Submitted:
30 May 2024
Posted:
31 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mechanisms of Action, Antibacterial Activity and Adverse Effects
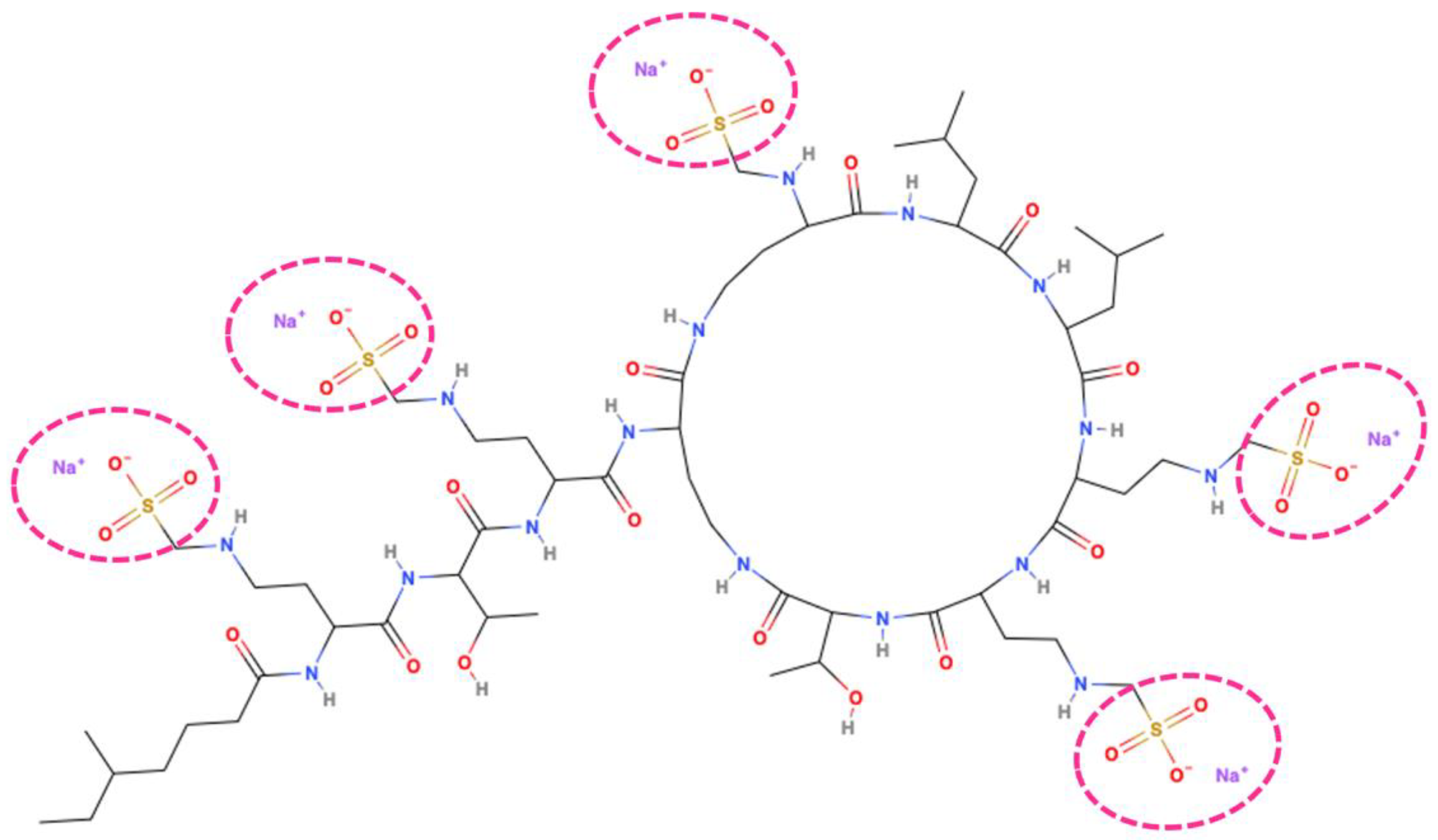
2.1. Structure and Mode of Action
2.2. Adverse Effects
2.3. In Vitro Antimicrobial Activity
2.4. Antimcirobial Susceptibility Testing
3. Mechanisms of Colistin Resistance
3.1. Modification of LPS Structure by Chromosomal Mutations
3.2. Loss of LPS Structure
3.3. Plasmid-Mediated Colistin Resistance
3.4. Overexpression of Efflux Pumps
4. Pharmacokinetic/Pharmacodynamic Features
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare (Basel) 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr Opin Microbiol 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria-A Review. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals (Basel) 2023, 16. [Google Scholar] [CrossRef]
- Walsh, C.T.; Wencewicz, T.A. Prospects for New Antibiotics: A Molecule-Centered Perspective. J Antibiot (Tokyo) 2014, 67, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Giani, T.; Bovo, F.; Lombardo, D.; Amadesi, S.; Lazzarotto, T.; Coppi, M.; Rossolini, G.M.; Ambretti, S. Resistance to Ceftazidime/Avibactam, Meropenem/Vaborbactam and Imipenem/Relebactam in Gram-Negative MDR Bacilli: Molecular Mechanisms and Susceptibility Testing. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef]
- El-Sayed Ahmed, M.A.E.-G.; Zhong, L.-L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.-B. Colistin and Its Role in the Era of Antibiotic Resistance: An Extended Review (2000–2019). Emerg Microbes Infect 2020, 9, 868–885. [Google Scholar] [CrossRef]
- Mondal, A.H.; Khare, K.; Saxena, P.; Debnath, P.; Mukhopadhyay, K.; Yadav, D. A Review on Colistin Resistance: An Antibiotic of Last Resort. Microorganisms 2024, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y. A New Antibiotic “Colistin” Produced by Spore-Forming Soil Bacteria. J Antibiot. 1950, 3, 457–458. [Google Scholar]
- Stansly, P.G.; Schlosser, M.E. Studies on Polymyxin: Isolation and Identification of Bacillus Polymyxa and Differentiation of Polymyxin from Certain Known Antibiotics. J Bacteriol 1947, 54, 549–556. [Google Scholar] [CrossRef]
- Hamel, M.; Rolain, J.-M.; Baron, S.A. The History of Colistin Resistance Mechanisms in Bacteria: Progress and Challenges. Microorganisms 2021, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- Bergen, P.J.; Li, J.; Rayner, C.R.; Nation, R.L. Colistin Methanesulfonate Is an Inactive Prodrug of Colistin against Pseudomonas Aeruginosa. Antimicrob Agents Chemother 2006, 50, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Ehrentraut, S.F.; Muenster, S.; Kreyer, S.; Theuerkauf, N.U.; Bode, C.; Steinhagen, F.; Ehrentraut, H.; Schewe, J.-C.; Weber, M.; Putensen, C.; et al. Extensive Therapeutic Drug Monitoring of Colistin in Critically Ill Patients Reveals Undetected Risks. Microorganisms 2020, 8, 415. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Why and How Are Peptide–Lipid Interactions Utilized for Self-Defense? Magainins and Tachyplesins as Archetypes. Biochimica et Biophysica Acta (BBA) - Biomembranes 1999, 1462, 1–10. [Google Scholar] [CrossRef]
- Shai, Y. Mechanism of the Binding, Insertion and Destabilization of Phospholipid Bilayer Membranes by α-Helical Antimicrobial and Cell Non-Selective Membrane-Lytic Peptides. Biochimica et Biophysica Acta (BBA) - Biomembranes 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Yang, L.; Weiss, T.M.; Lehrer, R.I.; Huang, H.W. Crystallization of Antimicrobial Pores in Membranes: Magainin and Protegrin. Biophys J 2000, 79, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lee, W.; Kwa, A.L. Polymyxin B versus Colistin: An Update. Expert Rev Anti Infect Ther 2015, 13, 1481–1497. [Google Scholar] [CrossRef] [PubMed]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E.W. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb Perspect Med 2016, 6, a025288. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.F.; Silva, D.; Rodrigues, A.; Pina-Vaz, C. Colistin Update on Its Mechanism of Action and Resistance, Present and Future Challenges. Microorganisms 2020, 8. [Google Scholar] [CrossRef]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure−Activity Relationships of Polymyxin Antibiotics. J Med Chem 2010, 53, 1898–1916. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clinical Infectious Diseases 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Pogue, J.M.; Tran, T.B.; Nation, R.L.; Li, J. Agents of Last Resort. Infect Dis Clin North Am 2016, 30, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Gurjar, M. Colistin for Lung Infection: An Update. J Intensive Care 2015, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Deris, Z.Z.; Akter, J.; Sivanesan, S.; Roberts, K.D.; Thompson, P.E.; Nation, R.L.; Li, J.; Velkov, T. A Secondary Mode of Action of Polymyxins against Gram-Negative Bacteria Involves the Inhibition of NADH-Quinone Oxidoreductase Activity. J Antibiot (Tokyo) 2014, 67, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chan, T.; Xu, C.; Zhu, L.; Zhou, Q.T.; Roberts, K.D.; Chan, H.-K.; Li, J.; Zhou, F. Human Oligopeptide Transporter 2 (PEPT2) Mediates Cellular Uptake of Polymyxins. J Antimicrob Chemother 2016, 71, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Visentin, M.; Gai, Z.; Torozi, A.; Hiller, C.; Kullak-Ublick, G.A. Colistin Is Substrate of the Carnitine/Organic Cation Transporter 2 (OCTN2, SLC22A5). Drug Metabolism and Disposition 2017, 45, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Samodelov, S.; Kullak-Ublick, G.; Visentin, M. Molecular Mechanisms of Colistin-Induced Nephrotoxicity. Molecules 2019, 24, 653. [Google Scholar] [CrossRef]
- Kilic, I.; Ayar, Y.; Ceylan, İ.; Kaya, P.K.; Caliskan, G. Nephrotoxicity Caused by Colistin Use in ICU: A Single Centre Experience. BMC Nephrol 2023, 24, 302. [Google Scholar] [CrossRef]
- Nation, R.L.; Li, J. Colistin in the 21st Century. Curr Opin Infect Dis 2009, 22, 535–543. [Google Scholar] [CrossRef]
- Akajagbor, D.S.; Wilson, S.L.; Shere-Wolfe, K.D.; Dakum, P.; Charurat, M.E.; Gilliam, B.L. Higher Incidence of Acute Kidney Injury With Intravenous Colistimethate Sodium Compared With Polymyxin B in Critically Ill Patients at a Tertiary Care Medical Center. Clinical Infectious Diseases 2013, 57, 1300–1303. [Google Scholar] [CrossRef]
- Dalfino, L.; Puntillo, F.; Ondok, M.J.M.; Mosca, A.; Monno, R.; Coppolecchia, S.; Spada, M.L.; Bruno, F.; Brienza, N. Colistin-Associated Acute Kidney Injury in Severely Ill Patients: A Step Toward a Better Renal Care? A Prospective Cohort Study. Clinical Infectious Diseases 2015, 61, 1771–1777. [Google Scholar] [CrossRef]
- ÖZKARAKAŞ, H.; KÖSE, I.; ZİNCİRCİOĞLU, Ç.; ERSAN, S.; ERSAN, G.; ŞENOĞLU, N.; KÖSE, Ş.; ERBAY, R.H. Risk Factors for Colistin-Associated Nephrotoxicity and Mortality in Critically Ill Patients. Turk J Med Sci 2017, 47, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, N.; Giannella, M.; Antonelli, M.; Antonini, M.; Barsic, B.; Belancic, L.; Inkaya A., C.; De Pascale, G.; Grilli, E.; Tumbarello, M.; et al. Clinical Experience of Colistin-Glycopeptide Combination in Critically Ill Patients Infected with Gram-Negative Bacteria. Antimicrob Agents Chemother 2014, 58, 851–858. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Tsiodras, S.; Michalopoulos, A. The Use of Intravenous and Aerosolized Polymyxins for the Treatment of Infections in Critically Ill Patients: A Review of the Recent Literature. Clin Med Res 2006, 4, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Spapen, H.; Jacobs, R.; Van Gorp, V.; Troubleyn, J.; Honoré, P.M. Renal and Neurological Side Effects of Colistin in Critically Ill Patients. Ann Intensive Care 2011, 1, 14. [Google Scholar] [CrossRef]
- KUBIKOWSKI, P.; SZRENIAWSKI, Z. THE MECHANISM OF THE NEUROMUSCULAR BLOCKADE BY ANTIBIOTICS. Arch Int Pharmacodyn Ther 1963, 146, 549–560. [Google Scholar] [PubMed]
- Wang, Y.; Li, H.; Xie, X.; Wu, X.; Li, X.; Zhao, Z.; Luo, S.; Wan, Z.; Liu, J.; Fu, L.; et al. In Vitro and in Vivo Assessment of the Antibacterial Activity of Colistin Alone and in Combination with Other Antibiotics against Acinetobacter Baumannii and Escherichia Coli. J Glob Antimicrob Resist 2020, 20, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Walkty, A.; DeCorby, M.; Nichol, K.; Karlowsky, J.A.; Hoban, D.J.; Zhanel, G.G. In Vitro Activity of Colistin (Polymyxin E) against 3,480 Isolates of Gram-Negative Bacilli Obtained from Patients in Canadian Hospitals in the CANWARD Study, 2007-2008. Antimicrob Agents Chemother 2009, 53, 4924–4926. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.M.; Rao, J.U.; Kaleem, F.; Hassan, A.; Khalid, A.; Anjum, R. In Vitro Efficacy of Colistin against Multi-Drug Resistant Pseudomonas Aeruginosa by Minimum Inhibitory Concentration. Pak J Pharm Sci 2013, 26, 7–10. [Google Scholar]
- Gaibani, P.; Lombardo, D.; Lewis, R.E.; Mercuri, M.; Bonora, S.; Landini, M.P.; Ambretti, S. In Vitro Activity and Post-Antibiotic Effects of Colistin in Combination with Other Antimicrobials against Colistin-Resistant KPC-Producing Klebsiella Pneumoniae Bloodstream Isolates. Journal of Antimicrobial Chemotherapy 2014, 69, 1856–1865. [Google Scholar] [CrossRef]
- Kheshti, R.; Pourabbas, B.; Mosayebi, M.; Vazin, A. In Vitro Activity of Colistin in Combination with Various Antimicrobials against <em>Acinetobacter Baumannii</Em> Species, a Report from South Iran. Infect Drug Resist 2018, Volume 12, 129–135. [Google Scholar] [CrossRef]
- Brennan-Krohn, T.; Grote, A.; Rodriguez, S.; Kirby, J.E.; Earl, A.M. Transcriptomics Reveals How Minocycline-Colistin Synergy Overcomes Antibiotic Resistance in Multidrug-Resistant Klebsiella Pneumoniae. Antimicrob Agents Chemother 2022, 66. [Google Scholar] [CrossRef] [PubMed]
- Rout, B.; Dash, S.K.; Sahu, K. kumar; Behera, B.; Praharaj, I.; Otta, S. Evaluation of Different Methods for in Vitro Susceptibility Testing of Colistin in Carbapenem Resistant Gram-Negative Bacilli. Access Microbiol 2023, 5. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI) Performance and Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100; CLSI: 950 West Wiley Road, Suite 2500. [Google Scholar]
- Simner, P.J.; Bergman, Y.; Trejo, M.; Roberts, A.A.; Marayan, R.; Tekle, T.; Campeau, S.; Kazmi, A.Q.; Bell, D.T.; Lewis, S.; et al. Two-Site Evaluation of the Colistin Broth Disk Elution Test To Determine Colistin In Vitro Activity against Gram-Negative Bacilli. J Clin Microbiol 2019, 57. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Trent, M.S. Fortifying the Barrier: The Impact of Lipid A Remodelling on Bacterial Pathogenesis. Nat Rev Microbiol 2013, 11, 467–481. [Google Scholar] [CrossRef]
- Novović, K.; Jovčić, B. Colistin Resistance in Acinetobacter Baumannii: Molecular Mechanisms and Epidemiology. Antibiotics 2023, 12, 516. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Hao, J.; Xiao, W.; Ye, C.; Xiao, X.; Jian, C.; Tang, M.; Li, G.; Liu, J.; Zeng, Z. Role of Efflux Pumps, Their Inhibitors, and Regulators in Colistin Resistance. Front Microbiol 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Aghapour, Z.; Gholizadeh, P.; Ganbarov, K.; bialvaei, A.Z.; Mahmood, S.S.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Yousefi, B.; Samadi Kafil, H. <p>Molecular Mechanisms Related to Colistin Resistance in Enterobacteriaceae</P>. Infect Drug Resist 2019, Volume 12, 965–975. [Google Scholar] [CrossRef]
- Zhang, H.; Srinivas, S.; Xu, Y.; Wei, W.; Feng, Y. Genetic and Biochemical Mechanisms for Bacterial Lipid A Modifiers Associated with Polymyxin Resistance. Trends Biochem Sci 2019, 44, 973–988. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.-M. Mechanisms of Polymyxin Resistance: Acquired and Intrinsic Resistance in Bacteria. Front Microbiol 2014, 5. [Google Scholar] [CrossRef]
- Pelletier, M.R.; Casella, L.G.; Jones, J.W.; Adams, M.D.; Zurawski, D. V.; Hazlett, K.R.O.; Doi, Y.; Ernst, R.K. Unique Structural Modifications Are Present in the Lipopolysaccharide from Colistin-Resistant Strains of Acinetobacter Baumannii. Antimicrob Agents Chemother 2013, 57, 4831–4840. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Rafailidis, P.I.; Matthaiou, D.K. Resistance to Polymyxins: Mechanisms, Frequency and Treatment Options. Drug Resistance Updates 2010, 13, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Ly, N.S.; Yang, J.; Bulitta, J.B.; Tsuji, B.T. Impact of Two-Component Regulatory Systems PhoP-PhoQ and PmrA-PmrB on Colistin Pharmacodynamics in Pseudomonas Aeruginosa. Antimicrob Agents Chemother 2012, 56, 3453–3456. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.S. The Salmonella PmrAB Regulon: Lipopolysaccharide Modifications, Antimicrobial Peptide Resistance and More. Trends Microbiol 2008, 16, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Dia, N.M.; Gautret, P.; Benkouiten, S.; Belhouchat, K.; Drali, T.; Parola, P.; Brouqui, P.; Memish, Z.; Raoult, D.; et al. Acquisition of Extended-Spectrum Cephalosporin- and Colistin-Resistant Salmonella Enterica Subsp. Enterica Serotype Newport by Pilgrims during Hajj. Int J Antimicrob Agents 2015, 45, 600–604. [Google Scholar] [CrossRef]
- Quesada, A.; Porrero, M.C.; Téllez, S.; Palomo, G.; García, M.; Domínguez, L. Polymorphism of Genes Encoding PmrAB in Colistin-Resistant Strains of Escherichia Coli and Salmonella Enterica Isolated from Poultry and Swine. Journal of Antimicrobial Chemotherapy 2015, 70, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Cannatelli, A.; Di Pilato, V.; Giani, T.; Arena, F.; Ambretti, S.; Gaibani, P.; D’Andrea, M.M.; Rossolini, G.M. In Vivo Evolution to Colistin Resistance by PmrB Sensor Kinase Mutation in KPC-Producing Klebsiella Pneumoniae Is Associated with Low-Dosage Colistin Treatment. Antimicrob Agents Chemother 2014, 58, 4399–4403. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Lin, T.-L.; Pan, Y.-J.; Wang, Y.-P.; Lin, Y.-T.; Wang, J.-T. Colistin Resistance Mechanisms in Klebsiella Pneumoniae Strains from Taiwan. Antimicrob Agents Chemother 2015, 59, 2909–2913. [Google Scholar] [CrossRef] [PubMed]
- Jayol, A.; Poirel, L.; Brink, A.; Villegas, M.-V.; Yilmaz, M.; Nordmann, P. Resistance to Colistin Associated with a Single Amino Acid Change in Protein PmrB among Klebsiella Pneumoniae Isolates of Worldwide Origin. Antimicrob Agents Chemother 2014, 58, 4762–4766. [Google Scholar] [CrossRef]
- Gerson, S.; Betts, J.W.; Lucaßen, K.; Nodari, C.S.; Wille, J.; Josten, M.; Göttig, S.; Nowak, J.; Stefanik, D.; Roca, I.; et al. Investigation of Novel PmrB and EptA Mutations in Isogenic Acinetobacter Baumannii Isolates Associated with Colistin Resistance and Increased Virulence In Vivo. Antimicrob Agents Chemother 2019, 63. [Google Scholar] [CrossRef]
- Park, Y.K.; Choi, J.Y.; Shin, D.; Ko, K.S. Correlation between Overexpression and Amino Acid Substitution of the PmrAB Locus and Colistin Resistance in Acinetobacter Baumannii. Int J Antimicrob Agents 2011, 37, 525–530. [Google Scholar] [CrossRef]
- Dahdouh, E.; Gómez-Gil, R.; Sanz, S.; González-Zorn, B.; Daoud, Z.; Mingorance, J.; Suárez, M. A Novel Mutation in PmrB Mediates Colistin Resistance during Therapy of Acinetobacter Baumannii. Int J Antimicrob Agents 2017, 49, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, S.M.; Brannon, M.K.; Dasgupta, N.; Pier, M.; Sgambati, N.; Miller, A.K.; Selgrade, S.E.; Miller, S.I.; Denton, M.; Conway, S.P.; et al. PmrB Mutations Promote Polymyxin Resistance of Pseudomonas Aeruginosa Isolated from Colistin-Treated Cystic Fibrosis Patients. Antimicrob Agents Chemother 2012, 56, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Anim, D.; Kwon, D.H. Differential Role of Two-Component Regulatory Systems (≪I≫PhoPQ≪/I≫ and ≪I≫PmrAB≪/I≫) in Polymyxin B Susceptibility of ≪I≫Pseudomonas Aeruginosa≪/I≫ Adv Microbiol 2012, 02, 31–36. Adv Microbiol. [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.-M. Emergence of Colistin-Resistant Bacteria in Humans without Colistin Usage: A New Worry and Cause for Vigilance. Int J Antimicrob Agents 2016, 47, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Siu, L.K.; Chang, F.-Y.; Tsai, Y.-K.; Lin, Y.-T.; Chiu, S.-K.; Huang, L.-Y.; Lin, J.-C. A Novel Deletion Mutation in PmrB Contributes to Concurrent Colistin Resistance in Carbapenem-Resistant Escherichia Coli Sequence Type 405 of Clinical Origin. Antimicrob Agents Chemother 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Jayol, A.; Nordmann, P.; Brink, A.; Poirel, L. Heteroresistance to Colistin in Klebsiella Pneumoniae Associated with Alterations in the PhoPQ Regulatory System. Antimicrob Agents Chemother 2015, 59, 2780–2784. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Diene, S.M.; Kempf, M.; Berrazeg, M.; Bakour, S.; Gupta, S.K.; Thongmalayvong, B.; Akkhavong, K.; Somphavong, S.; Paboriboune, P.; et al. Worldwide Emergence of Colistin Resistance in Klebsiella Pneumoniae from Healthy Humans and Patients in Lao PDR, Thailand, Israel, Nigeria and France Owing to Inactivation of the PhoP/PhoQ Regulator MgrB: An Epidemiological and Molecular Study. Int J Antimicrob Agents 2014, 44, 500–507. [Google Scholar] [CrossRef]
- Nordmann, P.; Jayol, A.; Poirel, L. Rapid Detection of Polymyxin Resistance in Enterobacteriaceae. Emerg Infect Dis 2016, 22, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Gutu, A.D.; Sgambati, N.; Strasbourger, P.; Brannon, M.K.; Jacobs, M.A.; Haugen, E.; Kaul, R.K.; Johansen, H.K.; Høiby, N.; Moskowitz, S.M. Polymyxin Resistance of Pseudomonas Aeruginosa PhoQ Mutants Is Dependent on Additional Two-Component Regulatory Systems. Antimicrob Agents Chemother 2013, 57, 2204–2215. [Google Scholar] [CrossRef]
- Lippa, A.M.; Goulian, M. Feedback Inhibition in the PhoQ/PhoP Signaling System by a Membrane Peptide. PLoS Genet 2009, 5, e1000788. [Google Scholar] [CrossRef]
- Cannatelli, A.; Giani, T.; D’Andrea, M.M.; Di Pilato, V.; Arena, F.; Conte, V.; Tryfinopoulou, K.; Vatopoulos, A.; Rossolini, G.M. MgrB Inactivation Is a Common Mechanism of Colistin Resistance in KPC-Producing Klebsiella Pneumoniae of Clinical Origin. Antimicrob Agents Chemother 2014, 58, 5696–5703. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.S.; Suzuki, Y.; Jones, M.B.; Marshall, S.H.; Rudin, S.D.; van Duin, D.; Kaye, K.; Jacobs, M.R.; Bonomo, R.A.; Adams, M.D. Genomic and Transcriptomic Analyses of Colistin-Resistant Clinical Isolates of Klebsiella Pneumoniae Reveal Multiple Pathways of Resistance. Antimicrob Agents Chemother 2015, 59, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Cannatelli, A.; D’Andrea, M.M.; Giani, T.; Di Pilato, V.; Arena, F.; Ambretti, S.; Gaibani, P.; Rossolini, G.M. In Vivo Emergence of Colistin Resistance in Klebsiella Pneumoniae Producing KPC-Type Carbapenemases Mediated by Insertional Inactivation of the PhoQ/PhoP MgrB Regulator. Antimicrob Agents Chemother 2013, 57, 5521–5526. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Camacho, E.; Gomez-Gil, R.; Tobes, R.; Manrique, M.; Lorenzo, M.; Galvan, B.; Salvarelli, E.; Moatassim, Y.; Salanueva, I.J.; Pareja, E.; et al. Genomic Analysis of the Emergence and Evolution of Multidrug Resistance during a Klebsiella Pneumoniae Outbreak Including Carbapenem and Colistin Resistance. Journal of Antimicrobial Chemotherapy 2014, 69, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Jayol, A.; Bontron, S.; Villegas, M.-V.; Ozdamar, M.; Turkoglu, S.; Nordmann, P. The MgrB Gene as a Key Target for Acquired Resistance to Colistin in Klebsiella Pneumoniae. Journal of Antimicrobial Chemotherapy 2015, 70, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Lin, T.-L.; Lin, Y.-T.; Wang, J.-T. Amino Acid Substitutions of CrrB Responsible for Resistance to Colistin through CrrC in Klebsiella Pneumoniae. Antimicrob Agents Chemother 2016, 60, 3709–3716. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.; Hadjadj, L.; Rolain, J.-M.; Olaitan, A.O. Molecular Mechanisms of Polymyxin Resistance: Knowns and Unknowns. Int J Antimicrob Agents 2016, 48, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Trebosc, V.; Gartenmann, S.; Tötzl, M.; Lucchini, V.; Schellhorn, B.; Pieren, M.; Lociuro, S.; Gitzinger, M.; Tigges, M.; Bumann, D.; et al. Dissecting Colistin Resistance Mechanisms in Extensively Drug-Resistant Acinetobacter Baumannii Clinical Isolates. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, J.H.; Harper, M.; Harrison, P.; Hale, J.D.F.; Vinogradov, E.; Seemann, T.; Henry, R.; Crane, B.; St. Michael, F.; Cox, A.D.; et al. Colistin Resistance in Acinetobacter Baumannii Is Mediated by Complete Loss of Lipopolysaccharide Production. Antimicrob Agents Chemother 2010, 54, 4971–4977. [Google Scholar] [CrossRef]
- Carretero-Ledesma, M.; García-Quintanilla, M.; Martín-Peña, R.; Pulido, M.R.; Pachón, J.; McConnell, M.J. Phenotypic Changes Associated with Colistin Resistance Due to Lipopolysaccharide Loss in Acinetobacter Baumannii. Virulence 2018, 9, 930–942. [Google Scholar] [CrossRef]
- Boinett, C.J.; Cain, A.K.; Hawkey, J.; Do Hoang, N.T.; Khanh, N.N.T.; Thanh, D.P.; Dordel, J.; Campbell, J.I.; Lan, N.P.H.; Mayho, M.; et al. Clinical and Laboratory-Induced Colistin-Resistance Mechanisms in Acinetobacter Baumannii. Microb Genom 2019, 5. [Google Scholar] [CrossRef]
- Lean, S.-S.; Yeo, C.C.; Suhaili, Z.; Thong, K.-L. Comparative Genomics of Two ST 195 Carbapenem-Resistant Acinetobacter Baumannii with Different Susceptibility to Polymyxin Revealed Underlying Resistance Mechanism. Front Microbiol 2016, 6. [Google Scholar] [CrossRef]
- Moffatt, J.H.; Harper, M.; Adler, B.; Nation, R.L.; Li, J.; Boyce, J.D. Insertion Sequence IS Aba11 Is Involved in Colistin Resistance and Loss of Lipopolysaccharide in Acinetobacter Baumannii. Antimicrob Agents Chemother 2011, 55, 3022–3024. [Google Scholar] [CrossRef] [PubMed]
- Kamoshida, G.; Yamada, N.; Nakamura, T.; Yamaguchi, D.; Kai, D.; Yamashita, M.; Hayashi, C.; Kanda, N.; Sakaguchi, M.; Morimoto, H.; et al. Preferential Selection of Low-Frequency, Lipopolysaccharide-Modified, Colistin-Resistant Mutants with a Combination of Antimicrobials in Acinetobacter Baumannii. Microbiol Spectr 2022, 10. [Google Scholar] [CrossRef]
- Beceiro, A.; Moreno, A.; Fernández, N.; Vallejo, J.A.; Aranda, J.; Adler, B.; Harper, M.; Boyce, J.D.; Bou, G. Biological Cost of Different Mechanisms of Colistin Resistance and Their Impact on Virulence in Acinetobacter Baumannii. Antimicrob Agents Chemother 2014, 58, 518–526. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect Dis 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, N.; Nordmann, P.; Poirel, L. Moraxella Species as Potential Sources of MCR-Like Polymyxin Resistance Determinants. Antimicrob Agents Chemother 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Xavier, B.B.; Lammens, C.; Ruhal, R.; Kumar-Singh, S.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Identification of a Novel Plasmid-Mediated Colistin-Resistance Gene, Mcr-2, in Escherichia Coli, Belgium, June 2016. Euro Surveill 2016, 21. [Google Scholar] [CrossRef]
- Yin, W.; Li, H.; Shen, Y.; Liu, Z.; Wang, S.; Shen, Z.; Zhang, R.; Walsh, T.R.; Shen, J.; Wang, Y. Novel Plasmid-Mediated Colistin Resistance Gene Mcr-3 in Escherichia Coli. mBio 2017, 8. [Google Scholar] [CrossRef]
- Carattoli, A.; Villa, L.; Feudi, C.; Curcio, L.; Orsini, S.; Luppi, A.; Pezzotti, G.; Magistrali, C.F. Novel Plasmid-Mediated Colistin Resistance Mcr-4 Gene in Salmonella and Escherichia Coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef]
- Borowiak, M.; Fischer, J.; Hammerl, J.A.; Hendriksen, R.S.; Szabo, I.; Malorny, B. Identification of a Novel Transposon-Associated Phosphoethanolamine Transferase Gene, Mcr-5, Conferring Colistin Resistance in d-Tartrate Fermenting Salmonella Enterica Subsp. Enterica Serovar Paratyphi B. Journal of Antimicrobial Chemotherapy 2017, 72, 3317–3324. [Google Scholar] [CrossRef] [PubMed]
- Zurfluh, K.; Tasara, T.; Poirel, L.; Nordmann, P.; Stephan, R. Draft Genome Sequence of Escherichia Coli S51, a Chicken Isolate Harboring a Chromosomally Encoded Mcr-1 Gene. Genome Announc 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Di Pilato, V.; Doi, Y.; Feldgarden, M.; Haft, D.H.; Klimke, W.; Kumar-Singh, S.; Liu, J.-H.; Malhotra-Kumar, S.; Prasad, A.; et al. Proposal for Assignment of Allele Numbers for Mobile Colistin Resistance (Mcr) Genes. Journal of Antimicrobial Chemotherapy 2018, 73, 2625–2630. [Google Scholar] [CrossRef] [PubMed]
- Nitz, F.; de Melo, B.O.; da Silva, L.C.N.; de Souza Monteiro, A.; Marques, S.G.; Monteiro-Neto, V.; de Jesus Gomes Turri, R.; Junior, A.D.S.; Conceição, P.C.R.; Magalhães, H.J.C.; et al. Molecular Detection of Drug-Resistance Genes of BlaOXA-23-BlaOXA-51 and Mcr-1 in Clinical Isolates of Pseudomonas Aeruginosa. Microorganisms 2021, 9, 786. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Baky, R.M.; Masoud, S.M.; Mohamed, D.S.; Waly, N.G.; Shafik, E.A.; Mohareb, D.A.; Elkady, A.; Elbadr, M.M.; Hetta, H.F. <p>Prevalence and Some Possible Mechanisms of Colistin Resistance Among Multidrug-Resistant and Extensively Drug-Resistant <em>Pseudomonas Aeruginosa</Em></P>. Infect Drug Resist 2020, Volume 13, 323–332. [Google Scholar] [CrossRef]
- Shabban, M.; Fahim, N.A.E.; Montasser, K.; El Magd, N.M.A. Resistance to Colistin Mediated by Mcr-1 among Multidrug Resistant Gram Negative Pathogens at a Tertiary Care Hospital, Egypt. J Pure Appl Microbiol 2020, 14, 1125–1132. [Google Scholar] [CrossRef]
- Hameed, F.; Khan, M.A.; Muhammad, H.; Sarwar, T.; Bilal, H.; Rehman, T.U. Plasmid-Mediated Mcr-1 Gene in Acinetobacter Baumannii and Pseudomonas Aeruginosa: First Report from Pakistan. Rev Soc Bras Med Trop 2019, 52. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.; Maboni, G.; Battisti, R.; da Costa, L.; Selva, H.L.; Levitzki, E.D.; Gressler, L.T. High Rates of Multidrug Resistance in Bacteria Associated with Small Animal Otitis: A Study of Cumulative Microbiological Culture and Antimicrobial Susceptibility. Microb Pathog 2022, 165, 105399. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.S.; Elshafiee, E.A.; Khalefa, H.S.; Kadry, M.; Hamza, D.A. Evidence of Colistin Resistance Genes (Mcr-1 and Mcr-2) in Wild Birds and Its Public Health Implication in Egypt. Antimicrob Resist Infect Control 2019, 8, 197. [Google Scholar] [CrossRef]
- Tartor, Y.H.; Gharieb, R.M.A.; Abd El-Aziz, N.K.; El Damaty, H.M.; Enany, S.; Khalifa, E.; Attia, A.S.A.; Abdellatif, S.S.; Ramadan, H. Virulence Determinants and Plasmid-Mediated Colistin Resistance Mcr Genes in Gram-Negative Bacteria Isolated From Bovine Milk. Front Cell Infect Microbiol 2021, 11. [Google Scholar] [CrossRef]
- Javed, H.; Saleem, S.; Zafar, A.; Ghafoor, A.; Shahzad, A. Bin; Ejaz, H.; Junaid, K.; Jahan, S. Emergence of Plasmid-Mediated Mcr Genes from Gram-Negative Bacteria at the Human-Animal Interface. Gut Pathog 2020, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, H.; Yamazoe, A.; Hosoyama, A.; Suenaga, H.; Kimura, N.; Hirose, J.; Watanabe, T.; Futagami, T.; Goto, M.; Furukawa, K. Draft Genome Sequence of Pseudomonas Aeruginosa KF702 (NBRC 110665), a Polychlorinated Biphenyl-Degrading Bacterium Isolated from Biphenyl-Contaminated Soil. Genome Announc 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Snesrud, E.; Maybank, R.; Kwak, Y.I.; Jones, A.R.; Hinkle, M.K.; McGann, P. Chromosomally Encoded Mcr-5 in Colistin-Nonsusceptible Pseudomonas Aeruginosa. Antimicrob Agents Chemother 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Martins-Sorenson, N.; Snesrud, E.; Xavier, D.E.; Cacci, L.C.; Iavarone, A.T.; McGann, P.; Riley, L.W.; Moreira, B.M. A Novel Plasmid-Encoded Mcr-4.3 Gene in a Colistin-Resistant Acinetobacter Baumannii Clinical Strain. Journal of Antimicrobial Chemotherapy 2020, 75, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Li, C.; Duan, R.; Qin, S.; Liang, J.; Xiao, M.; Lv, D.; Jing, H.; Wang, X. Retrospective Screening and Analysis of Mcr-1 and BlaNDM in Gram-Negative Bacteria in China, 2010–2019. Front Microbiol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Kalová, A.; Gelbíčová, T.; Overballe-Petersen, S.; Litrup, E.; Karpíšková, R. Characterisation of Colistin -Resistant Enterobacterales and Acinetobacter Strains Carrying Mcr Genes from Asian Aquaculture Products. Antibiotics 2021, 10, 838. [Google Scholar] [CrossRef] [PubMed]
- Bitar, I.; Medvecky, M.; Gelbicova, T.; Jakubu, V.; Hrabak, J.; Zemlickova, H.; Karpiskova, R.; Dolejska, M. Complete Nucleotide Sequences of Mcr-4.3 -Carrying Plasmids in Acinetobacter Baumannii Sequence Type 345 of Human and Food Origin from the Czech Republic, the First Case in Europe. Antimicrob Agents Chemother 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Al-Kadmy, I.M.S.; Ibrahim, S.A.; Al-Saryi, N.; Aziz, S.N.; Besinis, A.; Hetta, H.F. Prevalence of Genes Involved in Colistin Resistance in Acinetobacter Baumannii: First Report from Iraq. Microbial Drug Resistance 2020, 26, 616–622. [Google Scholar] [CrossRef]
- Srinivasan, V.B.; Rajamohan, G. KpnEF, a New Member of the Klebsiella Pneumoniae Cell Envelope Stress Response Regulon, Is an SMR-Type Efflux Pump Involved in Broad-Spectrum Antimicrobial Resistance. Antimicrob Agents Chemother 2013, 57, 4449–4462. [Google Scholar] [CrossRef]
- Warner, D.M.; Levy, S.B. Different Effects of Transcriptional Regulators MarA, SoxS and Rob on Susceptibility of Escherichia Coli to Cationic Antimicrobial Peptides (CAMPs): Rob-Dependent CAMP Induction of the MarRAB Operon. Microbiology (N Y) 2010, 156, 570–578. [Google Scholar] [CrossRef]
- Koutsolioutsou, A.; Peña-Llopis, S.; Demple, B. Constitutive SoxR Mutations Contribute to Multiple-Antibiotic Resistance in Clinical Escherichia Coli Isolates. Antimicrob Agents Chemother 2005, 49, 2746–2752. [Google Scholar] [CrossRef] [PubMed]
- Parra-Lopez, C.; Baer, M.T.; Groisman, E.A. Molecular Genetic Analysis of a Locus Required for Resistance to Antimicrobial Peptides in Salmonella Typhimurium. EMBO J 1993, 12, 4053–4062. [Google Scholar] [CrossRef]
- Lin, M.-F.; Lin, Y.-Y.; Lan, C.-Y. Contribution of EmrAB Efflux Pumps to Colistin Resistance in Acinetobacter Baumannii. Journal of Microbiology 2017, 55, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.; Lau, C.H.-F.; Gilmour, C.; Hao, Y.; Lam, J.S. Polymyxin Susceptibility in Pseudomonas Aeruginosa Linked to the MexXY-OprM Multidrug Efflux System. Antimicrob Agents Chemother 2015, 59, 7276–7289. [Google Scholar] [CrossRef]
- Goli, H.R.; Nahaei, M.R.; Ahangarzadeh Rezaee, M.; Hasani, A.; Samadi Kafil, H.; Aghazadeh, M. Emergence of Colistin Resistant Pseudomonas Aeruginosa at Tabriz Hospitals, Iran. Iran J Microbiol 2016, 8, 62–69. [Google Scholar] [PubMed]
- Ni, W.; Li, Y.; Guan, J.; Zhao, J.; Cui, J.; Wang, R.; Liu, Y. Effects of Efflux Pump Inhibitors on Colistin Resistance in Multidrug-Resistant Gram-Negative Bacteria. Antimicrob Agents Chemother 2016, 60, 3215–3218. [Google Scholar] [CrossRef] [PubMed]
- Bergen, P.J.; Li, J.; Nation, R.L. Dosing of Colistin—Back to Basic PK/PD. Curr Opin Pharmacol 2011, 11, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Dudhani, R. V.; Turnidge, J.D.; Coulthard, K.; Milne, R.W.; Rayner, C.R.; Li, J.; Nation, R.L. Elucidation of the Pharmacokinetic/Pharmacodynamic Determinant of Colistin Activity against Pseudomonas Aeruginosa in Murine Thigh and Lung Infection Models. Antimicrob Agents Chemother 2010, 54, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Dudhani, R. V.; Turnidge, J.D.; Nation, R.L.; Li, J. FAUC/MIC Is the Most Predictive Pharmacokinetic/Pharmacodynamic Index of Colistin against Acinetobacter Baumannii in Murine Thigh and Lung Infection Models. Journal of Antimicrobial Chemotherapy 2010, 65, 1984–1990. [Google Scholar] [CrossRef]
- Cheah, S.-E.; Wang, J.; Nguyen, V.T.T.; Turnidge, J.D.; Li, J.; Nation, R.L. New Pharmacokinetic/Pharmacodynamic Studies of Systemically Administered Colistin against Pseudomonas Aeruginosa and Acinetobacter Baumannii in Mouse Thigh and Lung Infection Models: Smaller Response in Lung Infection. Journal of Antimicrobial Chemotherapy 2015, dkv267. [Google Scholar] [CrossRef]
- Tsala, M.; Vourli, S.; Georgiou, P.-C.; Pournaras, S.; Tsakris, A.; Daikos, G.L.; Mouton, J.W.; Meletiadis, J. Exploring Colistin Pharmacodynamics against Klebsiella Pneumoniae: A Need to Revise Current Susceptibility Breakpoints. Journal of Antimicrobial Chemotherapy 2018, 73, 953–961. [Google Scholar] [CrossRef]
- Gautam, V.; Shafiq, N.; Mouton, J.; Malhotra, S.; Kaur, S.; Ray, P. Pharmacokinetics of Colistin in Patients with Multidrug-Resistant Gram-Negative Infections: A Pilot Study. Indian Journal of Medical Research 2018, 147, 407. [Google Scholar] [CrossRef] [PubMed]
- Sorlí, L.; Luque, S.; Li, J.; Campillo, N.; Danés, M.; Montero, M.; Segura, C.; Grau, S.; Horcajada, J.P. Colistin for the Treatment of Urinary Tract Infections Caused by Extremely Drug-Resistant Pseudomonas Aeruginosa: Dose Is Critical. Journal of Infection 2019, 79, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Imberti, R.; Cusato, M.; Villani, P.; Carnevale, L.; Iotti, G.A.; Langer, M.; Regazzi, M. Steady-State Pharmacokinetics and BAL Concentration of Colistin in Critically Ill Patients After IV Colistin Methanesulfonate Administration. Chest 2010, 138, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Markantonis, S.L.; Markou, N.; Fousteri, M.; Sakellaridis, N.; Karatzas, S.; Alamanos, I.; Dimopoulou, E.; Baltopoulos, G. Penetration of Colistin into Cerebrospinal Fluid. Antimicrob Agents Chemother 2009, 53, 4907–4910. [Google Scholar] [CrossRef] [PubMed]
- Ozcimen, M.; Ozcimen, S.; Sakarya, Y.; Sakarya, R.; Goktas, S.; Alpfidan, I.; Erdogan, E. Ocular Penetration of Intravenously Administered Colistin in Rabbit Uveitis Model. Journal of Ocular Pharmacology and Therapeutics 2014, 30, 681–685. [Google Scholar] [CrossRef]
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The Re-Emerging Antibiotic for Multidrug-Resistant Gram-Negative Bacterial Infections. Lancet Infect Dis 2006, 6, 589–601. [Google Scholar] [CrossRef]
| Site of infection |
Study design | Number of patients | Setting | Dose | Absolute tissue concentrations | Absolute plasmatic concentrations | Penetration rate (AUCtissue/AUCplasma) |
PK/PD target attainment | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Lung | Prospective observational | 13 | ICU VAP |
2 MU q8h IV | Undetectable | Cmin 1.03±0.69 mg/L AUC/MIC ratio 17.3±9.3 (for MIC=2 mg/L) |
0.00 | Suboptimal in ELF | [126] |
| CSF | Prospective observational | 5 | ICU | 2-3 MU q8h IV | Cmin 0.47 mg/L AUC 0.53 mg*h/L |
Cmin 9.26 mg/L AUC 10.4 mg*h/L |
0.05 | Optimal PK/PD target attainment only for P. aeruginosa and A. baumannii strains exhibiting MIC values up to 0.06 mg/L | [127] |
| Ocular | Preclinical rabbit uveitis model | 20 | Uveitis induced after endotoxin injection | 5 mg/kg IV |
Aqueous humor 0.62±0.07 (at 0.5h) 0.45±0.05 (at 3h) 0.38±0.08 (at 6h) Vitreous humor 0.02±0.01 (at 3h) |
9.84±2.0 (at 0.5h) 0.93±0.07 (at 3h) 0.24±0.08 (at 6h) |
0.07 (aqueous humor at 0.5h) 0.48 (aqueous humor at 3h) 1.58 (aqueous humor at 6h) 0.02 (vitreous humor at 3h) |
Not assessable | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





