Submitted:
29 May 2024
Posted:
30 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Catalyst Preparation
2.2.2. Catalyst Characterization
2.2.3. Catalytic Performance Evaluation
3. Results and Discussion
3.1. Catalyst Characterization
3.1.1. Morphology
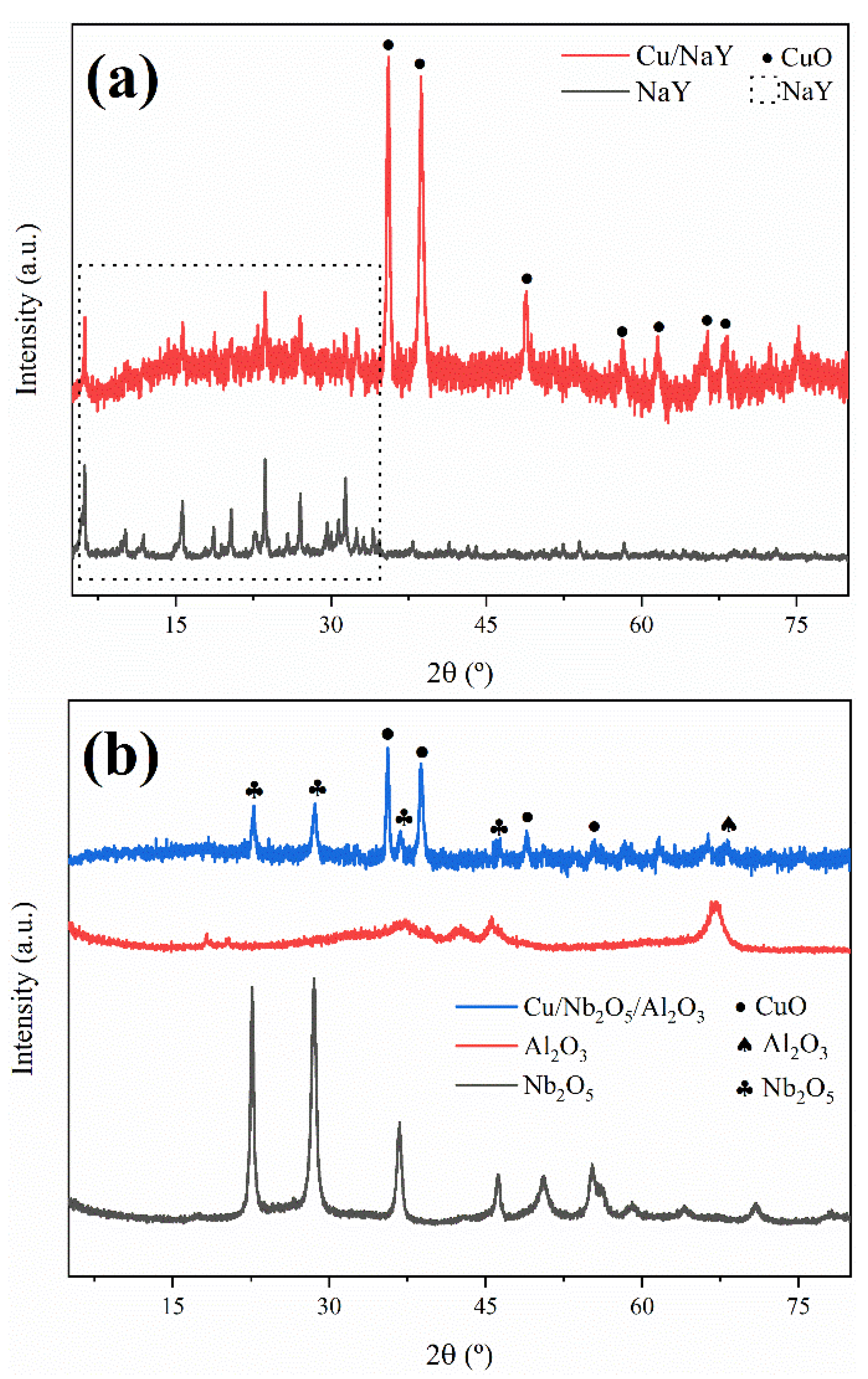
3.1.2. Crystallinity
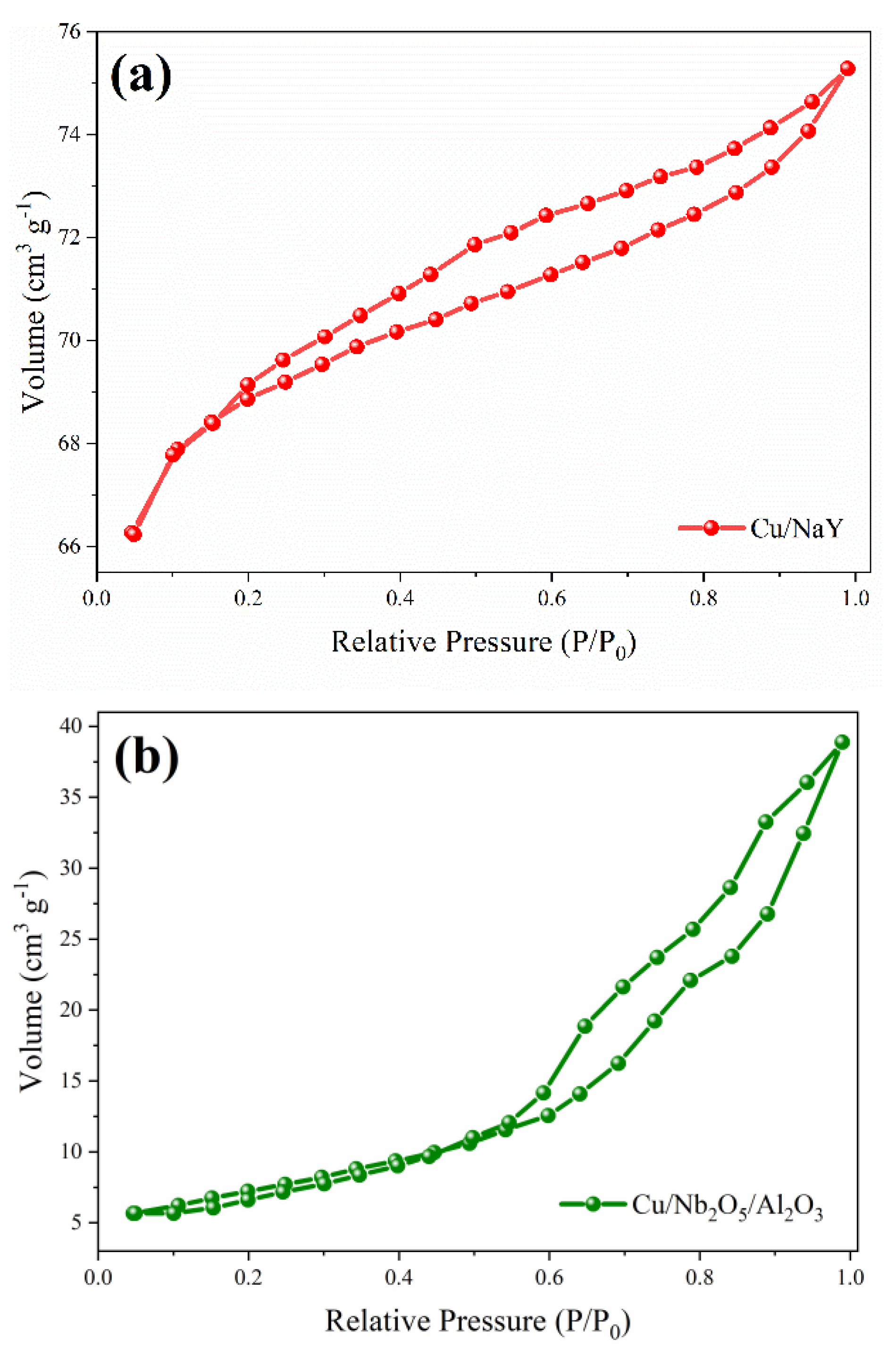
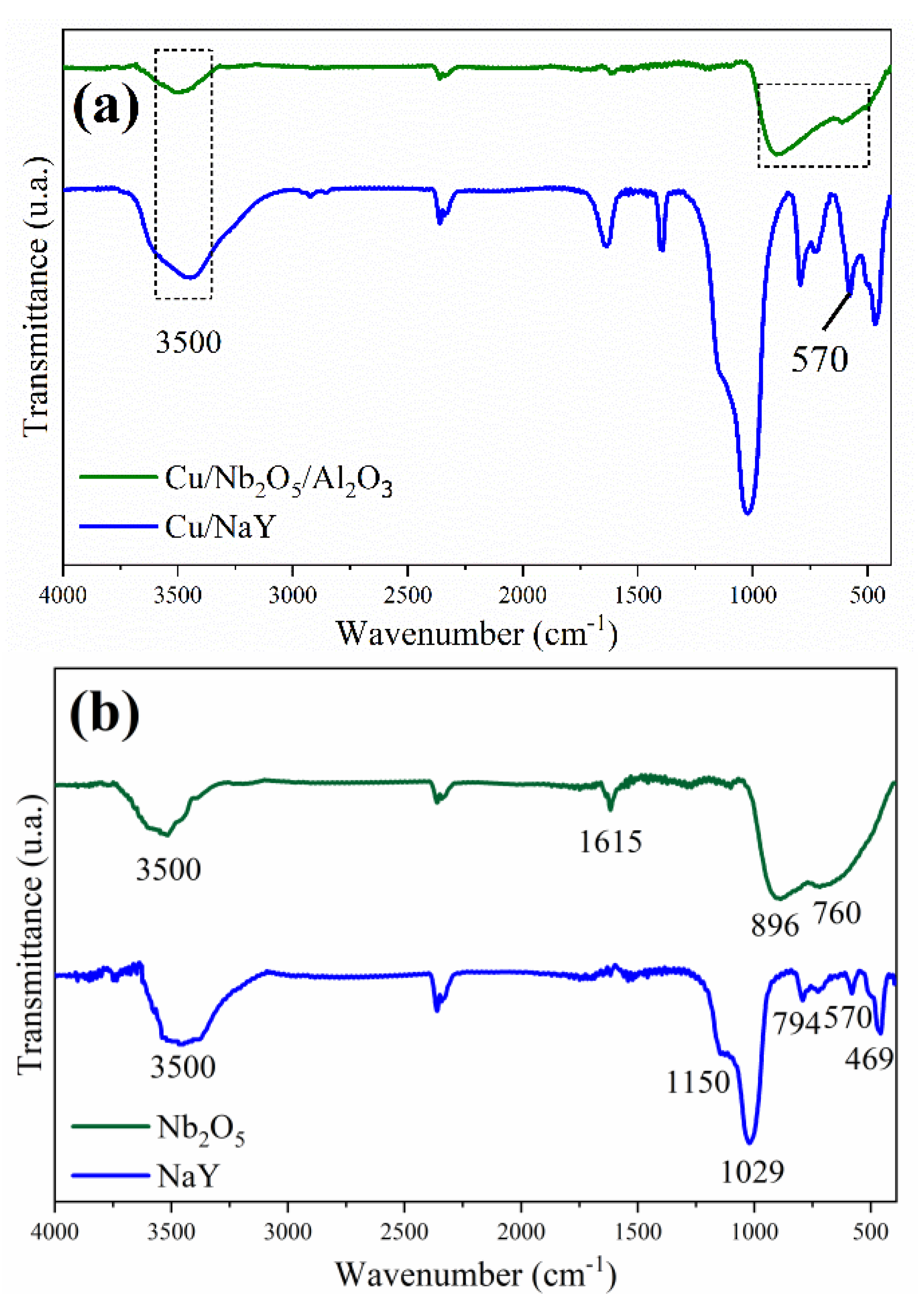
3.1.3. Textural Parameters
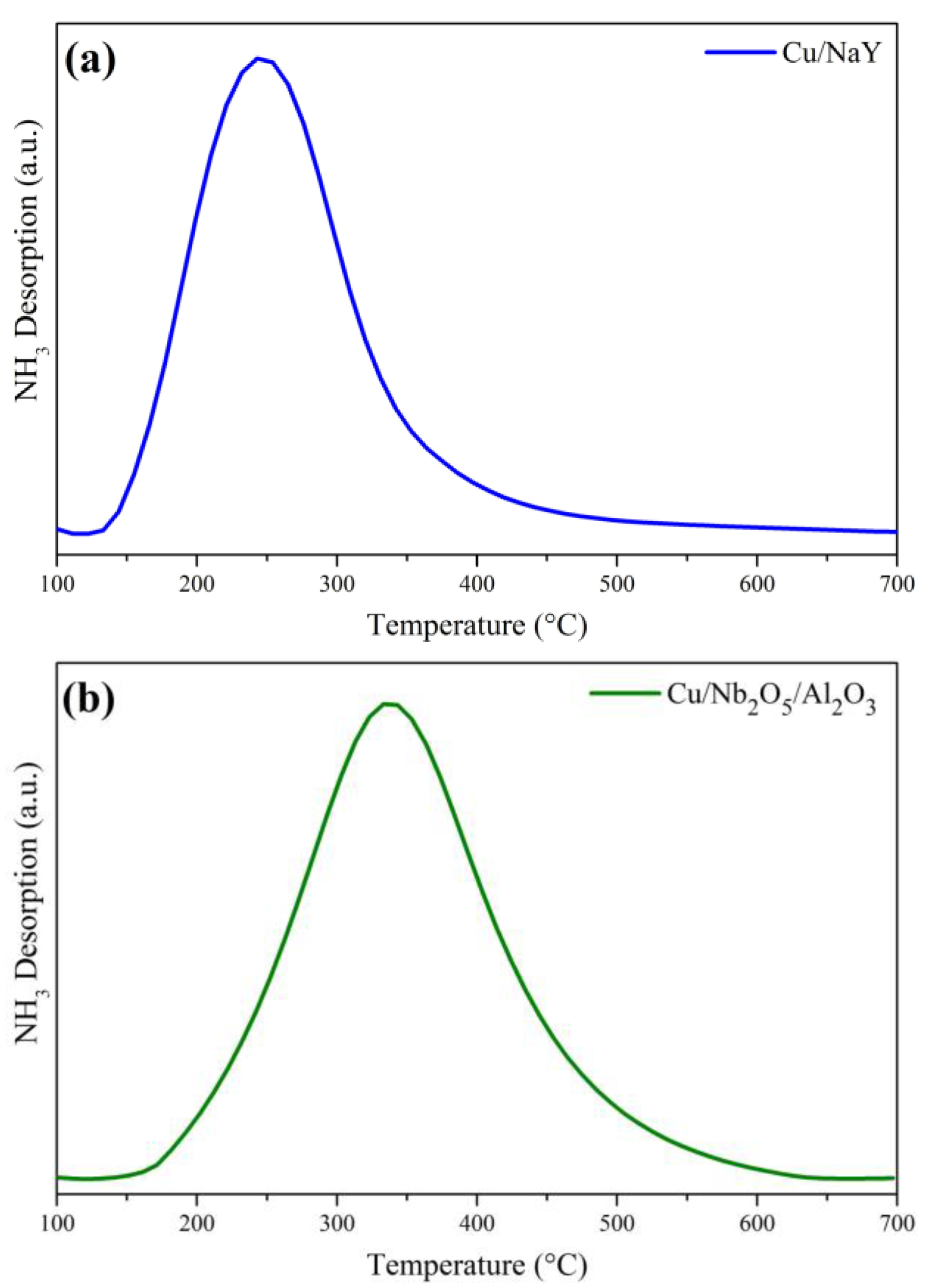
3.1.4. Temperature-Programmed Desorption (TPD)
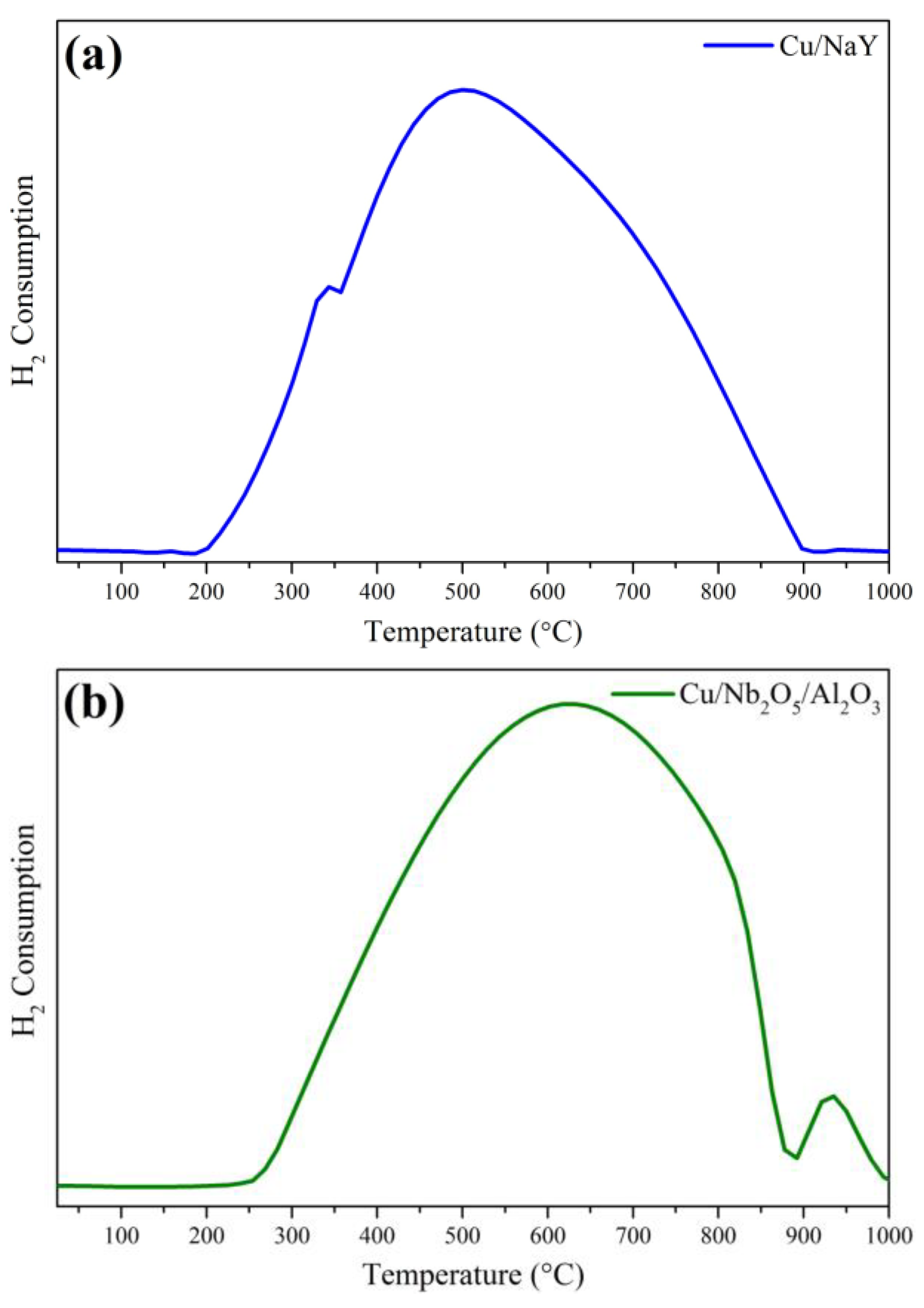
3.1.5. Temperature-Programmed Reduction (TPR)
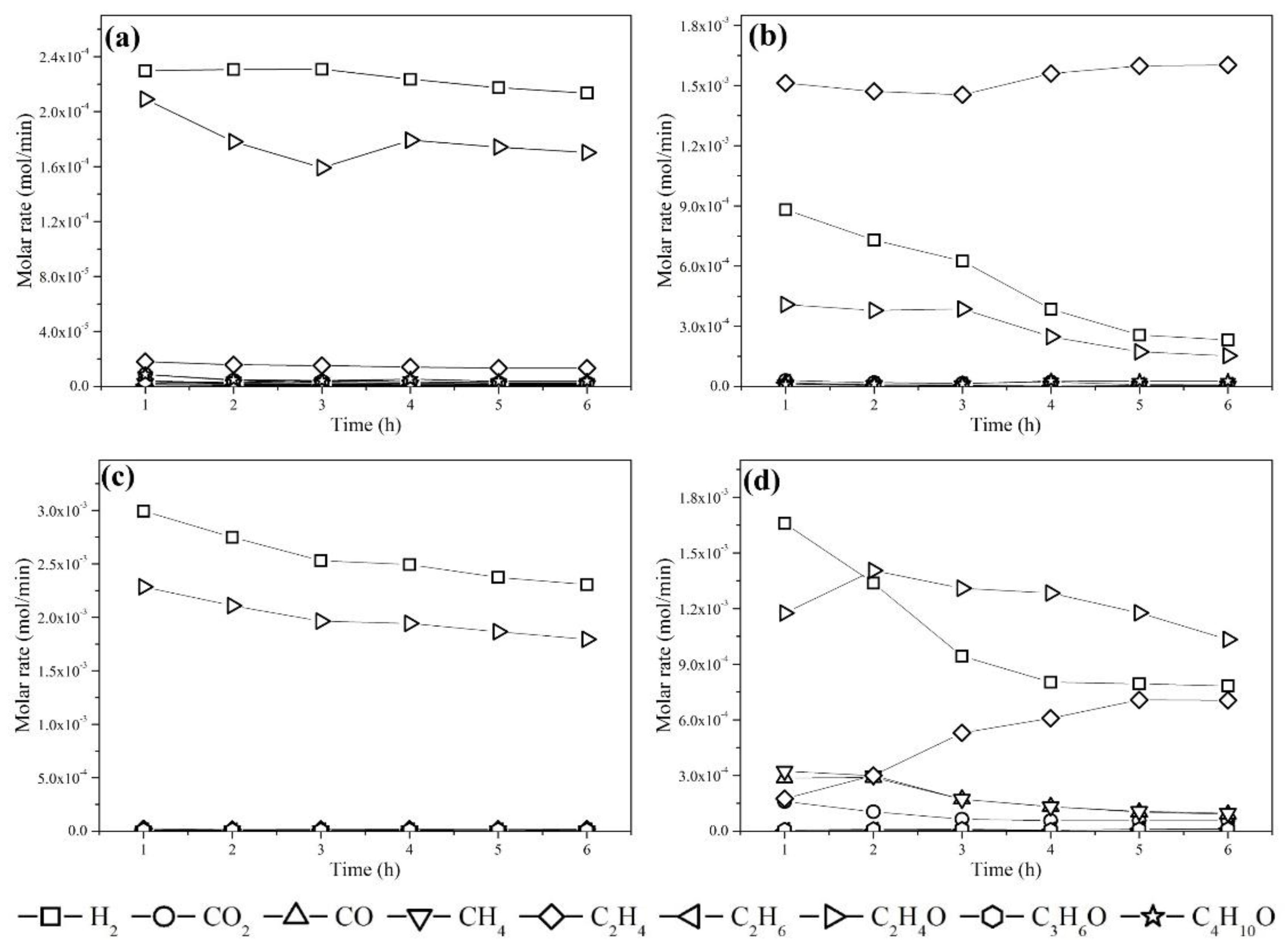
3.2. Catalytic Performance Evaluation
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Luo, M.; Li, S.; Di, Z.; Yang, Z.; Chou, W.; Shi, B. Fischer-Tropsch Synthesis: Effect of Nitric Acid Pretreatment on Graphene-Supported Cobalt Catalyst. Appl Catal A Gen 2020, 599, 117608. [CrossRef]
- Yousefian, F.; Babatabar, M.A.; Eshaghi, M.; Poor, S.M.; Tavasoli, A. Pyrolysis of Rice Husk, Coconut Shell, and Cladophora Glomerata Algae and Application of the Produced Biochars as Support for Cobalt Catalyst in Fischer–Tropsch Synthesis. Fuel Processing Technology 2023, 247, 107818. [CrossRef]
- Liu, Y.; Zou, R.; Qin, B.; Gan, J.; Peng, X. Energy-Efficient Monosaccharides Electrooxidation Coupled with Green Hydrogen Production by Bifunctional Co9S8/Ni3S2 Electrode. Chemical Engineering Journal 2022, 446, 136950. [CrossRef]
- Mert, M.E.; Edis, C.; Akyıldız, Ş.; Demir, B.N.; Nazligul, H.; Gurdal, Y.; Doğru Mert, B. Design and Performance Analysis of a PV-Assisted Alkaline Electrolysis for Hydrogen Production: An Experimental and Theoretical Study. Fuel 2024, 355, 129497. [CrossRef]
- Vadalà, M.; Kröll, E.; Küppers, M.; Lupascu, D.C.; Brunstermann, R. Hydrogen Production via Dark Fermentation by Bacteria Colonies on Porous PDMS-Scaffolds. Int J Hydrogen Energy 2023, 48, 25274–25284. [CrossRef]
- Öztan, H.; Çapoğlu, İ.K.; Uysal, D.; Doğan, Ö.M. A Parametric Study to Optimize the Temperature of Hazelnut and Walnut Shell Gasification for Hydrogen and Methane Production. Bioresour Technol Rep 2023, 23, 101581. [CrossRef]
- Guan, D.; Wang, F.; Zhang, X.; Dou, W.; Sun, Y. Comprehensive Study on Catalytic Coating Tubular Reactor with Electromagnetic Induction Heating for Hydrogen Production through Methanol Steam Reforming. Int J Hydrogen Energy 2024, 50, 1–17. [CrossRef]
- Hu, Y.; He, W.; Shen, Y. Recyclable NiMnOx/NaF Catalysts: Hydrogen Generation via Steam Reforming of Formaldehyde. Fuel 2023, 354, 129311. [CrossRef]
- Levikhin, A.A.; Boryaev, A.A. High-Temperature Reactor for Hydrogen Production by Partial Oxidation of Hydrocarbons. Int J Hydrogen Energy 2023, 48, 28187–28204. [CrossRef]
- da Silva, F.A.; Dancini-Pontes, I.; DeSouza, M.; Fernandes, N.R.C. Kinetics of Ethanol Steam Reforming over Cu–Ni/NbxOy Catalyst. Reaction Kinetics, Mechanisms and Catalysis 2017, 122, 557–574. [CrossRef]
- Shtyka, O.; Dimitrova, Z.; Ciesielski, R.; Kedziora, A.; Mitukiewicz, G.; Leyko, J.; Maniukewicz, W.; Czylkowska, A.; Maniecki, T. Steam Reforming of Ethanol for Hydrogen Production: Influence of Catalyst Composition (Ni/Al2O3, Ni/Al2O3–CeO2, Ni/Al2O3–ZnO) and Process Conditions. Reaction Kinetics, Mechanisms and Catalysis 2021, 132, 907–919. [CrossRef]
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy & Fuels 2005, 19, 2098–2106. [CrossRef]
- Zanchet, D.; Santos, J.B.O.; Damyanova, S.; Gallo, J.M.R.; Bueno, J.M.C. Toward Understanding Metal-Catalyzed Ethanol Reforming. ACS Catal 2015, 5, 3841–3863. [CrossRef]
- Trane-Restrup, R.; Dahl, S.; Jensen, A.D. Steam Reforming of Ethanol: Effects of Support and Additives on Ni-Based Catalysts. Int J Hydrogen Energy 2013, 38, 15105–15118. [CrossRef]
- Wurzler, G.T.; Rabelo-Neto, R.C.; Mattos, L. V.; Fraga, M.A.; Noronha, F.B. Steam Reforming of Ethanol for Hydrogen Production over MgO—Supported Ni-Based Catalysts. Appl Catal A Gen 2016, 518, 115–128. [CrossRef]
- Palma, V.; Ruocco, C.; Castaldo, F.; Ricca, A.; Boettge, D. Ethanol Steam Reforming over Bimetallic Coated Ceramic Foams: Effect of Reactor Configuration and Catalytic Support. Int J Hydrogen Energy 2015, 40, 12650–12662. [CrossRef]
- Alonso, C.G.; Furtado, A.C.; Cantão, M.P.; Andreo dos Santos, O.A.; Camargo Fernandes-Machado, N.R. Reactions over Cu/Nb2O5 Catalysts Promoted with Pd and Ru during Hydrogen Production from Ethanol. Int J Hydrogen Energy 2009, 34, 3333–3341. [CrossRef]
- Chen, F.; Tao, Y.; Ling, H.; Zhou, C.; Liu, Z.; Huang, J.; Yu, A. Ni-Cu Bimetallic Catalysts on Yttria-Stabilized Zirconia for Hydrogen Production from Ethanol Steam Reforming. Fuel 2020, 280, 118612. [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Steam Reforming of Ethanol for Hydrogen Production: Efficacy of Ceria Promoted Cu–Co on Mesoporous Cellular Foam Silica. Int J Hydrogen Energy 2023, 48, 31550–31570. [CrossRef]
- Guarido, C.E.M.; Cesar, D. V.; Souza, M.M.V.M.; Schmal, M. Ethanol Reforming and Partial Oxidation with Cu/Nb2O5 Catalyst. Catal Today 2009, 142, 252–257. [CrossRef]
- Hou, T.; Zhang, S.; Chen, Y.; Wang, D.; Cai, W. Hydrogen Production from Ethanol Reforming: Catalysts and Reaction Mechanism. Renewable and Sustainable Energy Reviews 2015, 44, 132–148. [CrossRef]
- Mariño, F.J.; Cerrella, E.G.; Duhalde, S.; Jobbagy, M.; Laborde, M.A. Hydrogen from Steam Reforming of Ethanol. Characterization and Performance of Copper-Nickel Supported Catalysts. Int J Hydrogen Energy 1998, 23, 1095–1101. [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A Review on Reforming Bio-Ethanol for Hydrogen Production. Int J Hydrogen Energy 2007, 32, 3238–3247. [CrossRef]
- Snytnikov, P.V.; Badmaev, S.D.; Volkova, G.G.; Potemkin, D.I.; Zyryanova, M.M.; Belyaev, V.D.; Sobyanin, V.A. Catalysts for Hydrogen Production in a Multifuel Processor by Methanol, Dimethyl Ether and Bioethanol Steam Reforming for Fuel Cell Applications. Int J Hydrogen Energy 2012, 37, 16388–16396. [CrossRef]
- Inokawa, H.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Difference in the Catalytic Activity of Transition Metals and Their Cations Loaded in Zeolite Y for Ethanol Steam Reforming. Int J Hydrogen Energy 2010, 35, 11719–11724. [CrossRef]
- Nippes, R.P.; Frederichi, D.; Olsen Scaliante, e M.H.N. Enhanced Photocatalytic Performance under Solar Radiation of ZnO through Hetero-Junction with Iron Functionalized Zeolite. J Photochem Photobiol A Chem 2021, 418, 113373. [CrossRef]
- Mohamed, R.M.; Mkhalid, I.A.; Barakat, M.A. Rice Husk Ash as a Renewable Source for the Production of Zeolite NaY and Its Characterization. Arabian Journal of Chemistry 2015, 8, 48–53. [CrossRef]
- Tolentino, C.M.C.; de Luna, M.D.G.; Futalan, C.M.; Choi, A.E.S.; Manegdeg, F.G.; Grisdanurak, N. Influence of Hydrocarbons on Hydrogen Chloride Removal from Refinery Off-Gas by Zeolite NaY Derived from Rice Husks. Science of The Total Environment 2020, 728, 138782. [CrossRef]
- Ali, M.M.M.; Ahmed, M.J.; Hameed, B.H. NaY Zeolite from Wheat (Triticum Aestivum L.) Straw Ash Used for the Adsorption of Tetracycline. J Clean Prod 2018, 172, 602–608. [CrossRef]
- Campos-Skrobot, F.C.; Rizzo-Domingues, R.C.P.; Fernandes-Machado, N.R.C.; Cantão, M.P. Novel Zeolite-Supported Rhodium Catalysts for Ethanol Steam Reforming. J Power Sources 2008, 183, 713–716. [CrossRef]
- Kwak, B.S.; Lee, J.S.; Lee, J.S.; Choi, B.-H.; Ji, M.J.; Kang, M. Hydrogen-Rich Gas Production from Ethanol Steam Reforming over Ni/Ga/Mg/Zeolite Y Catalysts at Mild Temperature. Appl Energy 2011, 88, 4366–4375. [CrossRef]
- Lee, J.-S.; Kim, J.-E.; Kang, M.-S. Hydrogen Production from Ethanol Steam Reforming over SnO 2 -K2O/Zeolite Y Catalyst. Bull Korean Chem Soc 2011, 32, 1912–1920. [CrossRef]
- Androulakis, A.; Yentekakis, I.V.; Panagiotopoulou, P. Dry Reforming of Methane over Supported Rh and Ru Catalysts: Effect of the Support (Al2O3, TiO2, ZrO2, YSZ) on the Activity and Reaction Pathway. Int J Hydrogen Energy 2023, 48, 33886–33902. [CrossRef]
- Kharaji, A.G.; Shariati, A.; Takassi, M.A. A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction. Chin J Chem Eng 2013, 21, 1007–1014. [CrossRef]
- Pastor-Pérez, L.; Shah, M.; le Saché, E.; Ramirez Reina, T. Improving Fe/Al2O3 Catalysts for the Reverse Water-Gas Shift Reaction: On the Effect of Cs as Activity/Selectivity Promoter. Catalysts 2018, 8, 608. [CrossRef]
- Ranjbar, A.; Aghamiri, S.F.; Irankhah, A. Effect of MgO/Al2O3 Ratio in the Support of Mesoporous Ni/MgO–Al2O3 Catalysts for CO2 Utilization via Reverse Water Gas Shift Reaction. Int J Hydrogen Energy 2023, 48, 19115–19125. [CrossRef]
- Xiao, T.; Xie, J.; Cheng, J.; Dai, X.; Lu, S.; Zuo, R.; Li, Z.; Yang, Z. Al2O3 Supported NiCu Alloy as a Stable Catalyst for Selective Hydrogenation of Phthalic Anhydride to Phthalide. Appl Catal A Gen 2023, 660, 119189. [CrossRef]
- Yao, X.; Gao, F.; Dong, L. The Application of Incorporation Model in γ-Al2O3 Supported Single and Dual Metal Oxide Catalysts: A Review. Chinese Journal of Catalysis 2013, 34, 1975–1985. [CrossRef]
- Ji, N.; Yin, J.; Rong, Y.; Li, H.; Yu, Z.; Lei, Y.; Wang, S.; Diao, X. More than a Support: The Unique Role of Nb2O5 in Supported Metal Catalysts for Lignin Hydrodeoxygenation. Catal Sci Technol 2022, 12, 3751–3766. [CrossRef]
- Zhang, C.; Wu, J.; Hua, C.; Zhu, L.; Qiu, K.; Wang, S. Hydrodeoxygenation Performance of Lignin-Derived Phenolics to Cycloalkanes: Insights into the Crystal Structures of the Nb2O5 Support. Energy & Fuels 2023, 37, 14006–14020. [CrossRef]
- Nippes, R.P.; Gomes, A.D.; Macruz, P.D.; de Souza, M. Photocatalytic Removal of 17β-Estradiol from Water Using a Novel Bimetallic NiCu/Nb2O5 Catalyst. Environmental Science and Pollution Research 2023, 30, 103731–103742. [CrossRef]
- Nippes, R.P.; Macruz, P.D.; Gomes, A.D.; Girotto, C.P.; Scaliante, M.H.N.O.; de Souza, M. Removal of Reactive Blue 250 Dye from Aqueous Medium Using Cu/Fe Catalyst Supported on Nb2O5 through Oxidation with H2O2. Reaction Kinetics, Mechanisms and Catalysis 2022, 135, 2697–2717. [CrossRef]
- Dancini-Pontes, I.; DeSouza, M.; Silva, F.A.; Scaliante, M.H.N.O.; Alonso, C.G.; Bianchi, G.S.; Medina-Neto, A.; Pereira, G.M.; Fernandes-Machado, N.R.C. Influence of the CeO2 and Nb2O5 Supports and the Inert Gas in Ethanol Steam Reforming for H2 Production. Chemical Engineering Journal 2015, 273, 66–74. [CrossRef]
- Menezes, J.P. da S.Q.; Manfro, R.L.; Souza, M.M.V.M. Hydrogen Production from Glycerol Steam Reforming over Nickel Catalysts Supported on Alumina and Niobia: Deactivation Process, Effect of Reaction Conditions and Kinetic Modeling. Int J Hydrogen Energy 2018, 43, 15064–15082. [CrossRef]
- Mortezaei, Z.; Zendehdel, M.; Bodaghifard, M.A. Cu Complex Grafted on the Porous Materials: Synthesis, Characterization and Comparison of Their Antibacterial Activity with Nano-Cu/NaY Zeolite. Journal of the Iranian Chemical Society 2020, 17, 283–295. [CrossRef]
- Gonçalves, J.F.; Souza, M.M.V.M. Ni/X%Nb2O5/Al2O3 Catalysts Prepared via Coprecipitation-Wet Impregnation Method for Methane Steam Reforming. Current Catalysis 2020, 9, 80–89. [CrossRef]
- Kugai, J.; Subramani, V.; Song, C.; Engelhard, M.; Chin, Y. Effects of Nanocrystalline CeO2 Supports on the Properties and Performance of Ni–Rh Bimetallic Catalyst for Oxidative Steam Reforming of Ethanol. J Catal 2006, 238, 430–440. [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A. V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure and Applied Chemistry 2015, 87, 1051–1069. [CrossRef]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.C.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of Caffeine on Mesoporous Activated Carbon Fibers Prepared from Pineapple Plant Leaves. Ecotoxicol Environ Saf 2018, 147, 64–71. [CrossRef]
- Zhang, H.; Liu, X.; He, G.; Zhang, X.; Bao, S.; Hu, W. Bioinspired Synthesis of Nitrogen/Sulfur Co-Doped Graphene as an Efficient Electrocatalyst for Oxygen Reduction Reaction. J Power Sources 2015, 279, 252–258. [CrossRef]
- Patdhanagul, N.; Srithanratana, T.; Rangsriwatananon, K.; Hengrasmee, S. Ethylene Adsorption on Cationic Surfactant Modified Zeolite NaY. Microporous and Mesoporous Materials 2010, 131, 97–102. [CrossRef]
- Zeng, Y.; Walker, H.; Zhu, Q. Reduction of Nitrate by NaY Zeolite Supported Fe, Cu/Fe and Mn/Fe Nanoparticles. J Hazard Mater 2017, 324, 605–616. [CrossRef]
- Chang, H.-Y.; Lai, G.-H.; Tsai, D.-H. Aerosol Route Synthesis of Ni-CeO2-Al2O3 Hybrid Nanoparticle Cluster for Catalysis of Reductive Amination of Polypropylene Glycol. Advanced Powder Technology 2019, 30, 2293–2298. [CrossRef]
- Fawaz, A.; Bizreh, Y.W.; Al-Hamoud, L. (NiO, Ag /Fe2O3-Al2O3-Bentonite) as Promising Catalyst for CO and HC Removal from Single-Cylinder Engine Exhaust Emissions. Catal Commun 2022, 171, 106521. [CrossRef]
- Morales-Pacheco, P.; Alvarez, F.; Bucio, L.; Domínguez, J.M. Synthesis and Structural Properties of Zeolitic Nanocrystals II: FAU-Type Zeolites. The Journal of Physical Chemistry C 2009, 113, 2247–2255. [CrossRef]
- Ameri, A.; Faramarzi, M.A.; Tarighi, S.; Shakibaie, M.; Ameri, A.; Ramezani-Sarbandi, A.; Forootanfar, H. Removal of Dyes by Trametes Versicolor Laccase Immobilized on NaY-Zeolite. Chemical Engineering Research and Design 2023, 197, 240–253. [CrossRef]
- El-Bahy, Z.M. Oxidation of Carbon Monoxide over Cu- and Ag-NaY Catalysts with Aqueous Hydrogen Peroxide. Mater Res Bull 2007, 42, 2170–2183. [CrossRef]
- Xu, Y.; Chen, D.; Jiao, X. Fabrication of CuO Pricky Microspheres with Tunable Size by a Simple Solution Route. J Phys Chem B 2005, 109, 13561–13566. [CrossRef]
- Khan, I.; Baig, N.; Qurashi, A. Graphitic Carbon Nitride Impregnated Niobium Oxide (g-C3N4/Nb2O5) Type (II) Heterojunctions and Its Synergetic Solar-Driven Hydrogen Generation. ACS Appl Energy Mater 2019, 2, 607–615. [CrossRef]
- da Conceição, L.R. V.; Carneiro, L.M.; Rivaldi, J.D.; de Castro, H.F. Solid Acid as Catalyst for Biodiesel Production via Simultaneous Esterification and Transesterification of Macaw Palm Oil. Ind Crops Prod 2016, 89, 416–424. [CrossRef]
- Fan, D.; Jiang, S.; Qiao, K.; Zhang, S.; Wang, H.; Bo, D.; Zhang, Y.; Yu, T.; Zhai, D.; Ren, G.; et al. Cuprous Species Distribution over CuCl/NaY Dependent on Acidity and Their CO Adsorption/Desorption Performance Study. Chemical Engineering Journal 2022, 433, 133763. [CrossRef]
- Padró, C.L.; Rey, E.A.; González Peña, L.F.; Apesteguía, C.R. Activity, Selectivity and Stability of Zn-Exchanged NaY and ZSM5 Zeolites for the Synthesis of o-Hydroxyacetophenone by Phenol Acylation. Microporous and Mesoporous Materials 2011, 143, 236–242. [CrossRef]
- Wang, Z.; Ma, R.; Song, W. Influence of HSAPO-34, HZSM-5, and NaY on Pyrolysis of Corn Straw Fermentation Residue via Py-GC/MS. J Anal Appl Pyrolysis 2016, 122, 183–190. [CrossRef]
- Ungureanu, A.; Dragoi, B.; Chirieac, A.; Ciotonea, C.; Royer, S.; Duprez, D.; Mamede, A.S.; Dumitriu, E. Composition-Dependent Morphostructural Properties of Ni–Cu Oxide Nanoparticles Confined within the Channels of Ordered Mesoporous SBA-15 Silica. ACS Appl Mater Interfaces 2013, 5, 3010–3025. [CrossRef]
- da Silva, F.A.; Pontes, I.D.; Wurzler, G.T.; Alonso, C.G.; Medina-Neto, A.; Scaliante, M.H.N.O.; Desouza, M.; Fernandes-Machado, N.R.C. Production of Hydrogen from Bioethanol in Cu-Ni/NbxOy Catalysts Obtained by Different Preparation Methods. Int J Hydrogen Energy 2016, 41, 8111–8119. [CrossRef]
- Lorenzut, B.; Montini, T.; De Rogatis, L.; Canton, P.; Benedetti, A.; Fornasiero, P. Hydrogen Production through Alcohol Steam Reforming on Cu/ZnO-Based Catalysts. Appl Catal B 2011, 101, 397–408. [CrossRef]
- Mattos, L. V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of Hydrogen from Ethanol: Review of Reaction Mechanism and Catalyst Deactivation. Chem Rev 2012, 112, 4094–4123. [CrossRef]
- Tang, S.; Li, F.; Liu, J.; Guo, B.; Tian, Z.; Lv, J. MgO/NaY as Modified Mesoporous Catalyst for Methanolysis of Polyethylene Terephthalate Wastes. J Environ Chem Eng 2022, 10, 107927. [CrossRef]
- Boonyoung, P.; Thongratkaew, S.; Rungtaweevoranit, B.; Pengsawang, A.; Praserthdam, P.; Sanpitakseree, C.; Faungnawakij, K. Formic Acid as a Sacrificial Agent for Byproduct Suppression in Glucose Dehydration to 5-Hydroxymethylfurfural Using NaY Zeolite Catalyst. Bioresour Technol 2024, 392, 130010. [CrossRef]
- Nagpure, A.S.; Mohture, V.M.; Kayarkar, A. Green Synthesis of Highly Dispersed Cu Metal Nanoparticles Catalysts. Inorg Chem Commun 2022, 146, 110118. [CrossRef]
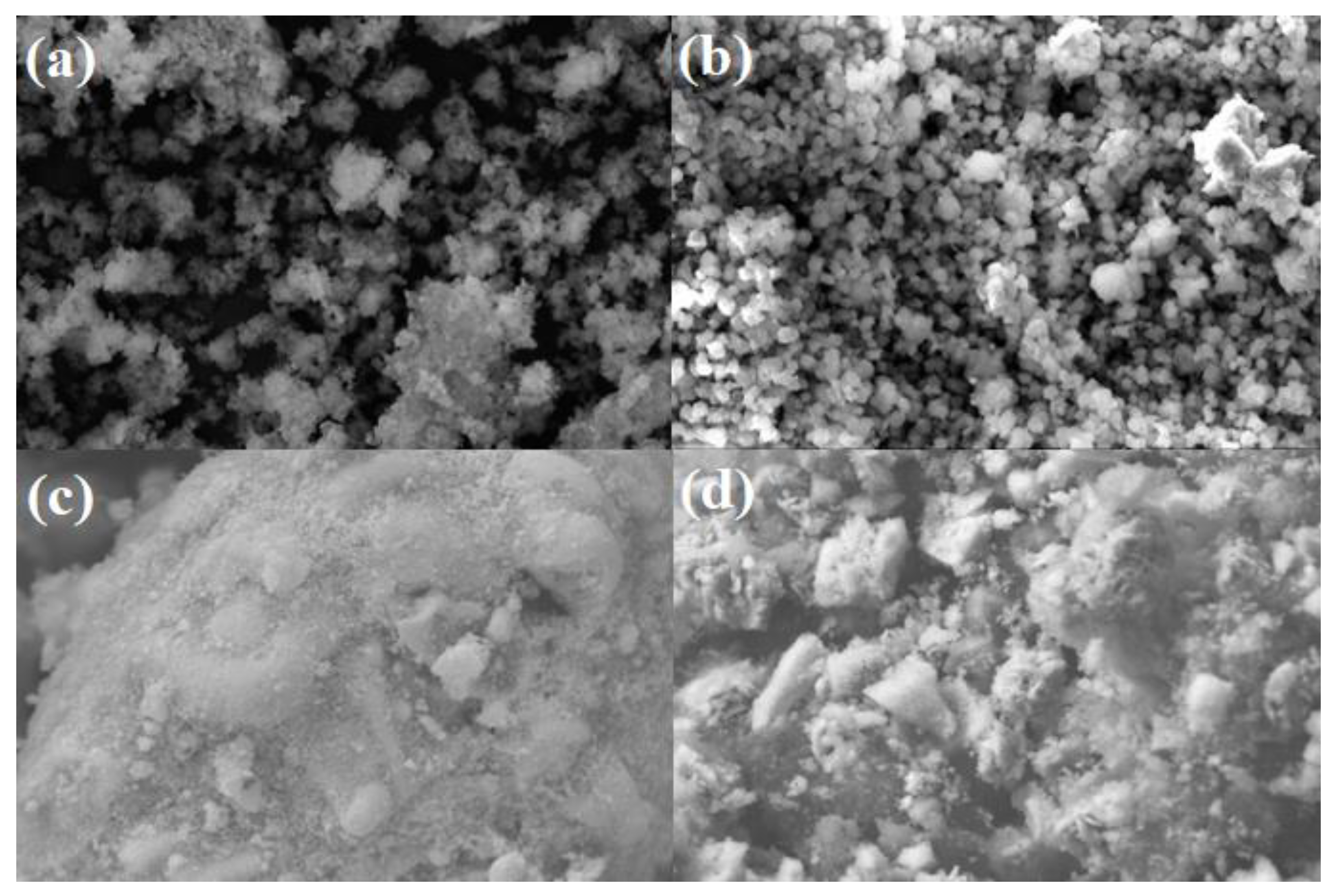
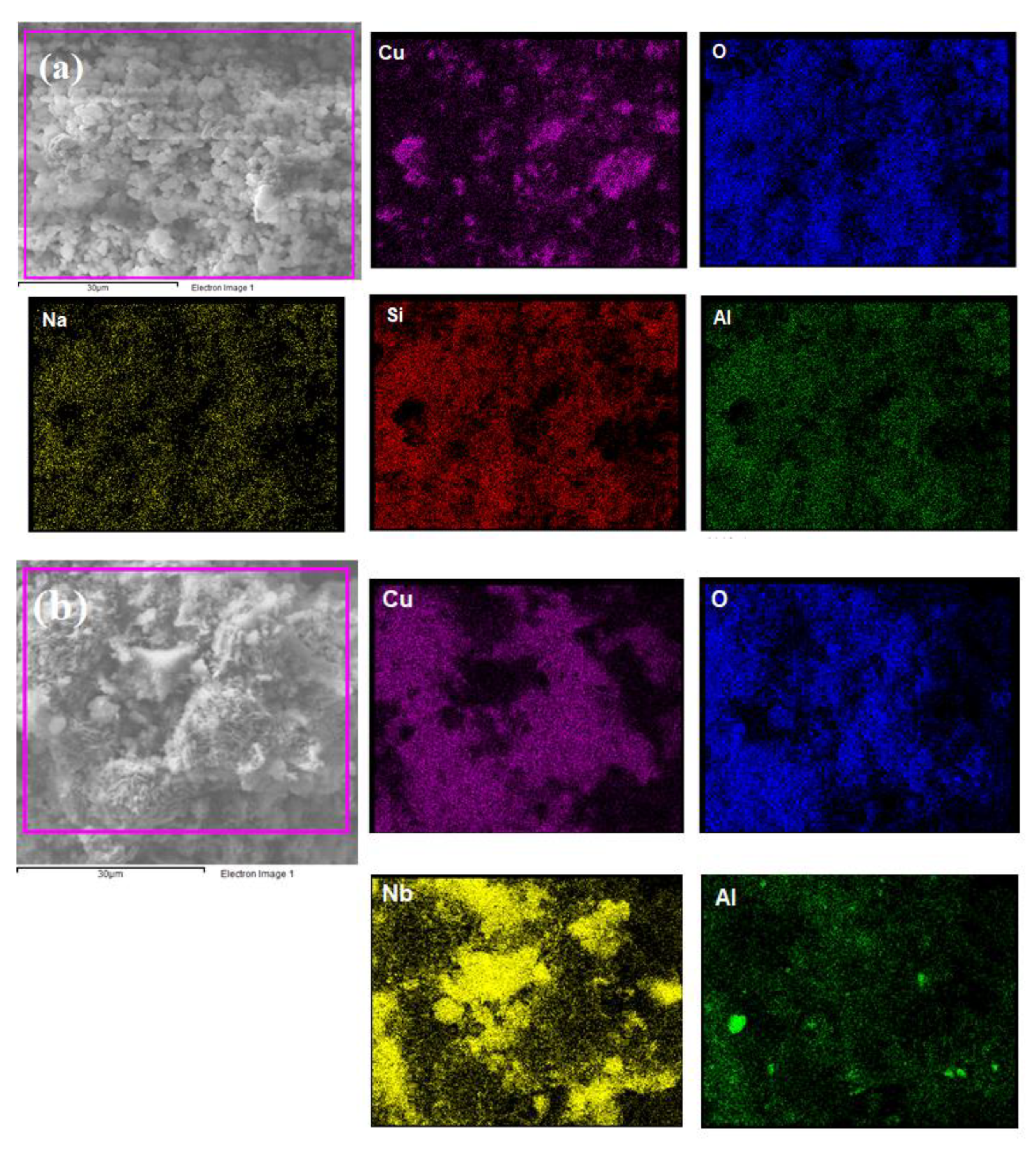

| Sample | Element (%) | ||||||
|---|---|---|---|---|---|---|---|
| Cu | Na | Al | Si | O | Nb | C | |
| Cu/NAY | 11.90 | 3.87 | 3.00 | 7.76 | 26.75 | - | 3.01 |
| Cu/Nb2O5/Al2O3 | 11.17 | - | 2.84 | - | 15.72 | 10.82 | 0.88 |
| Sample | Surface Area (m2 g-1) | Pore Volume (cm3 g-1) | Micropore | Pore Diameter (nm) |
|---|---|---|---|---|
| Volume (cm3 g-1) | ||||
| Nb2O5 | 71.73 | 0.37 | 0.32 | 8.24 |
| NaY | 588.49 | 0.1479 | 0.0281 | 1.74 |
| Al2O3 | 99.26 | 0.1799 | 0,0389 | 7.24 |
| Cu/NaY | 210.4 | 0.118 | 0.1114 | 2.24 |
| Cu/Nb2O5/Al2O3 | 26 | 0.0617 | 0.0106 | 9.56 |
| Sample | Chemisorbed NH3 (mmol/g) | Temperature (°C) |
|---|---|---|
| Cu/NaY | 1.598 | 247 |
| Cu/Nb2O5/Al2O3 | 0.059 | 336 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





