently, this CFS can be subjected to various purification methods to recover the bacteriocin in question in the purest form possible. Among the methods that are applied today are: Ion exchange chromatography, gel chromatography, HPLC. reverse phase chromatography, solvent fractionation, among other that allow the separation of the component of interest from contaminants and/or impurities, as well as other formed elements of the culture medium, all these methods have been described as effective by diverse research as reported by Ye and collaborators [
16].
Finally, after obtaining a purified bioproduct, the characterization methodologies can be applied. These tests are carried out with the purpose of understanding the molecular structure of the bacteriocin (Mass Spectroscopy or IR), knowing its stability (Enzymatic sensitivity tests, stability in pH gradient, thermostability, among other tests). These tests are carried out to outline the conditions under which the product could be used to perform the spectrum activity tests against microorganisms of medical, food preservation and/or biotechnological interest (
Figure 5) [
11,
16].
6. Uses and Potential Uses of Bacteriocins
About the application that these biomolecules can have; it is extremely important to emphasize their biotechnological and/or health potential when it comes to combating microorganisms with relatively low toxicity compared to regular antibiotics. Some of the potential uses are commented below.
6.1. Combat Antimicrobial Resistance: The first use that can be given to bacteriocins and that quickly comes to mind is medicinal use as antibiotic therapies against microorganisms that are not susceptible to current antibiotics [
12].
Within this area it is important to mention the global problem of antimicrobial resistance, where microorganisms become resistant to drugs to which they were previously susceptible, due to the selection of clones that have mechanisms and/or mutations, that allows them to survive their effects. The conditions in which this phenomenon occurs are normal, but its appearance is accelerated by the indiscriminate and empirical use of antibiotics for treatments, the misuse of these by patients, their use in other activities such as livestock farming and non-controlled disposal to natural ecosystems [
1].
On this regard the WHO (world health organization) determined a “priority” group which has demonstrated an accelerated development of resistance mechanisms, the one known as the ESKAPE group, composed of
Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp. It is in this area where research on the use of bacteriocins as medicinal therapy becomes important, since they have demonstrated effectiveness in the elimination and/or inhibition of the growth of these microorganisms, which opens the door to their use as part of the efforts to the fight against this global problem [
1]. However, an important issue is the safety use of them, turning it the task to solve.
6.2. Use As Preservative Agents: On the side of food biotechnology, recent studies have shown that the presence of non-pathogenic groups of bacteria, such as Lactobacillus, plays an important role when it comes to food preservation [
7]. Within this, it has been shown that the presence of strains that produce some type of bacteriocin can inhibit the growth of microorganisms harmful to health on the surface of foods such as cheese, beef, ham and prepared food items as cheonggukjang (a traditional Korea dish), additionally the usage of nanometric systems on the food item preparation could also provide extremely good effects on prepared drinks as wines and fruit juices. All the mentioned have proven to extend the item shell life by around 30 days compared to non-bacteriocin containing items [
7,
40].
An example of practical applications that have been developed over the years corresponds to the application of coatings supplemented with
Lactobacillus strains in food preservation, which have demonstrated effectiveness in inhibiting the growth of
Listeria monocytogenes, a pathogenic bacteria known for causing severe food born illnesses [
7].
6.3. Restoration of the Balance of the Microbiota: A final use to highlight that has been elucidated for these biomolecules is based on their regulatory capacity of the microbiome. The commensal microbiome of the various areas of the human body, such as the digestive tract, plays an important and first-line role in the defense against pathogenic microorganisms [
15,
17].
However, when the balance between the microorganisms present is lost, either due to prolonged antibiotic treatments, poor diet and/or colonization of harmful microorganisms, a condition known as “dysbiosis” occurs, which has been associated with dangerous diseases such as chronic infections by microorganisms such as
Clostridioides difficile, producing a chronic inflammatory disorder and even the development of cancer [
15].
Considering this, the development of probiotic formulations based on lactic acid bacteria that produce bacteriocins, as well as the transplantation of a healthy microbiome (commonly through fecal transplants), have gained relevance in the safe and effective treatment of dysbiosis, achieving equal results, or even superior to conventional antibiotic therapy [
15].
It should be noted that these described uses do not cover the absolute map of what these biomolecules can do for biotechnological purposes.
7. Nisin: The First Bacteriocin Approved for Use:
Speaking of historical terms, the discovery of the first bacteriocin currently approved by the FDA dates to 1928, the same time in which Alexander Fleming would discover penicillin, in this year the scientists Rogers and Whittier would report the ability of a bacterial strain, at that time known as group N
Streptococcus, to produce metabolites that inhibit the development of pathogens. This biomolecule ended up being named “Group N
Streptococcus Inhibitory Substance” which is abbreviated to
nisin by adding the suffix -in [
5].
Although at that time its activity against relevant pathogenic microorganisms such as
Mycobacterium tuberculosis was demonstrated, it was determined to be of little use due to its poor solubility and fragility against enzymes [
5]. However, in the 1950s, its usefulness as a food preservative was determined, due to its ability to be added to foods, inhibiting bacterial genera such as
Clostridium, Staphylococcus, Bacillus, Listeria, among other gram positives without altering the flavor of the food, and without entailing adverse effects in its consumption, a fact that earned it authorization by the FDA as a food preservative, being the first bacteriocin authorized for use by this institution [
5,
6].
This bacteriocin being a desirable product for both the food and biotechnology industries, has been studied over the years, achieving the production of modified
nisins (named with a letter code) (
Figure 6) that give them better physicochemical properties, as well as its conjugation to nanometric systems which have allowed extending the spectrum of action to gram-negative microorganisms [
5,
6].
8. New Technological Trends for the Use of Bacteriocins:
Finally, the last topic to be addressed in this review corresponds to the new technological trends that have been developed in the current decade for the use of bacteriocins from a biotechnological level.
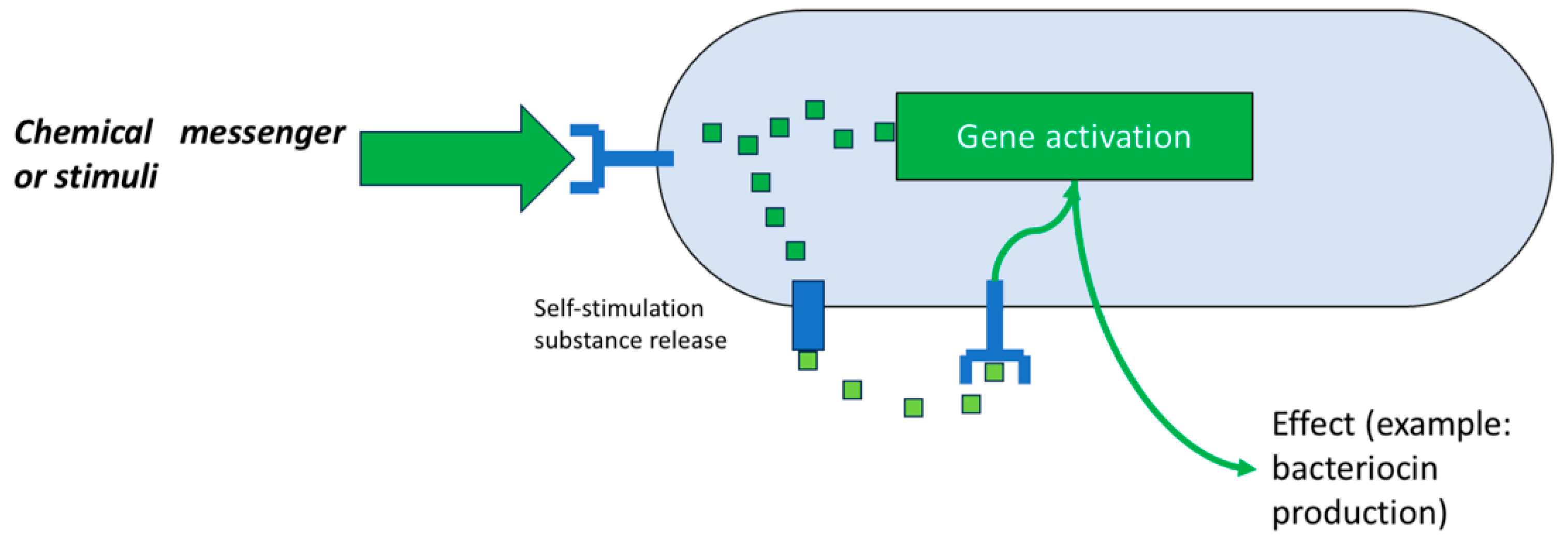
The first system used is based on the regulatory aspect of its production by microorganisms, that is, the “
quorum sensing” system (
Figure 7). Various studies have sought ways to generate “optimal” conditions that induce the producing bacteria to synthesize bacteriocins for their subsequent recovery [
9].
Research has shown that the main factors that cause a bacteriocin-producing strain to synthesize it and release it into the environment are the presence of competing strains, a shortage of nutrients, and the presence of sufficient clones of the producing microorganism. Therefore, a bioreactor capable of controlling these factors is an attractive objective for study and development [
9].
Finally, another technological trend that has been widely used to optimize the effect and/or expand the spectrum of activity of bacteriocins corresponds to nanometric conjugate systems [
10]. This technological addition is made seeking to emulate natural mechanisms that can be observed in some
Lactobacillus acidophilus strains, a microorganism that can generate membrane vesicles and use as a “vehicle” for delivering the bacteriocins it produces [
8].
9. Production Earnings of Actual Bacteriocins:
On the topic of the possible profits that arise from the usage and/or production of bacteriocins, is important to understand that as in 2019, antibiotic industry generated a profit of an estimated 59,000 million dollars worldwide, being forecasted to amass a profit of 20 million dollars by 2027 [
43]. The last considering all the possible applications where bacteriocins could pose an cheaper to produce alternative, said applications enroll the applications of antimicrobials on the food industry, where the usage of these molecules for both the enhance of production (on which the applications of antimicrobials either as additives to fertilizers, or as products given to cattle animals as antibiotics or probiotics are highly profitable for farmers) and the preservation of food items (applied or present on different food items, or used as antimicrobials for cooking items) [
41,
42]. On the last point, it is estimated by the Food and Agriculture Organization of the United Nations that around 1/3 of the food items go to waste, meaning that the successful application of bacteriocins as novel and more effective food preservatives would amount for a huge economic impact regarding the avoidance of loss of food.
With all discussed, the potential application of bacteriocins as antimicrobial, could pose an equal, if not larger income compared to the current antibiotic industry profit, this considering the possibility of cheaper production and the relative safety associated to their use.
10. Perspectives:
10.1. Synergy Studies among Bacteriocins and Classical Antibiotics or other Bioactive Compounds:
Considering everything described on this review, bacteriocins shown promising characteristics which opens the possibility for their individual use as antimicrobial agents, but recent research has found that the association of newly described bacteriocins with other bio active compounds shows possible positive synergic effects compared to their basal effects, making said combinations an desirable alternative to either reduce the quantity of bacteriocin used to achieve an desirable effect or to enhance the activity of already defined antibiotic/antimicrobial consortia, an example of the first can be seen on the research conducted by Soltani and collaborators which deduced that the use of the bacteriocin
reuterin combined with other bioactive compounds such as organic acids shown an synergic effect that allowed for the desired antimicrobial effect on pathogens using a lower concentration of
reuterin [
39].
Speaking of the enhancing of the activity of bioactive compounds or mixtures already used today research has shown that the adding of bacteriocins with known antimicrobial effect such as antibiotics, can amount for a synergy which allows for the treatment of microorganisms which previously developed some kind of resistance, an example of this can be the use of bacteriocins produced by
Enterococcus faecium alongside antibiotics as vancomycin and ciprofloxacin against
Listeria monocytogenes, which shown an increased effect compared to that of the individual compounds [
38]. Moreover, research involving common
Enterococcus species associated to urinary tract infections has shown that the usage of bacteriocins as AS-48, which provides effect at concentrations below 10 mg/L, alongside 20 common antibiotics used for the treatment of this infections as gentamicin and amoxicillin/clavulanate shows synergic effect that amounts for a 100-fold increase of the antimicrobial minimal inhibitory concentration, result which is highly promising for the clinical field as it can amount for a therapeutic success using less antibiotics for this kinds of infections [
37].
Another potential approach is the chemical modification of each bacteriocin that could enhance their activity and use the machine learning or artificial intelligence to improve the action on the bacterial target and wide this activity to current resistant microorganisms and reduce at the same time the toxicity of such compounds.
10. Conclusions:
For closure, bacteriocins, although they are not yet a 100% understood topic, do correspond to a group of bacterial metabolites of great interest to the pharmaceutical, food and biotechnology industries. This is due to its ability to disrupt the microbial development of many microorganisms of interest, in addition to their apparent few adverse effects.
It is for this reason that more efforts must be made to be able to take advantage of them optimally, such as the final outline of a classification system for these biomolecules, in addition to the determination of the mechanisms of action that even today are not fully described for some discovered bacteriocins. However, it is important that their future use is carried out responsibly to avoid, as is the case with antibiotics, for them to end up becoming obsolete for the treatment and elimination of the microorganisms against which they have the promise of action.
References
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Miller, J.H. BAPRES: a software tool for predicting bacteriocins using an optimal set of features. BMC Bioinformatics 2023, 24. [Google Scholar] [CrossRef]
- Yadav, M.; Tiwari, S.K. Methods for determination of antimicrobial activity of bacteriocins of lactic acid bacteria. Microbiology 2023, 92, 745–765. [Google Scholar] [CrossRef]
- Fernández-Fernández, R.; Lozano, C.; Fernández-Pérez, R.; Zarazaga, M.; Peschel, A.; Krismer, B.; Torres, C. Detection and evaluation of the antimicrobial activity of micrococcin P1 isolated from commensal and environmental staphylococcal isolates against MRSA. International Journal of Antimicrobial Agents 2023, 62, 106965. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; De Ullivarri, M.F.; Ross, R.P.; Field, D. After a century of Nisin research - where are we now? Fems Microbiology Reviews 2023, 47. [Google Scholar] [CrossRef] [PubMed]
- Brum, L.F.W.; Santos, C.D.; Santos, J.H.Z.D.; Brandelli, A. Structured silica materials as innovative delivery systems for the bacteriocin nisin. Food Chemistry 2022, 366, 130599. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.P.M.; Ribeiro, S.C.; Teixeira, J.A.; Silva, C. Application of an alginate-based edible coating with bacteriocin-producing lactococcus strains in fresh cheese preservation. LWT 2022, 153, 112486. [Google Scholar] [CrossRef]
- Dean, S.N.; Rimmer, M.A.; Turner, K.B.; Phillips, D.A.; Caruana, J.; Hervey, W.J.; Leary, D.H.; Walper, S.A. Lactobacillus acidophilus membrane vesicles as a vehicle of bacteriocin delivery. Frontiers in Microbiology 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Barragán, A.; West, S.A. The cost and benefit of quorum sensing-controlled bacteriocin production in lactobacillus plantarum. Journal of Evolutionary Biology 2019, 33, 101–111. [Google Scholar] [CrossRef]
- Sulthana, R.; Archer, A.C. Bacteriocin nanoconjugates: boon to medical and food industry. Journal of Applied Microbiology 2021, 131, 1056–1071. [Google Scholar] [CrossRef]
- Li, H.; Xiang, Y.; Zhang, M.; Jiang, Y.-H.; Zhang, Y.; Liu, Y.-Y.; Lü, L.; Zhang, Q. A novel bacteriocin from Lactobacillus salivarius against Staphylococcus aureus: isolation, purification, identification, antibacterial and antibiofilm activity. LWT 2021, 140, 110826. [Google Scholar] [CrossRef]
- Leslie, V.A.; Alarjani, K.M.; Malaisamy, A.; Balasubramanian, B. Bacteriocin producing microbes with bactericidal activity against multidrug resistant pathogens. Journal of Infection and Public Health 2021, 14, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.; Spellerberg, B. Bacteriocin production by Beta-Hemolytic streptococci. Pathogens 2021, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fu, Y.; Liu, F.; Liu, Z.; Ma, J.; Jiang, R.; Song, C.; Jiang, Z. Purification and antimicrobial mechanism of a novel bacteriocin produced by Lactobacillus rhamnosus 1.0320. LWT 2021, 137, 110338. [Google Scholar] [CrossRef]
- Anjana; Tiwari, S.K. Bacteriocin-Producing probiotic lactic acid bacteria in controlling dysbiosis of the gut microbiota. Frontiers in Cellular and Infection Microbiology 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Wang, J.; Liu, M.; Li, P.; Gu, Q. Purification and characterization of a novel bacteriocin from Lactobacillus paracasei ZFM54. LWT 2021, 143, 111125. [Google Scholar] [CrossRef]
- Heilbronner, S.; Krismer, B.; Brötz-Oesterhelt, H.; Peschel, A. The microbiome-shaping roles of bacteriocins. Nature Reviews Microbiology 2021, 19, 726–739. [Google Scholar] [CrossRef]
- Negash, A.W.; Tsehai, B.A. Current applications of bacteriocin. International Journal of Microbiology 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Mercado, V.; Olmos, J. Bacteriocin production by bacillus species: isolation, characterization, and application. Probiotics and Antimicrobial Proteins 2022, 14, 1151–1169. [Google Scholar] [CrossRef]
- García-Curiel, L.; Del Rocío López-Cuellar, Ma.; Rodríguez-Hernández, A.-I.; Chavarría-Hernández, N. Toward understanding the signals of bacteriocin production by Streptococcus SPP. and their importance in current applications. World Journal of Microbiology & Biotechnology 2021, 37. [Google Scholar] [CrossRef]
- Sharma, K.; Kaur, S.; Singh, R.; Kumar, N. Classification and mechanism of bacteriocin induced cell death: a review. The Journal of Microbiology, Biotechnology and Food Sciences 2021, 11, e3733. [Google Scholar] [CrossRef]
- Li, Y.; Yan, J.; Chen, Z.; Gu, Q.; Li, P. Antibacterial effects of bacteriocin PLNC8 against Helicobacter pylori and its potential mechanism of action. Foods 2022, 11, 1235. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramos, A.; Madi-Moussa, D.; Coucheney, F.; Drider, D. Current knowledge of the mode of action and immunity mechanisms of LAB-Bacteriocins. Microorganisms 2021, 9, 2107. [Google Scholar] [CrossRef]
- Marković, K.G.; Grujović, M. Ž.; Koraćević, M.G.; Nikodijević, D.D.; Milutinović, M.; Semedo-Lemsaddek, T.; Djilas, M. Colicins and microcins produced by enterobacteriaceae: characterization, mode of action, and putative applications. International Journal of Environmental Research and Public Health 2022, 19, 11825. [Google Scholar] [CrossRef] [PubMed]
- Salini, F.; Iacumin, L.; Comi, G.; Dicks, L.M.T. Thermophilin 13: In silico analysis provides new insight in genes involved in bacteriocin production. Microorganisms 2023, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Zendo, T.; Sonomoto, K. Multiple bacteriocin production in lactic acid bacteria. Journal of Bioscience and Bioengineering 2022, 134, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-Sensing regulation of antimicrobial resistance in bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef]
- Twomey, E.; Hill, C.; Field, D.; Begley, M. Recipe for Success: Suggestions and recommendations for the isolation and characterisation of bacteriocins. International Journal of Microbiology 2021, 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nisa, M.; Dar, R.A.; Fomda, B.A.; Nazir, R. Combating food spoilage and pathogenic microbes via bacteriocins: A natural and eco-friendly substitute to antibiotics. Food Control 2023, 149, 109710. [Google Scholar] [CrossRef]
- Fernandes, A.; Jobby, R. Bacteriocins from lactic acid bacteria and their potential clinical applications. Applied Biochemistry and Biotechnology 2022, 194, 4377–4399. [Google Scholar] [CrossRef]
- Antoshina, D.V.; Balandin, S.V.; Ovchinnikova, T.V. Structural features, mechanisms of action, and prospects for practical application of Class II bacteriocins. Biochemistry (Moscow) 2022, 87, 1387–1403. [Google Scholar] [CrossRef]
- Mercado, V.; Olmos, J. Bacteriocin production by bacillus species: isolation, characterization, and application. Probiotics and Antimicrobial Proteins 2022, 14, 1151–1169. [Google Scholar] [CrossRef]
- Marković, K.G.; Grujović, M.; Koraćević, M.G.; Nikodijević, D.D.; Milutinović, M.; Semedo-Lemsaddek, T.; Djilas, M. Colicins and microcins produced by enterobacteriaceae: characterization, mode of action, and putative applications. International Journal of Environmental Research and Public Health 2022, 19, 11825. [Google Scholar] [CrossRef] [PubMed]
- Lozo, J.; Topisirović, L.; Kojić, M. Natural bacterial isolates as an inexhaustible source of new bacteriocins. Applied Microbiology and Biotechnology 2021, 105, 477–492. [Google Scholar] [CrossRef]
- Drider, D. Gut Microbiota is an Important Source of Bacteriocins and Their In Situ Expression Can Be Explored for Treatment of Bacterial Infections. Probiotics and Antimicrobial Proteins 2021, 13, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Yi, E.S.; Kim, A.-J. Antimicrobial and Antibiofilm Effect of Bacteriocin-Producing Pediococcus inopinatus K35 Isolated from Kimchi against Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics 2023, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Montalbán-López, M.; Cebrián, R.; Galera, R.; Mingorance, L.; Martín-Platero, A.M.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M. Synergy of the Bacteriocin AS-48 and Antibiotics against Uropathogenic Enterococci. Antibiotics 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Fugaban, J.I.I.; Bucheli, J.E.V.; Holzapfel, W.H.; Todorov, S.D. Assessment of Bacteriocin-Antibiotic Synergy for the Inhibition and Disruption of Biofilms of Listeria monocytogenes and Vancomycin-Resistant Enterococcus. Microbiology Research 2022, 13, 480–499. [Google Scholar] [CrossRef]
- Soltani, S.; Biron, É.; Said, L.B.; Subirade, M.; Fliss, I. Bacteriocin-Based Synergetic Consortia: a Promising Strategy to Enhance Antimicrobial Activity and Broaden the Spectrum of Inhibition. Microbiology Spectrum 2022, 10. [Google Scholar] [CrossRef]
- Verma, D.K.; Thakur, M.; Singh, S.; Tripathy, S.; Gupta, A.; Baranwal, D.; Patel, A.; Shah, N.; Utama, G.L.; Niamah, A.K.; Chávez-González, M.L.; Gallegos, C.F.; Aguilar, C.N.; Srivastav, P.P. Bacteriocins as antimicrobial and preservative agents in food: Biosynthesis, separation and application. Food Bioscience 2022, 46, 101594. [Google Scholar] [CrossRef]
- Eveno, M.; Savard, P.; Belguesmia, Y.; Bazinet, L.; Gancel, F.; Drider, D.; Fliss, I. Compatibility, cytotoxicity, and gastrointestinal tenacity of Bacteriocin-Producing bacteria selected for a consortium probiotic formulation to be used in livestock feed. Probiotics and Antimicrobial Proteins 2020, 13, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Archacka, M.; Celińska, E.; Białas, W. Techno-economic analysis for probiotics preparation production using optimized corn flour medium and spray-drying protective blends. Food and Bioproducts Processing 2020, 123, 354–366. [Google Scholar] [CrossRef]
- Rees; European Pharmaceutical Review. Antibiotics market set to generate revenue of $58,800 million by 2027. European Pharmaceutical Review. Available online: https://www.europeanpharmaceuticalreview.com/news/139468/antibiotics-market-set-to-generate-revenue-of-58800-million-by-2027/.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).