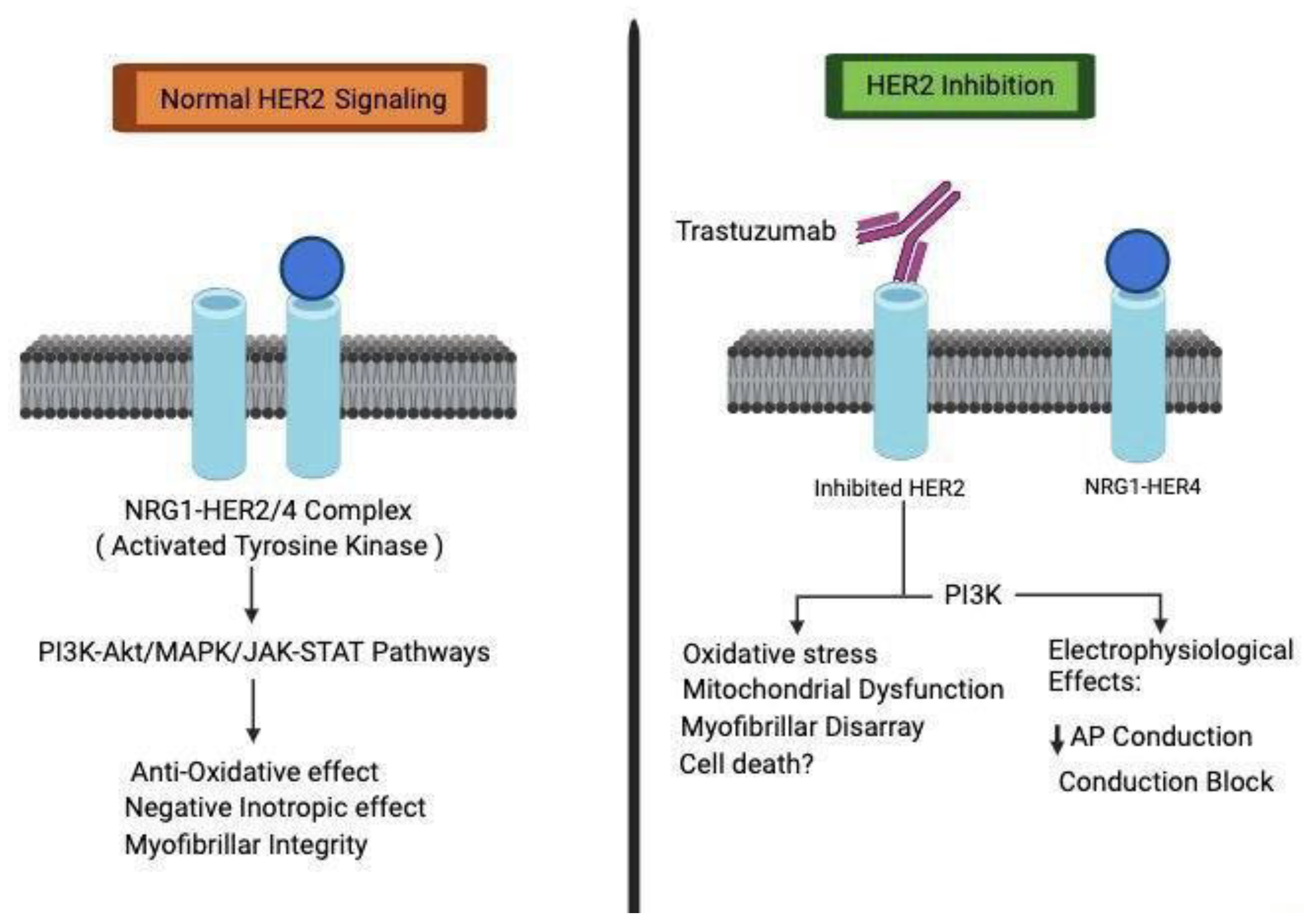
1. Introduction and Background
Transtuzumab was first discovered at Genentech, Inc. in San Francisco, California is a monoclonal antibody targeting HER2 and upregulates cell cycle inhibitors like P21 and P27. Trastuzumab, a humanized monoclonal antibody targeting HER2, is approved for treating HER2-positive breast cancers and gastric cancer [
1]. While HER2 overexpression in breast cancer is associated with aggressiveness, Trastuzumab's mechanisms of action are multifaceted, including antibody-dependent cell-mediated cytotoxicity, inhibition of cleavage, and interference with oncogenic signaling. Trastuzumab's effects on HER2 phosphorylation at Y1248 enhance its interaction with a negative regulator, Csk-homologous kinase, inhibiting breast cancer cell growth. Clinical evidence supports the importance of HER2 phosphorylation at Y1248 in predicting Trastuzumab's efficacy in neoadjuvant treatment [
2]. This evolving understanding of Trastuzumab's mechanisms underscores the complexities of HER2-targeted therapies. The central role of HER2 signaling in cardiac development is firmly established as shown in
Figure 1, evidenced by studies in conditional mutant mice with cardiac-specific HER2 deletion that exhibit various hallmarks of dilated cardiomyopathy. Our recent investigations have strengthened the direct association between impaired HER2 signaling and the onset of cardiotoxicity induced by trastuzumab. Trastuzumab disrupts HER signaling by phosphorylating specific sites, 845 and 1248, on HER1 and HER2, respectively.
This initiates the Erk/mTOR/Ulk 1 signaling cascade, hindering autophagy and resulting in the accumulation of reactive oxygen species within cardiomyocytes, contributing to cardiotoxicity. Notably, our research underscores that pertuzumab, an alternative anti-HER2 monoclonal antibody with a distinct epitope, does not impact HER2 signaling. This finding is consistent in both in vitro cardiomyocyte studies and is corroborated by clinical investigations [
3,
4,
5]. In contrast to trastuzumab, pertuzumab does not interfere with Akt activity, a pathway downregulated by trastuzumab in HER2-positive breast cancer cells. The differential regulation of HER2 signaling pathways in human cardiomyocytes and tumor cells following trastuzumab binding holds promise for unraveling the intricate mechanisms responsible for trastuzumab-associated cardiotoxicity [
5]. It is preferably used for cancers that are HER2 receptor-positive breast and gastrointestinal malignancies. It can be used alone or with some other chemotherapy medication. The route of administration is either slow intravenous or subcutaneous with common side effects of fever, headaches, rash, and severe side effects including heart failure or lung disease. Breast cancer is a heterogeneous disease with distinct behavior and one of the leading causes of cancer-related death in the United States amongst females [
6]. Though metastatic breast cancer is unlikely to be cured improvements in survival along with the addition of new systemic therapies. Respective options for treatment are if the tumor is hormone receptor-positive or HER2 is overexpressed. Even though Trastuzumab considerably improves the prognosis of HER2-positive breast cancer, its cardiotoxicity commonly results in inescapable treatment Interference [
7,
8].
Early-phase I/II trials with trastuzumab, alone or with cisplatin, showed good tolerance. Yet, a pivotal phase III trial combining trastuzumab with anthracyclines revealed cardiac dysfunction in 27% of HER2-positive breast cancer patients, contrasting with <7% in the anthracycline-only group. Retrospective analyses suggested a 1 in 4 risk of left ventricular dysfunction post-trastuzumab in the adjuvant setting. Large studies affirmed heightened cardiotoxicity with trastuzumab. Subsequent trials adapted, adopting sequential trastuzumab-chemotherapy, rigorous ejection fraction monitoring, and excluding cardiovascularly compromised patients. Cardioprotective agents showed limited benefits initially, but recent data hint at reduced cardiotoxic events. Despite adjustments, trastuzumab regimens remain a significant cardiotoxicity concern [
9,
10].
The combination of trastuzumab and anthracycline-based chemotherapy, such as doxorubicin, is a standard approach for breast cancer treatment at various stages. However, the precise mechanisms underlying cardiotoxicity from this combination therapy remain poorly understood. Both doxorubicin and trastuzumab, whether administered individually or together, have been associated with severe cardiotoxic effects [
11]. Doxorubicin's primary target for cardiotoxicity is DNA topoisomerase IIB (TOP2B), resulting in double strand DNA breaks, DNA damage response activation, and programmed cell death in cardiomyocytes. It is hypothesized that in the absence of trastuzumab, anthracycline-induced damage to TOP2B might be repaired through unidentified mechanisms associated with normal HER2 function in cardiomyocytes. However, the presence of trastuzumab, as an anti-HER2 agent, could compromise HER2 function, potentially leading to significant cardiomyocyte damage. Although this "repair-interfering model" is plausible, it lacks substantial experimental evidence supporting its role in trastuzumab/anthracycline combination therapy-induced cytotoxicity [
12,
13].
2. Review
Unmasking the Potential for Cardiotoxicity with Immune Checkpoint Inhibitors and Trastuzumab. Immune checkpoint proteins, such as CTLA-4 and PD-1, exert critical roles in T cell regulation by interacting with CD80/CD86 and PD-L1. These interactions suppress anti-tumor T cell responses, aiding tumor cells in evading immune-mediated destruction. Notably, various monoclonal antibodies targeting immune checkpoints, like anti-CTLA-4 (ipilimumab and tremelimumab), anti-PD-1 (nivolumab and pembrolizumab), and anti-PD-L1 (atezolizumab, avelumab, and durvalumab), have received FDA approval in recent years. While these immune checkpoint inhibitors have transformed the management of advanced malignancies, their activation of the immune system also brings forth a spectrum of adverse events, including myocarditis, cardiomyopathy, myocardial fibrosis, and heart failure, the mechanisms of which are yet to be fully elucidated [
14]. In a study called PANACEA, they looked at using immune checkpoint antibodies alone and with trastuzumab and anthracycline-based treatments. For those getting trastuzumab and pembrolizumab, they didn't find heart issues initially, but 19% had immune-related side effects. More research from this study and others is really important to know how safe it is to use trastuzumab with immune checkpoint inhibitors for the heart [
15].
Now, more and more cancer patients are getting treatments that target HER2 and use immune checkpoint drugs. This raises the chance of heart problems. We urgently need to figure out why these drugs might harm the heart and if using them together makes this risk higher. It's crucial to identify specific things about a person's immune system that might make them more likely to have heart issues because of these treatments [
16,
17]. The development of time-sensitive and precise bioassays and innovative non-invasive detection methods is an immediate necessity to facilitate the early identification of cardiotoxicity. These tools will support robust post-marketing surveillance programs for these drugs and ultimately enhance the quality of life for cancer patients [
17]. Data from adjuvant trastuzumab trials show that asymptomatic LVEF declines occur in 4.1% to 30.1% of patients, while symptomatic CHF rates are lower at 0.6% to 3.8%. In the HERA trial, 2-year and 1-year trastuzumab arms had higher CHF (0.8% and 0.8%) and LVEF decline (7.2% and 4.1%) compared to observation. Long-term follow-up (7-9 years) didn't reveal delayed cardiotoxicity. In NSABP B-31, 2.6% with trastuzumab and 0.9% in the control group developed CHF, associated with age ≥60, baseline LVEF 50-55%, and antihypertensive use. LVEF decline was noted in 15.4%, 31.1%, and 27.1% in different treatment groups. APT trial (trastuzumab and paclitaxel) had lower CHF (0.5%) and LVEF decline (3.2%). BCIRG 006 revealed higher CHF in AC-DH (2.0%) than AC-D (0.7%) or TCH (0.4%). A meta-analysis confirmed an increased risk of grade 3 and 4 CHF with trastuzumab but low absolute rates. Most LVEF declines resolve after therapy cessation [
18].
The necessity for evidence-based guidelines in cardio-oncology arises due to significant knowledge gaps in cardiotoxicity screening. It is imperative to identify a subset of patients at high risk for cardiotoxicity, although current risk factors such as age, baseline LVEF, hypertension, and anthracycline exposure require validation. Randomized trials and unbiased registries are crucial for optimizing monitoring schedules and validating intervention effectiveness. For patients on anthracycline-free trastuzumab regimens without cardiotoxicity risks, pivotal randomized trials comparing serial monitoring to reduced testing are needed. Ongoing studies like SAFE-HEART explore the safety of prolonged anti-HER2 therapy in those with asymptomatic LVEF decline. The lack of well-designed studies hinders precise screening and intervention guidelines, emphasizing the need for collaborative efforts among medical organizations. Before establishing quality indicators, informative studies are essential, underscoring the importance of a robust research foundation in guiding oncology and medical practices [
19,
20].
The clinical utility of routine serial LVEF monitoring in early-stage breast cancer patients receiving trastuzumab-based therapy is under discussion. The association between asymptomatic LVEF decline and heart failure in this patient population is not entirely clear, with nearly a decade of follow-up data failing to establish a strong predictive link. Current evidence lacks well-designed trials demonstrating that early intervention in asymptomatic patients can prevent the development of symptomatic heart failure [
21]. Routine LVEF monitoring has limitations, including error rates, and false-positive findings can lead to unnecessary diagnostic efforts. This may result in patients being incorrectly identified as having cardiotoxicity, potentially compromising their curative treatment. The rates of both symptomatic and asymptomatic LVEF decline are remarkably low in such cases. The correlation between low baseline LVEF and adverse cardiac outcomes suggests that tailored monitoring may be more appropriate. In conclusion, the clinical utility of routine serial LVEF monitoring for all early-stage breast cancer patients on trastuzumab-based therapy is uncertain, especially in the absence of anthracycline treatment. Individualized risk assessment and monitoring strategies may be more suitable to optimize care, minimize harm, and control costs [
21].
2.1. Clinical Utility of HER2 Testing
HER2 status is one of the most important and anticipating factors in breast cancer and is recommended for routine testing on newly diagnosed and metastatic breast cancers. Because HER2 status is a predictive factor in breast cancer, we recommend routine testing of HER2 expression on newly diagnosed, invasive, and metastatic breast cancers, following several guideline bodies. High levels of HER2 expression identify those women who benefit from treatment with agents that target HER2. HER2 overexpression was associated with high rates of disease recurrence and death in the absence of adjuvant systemic therapy. However, the value of this prognostic information in clinical practice is questionable, particularly with the earlier use of HER2-directed agents in the neoadjuvant and adjuvant setting. For women with hormone receptor-positive breast cancer, HER2 gene expression is an important component of the 21-gene recurrence score assay [
22].
2.2. Recent Cardiotoxicity Surveillance
Current recommendations for monitoring cardiotoxicity during breast cancer treatment advise assessing left ventricular ejection fraction (LVEF) at baseline and throughout therapy, although the supportive data are limited. In a case-control study of 53 HER2-positive breast cancer patients with cardiotoxicity and 159 controls, it was revealed that any reduction in LVEF to less than 55% at any surveillance point during treatment was associated with an increased risk of heart failure. Early identification of diminished LVEF during routine surveillance may enable the implementation of preventative heart failure strategies [
23,
24,
25].
New evidence shows that checking global longitudinal strain (GLS) helps find early heart problems during cancer treatment. The SUCCOUR trial studied if using GLS to guide early heart protection could stop decreases in left ventricular ejection fraction (LVEF) for patients on heart-harming chemotherapy, compared to the usual LVEF-based approach. While the trial didn't find a big difference in the main result (LVEF change from start to one year between the groups), the GLS-guided approach had fewer cases of heart problems over time (5.8% vs. 13.7%, p=0.02) [
25]. More research is needed to know if using GLS in this way helps in the long run for cancer or heart health.
Cardiotoxicity resulting from anthracycline chemotherapy is a significant and potentially life-threatening issue, diminishing the quality of life and limiting the effectiveness of this treatment. Randomized controlled trials (RCTs) were identified for various interventions, including N-acetylcysteine, phenethylamines, coenzyme Q10, the combination of vitamin E, vitamin C, and N-acetylcysteine, L-carnitine, carvedilol, amifostine, and dexrazoxane. However, for other potential cardioprotective measures, no RCTs were found, and non-randomized studies and case reports, which carry a high risk of bias, were excluded [
26,
27]. Pooling results for several interventions was not feasible due to the limited number of RCTs, noncomparable heart failure definitions, and methodological limitations. None of the included RCTs demonstrated a statistically significant difference in the incidence of heart failure, which may be attributed to small sample sizes and varying anthracycline dosages. The effectiveness of these cardioprotective interventions in preventing anthracycline-induced heart damage remains inconclusive. A more robust and comprehensive investigation is needed to address this critical issue, especially for dexrazoxane, which showed promise in multiple RCTs involving adult breast cancer patients, while subgroup analyses for different patient populations were challenging [
28,
29].
3. Conclusions
Trastuzumab-induced cardiomyopathy poses a significant challenge in HER2-positive cancer therapy. Despite its efficacy, it often leads to treatment discontinuation due to left ventricular systolic dysfunction. This review explores the multifaceted aspects of Trastuzumab-induced cardiomyopathy, from its intricate mechanisms to diagnostic strategies and potential cardioprotective interventions.
Understanding Trastuzumab's complex mechanisms, including HER2 phosphorylation and signaling, highlights the need to balance effective cancer treatment with cardiotoxicity prevention. The use of Trastuzumab alongside anthracycline-based chemotherapy further complicates the landscape, necessitating additional research on the "repair-interfering model." The introduction of immune checkpoint inhibitors adds another layer of complexity, emphasizing the importance of rigorous cardiac risk assessment and monitoring as their use in conjunction with Trastuzumab becomes more common.
Innovative cardiotoxicity surveillance methods, like global longitudinal strain (GLS), offer hope for early intervention, especially with the growing patient population exposed to Trastuzumab and immune checkpoint inhibitors. Collaboration among cardiologists, oncologists, and researchers is vital to optimize patient outcomes in the Trastuzumab therapy era. Evidence-based guidelines and personalized monitoring are essential to strike the right balance between effective cancer treatment and cardiovascular well-being. Addressing Trastuzumab-induced cardiomyopathy complexities can enhance the quality of life for cancer patients and improve treatment safety and efficacy.
Conflicts of interest
In compliance with the ICMJE uniform disclosure form, all authors declare the following:.
Payment/services info
All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships
All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships
All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
- Barish R, Gates E, Barac A: Trastuzumab-Induced Cardiomyopathy. Cardiol Clin. 2019, 37:407-418. [CrossRef]
- Ye T, Yang W, Gao T, et al.: Trastuzumab-induced cardiomyopathy via ferroptosis-mediated mitochondrial dysfunction. Free Radic Biol Med. 2023, 206:143-161. [CrossRef]
- Nowsheen S, Viscuse PV, O'Sullivan CC, et al.: Incidence, Diagnosis, and Treatment of Cardiac Toxicity from Trastuzumab in Patients with Breast Cancer. Curr Breast Cancer Rep. 2017, 9:173-182. [CrossRef]
- Dempsey N, Rosenthal A, Dabas N, Kropotova Y, Lippman M, Bishopric NH: Trastuzumab-induced cardiotoxicity: a review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2- directed therapies. Breast Cancer Res Treat. 2021. [CrossRef]
- 188:21-36. [CrossRef]
- Conte P, Frassoldati A, Bisagni G, et al.: Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: final results of the phase III randomized Short-HER studydouble dagger. Ann Oncol. 2018, 29:2328-33. [CrossRef]
- Baselga J, Carbonell X, Castañeda-Soto NJ, et al.: Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005, 1:2162-71. [CrossRef]
- Slamon DJ, Leyland-Jones B, Shak S, et al.: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001, 15:783-92. [CrossRef]
- Romond EH, Perez EA, Bryant J, et al.: Trastuzumab plus adjuvant chemotherapy for operable HER2- positive breast cancer. N Engl J Med. 2005, 20:1673-84. [CrossRef]
- Baselga J, Perez EA, Pienkowski T, Bell R: Adjuvant trastuzumab: a milestone in the treatment of HER-2- positive early breast cancer. Oncologist. 200611, 1:4-12. [CrossRef]
- Cobleigh MA, Vogel CL, Tripathy D, et al.: Multinational study of the efficacy and safety of humanized antiHER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999, 17:2639-48. [CrossRef]
- Copeland-Halperin RS, Liu JE, Yu AF: Cardiotoxicity of HER2-targeted therapies . Curr Opin Cardiol. 2019, 34:451-458. [CrossRef]
- Dang C, Guo H, Najita J, et al.: Groarke J, Moslehi J, Krop I, Burstein HJ, Hudis C, Winer EP, Tolaney SM. Cardiac Outcomes of Patients Receiving Adjuvant Weekly Paclitaxel and Trastuzumab for Node-Negative, ERBB2-Positive Breast Cancer. JAMA Oncol. 2016, 2:29-36. [CrossRef]
- Gabani M, Castañeda D, Nguyen QM, et al.: Association of Cardiotoxicity With Doxorubicin and Trastuzumab: A Double-Edged Sword in Chemotherapy. Cureus. 2021, 22:18194. [CrossRef]
- Shakir DK, Rasul KI: Chemotherapy induced cardiomyopathy: pathogenesis, monitoring and management. J Clin Med Res. 2009, 1:8-12. Epub 2009 Mar 24. [CrossRef]
- Von Hoff DD, Rozencweig M, Layard M, Slavik M, Muggia FM: Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am J Med. 1977, 62:200-8. [CrossRef]
- DJ Slamon, B Leyland-Jones, S Shak , etal: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 344, 783:792. [CrossRef]
- Lee L, Cheung WY, Atkinson E, Krzyzanowska MK: Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol. 2011, 1:106-17. [CrossRef]
- Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM: Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies are clinicians responding optimally?. J Am Coll Cardiol. 2010, 9:1644- 50. [CrossRef]
- Mata Caballero R, Serrano Antolín JM, Jiménez Hernández RM, et al.: Incidence of long-term cardiotoxicity and evolution of the systolic function in patients with breast cancer treated with anthracyclines. Cardiol J. 2022:228-234. [CrossRef]
- van Dalen EC, Caron HN, Dickinson HO, Kremer LC: Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011, 15:003917. [CrossRef]
- Asselin BL, Devidas M, Chen L, et al.: Cardioprotection and Safety of Dexrazoxane in Patients Treated for Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Advanced-Stage Lymphoblastic Non-Hodgkin Lymphoma: A Report of the Children's Oncology Group Randomized Trial. Pediatric Oncology Group 9404. J Clin Oncol. 2016, 10:854-62. Epub 2015 Dec 23. Erratum in: J. [CrossRef]
- Akpek M, Ozdogru I, Sahin O, et al.: Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur J Heart Fail. 2015, 17:81-9. [CrossRef]
- Lenihan DJ, Oliva S, Chow EJ, Cardinale D: Cardiac toxicity in cancer survivors . Cancer. 20131119, 11:2131- 42. [CrossRef]
- Philip LJ, Findlay SG, Gill JH: Baseline blood pressure and development of cardiotoxicity in patients treated with anthracyclines: A systematic review. Int J Cardiol Cardiovasc Risk Prev. 2022, 13:200153. [CrossRef]
- McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM: Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc Drugs Ther. 2017, 31:63-75. [CrossRef]
- Dempsey, N., Rosenthal, et al.: Trastuzumab-induced cardiotoxicity: a review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies. 188:21-36. [CrossRef]
- Herrmann, J: Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia . Nat Rev Cardiol. 17:474-502. [CrossRef]
- Li MY, Peng LM, Chen XP: Pharmacogenomics in drug-induced cardiotoxicity: Current status and the future. Front Cardiovasc Med. 2022, 13:966261. [CrossRef]
- Sardesai S, Sukumar J, Kassem M, et al.: Clinical impact of interruption in adjuvant Trastuzumab therapy in patients with operable HER-2 positive breast cancer. Cardiooncology. 2020, 5:26-10. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).