Submitted:
30 May 2024
Posted:
31 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
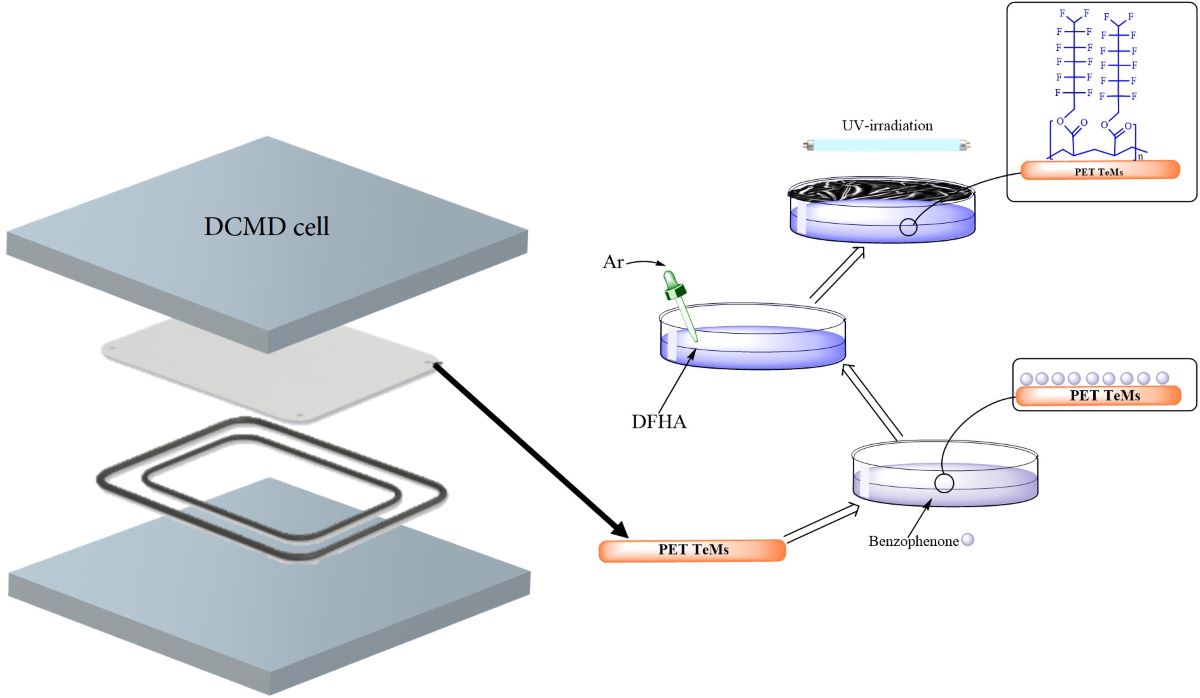
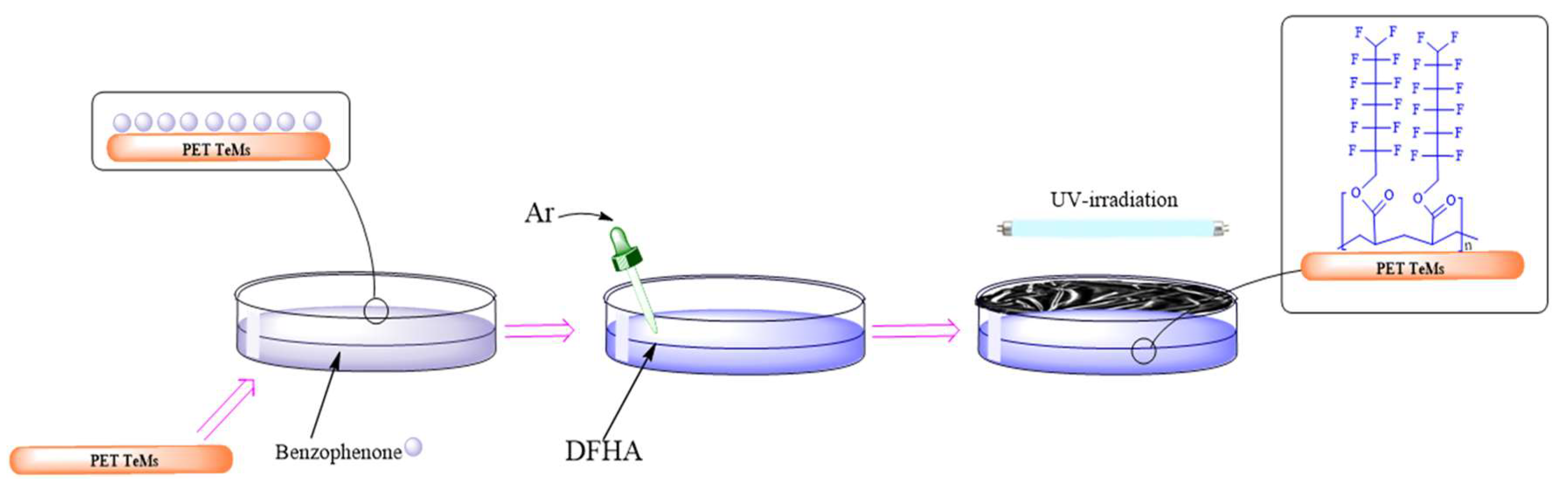
2.2. Method of Producing and Modification of PET TeMs
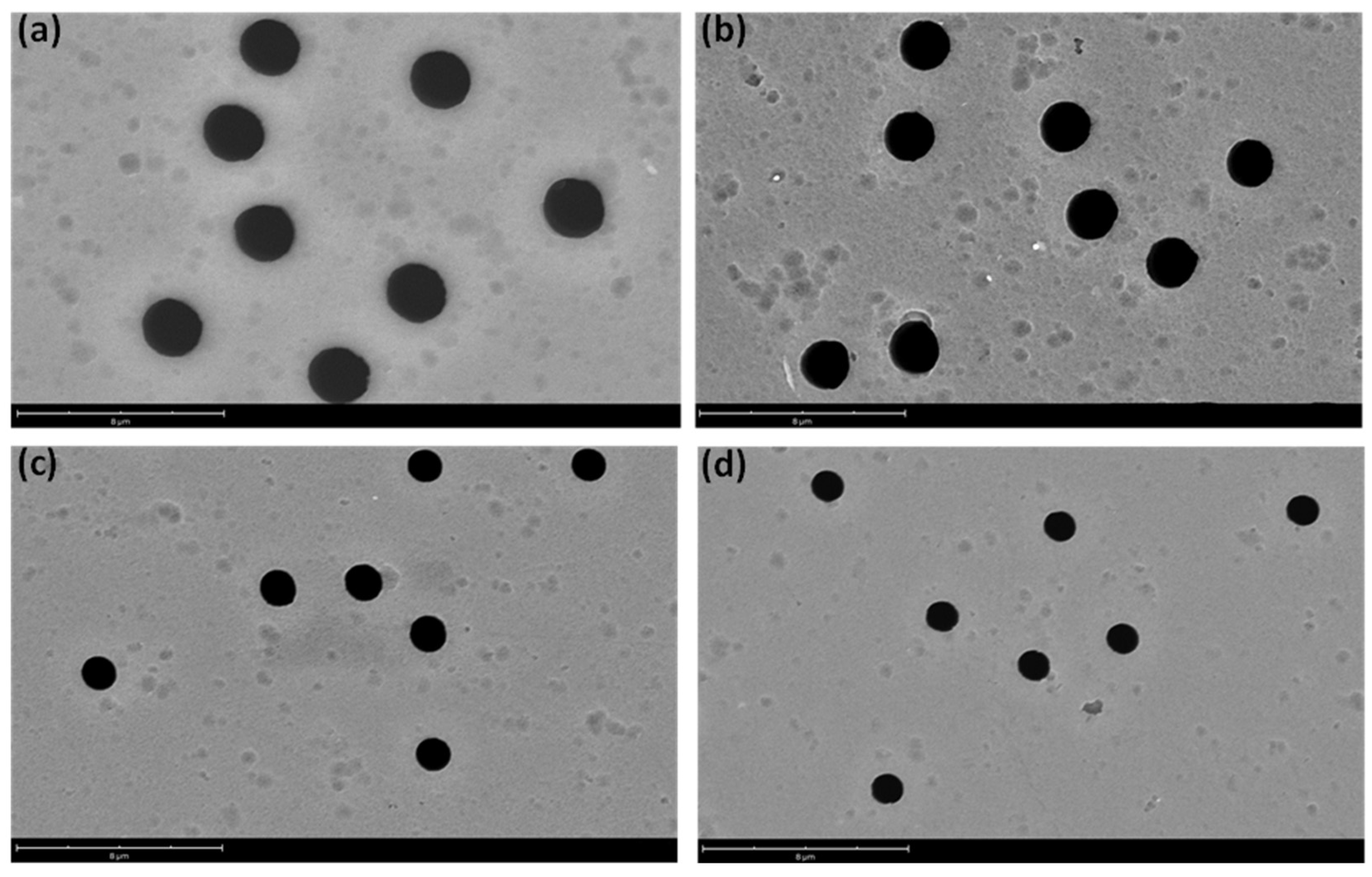
2.3. Characterization Technics
2.4. Membrane Distillation Tests
3. Results and Discussion
3.1. Fabrication of Hydrophobic PET TeMs
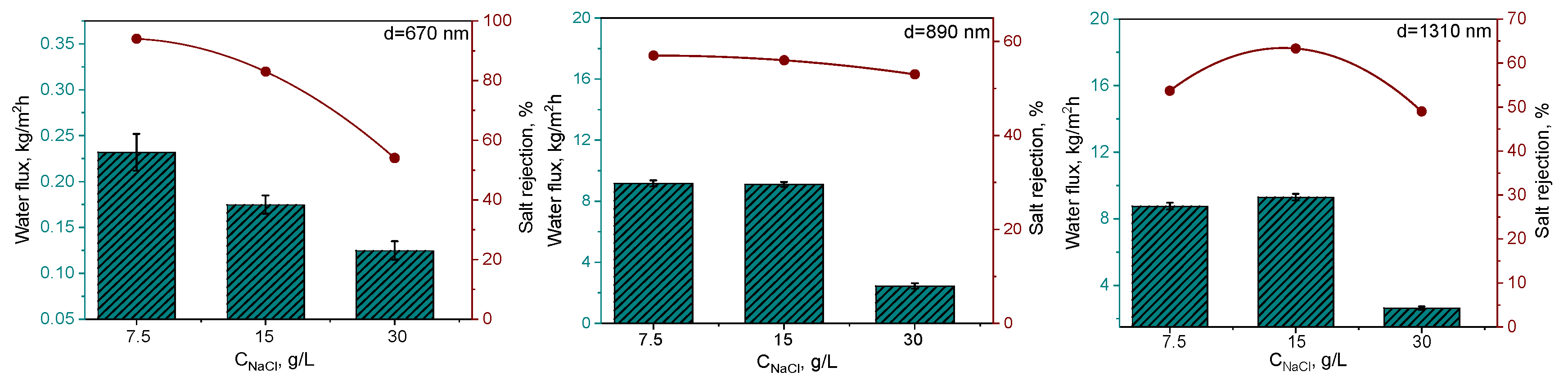
3.2. Desalination by DCMD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schlosser, C.A.; Strzepek, K.; Gao, X.; Fant, C.; Blanc, É.; Paltsev, S.; Jacoby, H.; Reilly, J.; Gueneau, A. The Future of Global Water Stress: An Integrated Assessment. Earth’s Futur. 2014, 2, 341–361. [Google Scholar] [CrossRef]
- UNICEF 2015 Update and MDG Assessment. Prog. Sanit. Drink. Water 2015, 90.
- Yeszhanov, A.B.; Korolkov, I. V.; Dosmagambetova, S.S.; Zdorovets, M. V.; Güven, O. Recent Progress in the Membrane Distillation and Impact of Track-Etched Membranes. Polymers (Basel). 2021, 13, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasoul, A. (Ed.). Advances in Membrane Technologies; 2020.
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse Osmosis Desalination: Water Sources, Technology, and Today’s Challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Souhaimi, M.K.; Matsuura, T. Membrane Distillation; Elsevier, 2011; ISBN 9780444531261.
- Korolkov, I. V.; Yeszhanov, A.B.; Zdorovets, M. V.; Gorin, Y.G.; Güven, O.; Dosmagambetova, S.S.; Khlebnikov, N.A.; Serkov, K. V.; Krasnopyorova, M. V.; Milts, O.S.; et al. Modification of PET Ion Track Membranes for Membrane Distillation of Low-Level Liquid Radioactive Wastes and Salt Solutions. Sep. Purif. Technol. 2019, 227, 115694. [Google Scholar] [CrossRef]
- Jiang, M.; Fang, Z.; Liu, Z.; Huang, X.; Wei, H.; Yu, C.Y. Application of Membrane Distillation for Purification of Radioactive Liquid. Clean. Eng. Technol. 2023, 12. [Google Scholar] [CrossRef]
- Alves, V.D.; Coelhoso, I.M. Orange Juice Concentration by Osmotic Evaporation and Membrane Distillation: A Comparative Study. J. Food Eng. 2006, 74, 125–133. [Google Scholar] [CrossRef]
- Boukhriss, M.; Timoumi, M.; Bacha, H. Ben Experimental of Membrane Distillation Unit Coupled with a DCMD Using Solar Energy. Sol. Compass 2023, 7, 100055. [Google Scholar] [CrossRef]
- Parani, S.; Oluwafemi, O.S. Membrane Distillation: Recent Configurations, Membrane Surface Engineering, and Applications. Membranes (Basel). 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Wang, H.; Ding, F.; Jin, G.; Li, C.; Meng, H. Superhydrophobic Modification of Ceramic Membranes for Vacuum Membrane Distillation. Chinese J. Chem. Eng. 2017, 25, 1395–1401. [Google Scholar] [CrossRef]
- Dong, S.; Yun, Y.; Wang, M.; Li, C.; Fu, H.; Li, X.; Yang, W.; Liu, G. Superhydrophobic Alumina Hollow Ceramic Membrane Modified by TiO2 Nanorod Array for Vacuum Membrane Distillation. J. Taiwan Inst. Chem. Eng. 2020, 117, 56–62. [Google Scholar] [CrossRef]
- Al-Sairfi, H.; Koshuriyan, M.Z.A.; Ahmed, M. Membrane Distillation of Saline Feeds and Produced Water: A Comparative Study of an Air-Gap and Vacuum-Driven Modules. Desalin. Water Treat. 2024, 317, 100145. [Google Scholar] [CrossRef]
- Julian, H.; Nurgirisia, N.; Qiu, G.; Ting, Y.P.; Wenten, I.G. Membrane Distillation for Wastewater Treatment: Current Trends, Challenges and Prospects of Dense Membrane Distillation. J. Water Process Eng. 2022, 46, 102615. [Google Scholar] [CrossRef]
- Ravi, J.; Othman, M.H.D.; Matsuura, T.; Ro’il Bilad, M.; El-badawy, T.H.; Aziz, F.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Polymeric Membranes for Desalination Using Membrane Distillation: A Review. Desalination 2020, 490, 114530. [Google Scholar] [CrossRef]
- Abid, M. Bin; Wahab, R.A.; Salam, M.A.; Gzara, L.; Moujdin, I.A. Desalination Technologies, Membrane Distillation, and Electrospinning, an Overview. Heliyon 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Qtaishat, M.R.; Matsuura, T. Modelling of Pore Wetting in Membrane Distillation Compared with Pervaporation; Elsevier Ltd, 2015; ISBN 9781782422563.
- Shakayeva, A.K.; Munasbaeva, K.K.; Zhumazhanova, A.T.; Zdorovets, M. V.; Korolkov, I. V. Electrochemical Sensors Based on Modified Track–Etched Membrane for Non-Enzymatic Glucose Determination. Microchem. J. 2023, 193. [Google Scholar] [CrossRef]
- Muslimova, I.B.; Zhumanazar, N.; Melnikova, G.B.; Yeszhanov, A.B.; Zhatkanbayeva, Z.K.; Chizhik, S.A.; Zdorovets, M. V.; Güven, O.; Korolkov, I. V. Preparation and Application of Stimuli-Responsive PET TeMs: RAFT Graft Block Copolymerisation of Styrene and Acrylic Acid for the Separation of Water–Oil Emulsions. RSC Adv. 2024, 14, 14425–14437. [Google Scholar] [CrossRef] [PubMed]
- Mashentseva, A.A.; Barsbay, M.; Aimanova, N.A.; Zdorovets, M. V. Application of Silver-Loaded Composite Track-Etched Membranes for Photocatalytic Decomposition of Methylene Blue under Visible Light. Membr. 2021, Vol. 11, Page 60 2021, 11, 60. [CrossRef]
- Apel, P. Track Etching Technique in Membrane Technology. Radiat. Meas. 2001, 34, 559–566. [Google Scholar] [CrossRef]
- Ma, T.; Janot, J.M.; Balme, S. Track-Etched Nanopore/Membrane: From Fundamental to Applications. Small Methods 2020, 4, 2000366. [Google Scholar] [CrossRef]
- Alsebaeai, M.K.; Ahmad, A.L. Membrane Distillation: Progress in the Improvement of Dedicated Membranes for Enhanced Hydrophobicity and Desalination Performance. J. Ind. Eng. Chem. 2020. [Google Scholar] [CrossRef]
- Li, X.; Pan, J.; Macedonio, F.; Ursino, C.; Carraro, M.; Bonchio, M.; Drioli, E.; Figoli, A.; Wang, Z.; Cui, Z. Fluoropolymer Membranes for Membrane Distillation and Membrane Crystallization. Polymers (Basel). 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, R.; Nettis, E.; Vitale, A. Fluoropolymers for Oil/Water Membrane Separation. Oppor. Fluoropolymers 2020, 209–246. [Google Scholar] [CrossRef]
- Kujawa, J.; Rozicka, A.; Cerneaux, S.; Kujawski, W. The Influence of Surface Modification on the Physicochemical Properties of Ceramic Membranes. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 443, 567–575. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Y.; Liu, J.; Li, X.; Li, B.; Wang, S. Preparation of Re-Entrant and Anti-Fouling PVDF Composite Membrane with Omniphobicity for Membrane Distillation. J. Memb. Sci. 2020, 595, 117563. [Google Scholar] [CrossRef]
- Korolkov, I. V.; Yeszhanov, A.B.; Gorin, Y.G.; Zdorovets, M. V.; Khlebnikov, N.A.; Serkov, K. V. Hydrophobization of PET Track-Etched Membranes for Direct Contact Membrane Distillation. Mater. Res. Express 2018, 5, 065317. [Google Scholar] [CrossRef]
- Yeszhanov, A.B.; Korolkov, I. V.; Güven, O.; Melnikova, G.B.; Dosmagambetova, S.S.; Borissenko, A.N.; Nurkassimov, A.K.; Kassymzhanov, M.T.; Zdorovets, M. V. Effect of Hydrophobized PET TeMs Membrane Pore-Size on Saline Water Treatment by Direct Contact Membrane Distillation. RSC Adv. 2024, 14, 4034–4042. [Google Scholar] [CrossRef] [PubMed]
- Korolkov, I. V.; Mashentseva, A.A.; Güven, O.; Niyazova, D.T.; Barsbay, M.; Zdorovets, M. V. The Effect of Oxidizing Agents/Systems on the Properties of Track-Etched PET Membranes. Polym. Degrad. Stab. 2014, 107, 150–157. [Google Scholar] [CrossRef]
- Korolkov, I. V.; Mashentseva, A.A.; Güven, O.; Taltenov, A.A. UV-Induced Graft Polymerization of Acrylic Acid in the Sub-Micronchannels of Oxidized PET Track-Etched Membrane. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. with Mater. Atoms 2015, 365, 419–423. [Google Scholar] [CrossRef]
- Yeszhanov, A.B.; Korolkov, I. V; Kh Shakayeva, A.; Lissovskaya, L.I.; Zdorovets, M. V Preparation of Poly(Ethylene Terephthalate) Track-Etched Membranes for the Separation of Water-Oil Emulsions. Eurasian J. Chem. 2023, 110, 131–138. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Grafting in Confined Spaces: Functionalization of Nanochannels of Track-Etched Membranes. 2014. [Google Scholar] [CrossRef]
- Güven, O. Established and Emerging Applications of Radiation-Induced Graft Polymerization. Appl. Ioniz. Radiat. Mater. Process. 2017, Volume, 355–373. [Google Scholar]
- Kuşçuoğlu, C.K.; Güner, H.; Söylemez, M.A.; Güven, O.; Barsbay, M. A Smartphone-Based Colorimetric PET Sensor Platform with Molecular Recognition via Thermally Initiated RAFT-Mediated Graft Copolymerization. Sensors Actuators, B Chem. 2019, 296. [Google Scholar] [CrossRef]
- Korolkov, I. V.; Mashentseva, A.A.; Güven, O.; Gorin, Y.G.; Zdorovets, M. V. Protein Fouling of Modified Microporous PET Track-Etched Membranes. Radiat. Phys. Chem. 2018, 151, 141–148. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Borgekov, D.; Kenzhina, I.; Zdorovets, M.; Korolkov, I.; Kaniukov, E.; Kutuzau, M.; Shumskaya, A. PET Ion-Track Membranes: Formation Features and Basic Applications. Springer Proc. Phys. 2019, 221, 461–479. [Google Scholar]
- Luo, Z. hong; He, T. yun Synthesis and Characterization of Poly(Dimethylsiloxane)-Block-Poly(2,2,3,3,4,4,4-Heptafluorobutyl Methacrylate) Diblock Copolymers with Low Surface Energy Prepared by Atom Transfer Radical Polymerization. React. Funct. Polym. 2008, 68, 931–942. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Lienhard V, J.H.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting Phenomena in Membrane Distillation: Mechanisms, Reversal, and Prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Dr. Nasreen Ocean Salinity. Int. J. Mod. Trends Sci. Technol. 2022, 8, 296–302. [Google Scholar]
- Zou, L.; Zhang, Y.; Yu, J.; Zha, S.; Guan, R.; Jiang, S. Study of a Poly(Vinylidene Fluoride)/Hydrophobic Silica Sol Hybrid Hollow Fiber Membrane for Treatment of Produced Water via Direct Contact Membrane Distillation. J. Water Process Eng. 2021, 44, 102345. [Google Scholar] [CrossRef]
- Hussein, S.S.; Ibrahim, S.S.; Toma, M.A.; Alsalhy, Q.F.; Drioli, E. Novel Chemical Modification of Polyvinyl Chloride Membrane by Free Radical Graft Copolymerization for Direct Contact Membrane Distillation (DCMD) Application. J. Memb. Sci. 2020, 611, 118266. [Google Scholar] [CrossRef]
- Nambikkattu, J.; Kaleekkal, N.J. Fluoroalkylsilane Grafted FeOOH Nanorods Impregnated PVDF-Co-HFP Membranes with Enhanced Wetting and Fouling Resistance for Direct Contact Membrane Distillation. J. Environ. Chem. Eng. 2023, 11, 109624. [Google Scholar] [CrossRef]
- Rahimnia, R.; Pakizeh, M. Preparation and Characterization of PPO/PS Porous Membrane for Desalination via Direct Contact Membrane Distillation (DCMD). J. Memb. Sci. 2023, 669, 121297. [Google Scholar] [CrossRef]
- Ursino, C.; Ounifi, I.; Di Nicolò, E.; Cheng, X.Q.; Shao, L.; Zhang, Y.; Drioli, E.; Criscuoli, A.; Figoli, A. Development of Non-Woven Fabric-Based ECTFE Membranes for Direct Contact Membrane Distillation Application. Desalination 2021, 500. [Google Scholar] [CrossRef]
- Niknejad, A.S.; Bazgir, S.; Sadeghzadeh, A.; Shirazi, M.M.A. Styrene-Acrylonitrile (SAN) Nanofibrous Membranes with Unique Properties for Desalination by Direct Contact Membrane Distillation (DCMD) Process. Desalination 2020, 488, 114502. [Google Scholar] [CrossRef]
- Siyal, M.I.; Kim, J.O. Fluorographite-Co-Polydimethylsiloxane Coated Polyvinylidene-Fluoride Membrane for Desalination of Highly Saline Water with Humic Acid in Direct Contact Membrane Distillation. Environ. Res. 2018, 167, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shen, F.; Zhang, B.; Jiang, H.; Li, J.; Luo, J.; Wu, H.; Khan, R.; Wan, Y. Fabrication of PES-Based Membranes with a High and Stable Desalination Performance for Membrane Distillation. RSC Adv. 2016, 6, 107840–107850. [Google Scholar] [CrossRef]
- Talukder, E.; Talukder, R.; Pervez, N.; Song, H.; Naddeo, V. Bead-Containing Superhydrophobic Nanofiber Membrane for Membrane Distillation. 2024, 1–14.


| № Sample | Polymerization Time, min | Monomer Concentration, % | Absorbance Value (A950/A1410) |
|---|---|---|---|
| 1 | 30 | 30 | 0.495±0.042 |
| 2 | 45 | 30 | 0.539±0.024 |
| 3 | 60 | 30 | 0.572±0.014 |
| 4 | 60 | 20 | 0.519±0.064 |
| 5 | 60 | 10 | 0.491±0.002 |
| № Sample | Polymerization Time, min | DFHA Concentration, % | CA, ° ±5° |
Pore Size (from SEM Analysis), nm | Concentration of F, % |
|---|---|---|---|---|---|
| 1 | 0 | - | 51 | 2510±560 | - |
| 2 | 60 | 10 | 68 | 2420±683 | 0.42±0.2 |
| 3 | 60 | 20 | 69 | 2175±124 | 0.91±0.5 |
| 4 | 60 | 30 | 97 | 1295±640 | 0.94±0.48 |
| 5 | 45 | 30 | 73 | 1333±133 | 0.86±0.15 |
| 6 | 30 | 30 | 62 | 2091±136 | 0.6±0.4 |
| Membrane Material | CA (°) | DCMD Conditions | R, % | Ref. |
|---|---|---|---|---|
| DHPVC-graft-PEA | 95.48±0.79 | NaCl 35, 70 and 100 g/L | 99.9 | [43] |
| PVDF-co-HFP with F-g-FeOOH | 132±1.6 | NaCl 10000 ppm | 99.9 | [44] |
| PPO/PS | - | NaCl 35000 ppm | 99.9 | [45] |
| LMP ECTFE | 116±2 | NaCl 0.6 M | 94.95 – 99.8 | [46] |
| SAN electrospun nanofibers | 139.8±0.8 | NaCl 35 and 70 g/L | 99.9 | [47] |
| PVDF@FGi particles | 105 - 140 | NaCl 1 M | 99.8 | [48] |
| PES | ~102 | NaCl 35 g/L | 69.8 | [49] |
| SPES@MWCNTs | 145 | NaCl 3.5% | 99.8 | [50] |
| Pure SPES | 70 | NaCl 3.5% | 95.2 | |
| PET TeMs-graft-LMA (700-1300 nm) | 94±4 | NaCl 7.5, 15 and 30 g/L | 91.4 | [30] |
| PET TeMs-graft-DFHA (700-1300 nm) | 97±4 | NaCl 7.5, 15 and 30 g/L | 94 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





