1. Introduction
Multiple sclerosis (MS) is a neurodegenerative demyelinating inflammatory disease related to axonal destruction, which is the most influential factor in the disability of people with MS (PwMS). This axonal neurodegeneration process releases proteins that form part of the cytoskeleton, such as neurofilament light chain in the cerebrospinal fluid (CSF) and serum [
1]. Among biomarkers under investigation for the implementation of personalized medicine [
2], serum neurofilament light chain (sNfL) is a promising candidate to assess the activity, progression, prognosis, and response to treatment of PwMS [
3]. Baseline sNfL levels have also been proposed as a potential predictive biomarker of disability in clinically isolated demyelinating syndrome [
4].
CSF and serum neurofilament light chain levels correlate with each other [
4], and their analysis is more accessible in serum by means of Single Molecule Array (SIMOA) technology [
5]. Researchers are using SIMOA to investigate the relationship of sNfL levels with inflammation and neurodegeneration in PwMS [
6], although no agreement has yet been reached on the sNfL levels associated with a worse prognosis. Elevated sNfL levels have been reported in PwMS with a greater frequency and severity of relapses [
7] and in those with higher radiological activity [
8], while they have also been associated with neurodegeneration and a greater risk of disability [
9] and brain atrophy [
10]. sNfL levels might therefore act as a predictive biomarker of clinical and radiological activity and of the response to different disease modifying treatments (DMTs), which is essential to determine suboptimal responses [
11] and enhance the precision of MS therapies [
12].
sNfLs are not MS-specific, also being elevated in active neuroaxonal lesions after traumatic brain injury, stroke, and other neurodegenerative diseases [
13]. Physiologically, sNfL levels increase from the age of 60 years onwards, and reference values are given by age [
14]. In the meta-analysis published by Cai et al., reported sNfL levels ranged between 9 and 35.9 pg/mL [
15].
There is a need to establish sNfL cutoff levels by age group to differentiate between pathological and non-pathological conditions. Accordingly, a z-score was calculated, based on 10,133 samples from healthy individuals and adjusting sNfL levels by age and body mass index (BMI), reporting an association between a sNfL z-score >1.5 (percentile ≥94%) and a greater risk of clinical or radiological disease activity and/or worsening of the expanded disability status scale (EDSS) score in the following year [
16]. It has been concluded that neurofilament light chain levels are more closely associated with inflammatory activity than with progression [
17].
Ocrelizumab (OCR) is a recombinant humanized monoclonal antibody that selectively acts on B lymphocytes with surface expression of CD20 [
18]. OCR has been found to significantly reduce the clinical activity and partially limit the progression of disability in PwMS [
19]. OCR was reported to reduce sNfL levels in progressive forms in the ORATORIO trial [
20] and in relapse forms in the OPERA trial [
21], while another pivotal study observed an association between sNfL levels at 48 weeks and the risk of disability progression up to 9 years later [
22]. In the ORATORIO trial, a 10-fold increase in baseline sNfL levels in the control group was associated with greater risk of progression
as assessed by the Nine Hole Peg Test (9-HPT) (HR = 2.33, p = 0.036) and Timed 25-Foot Walk Test
(T25FWT) (HR = 5.35, p = 0.003). The significant reduction in sNfL, independently of inflammatory activity, indicates that OCR treatment can also limit neuroaxonal damage.
The Ocrelizumab Biomarker Outcome Evaluation (OBOE) study (NCT02688985) reported a 30.8% reduction in sNfL levels after 12 months of OCR treatment and a correlation between sNfL levels and the number of Gd+ active lesions and/or increased T2 lesions in brain magnetic resonance imaging (MRI) [
23]. The reduction in sNfL levels obtained by OCR was also
found to be independent of baseline clinical or radiological activity [
24]. A study in PwMS found no significant difference in sNfL values between OCR and rituximab (RTX) treatments (
18.32 vs. 18.28 pg/mL, respectively) [
25]
.
Various studies of PwMS have described a reduction in sNfL levels with the start of DMTs, which have demonstrated moderate (teriflunomide (TFL) [
26], dimethyl fumarate (DMF) [
27] or high natalizumab (NTZ) [
28], fingolimod (FGL) [
29]], alemtuzumab (ALM) [
30], cladribine (CLD) [
31] OCR [
22], ofatumumab (OFT) [
32] effectiveness.
Delcoigne et al. studied sNFL levels in 1,261 patients receiving ALM, DMF, FGL, NTZ, TFL, or RTX and observed that baseline sNfL concentrations were positively associated with relapses and EDSS score, with all DMTs achieving a reduction in sNfL levels, which was highest with ALM and lowest with TFL [
33].
sNfL levels have been considered as a biomarker of disability progression. A study of 578 patients followed for seven years associated levels >10 pg/mL with a greater risk of progression and, independently, with a greater risk of disability during the first six months. In a multicenter study based on
Real-Life Experience, PwMS with sNfL levels >8 pg/mL had a 2.8-fold greater risk of deterioration, 4-fold greater risk of new lesions in T2, and 3.3-fold greater risk of relapse within two years [
34]
. However, other authors found no increased disability progression in PwMS with elevated sNfL levels treated with highly effective drugs [
35]. Pivotal clinical trials, extension studies, and real-life studies have shown that sNfL levels can be reduced by treatment with both moderately and highly effective DMTs [
36]. However, most of this evidence has been based on selected cohorts, largely in clinical trials, and few data have been published on populational cohorts [
37]
.
The possibility to determine sNfL in peripheral blood has resulted in some research on sNfL levels in the clinical setting over recent years. However, the availability of SIMOA technology has been limited to date, and further research is required to determine the sensitivity and specificity of sNfL as a biomarker and to establish reference values and standardized protocols [
1]. The objectives of this real-life study were: to determine sNfL levels in PwMS before starting OCR treatment (baseline) and every 3 months for 12 months; to evaluate the effectiveness of OCR; to assess the usefulness of sNfL levels as a biomarker for short-term prognosis; and to explore their relationship with other clinical-demographic variables.
2. Materials and Methods
This prospective observational study included 30 PwMS prescribed OCR between 2021 and 2022 in three public hospitals in Southern Spain (San Cecilio Clinical and Virgen de las Nieves University Hospitals in Granada and Torrecardenas University Hospital in Almeria). OCR was prescribed following the routine clinical protocol at the hospitals, and MS was diagnosed in accordance with the 2017 McDonald criteria [
38] Data were gathered on demographics (age, sex, BMI) and clinical variables (age at disease onset, time with disease, number of DMTs received, the most recent DMT before OCR, and the reason for prescribing OCR). The effectiveness of treatment was assessed by results obtained at baseline and 12 months for the EDSS [
39], annualized relapse rate (ARR), T25FW [
40], 9HPT [
41], and brain MRI, considering T1-weighted images before and after contrast (gadolinium-Gd) administration and T2/fluid-attenuated inversion recovery (FLAIR) sequences. No evidence of active disease (NEDA-3), recorded at baseline and 12 months, was defined by the absence of clinical relapse and sustained disability worsening (i.e., 1.5-point increase from baseline EDSS of 0, 1-point increase from EDSS of 1- 5.5, and 0.5-point increase from EDSS >5.5), and the lack of MRI activity [
42].
Venous blood sNfL levels were measured at baseline and at 3, 6, 9, and 12 months using SIMOA technology (Quanterix, Billerica, MA) with an SR-X instrument (Quanterix, Lexington, MA) and NF-light Advantage Kit (Quanterix, Billerica, MA); these analyses were conducted by the Immunology Department of the Ramón y Cajal Hospital in Madrid (Spain). The post-OCR response of sNfL levels was evaluated by calculating absolute mean sNFL values at baseline (sNfL-b) and 12 months (sNFL-12 m) and considering the ratio (sNfL-12 m/sNfL-b) and relative change in sNfL levels. Levels were also expressed as percentiles, and z-scores were calculated according to age- and BMI-adjusted standard reference values [
16], with a z-score of sNfL >1.5 (percentile ≥94) being associated with a higher risk of disease activity.
SPSS 28.0 (IBM SPSS, Armonk NY) was used for data analyses. Median values with interquartile range (IQR) were calculated for continuous variables and non-parametric tests were therefore applied, using the Mann–Whitney U test for unpaired data and the Wilcoxon signed-rank test for paired data. Spearman’s rank-order correlation coefficient was applied to determine monotonic relationships between sNfL and clinical or demographic variables. Frequencies and percentages were calculated for categorical variables, applying the chi-square test or Fisher’s exact test. Clinical outcome and confounding variables were analyzed by means of mixed linear regression models, with clinical variable as dependent variable and time and selected demographic data as independent variables. Time was considered as a continuous variable, with time zero corresponding to the start of the study. Demographic variables were entered in models by a stepwise procedure, using the Akaike information criterion in ANOVA to evaluate their significance. The response variable was transformed to improve the parameters of models (mainly to solve heteroscedasticity). Categorical variables were analyzed using generalized mixed logistic regression models. P-≤0.05 was considered statistically significant in all tests.
Ethical considerations. The study was performed in accordance with Good Clinical Practice and the Helsinki Declaration and was approved by the biomedical research ethics committee of Andalusia. Informed consent was obtained from all study participants
3. Results
The study included 30 PwMS with forms of relapsing-remitting MS (RRMS) who had received OCR treatment for at least 12 months. At the onset of OCR treatment (baseline), the mean age was 40.8±10.5 years, the mean BMI was 24.5±3.81 (n=21), and 57% were female; male and female participants did not significantly differ in sNFL levels (16.9 vs. 9.46 pg/mL, respectively; p=0.368).
3.1. Baseline Clinical Characteristics and Follow Up
Table 1 lists the baseline clinical characteristics of the PwMS before OCR treatment: seven (23.3%) were naïve, seven (23.3%) switched from fingolimod, four (13.3%) from natalizumab, four (13.3%) from cladribine, three (10%) from interferon-beta, two (6.7%) from dimethyl fumarate, two (6.7%) from alemtuzumab, and one (3.3%) from teriflunomide. The switch was due to a suboptimal response in 29 (96.7%), relapse in 11 (36.7%), and clinical/radiological activity in 18 (60%), and for safety reasons in 1 patient. The mean number of previous treatments was 1.53±1.33: one previous DMT had been received by 47.8%, two by 21.8%, and three by 30.4%.
At the 12-month follow-up, EDSS worsening was observed in one participant (3.3%), relapse in seven (23.3%), and radiological activity (new lesions in T2, no Gd+ activity) in two (8.3%). NEDA-3 was recorded in 70.8% of participants, a reduced ARR in 83.9% (0.23±0.42 at 12 months vs. 1.43±1.01 at baseline, p<0.01). The mean EDSS (2.98±1.89) did not significantly differ versus baseline (3.1±1.69), while active radiological lesions were detected in two participants (8.3%) versus ten (34.5%) at baseline, an 80% reduction.
Table 1.
Clinical-demographic characteristics before starting OCR and at one year of treatment.
Table 1.
Clinical-demographic characteristics before starting OCR and at one year of treatment.
| |
Before OCR |
After year OCR |
p value |
| MS onset age (y), mean (SD) |
30,1(9,9) |
|
|
| Sex, female/male, n (%) |
17/13 (56,5/43,3) |
|
|
| Disease duration (y), mean (SD) |
12,2(9,39) |
|
|
| Age (y), mean (SD) |
40,8(10,5) |
|
|
| Time with OCR, (m), mean (SD) |
24,3(7,94) |
|
|
| Baseline EDSS, median (range) |
2,5 (1-6,5) |
2,5 (1-7) |
p = 0,072 |
| ARR mean (SD) |
1,43(1) |
0,23(0,43) |
p< 0,001 |
|
T25FW (n=20) (s), mean (SD) |
8,08(5,78) |
8,69(7,5) |
p = 0,649 |
|
9HPT- (s) (n=24) mean (SD) |
29,2(13,3) |
29,4(23,83)
|
p = 0,989 |
|
9HPT-nd (s) (n=24) mean (SD) |
29(9.98) |
28,30(12,48)
|
p = 0,187 |
| T2 lesion on MRI (n = 29), n(%) |
|
|
|
| >20 |
19 (65,5) |
|
|
| 9-20 |
9 (31) |
|
|
| < 9 |
1 (3,4) |
|
|
| T1 gadolinium enhancement. n (%) |
10 (34,5%)
(n = 29) |
0
(n =24) |
p = 0,011 |
| New or enlarged T2 lesions, (n =24), n (%) |
|
2 (8,3%)
|
|
| PwMS with DMT before OCR, n, (%) |
23(76,7) |
|
|
| High-efficacy therapies |
17 (73,9) |
|
|
| Moderate-efficacy therapies |
6 (26,1) |
|
|
| PwMS Navie |
7 (23,3) |
|
|
Table 1: Annualised relapse rate (ARR), dominat (d), disease-modifying treatment (DMT), expanded disability status scale (EDSS), magnetic resonance imaging (MRI) month (m), nine hole peg test (9HPT), non dominat (nd) ocrelizumab (OCR), people with multiple sclerosis (PwMS), seconds (s) standard deviation (SD), timed 25-foot walk
(T25FW), year (y).
3.2. sNfL Levels and Clinical Outcomes
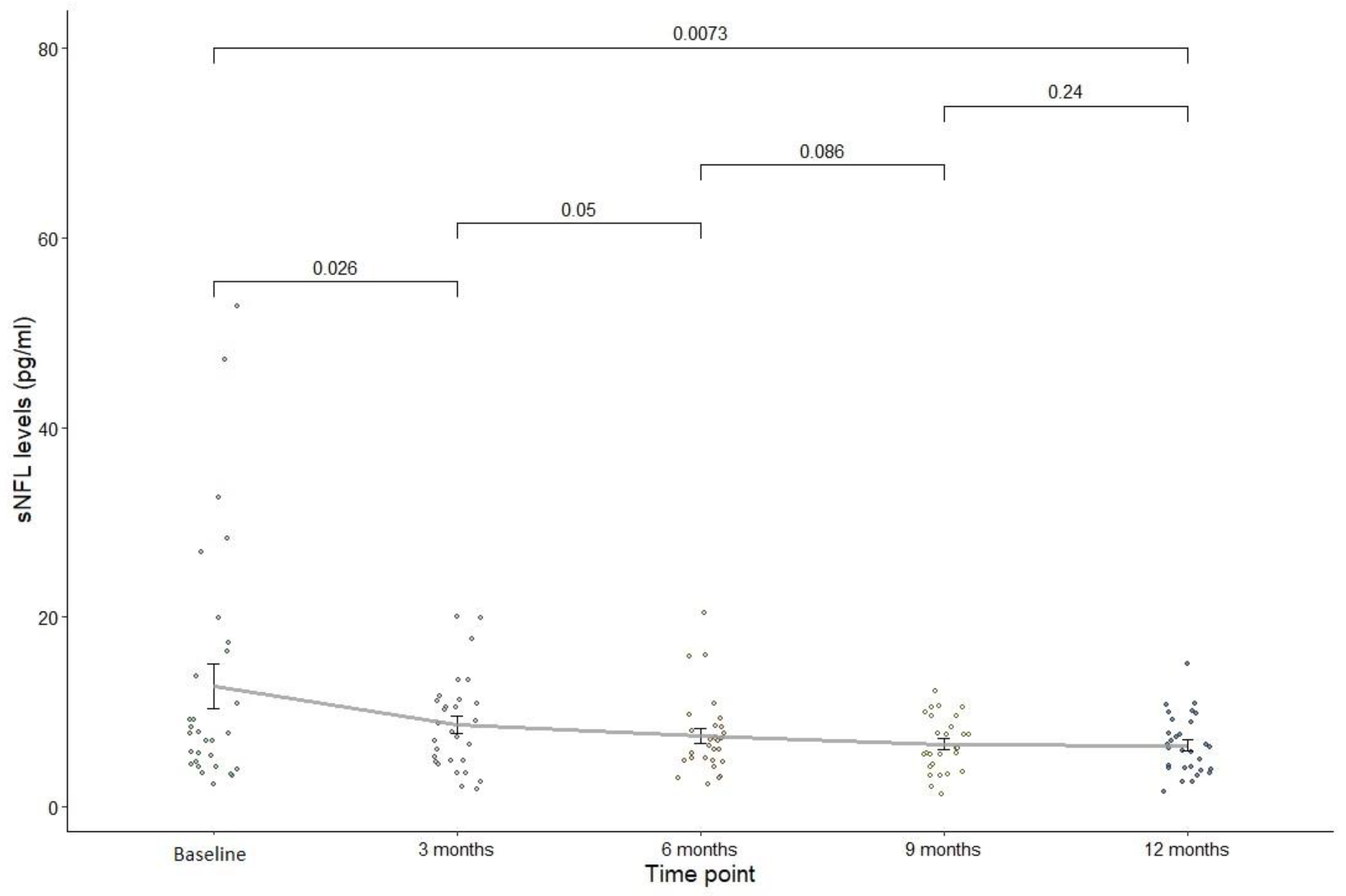
Figure 1 plots sNFL levels against measurement timepoints (baseline and 3, 6, 9, and 12 months). Each point represents an individual observation, with a line showing the trend of sNFL changes at each timepoint. Mean values with standard deviations were calculated for sNFL levels at each timepoint. The mean reduction in sNfL levels over 12 months was 6.21±11.8 points, with a mean percentage sNfL reduction of 16.3±55.5. The mean sNFL level was 12.7±12.8 pg/mL at baseline
versus 6.48±3.07 pg/mL at 12 months (p=0.007), the mean z-score was 0.26±1.99 at baseline
versus -0.615± 1.26 at 12 months (p=0.008). At baseline, the sNfL level was >10 pg/mL in 33.3% at baseline
versus 13.3% at 12 months, and the z-score was >1.5 in 40% at baseline
versus 3.3% at 12 months (p=0.003). Among PwMS who had relapsed, 85.7% had shown an increase in sNfL levels during the three months before the relapse.
The median EDSS was higher in previously treated versus naïve patients (3.5 vs. 1.5, p=0.002). In comparison to the former, naïve patients had more relapses during the previous year (2.43±1.62 vs. 1.13±0.46, p=0.018) and higher radiological activity (mean of 1.43±1.72 vs. 0.727±1.70 Gd+ lesions, p=0.047). At baseline, sNfL levels were similar between the groups (19.1 vs. 10.7 pg/mL; p=0.174), although the naïve group had a higher mean sNfL z-score (1.55 ±1.5 vs. -0.134±1.97, p=0.033) and sNfL-b/sNfL-12 m ratio (p=0.012).
The sample was divided between patients with and without NEDA-3 at 12 months to evaluate the influence of baseline sNfL levels on clinical and radiological outcomes.
Table 2 exhibits demographic, clinical, and outcome data for the two groups. No significant between-group difference was found in baseline sNfL level (p=0.710), baseline z-score (p=0.26), sNfL level at 12 months (p=0.153), z-score at 12 months (p=0.852), reduction in sNfL (p=0.114), percentage sNfL reduction (p=0.065), or sNfL ratio (p=0.065).
Table 2: Annualised relapse rate (ARR), dominat (d), disease-modifying treatment (DMT), expanded disability status scale (EDSS), magnetic resonance imaging (MRI) month (m), nine hole peg test (9HPT), non dominat (nd) ocrelizumab (OCR), people with multiple sclerosis (PwMS), seconds (s) standard deviation (SD), timed 25-foot walk (
T25FW) year (y).
Likewise, no significant difference was found between patients with versus without relapse during the 12-month follow-up in baseline sNfL levels (p=0.441), baseline z-score (p=0.532), sNfL levels at 12 months (p=0.364), z-score at 12 months (p=0.544), reduction in sNfL (p=0.848), percentage sNfL reduction (p=0.848), or sNfl ratio (p=0.848).
Correlation analysis (Spearman`s rho) showed no relationship of baseline sNfL levels with age at baseline (-0.062; p= 0.746), age at disease onset (-0.045; p=0.812), time with the disease (-0172; p=0.364), relapses during the previous year (-0.086; p=0.653), or EDSS score at baseline (-0.729; p=0.136). A moderate correlation was found between baseline sNfL levels and radiological activity (Gd+ lesions) (0.458; p=0.012).
No difference was found between patients with versus without radiological activity during the 12-month follow-up in baseline sNfL levels (p=0.116), baseline z-score (p=0.116), sNfL levels at 12 months (p=0.958), z-score at 12 months (p=0.587), reduction in sNfL (p=0.116), percentage sNfL reduction (p=0.087), or sNfL ratio (p=0.087).
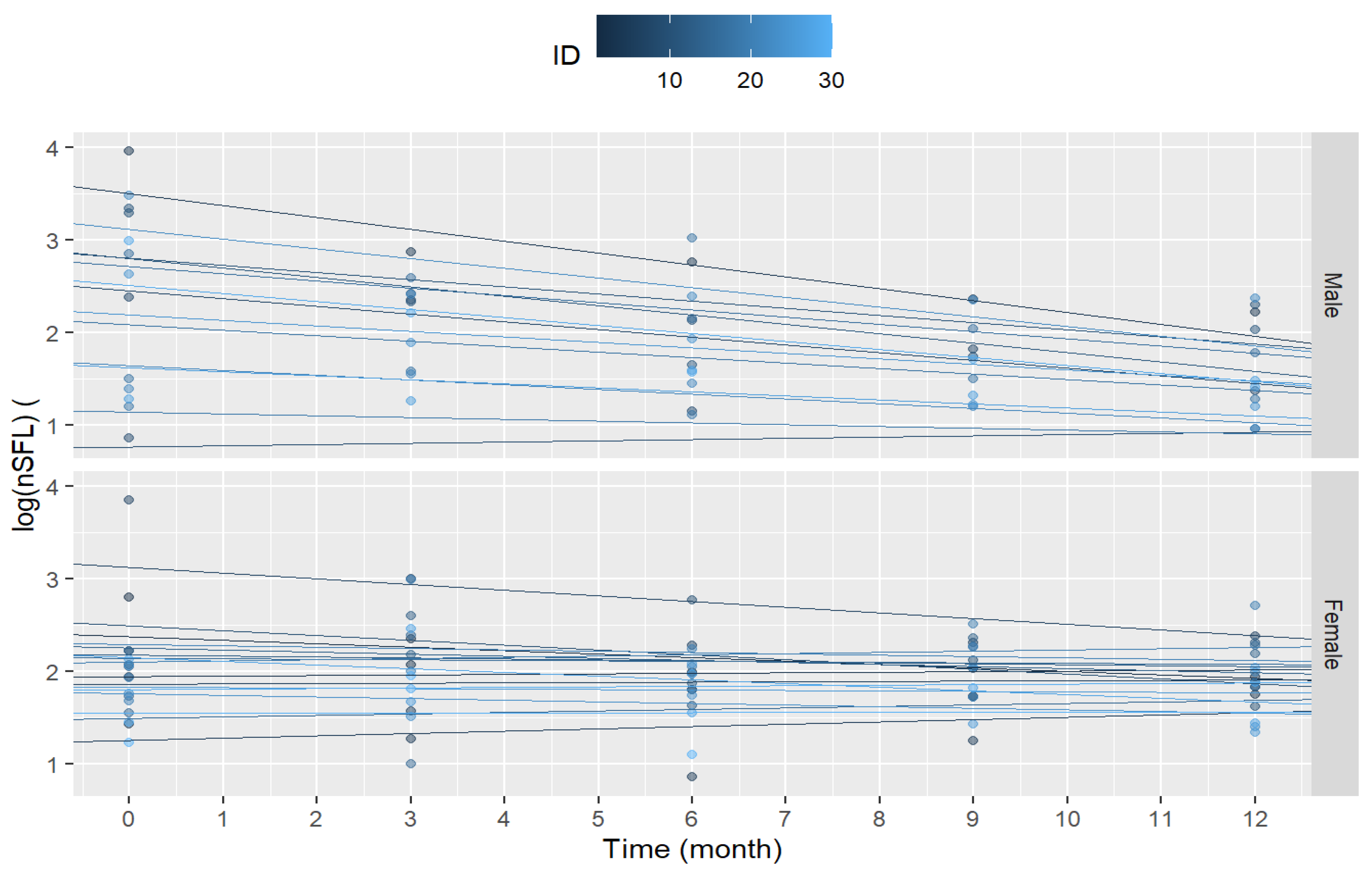
Clinical outcome and confounding variables were analyzed in mixed linear regression models. The final model included the sex and age of participants at baseline. The adjusted model permits random intercepts and random slopes for each participant to improve the depiction of individual variability over time. OCR treatment emerged as more effective to reduce blood sNFL levels in young males than in other groups, indicating that the treatment has a greater impact on these patients (
Figure 2). In the mixed effects binary logistic model on the most influential factors in relapses, the best fit was obtained for the sNFL levels at 3 and 9 months: with the odds ratio of a relapse rising to 0.386 per one-unit increase in sNFL at 3 months and to 2.035 per one-unit increase at 9 months.
4. Discussion
This real-world study followed PwMS treated with OCR for 12 months to determine the impact of this treatment on sNfL levels and other clinical, radiological, and disability outcomes, exploring the factors that influence this effect. Information provided by this type of study in a non-selected population complements data obtained in clinical trials [
32,
43].
In comparison to previous studies, the mean age at OCR onset was slightly higher (40 years), the time with disease was longer (around 12 years), the mean EDSS was lower, and the number of treatments was smaller (1.5 vs. 2.2), while ARR and Gd+ lesion load values were similar [
33,
44]. The main indication for this high-efficacy drug was a suboptimal response to moderate-efficacy (26% of participants) or high-efficacy (74%) drugs. ARR, disability progression (by EDSS, T25FW, and 9HPT), and MRI activity at 12 months were not associated with sNfL levels at baseline. The MRI finding of Gd+ lesions at OCR onset was moderately correlated with baseline sNfL levels (0.458. p=0.012), in line with the study by Cross et al. [
23]
NEDA-3 was reached in 70% of the PwMS, very similar to other results obtained using high-efficacy DMTs [
31,
44]. After 12 months of OCR treatment, the ARR was reduced in 83%, the EDSS was maintained, and there was an 80% reduction in those with radiological activity. In comparison, Sandgren et al. reported that 33% of ALM-treated patients reached NEDA-3 after a five-year follow-up, with a lower reduction in ARR.
Higher sNFL concentrations in CSF or serum have been associated with relapses, the emergence of new lesions in neuroimaging, and a worsening of the disease [
45]. Clinical trials have determined sNfL levels in order to evaluate the effectiveness of different DMTs, but less information has been published on their usefulness for DMT follow-up in the routine clinical setting [
16].
The significant reduction in sNfL levels verifies the molecular effect of OCR on neurodegeneration in PwMS and was observed at three months after the first infusion, earlier than reported for other DMTs [
27]. The 16% decrease in sNfL levels at 12 months was lesser than described in the OBOE study with OCR [
23] or in the study with CLD [
31].
The clinical and/or radiological activity of this disease is associated with elevated sNfL levels and z-scores [
46]. Interindividual differences in sNFL levels were minimized by comparing sNFL levels and z-scores [
16], obtaining similar results for both. Besides the reduction in mean sNfL, the z-score was also decreased at 12 months
versus baseline, and the sNfL level reduction was maintained for at least 12 months. Most studies of PwMS have described a reduction in sNfL levels during the first 12 months, being greater and earlier with high-efficacy treatments (anti CD20, CD52, and integrin α4β1 antibodies) than with other oral therapies (S1P-receptor inhibitors, DMF, and TFL) or “platform” drugs [
16,
26,
33,
47].
Baseline sNfL levels were not associated with EDSS-assessed worsening, in agreement with previous findings [
48]. sNfL levels in PwMS have been reported to rise during relapses and peak at three weeks [
17]. In the present study, sNfL levels increased over the three months before relapse in 85.7% of the PwMS with relapses, compared with 38% of those treated with ALM [
49]. As noted by other authors [
5,
50,
51], not all PwMS with clinical “relapse” have a higher sNfL level than determined in the three previous months. Indeed, sNfL levels were not increased before the relapse in one out of six patients in the present population.
Unlike in another study [
52], no association was observed between increased sNfL levels and greater disability assessed by EDSS, T25FW, or 9HPT test scores or between sNfL levels or baseline z-score and NEDA-3 at 12 months. This difference may be explained by the shorter follow-up time and/or the administration of a high-efficacy drug (OCR), given that Meier and coworkers found no association between sNfL levels and progression worsening in patients treated with anti-CD20 [
53]. A real-life study of 14 cladribine-treated PwMS also found no relationship between
sNfL levels and relapse, neuroimaging activity, EDSS progression, or NEDA-3 status [
31]. Likewise
, no correlation was reported between sNfL levels and NEDA-3 in 74 PwMS treated with CLD [
54] or in 52 PwMS treated with DMF after 12 months of follow up [
27]. However, studies with longer follow-up periods have demonstrated an association between baseline sNfL levels and the presence of clinical and radiological activity or EDSS progression [
9,
30,
33,
35,
49,
55]. Other authors have reported a modest association between sNfL levels and the degree of disability but not the frequency of relapses [
37]. After a 12-year follow-up period, the only association observed by Canto et al. was between sNfL levels and EDSS scores [
56]. Baseline ARR, EDSS, and radiological activity values were higher in naïve
versus previously treated patients, who had similar sNfL levels (19.1 vs. 10.7 pg/mL, respectively); however, the baseline level (1.55 vs. -0.134) and sNfL-b/sNfL-12 m ratio were higher in the naive group after adjustment by age and BMI (z-score). This may reflect the effect of DMTs on sNfL levels before the switch to OCR. Sandgren et al. also obtained higher sNfL levels in naive
versus previously treated PwMS group [
30].
Study limitations include the modest sample size and follow-up period, preventing study of the mid- to long-term prognosis. In addition, it is more challenging to obtain short-term differences in sNFL levels and their relationship with relapses or disease progression in patients treated with a very highly effective drug such as OCR.
Despite the prognostic and monitoring value of serial determinations of sNfL levels, consensus groups recently concluded that these analyses will not replace MRI over the short term but will be used as a complementary technique or with a greater frequency, given their lower cost, thereby optimizing neuroimaging [
57,
58].
5. Conclusions
Given the unpredictable course and heterogeneous development of MS, biomarkers are needed to improve follow-up of the disease and the response to DMTs. OCR is a highly effective drug, as confirmed in this study, obtaining an ARR reduction in 83%, maintaining the EDSS score, and reducing radiological activity in 80% at 12 months of follow up, when 70% of the PwMS reached NEDA-3. The OCR treatment produced an early reduction in sNfL levels at three months that was maintained over the follow up period. Gd+ activity on the baseline MRI scan was associated with higher sNfL levels. The evaluation of sNfL levels during the first year of OCR treatment did not prove able to predict a suboptimal response or a persistence of disease control. The long-term evaluation of sNfL levels in wider studies is warranted to determine the predictive value of sNfL levels in PwMS treated with high-efficacy drugs.
Author Contributions
Conceptualization, FJBH; formal analysis: AJGM,; investigation, RPM, ARM, VGM, JMBL, CMF, and AAdM; resources, MARR.; writing—original draft preparation, FJBH.; writing—review and editing, FJBH and RPM. funding acquisition, FJBH. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding for the determination of sNfL using SIMOA technology (Quanterix, Billerica, MA) by Roche pharma.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of ethics committee of the autonomous community of Andalusia (protocol 1.1. March 9, 2022), for studies involving humans. .
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Acknowledgments
We thank Luisa M. Villar, head of the immunology department of the Ramon y Cajal University Hospital in Madrid where the sNfL measurement was performed. We are grateful to the participants in this study, without whom this work would not have been possible.
Conflicts of Interest
FJBH received compensation for consulting services and speaking honoraria from Almirall, Biogen, Bristol Myers Squibb, Genzyme, Johnson & Johnson, Merck, Novartis, Roche, Sanofi, Teva. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”. CMF: received economic compensation from the pharmaceutical industry from Novartis, Sanofi-Genzyme, Roche, Merck and Biogen for informative conferences, and economic compensation for observational studies from Merk and Sanofi-Genzyme. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. ARV, VGM, AGdM, JMBL, MARR and AJGM: Theses authors declare no conflicts of interest. RPM: Received compensation for consulting services and speaking honoraria from Biogen, Genzyme, Johnson & Johnson, Merck, Novartis, Roche, Sanofi. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Leppert, D.; Kuhle, J. Blood Neurofilament Light Chain at the Doorstep of Clinical Application. Neurol Neuroimmunol Neuroinflamm 2019, 6, e599. [CrossRef]
- Sen, M.K.; Hossain, M.J.; Mahns, D.A.; Brew, B.J. Validity of Serum Neurofilament Light Chain as a Prognostic Biomarker of Disease Activity in Multiple Sclerosis. J Neurol 2023, 270, 1908–1930. [CrossRef]
- Varhaug, K.N.; Torkildsen, Ø.; Myhr, K.-M.; Vedeler, C.A. Neurofilament Light Chain as a Biomarker in Multiple Sclerosis. Front Neurol 2019, 10, 338. [CrossRef]
- Disanto, G.; Adiutori, R.; Dobson, R.; Martinelli, V.; Dalla Costa, G.; Runia, T.; Evdoshenko, E.; Thouvenot, E.; Trojano, M.; Norgren, N.; et al. Serum Neurofilament Light Chain Levels Are Increased in Patients with a Clinically Isolated Syndrome. J Neurol Neurosurg Psychiatry 2016, 87, 126–129. [CrossRef]
- Kuhle, J.; Barro, C.; Disanto, G.; Mathias, A.; Soneson, C.; Bonnier, G.; Yaldizli, Ö.; Regeniter, A.; Derfuss, T.; Canales, M.; et al. Serum Neurofilament Light Chain in Early Relapsing Remitting MS Is Increased and Correlates with CSF Levels and with MRI Measures of Disease Severity. Mult Scler 2016, 22, 1550–1559. [CrossRef]
- Ferrazzano, G.; Crisafulli, S.G.; Baione, V.; Tartaglia, M.; Cortese, A.; Frontoni, M.; Altieri, M.; Pauri, F.; Millefiorini, E.; Conte, A. Early Diagnosis of Secondary Progressive Multiple Sclerosis: Focus on Fluid and Neurophysiological Biomarkers. J Neurol 2021, 268, 3626–3645. [CrossRef]
- Preziosa, P.; Rocca, M.A.; Filippi, M. Current State-of-Art of the Application of Serum Neurofilaments in Multiple Sclerosis Diagnosis and Monitoring. Expert Rev Neurother 2020, 20, 747–769. [CrossRef]
- Rosso, M.; Gonzalez, C.T.; Healy, B.C.; Saxena, S.; Paul, A.; Bjornevik, K.; Kuhle, J.; Benkert, P.; Leppert, D.; Guttmann, C.; et al. Temporal Association of sNfL and Gad-Enhancing Lesions in Multiple Sclerosis. Ann Clin Transl Neurol 2020, 7, 945–955. [CrossRef]
- Barro, C.; Benkert, P.; Disanto, G.; Tsagkas, C.; Amann, M.; Naegelin, Y.; Leppert, D.; Gobbi, C.; Granziera, C.; Yaldizli, Ö.; et al. Serum Neurofilament as a Predictor of Disease Worsening and Brain and Spinal Cord Atrophy in Multiple Sclerosis. Brain 2018, 141, 2382–2391. [CrossRef]
- Comabella, M.; Sastre-Garriga, J.; Montalban, X. Precision Medicine in Multiple Sclerosis: Biomarkers for Diagnosis, Prognosis, and Treatment Response. Curr Opin Neurol 2016, 29, 254–262. [CrossRef]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood Neurofilament Light Chain as a Biomarker of MS Disease Activity and Treatment Response. Neurology 2019, 92, e1007–e1015. [CrossRef]
- Berger, T.; Stüve, O. Neurofilament Light Chain: An Important Step toward a Disease Biomarker in Multiple Sclerosis. Neurology 2019, 92, 451–452. [CrossRef]
- Delaby, C.; Bousiges, O.; Bouvier, D.; Fillée, C.; Fourier, A.; Mondésert, E.; Nezry, N.; Omar, S.; Quadrio, I.; Rucheton, B.; et al. Neurofilaments Contribution in Clinic: State of the Art. Front Aging Neurosci 2022, 14, 1034684. [CrossRef]
- Khalil, M.; Pirpamer, L.; Hofer, E.; Voortman, M.M.; Barro, C.; Leppert, D.; Benkert, P.; Ropele, S.; Enzinger, C.; Fazekas, F.; et al. Serum Neurofilament Light Levels in Normal Aging and Their Association with Morphologic Brain Changes. Nat Commun 2020, 11, 812. [CrossRef]
- Cai, L.; Huang, J. Neurofilament Light Chain as a Biological Marker for Multiple Sclerosis: A Meta-Analysis Study. Neuropsychiatr Dis Treat 2018, 14, 2241–2254. [CrossRef]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, Ö.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. Serum Neurofilament Light Chain for Individual Prognostication of Disease Activity in People with Multiple Sclerosis: A Retrospective Modelling and Validation Study. Lancet Neurol 2022, 21, 246–257. [CrossRef]
- Martin, S.-J.; McGlasson, S.; Hunt, D.; Overell, J. Cerebrospinal Fluid Neurofilament Light Chain in Multiple Sclerosis and Its Subtypes: A Meta-Analysis of Case-Control Studies. J Neurol Neurosurg Psychiatry 2019, 90, 1059–1067. [CrossRef]
- Ocrevus | European Medicines Agency Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ocrevus (accessed on 28 January 2024).
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med 2017, 376, 221–234. [CrossRef]
- Wolinsky, J.S.; Arnold, D.L.; Brochet, B.; Hartung, H.-P.; Montalban, X.; Naismith, R.T.; Manfrini, M.; Overell, J.; Koendgen, H.; Sauter, A.; et al. Long-Term Follow-up from the ORATORIO Trial of Ocrelizumab for Primary Progressive Multiple Sclerosis: A Post-Hoc Analysis from the Ongoing Open-Label Extension of the Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Neurol 2020, 19, 998–1009. [CrossRef]
- Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Brochet, B.; Naismith, R.T.; Traboulsee, A.; Wolinsky, J.S.; Belachew, S.; Koendgen, H.; et al. Five Years of Ocrelizumab in Relapsing Multiple Sclerosis: OPERA Studies Open-Label Extension. Neurology 2020, 95, e1854–e1867. [CrossRef]
- Bar-Or, A.; Thanei, G.-A.; Harp, C.; Bernasconi, C.; Bonati, U.; Cross, A.H.; Fischer, S.; Gaetano, L.; Hauser, S.L.; Hendricks, R.; et al. Blood Neurofilament Light Levels Predict Non-Relapsing Progression Following Anti-CD20 Therapy in Relapsing and Primary Progressive Multiple Sclerosis: Findings from the Ocrelizumab Randomised, Double-Blind Phase 3 Clinical Trials. EBioMedicine 2023, 93, 104662. [CrossRef]
- Cross A, Bennett J, von Budingen HC, et al. Ocrelizumab Treatment Reduced Levels of Neurofilament Light Chain and Numbers of B Cells in the Cerebrospinal Fluid of Patients with Relapsing Multiple Sclerosis in the OBOE Study [Abstract No. S56.008]. In: 71st Annual Meeting of the American Academy of Neurology. Neurology 2019, 92, S56.008.
- (Bar-Or A, Thanei G, Harp C, Bernasconi C, Bonati U, Cross AH, Ocrelizumab Treatment Induces a Sustained Blood NfL Reduction in Patients with PPMS and RMS. Mult Scler J 2021, 26, 178–179.
- Alcalá, C.; Quintanilla-Bordás, C.; Gascón, F.; Sempere, Á.P.; Navarro, L.; Carcelén-Gadea, M.; Landete, L.; Mallada, J.; Cañizares, E.; Belenguer, A.; et al. Effectiveness of Rituximab vs. Ocrelizumab for the Treatment of Primary Progressive Multiple Sclerosis: A Real-World Observational Study. J Neurol 2022, 269, 3676–3681. [CrossRef]
- Zhou, R.; Li, H.; Yang, H.; Jiang, F.; Cai, H.; Li, J.; Chen, S.; Fang, L.; Yin, J.; Zeng, Q. Serological Markers Exploration and Real-World Effectiveness and Safety of Teriflunomide in South Chinese Patients with Multiple Sclerosis. Mult Scler Relat Disord 2022, 58, 103446. [CrossRef]
- Sejbaek, T.; Nielsen, H.H.; Penner, N.; Plavina, T.; Mendoza, J.P.; Martin, N.A.; Elkjaer, M.L.; Ravnborg, M.H.; Illes, Z. Dimethyl Fumarate Decreases Neurofilament Light Chain in CSF and Blood of Treatment Naïve Relapsing MS Patients. J Neurol Neurosurg Psychiatry 2019, 90, 1324–1330. [CrossRef]
- Gunnarsson, M.; Malmeström, C.; Axelsson, M.; Sundström, P.; Dahle, C.; Vrethem, M.; Olsson, T.; Piehl, F.; Norgren, N.; Rosengren, L.; et al. Axonal Damage in Relapsing Multiple Sclerosis Is Markedly Reduced by Natalizumab. Ann Neurol 2011, 69, 83–89. [CrossRef]
- Novakova, L.; Axelsson, M.; Khademi, M.; Zetterberg, H.; Blennow, K.; Malmeström, C.; Piehl, F.; Olsson, T.; Lycke, J. Cerebrospinal Fluid Biomarkers of Inflammation and Degeneration as Measures of Fingolimod Efficacy in Multiple Sclerosis. Mult Scler 2017, 23, 62–71. [CrossRef]
- Sandgren, S.; Novakova, L.; Nordin, A.; Axelsson, M.; Malmeström, C.; Zetterberg, H.; Lycke, J. A Five-Year Observational Prospective Mono-Center Study of the Efficacy of Alemtuzumab in a Real-World Cohort of Patients with Multiple Sclerosis. Front Neurol 2023, 14, 1265354. [CrossRef]
- Seiberl, M.; Feige, J.; Hilpold, P.; Hitzl, W.; Machegger, L.; Buchmann, A.; Khalil, M.; Trinka, E.; Harrer, A.; Wipfler, P.; et al. Serum Neurofilament Light Chain as Biomarker for Cladribine-Treated Multiple Sclerosis Patients in a Real-World Setting. Int J Mol Sci 2023, 24, 4067. [CrossRef]
- Ziemssen, T.; Arnold, D.L.; Alvarez, E.; Cross, A.H.; Willi, R.; Li, B.; Kukkaro, P.; Kropshofer, H.; Ramanathan, K.; Merschhemke, M.; et al. Prognostic Value of Serum Neurofilament Light Chain for Disease Activity and Worsening in Patients With Relapsing Multiple Sclerosis: Results From the Phase 3 ASCLEPIOS I and II Trials. Front Immunol 2022, 13, 852563. [CrossRef]
- Delcoigne, B.; Manouchehrinia, A.; Barro, C.; Benkert, P.; Michalak, Z.; Kappos, L.; Leppert, D.; Tsai, J.A.; Plavina, T.; Kieseier, B.C.; et al. Blood Neurofilament Light Levels Segregate Treatment Effects in Multiple Sclerosis. Neurology 2020, 94, e1201–e1212. [CrossRef]
- Brune, S.; Høgestøl, E.A.; de Rodez Benavent, S.A.; Berg-Hansen, P.; Beyer, M.K.; Leikfoss, I.S.; Bos, S.D.; Sowa, P.; Brunborg, C.; Andorra, M.; et al. Serum Neurofilament Light Chain Concentration Predicts Disease Worsening in Multiple Sclerosis. Mult Scler 2022, 28, 1859–1870. [CrossRef]
- Monreal, E.; Fernández-Velasco, J.I.; García-Sánchez, M.I.; Sainz de la Maza, S.; Llufriu, S.; Álvarez-Lafuente, R.; Casanova, B.; Comabella, M.; Ramió-Torrentà, L.; Martínez-Rodríguez, J.E.; et al. Association of Serum Neurofilament Light Chain Levels at Disease Onset With Disability Worsening in Patients With a First Demyelinating Multiple Sclerosis Event Not Treated With High-Efficacy Drugs. JAMA Neurol 2023, 80, 397–403. [CrossRef]
- Meca-Lallana, V.; Rodríguez-Antigüedad, A.; Llaneza, M.A.; Meca-Lallana, J.E. [Plasma determination of neurofilaments as biomarkers in multiple sclerosis: conclusions of the EMotion Forum]. Rev Neurol 2021, 73, 101–110. [CrossRef]
- Anderson, V.; Bentley, E.; Loveless, S.; Bianchi, L.; Harding, K.E.; Wynford-Thomas, R.A.; Joseph, F.; Giovannoni, G.; Gnanapavan, S.; Robertson, N.P.; et al. Serum Neurofilament-Light Concentration and Real-World Outcome in MS. J Neurol Sci 2020, 417, 117079. [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol 2018, 17, 162–173. [CrossRef]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444–1452. [CrossRef]
- Kalinowski, A.; Cutter, G.; Bozinov, N.; Hinman, J.A.; Hittle, M.; Motl, R.; Odden, M.; Nelson, L.M. The Timed 25-Foot Walk in a Large Cohort of Multiple Sclerosis Patients. Mult Scler 2022, 28, 289–299. [CrossRef]
- Feys, P.; Lamers, I.; Francis, G.; Benedict, R.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R.; Multiple Sclerosis Outcome Assessments Consortium The Nine-Hole Peg Test as a Manual Dexterity Performance Measure for Multiple Sclerosis. Mult Scler 2017, 23, 711–720. [CrossRef]
- Giovannoni, G.; Turner, B.; Gnanapavan, S.; Offiah, C.; Schmierer, K.; Marta, M. Is It Time to Target No Evident Disease Activity (NEDA) in Multiple Sclerosis? Mult Scler Relat Disord 2015, 4, 329–333. [CrossRef]
- Bittner, S.; Oh, J.; Havrdová, E.K.; Tintoré, M.; Zipp, F. The Potential of Serum Neurofilament as Biomarker for Multiple Sclerosis. Brain 2021, 144, 2954–2963. [CrossRef]
- Pape, K.; Rolfes, L.; Steffen, F.; Muthuraman, M.; Korsen, M.; Meuth, S.G.; Zipp, F.; Bittner, S. Comparative Effectiveness of Natalizumab versus Ocrelizumab in Multiple Sclerosis: A Real-World Propensity Score-Matched Study. Ther Adv Neurol Disord 2022, 15, 17562864221142924. [CrossRef]
- Novakova, L.; Zetterberg, H.; Sundström, P.; Axelsson, M.; Khademi, M.; Gunnarsson, M.; Malmeström, C.; Svenningsson, A.; Olsson, T.; Piehl, F.; et al. Monitoring Disease Activity in Multiple Sclerosis Using Serum Neurofilament Light Protein. Neurology 2017, 89, 2230–2237. [CrossRef]
- Solís-Tarazona, L.; Raket, L.L.; Cabello-Murgui, J.; Reddam, S.; Navarro-Quevedo, S.; Gil-Perotin, S. Predictive Value of Individual Serum Neurofilament Light Chain Levels in Short-Term Disease Activity in Relapsing Multiple Sclerosis. Front Neurol 2024, 15, 1354431. [CrossRef]
- Khalil, M.; Teunissen, C.E.; Lehmann, S.; Otto, M.; Piehl, F.; Ziemssen, T.; Bittner, S.; Sormani, M.P.; Gattringer, T.; Abu-Rumeileh, S.; et al. Neurofilaments as Biomarkers in Neurological Disorders - towards Clinical Application. Nat Rev Neurol 2024. [CrossRef]
- Gaetani, L.; Salvadori, N.; Lisetti, V.; Eusebi, P.; Mancini, A.; Gentili, L.; Borrelli, A.; Portaccio, E.; Sarchielli, P.; Blennow, K.; et al. Cerebrospinal Fluid Neurofilament Light Chain Tracks Cognitive Impairment in Multiple Sclerosis. J Neurol 2019, 266, 2157–2163. [CrossRef]
- Akgün, K.; Kretschmann, N.; Haase, R.; Proschmann, U.; Kitzler, H.H.; Reichmann, H.; Ziemssen, T. Profiling Individual Clinical Responses by High-Frequency Serum Neurofilament Assessment in MS. Neurol Neuroimmunol Neuroinflamm 2019, 6, e555. [CrossRef]
- Calabresi PA, Arnold DL, Kinkel RP, et al. Serum Neurofilament Light (NfL): Towards a Blood Test for Prognosis and Disease/Treatment Monitoring in Multiple Sclerosis Patients (S24.003). Neurology 2018, 90, S24003.
- Johnsson, M.; Stenberg, Y.T.; Farman, H.H.; Blennow, K.; Zetterberg, H.; Malmeström, C.; Sandgren, S.; Rosenstein, I.; Lycke, J.; Axelsson, M.; et al. Serum Neurofilament Light for Detecting Disease Activity in Individual Patients in Multiple Sclerosis: A 48-Week Prospective Single-Center Study. Mult Scler 2024, 13524585241237388. [CrossRef]
- Jakimovski, D.; Zivadinov, R.; Ramanthan, M.; Hagemeier, J.; Weinstock-Guttman, B.; Tomic, D.; Kropshofer, H.; Fuchs, T.A.; Barro, C.; Leppert, D.; et al. Serum Neurofilament Light Chain Level Associations with Clinical and Cognitive Performance in Multiple Sclerosis: A Longitudinal Retrospective 5-Year Study. Mult Scler 2020, 26, 1670–1681. [CrossRef]
- Meier, S.; Willemse, E.A.J.; Schaedelin, S.; Oechtering, J.; Lorscheider, J.; Melie-Garcia, L.; Cagol, A.; Barakovic, M.; Galbusera, R.; Subramaniam, S.; et al. Serum Glial Fibrillary Acidic Protein Compared With Neurofilament Light Chain as a Biomarker for Disease Progression in Multiple Sclerosis. JAMA Neurol 2023, 80, 287–297. [CrossRef]
- Paolicelli, D.; Ruggieri, M.; Manni, A.; Gargano, C.D.; Carleo, G.; Palazzo, C.; Iaffaldano, A.; Bollo, L.; Guerra, T.; Saracino, A.; et al. Real-Life Experience of the Effects of Cladribine Tablets on Lymphocyte Subsets and Serum Neurofilament Light Chain Levels in Relapsing Multiple Sclerosis Patients. Brain Sci 2022, 12, 1595. [CrossRef]
- Hyun, J.-W.; Kim, Y.; Kim, G.; Kim, S.-H.; Kim, H.J. Longitudinal Analysis of Serum Neurofilament Light Chain: A Potential Therapeutic Monitoring Biomarker for Multiple Sclerosis. Mult Scler 2020, 26, 659–667. [CrossRef]
- Cantó, E.; Barro, C.; Zhao, C.; Caillier, S.J.; Michalak, Z.; Bove, R.; Tomic, D.; Santaniello, A.; Häring, D.A.; Hollenbach, J.; et al. Association Between Serum Neurofilament Light Chain Levels and Long-Term Disease Course Among Patients With Multiple Sclerosis Followed up for 12 Years. JAMA Neurol 2019, 76, 1359–1366. [CrossRef]
- Moccia, M.; Terracciano, D.; Brescia Morra, V.; Castaldo, G. Neurofilament in Clinical Practice: Is the Multiple Sclerosis Community Ready? Mult Scler 2024, 13524585241246536. [CrossRef]
- Bose, G.; Healy, B.C.; Barro, C.; Moreira Ferreira, V.F.; Saxena, S.; Glanz, B.I.; Lokhande, H.A.; Polgar-Turcsanyi, M.; Bakshi, R.; Weiner, H.L.; et al. Accuracy of Serum Neurofilament Light to Identify Contrast-Enhancing Lesions in Multiple Sclerosis. Mult Scler 2023, 29, 1418–1427. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).