1. Introduction
Overweight and obesity are defined as abnormal or excessive fat accumulation, which is increasing in prevalence and now considered to be a global epidemic. In addition, it has been reported that overweight and obesity are associated with several major diseases, such as cardiovascular disease, diabetes, and hypertension [
1]. In 2019 in Japan, the National Health and Nutrition Examination Survey reported overweight or obesity in 33.0% and 22.3% of males and females aged ≥ 20 years, respectively [
2]. In addition, people with overweight and obesity have been reported to have higher medical expenditures compared with those without [
3]. In light of this critical national health situation in Japan, a national program for preventing obesity is considered very important.
Oral health is important because it not only helps maintain healthy teeth and gingiva, but also contributes to general health. Several studies have focused on the risk of overweight and obesity from the viewpoint of oral health status, including chewing status. For instance, a systematic review revealed that poorer mastication was associated with overweight and obesity in 12 of 16 cross-sectional studies [
4]. It is also known that the number of opposing posterior teeth is related to overweight and obesity [
5]. These findings indicate that chewing status is a potential factor related to overweight and obesity. However, to our knowledge, only a few studies have investigated the longitudinal relationship between chewing status and overweight/obesity. Further clarification of this longitudinal relationship could help promote the development of improved methods for obesity prevention.
Waist circumference (WC) is a simple method to assess abdominal adiposity that is easy to assess, and is therefore the most commonly accepted first step to determine an individual’s degree of overweight or obesity [
6]. The International Atherosclerosis Society and the International Chair on Cardiometabolic Risk Working Group on Visceral Obesity have recommended the adoption of WC as a routine measurement in clinical practice to classify overweight/obesity [
7]. Evaluating changes in WC could therefore be useful to clarify the longitudinal relationship between chewing status and overweight/obesity.
In Japan, the Ministry of Health, Labour and Welfare requires medical insurers to conduct specific health checkups for insured persons aged 40–74 years [
8]. These checkups focus on the accumulation of visceral fat and assume that a WC exceeding a certain standard value increases the risk of lifestyle-related diseases. Unfortunately, dental checkups are not included in standard health checkups. However, the medical questionnaire used in these health checkups includes an item on chewing status. These kinds of health checkups are a core health policy in Japan for more than 30 million people, and if it were to become clear that chewing status is involved in changes in WC, it would provide a new screening method for the prevention of overweight and obesity in Japan.
Specific health checkups are carried out once a year. If it is known at the time of the specific health checkup how an individual’s chewing status is related to changes in WC 1 year later, this fact can easily be introduced into health guidance for overweight/obesity prevention. Given this background, in the present study, we hypothesized that chewing status might be related to increases in WC after 1 year. Therefore, a 1-year follow-up longitudinal study was conducted to clarify the relationship between self-reported chewing status and WC in Japanese adults who received a specific health checkup.
2. Materials and Methods
2.1. Participants
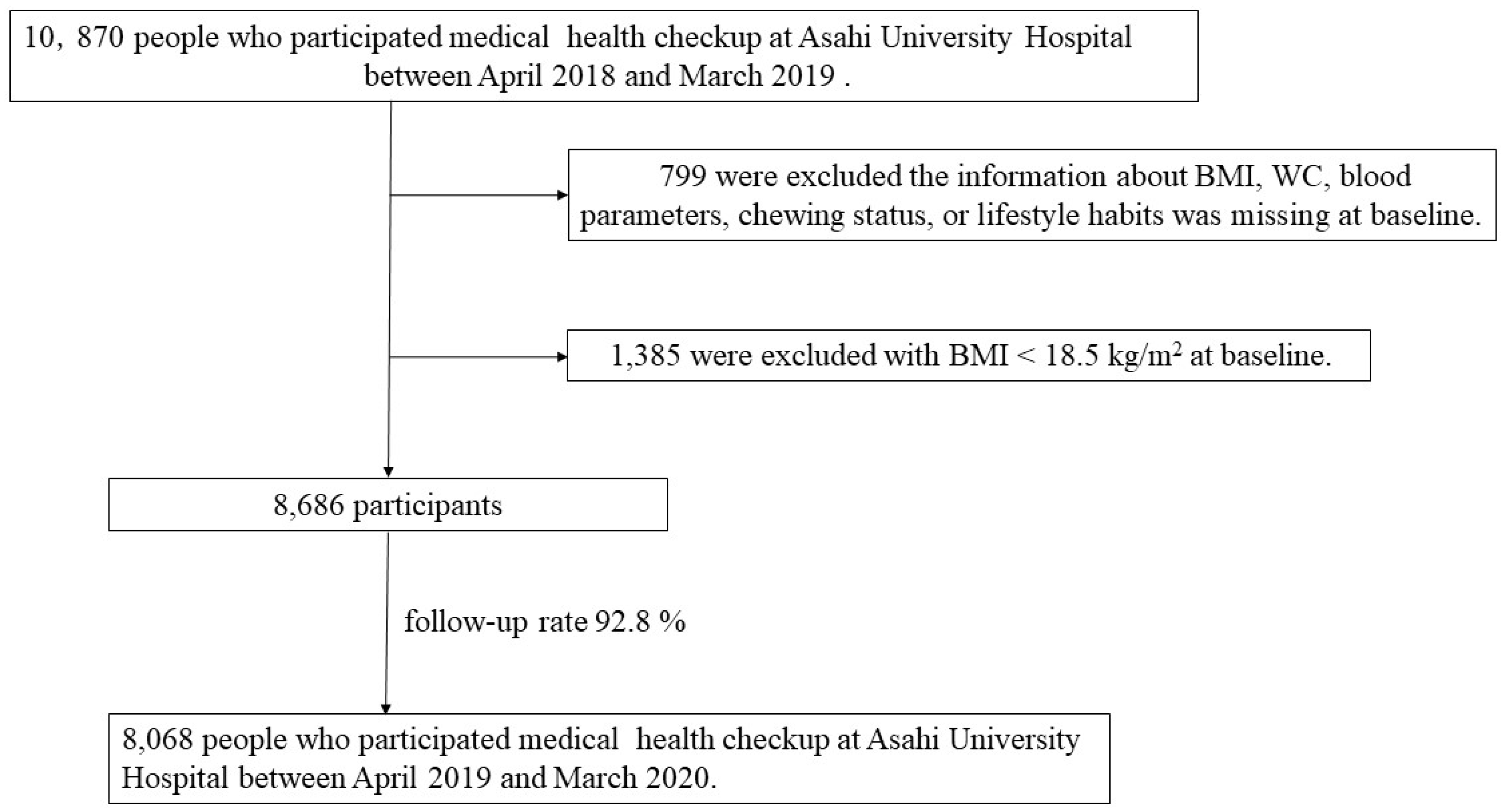
All study participants had received a specific health checkup at Asahi University Hospital Human Health Center between April 2018 and March 2019 (baseline). A total of 10,870 people were recruited, of who, 799 were excluded because information on body mass index (BMI), WC, blood parameters, chewing status, or lifestyle habits was missing at baseline or after the 1-year follow-up visit. In addition, 1,385 people with a BMI < 18.5 kg/m2 were excluded [
9]. Accordingly, 8,068 participants (5,138 males and 2,973 females; mean age, 51.0 years) were included in the final analysis between April 2019 and March 2020 (follow-up rate: 92.8%) (
Figure 1). This study was approved by the Ethics Committee of Asahi University (No. 30018; approved 2018) and performed in accordance with the Declaration of Helsinki. All participants provided informed consent. This study followed the STROBE guidelines.
2.2. Assessment of BMI and WC
Trained nurses measured each participant’s height (in centimeters to the nearest 0.1 cm) and body weight (in kilograms to the nearest 0.1 kg). BMI was calculated as weight divided by height in meters squared (kg/m2). WC (in centimeters to the nearest 0.5 cm) was measured horizontally at the umbilical level at the end of normal expiration [
10,
11].
2.3. Measurement of Blood Pressure
Blood pressure was measured using a standard sphygmomanometer (HBP-9030; OMRON, Kyoto, Japan) with the participants sitting on a chair after a minimum of 5 minutes of rest [
12,
13].
2.4. Measurement of Blood Parameters
Venous fasting blood samples were collected, and serum levels of triglyceride (TG), HDL cholesterol, and hemoglobin A1c (HbA1c) were determined using an automatic analyzer (Dimension Vista 1500; Siemens Healthineers Japan, Tokyo, Japan; DM-JACK; Kyowa Medex, Tokyo, Japan) [
14,
15].
2.5. Evaluation of Chewing Status
To evaluate chewing status, we used the questionnaire from the specific medical checkups in Japan. The responses to the relevant questionnaire item are as follows: “I can eat anything”, “Sometimes it is difficult to chew because of dental problems such as dental caries and periodontal disease”, and “I can hardly chew” [
16]. Respondents who answered “Sometimes it is difficult to chew because of dental problems such as dental caries and periodontal disease” or “I can hardly chew” were considered to have poor chewing status [
17].
2.6. Other Items Surveyed from the Self-Administered Questionnaire
The self-administered questionnaire used in specific medical checkups was used to collect information on the participants’ gender, age, smoking habits, amount of drinking, exercise habits, and sleep status. Smoking habits were classified as being present if the participant smoked at least one cigarette per day [
18]. Participants who drank > 180 mL of sake, > 500 mL of beer, > 80 mL of shochu, > 60 mL of whiskey double, or > 240 mL of wine were defined as heavy drinkers [
19]. Participants who exercised ≥ 30 minutes ≥ 2 days/week were defined as having a regular exercise habit [
17]. Sleep status was categorized as poor or good [
20].
2.7. Statistical Analysis
The outcome of interest was the 1-year change in WC from baseline to the follow-up visit (referred to as the change in WC). Changes in WC were calculated by subtracting WC at baseline from WC at follow-up. A 5-cm increase in WC is associated with increased cardiovascular risk factors [
21]. Therefore, in this study, an increase in WC of ≥ 5 cm was defined as an unhealthy change. Because continuous variables (age, blood pressure, HbA1c, TG, HDL cholesterol, and WC) are not normally distributed, data were presented as the median (25%, 75% percentiles). Differences between the two groups were evaluated using the Mann–Whitney U test for continuous variables and the ci-square test for categorical variables. In the multivariate logistic regression, variables with p-values > 0.05 were excluded in the model, the third category of variables related to the sample (age, gender, WC, and chewing status), and variables with significant differences in univariate logistic analysis, which were adjusted for in these analyses. All data were analyzed using SPSS (version 27; IBM Japan, Tokyo, Japan). All p-values < 0.05 were considered statistically significant.
3. Results
Table 1 shows the baseline characteristics of the participants with a chewing status of poor or good. In this study, 1,080 participants (13%) were diagnosed as having poor chewing status. Participants with poor chewing status were significantly older (p < 0.001) and had higher HbA1c (p = 0.004), and TG levels (p = 0.013) than did those with good chewing status. Participants with poor chewing status also had significantly lower levels of HDL cholesterol (p < 0.001) than did those with good chewing status. Furthermore, a significantly higher proportion of participants with poor compared with good chewing status had a smoking habit (p < 0.001), were heavy drinkers (p = 0.027), had regular exercise habits (p = 0.003), and had poor sleeping status (p < 0.001).
Table 2 shows the results of a comparison of WC by chewing status at baseline and after 1 year. The median (25%, 75% percentiles) WC in the good chewing status group was 80.0 cm (75.0, 86.0) at both baseline and after 1 year. By contrast, the median (25%, 75% percentiles) WC in the poor chewing status group was 80.0 cm (75.0, 87.0) at baseline and 81.5 cm (76.0, 87.5) after 1 year. The WC values in the poor chewing status group were significantly higher than those in the good chewing status group at both baseline (p = 0.017) and after 1 year (p < 0.001).
Table 3 shows the characteristics of changes in WC after 1 year by chewing status. Of the participants with good chewing status, 510 (7%) had an increase in WC of ≥ 5 cm after 1 year, whereas among those with poor chewing status, 103 (10%) had an increase in WC of ≥ 5 cm after 1 year
Table 4 shows the crude odds ratios (ORs) and 95% confidence intervals (CIs) for an increase in WC of ≥ 5 cm after 1 year. The results showed that the risk of a ≥ 5 cm increase in WC after 1 year was significantly correlated with gender (females: ORs: 1.264; 95% CIs: 1.069–1.494), BMI (≥ 25 kg/m2: ORs: 1.369; 95% CIs: 1.148–1.632), and chewing status (poor: ORs: 1.339; 95% CIs: 1.072–1.672) at baseline.
Table 5 shows the adjusted ORs and 95% CIs for a ≥ 5 cm increase in WC after 1 year. After adjusting for age, gender, WC, BMI, and chewing status, the risk of an increase in WC of ≥ 5 cm after 1 year was significantly correlated with gender (females: ORs: 1.206; 95% CIs: 1.008–1.443), WC (ORs: 0.967; 95% CIs: 0.954–0.981), BMI (≥ 25 kg/m2: ORs: 2.194; 95% CIs: 1.715–2.808), and chewing status (poor: ORs: 1.356; 95% CIs: 1.084–1.697) at baseline.
4. Discussion
To our knowledge, this is the first study to examine the association between chewing status and increased WC after 1 year. The median WC in the poor chewing status group was higher than that in the good chewing status group at both baseline and follow-up. The results of the multivariate logistic regression showed that a ≥ 5 cm increase in WC after 1 year was associated with poor chewing status at baseline (ORs: 1.356; 95% CIs: 1.084–1.697) after adjusting for age, gender, WC, and BMI. A previous study reported that a ≥ 5 cm increase in WC was associated with the development of cardiovascular disease [
1], suggesting that poor chewing status is a risk factor for a future unhealthy increase in WC. Furthermore, it is accepted that an increase in WC reflects an increase in visceral fat [
6]. The present results indicate that poor chewing status could be a risk factor for an unhealthy increase in visceral fat.
Several studies have examined the relationships among chewing status, weight change, and BMI. For instance, a cross-sectional study showed that the categorical chewing number was related independently to body weight increments of > 10 kg from 20 years of age in persons aged 35–61 years [
22]. Another cross-sectional study reported statistically significant negative correlations between BMI and the number of chewing cycles (r = –0.296, p = 0.020) and chewing duration (r = –0.354, p = 0.005) in fully dentate healthy adults [
23]. These findings suggest that chewing status is associated with a risk for overweight/obesity via changes in weight and BMI. In the present study, poor chewing status was associated with an unhealthy increase in WC. Therefore, the previous and present studies all support the concept that chewing status is a target factor for overweight/obesity prevention.
Specified medical checkups are conducted once a year in Japan. The present study was a 1-year longitudinal study conducted to evaluate changes until the next specified medical checkup. In other words, the results of this study indicated that respondents with poor mastication status in the specified medical checkups were at high risk of unhealthy increases in WC by the next specified medical checkup. Therefore, in order to prevent unhealthy accumulation of visceral fat, it is considered necessary not to leave participants who responded with poor chewing status until the next specified medical checkup. Self-reported chewing status has been shown to be associated with the number of existing teeth, the occlusal status of the molars, and the number of functional teeth [
24]. Therefore, dental health guidance for respondents with poor chewing status may be important in terms of preventing unhealthy increases in WC.
There are several possible mechanisms that may explain the association between chewing status and increases in WC. People with poor compared with good chewing status tend to consume fewer fruits and vegetables and more high-energy foods, which may lead to unhealthy increases in WC [
25]. Furthermore, it has been reported that chewing promotion increases the partial pressure of oxygen in the brain and activates brain function, thereby increasing the number of calories consumed in the body [
26,
27]. Therefore, the participants with poor chewing status in the present study may have had a reduced total number of calories, thereby leading to inhibited brain function, which may be associated with future increases in WC.
In the present study, a significant association was found between increases in WC and female gender. This finding is consistent with previous studies reporting that females accumulate fat more readily than males [
28,
29]. The reason that females are more likely to accumulate fat than males may be related to their lower basal metabolic rate and the influence of female hormones [
28,
29].
On the other hand, no association was found between increases in WC and age in this study. By contrast, a previous study reported finding an association between increased WC and older age [
30]. This discrepancy may be related to the difference in the age distribution of the participants in the previous and present studies; the 2019 National Health and Nutrition Survey reported that the rate of increase in WC was significantly higher among those aged ≥ 60 years [
2]. The participants in another previous study [
30] were older than those in the present study, with a mean age of 65 years (vs. 51.0 years in the present study).
In Japan, a BMI ≥ 25 kg/m2 is considered an indicator of obesity [
31]. Therefore, the present findings suggest that participants with obesity are more likely to have an increased WC after 1 year. This supports a previous study showing that participants with a higher BMI were more likely to have an increased WC [
32].
This study has several limitations. First, socioeconomic factors such as the participants’ income and educational levels were not considered. Socioeconomic factors are reportedly involved in unhealthy conditions such as increases in WC [
33]; however, these were unclear in the present study. Second, this study included only participants who received a specific health checkup at Asahi University Hospital Human Health Center. According to the 2018 National Health and Nutrition Survey, the proportion of people with obesity in their 50s was 25.9% [
2], which is higher than the proportion of participants in the present study (20.2%). Therefore, results may differ for different health populations, and external validity should be considered. On the other hand, a major strength of the present study is its sample size of more than 8,000 Japanese individuals. This sample size should be sufficient to show the longitudinal relationship between chewing status and increases in WC, which may be useful for inferring factors that contribute to the unhealthy accumulation of visceral fat in Japanese adults.
5. Conclusions
The results of the present study revealed an association between self-reported poor chewing status and an increase in WC of ≥ 5 cm after 1 year. Therefore, poor chewing status may be an important risk factor indicating the unhealthy accumulation of visceral fat in Japanese adults.
Author Contributions
The present study was carried out with the collaboration of all authors. R.Y., N.K., and T.T. conceived the study. K. I, T.A., T.Y., Y.S., K. W., A. O., F. D., T. K, and W. T. collected the data. R. Y., K.I. and T.T. analyzed the data, interpreted the results, and wrote the manuscript. All authors approved the final manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Asahi University (No. 30018; approved 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Acknowledgments
We are grateful to the Asahi University Hospital Human Health Center for providing us with the health checkup data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sowers, J.R. Obesity as a cardiovascular risk factor. Am J Med 2003, 115, 37S–41S. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. National Health and Nutrition Survey 2019. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/r1-houkoku_00002.html. (accessed on 30 October 2023).
- Arterburn, D.E.; Maciejewski, M.L.; Tsevat, J. Impact of morbid obesity on medical expenditures in adults. Int J Obes (Lond) 2005, 29, 334–339. [Google Scholar] [CrossRef]
- Tada, A.; Miura, H. Association of mastication and factors affecting masticatory function with obesity in adults: a systematic review. BMC Oral Health 2018, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Fukuda, H.; Kitamura, M.; Kawashita, Y.; Hayashida, H.; Furugen, R.; Koyama, Z.; Ando, Y.; Saito, T. Association between number of pairs of opposing posterior teeth, metabolic syndrome, and obesity. Odontology 2019, 107, 111–117. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Blom, D.; Libby, P.; Santos, R.; Obesity management: The window of opportunity for cardiometabolic clinicians. International Atherosclerosis Society. Available online: https://ias-education.athero.org/courses/obesity-management-the-window-of-opportunity-for-cardiometabolic-clinicians/ (accessed on 23 May 2024).
- Suzuki, S.; Sano, Y. Guidebook for specified health examination and specified health guidance leading to results. Chuohoki: TOkyo, Japan, 2014.
- Mir, I.A.; Soni, R.; Srivastav, S.K.; Bhavya, I.; Dar, W.Q.; Farooq, M.D.; Chawla, V.; Nadeem, M. Obesity as an Important Marker of the COVID-19 Pandemic. Cureus 2022, 14, e21403. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Azuma, T.; Yonenaga, T.; Sasai, Y.; Watanabe, K.; Deguchi, F.; Obora, A.; Kojima, T.; Tomofuji, T. Relationship between chewing status and fatty liver diagnosed by liver/spleen attenuation ratio: A cross-sectional study. Int J Environ Res Public Health 2022, 20, 307. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Mehrnezhad, A.; Razaviarab, N.; Barbosa-Silva, T.G.; Heymsfield, S.B. Calf circumference: cutoff values from the NHANES 1999-2006. Am J Clin Nutr 2021, 113, 1679–1687. [Google Scholar] [CrossRef]
- Hypertension Treatment Guidelines Development Committee. Hypertension treatment guidelines 2019. Available online: https://www.jpnsh.jp/guideline.html. (accessed on 30 October 2023).
- Kim, J.K.; Crimmins, E.M. Blood pressure and mortality: Joint effect of blood pressure measures. J Clin Cardiol Cardiovasc Ther 2020, 2, 1009. [Google Scholar] [CrossRef]
- Kawahara, T.; Imawatari, R.; Kawahara, C.; Inazu, T.; Suzuki, G. Incidence of type 2 diabetes in pre-diabetic Japanese individuals categorized by HbA1c levels: a historical cohort study. PLoS One 2015, 10, e0122698. [Google Scholar] [CrossRef]
- Nagahara, M.; Higuchi, Y.; Akatsu, J.; Tani, N.; Yamamoto, R.; Ohta, M. Verification of the effects of three percent weight loss at 6 months and application possibility of assessment at 3 months after the specific health guidance for male workers. Sangyo Eiseigaku Zasshi 2021, 63, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Irie, K.; Watanabe, K.; Deguchi, F.; Kojima, T.; Obora, A.; Tomofuji, T. Association between chewing problems and sleep among Japanese adults. Int J Dent 2019, 2019, 8196410. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Azuma, T.; Yonenaga, T.; Ekuni, D.; Watanabe, K.; Obora, A.; Deguchi, F.; Kojima, T.; Morita, M.; Tomofuji, T. Association between self-reported chewing status and glycemic control in Japanese adults. Int J Environ Res Public Health 2021, 18, 9548. [Google Scholar] [CrossRef] [PubMed]
- Qanash, S.; Alemam, S.; Mahdi, E.; Softah, J.; Touman, A.A.; Alsulami, A. Electronic cigarette among health science students in Saudi Arabia. Ann Thorac Med 2019, 14, 56–62. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Standard health examination and health guidance program [Fiscal year 2018 edition]. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000194155.html. (accessed on 30 October 2023).
- Kudo, A.; Asahi, K.; Satoh, H.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Fujimoto, S.; Narita, I.; Konta, T.; et al. Fast eating is a strong risk factor for new-onset diabetes among the Japanese general population. Sci Rep 2019, 9, 8210. [Google Scholar] [CrossRef] [PubMed]
- Suka, M.; Miwa, Y.; Ono, Y.; Yanagisawa, H. BMI, waist circumference, and clustering of cardiovascular risk factors in Japanese adults. Environ Health Prev Med 2011, 16, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Saito, T.; Mizuta, M.; Moromugi, S.; Ishimatsu, T.; Nishikado, S.; Takagi, H.; Konomi, Y. Chewing number is related to incremental increases in body weight from 20 years of age in Japanese middle-aged adults. Gerodontology 2013, 30, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hollis, J.H. Relationship between chewing behavior and body weight status in fully dentate healthy adults. Int J Food Sci Nutr 2015, 66, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Ueno, M.; Shinada, K. : Ohara, S.; Kawaguchi, Y. Validity of self-reported masticatory function in a Japanese population. J Dent Health 2010, 60, 214–223. [Google Scholar]
- Tada, A.; Miura, H. Systematic review of the association of mastication with food and nutrient intake in the independent elderly. Arch Gerontol Geriatr 2014, 59, 497–505. [Google Scholar] [CrossRef]
- Okuda, K.; Sakuma, Y.; Maeda, T.; Okazaki, J. Effects of masticatory dysfunction on brain function. J Orrofacial Pain. 2012; 5. [Google Scholar]
- Hetherington, M.M.; Regan, M.F. Effects of chewing gum on short-term appetite regulation in moderately restrained eaters. Appetite 2011, 57, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 2001, 4, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Jung, Y. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients 2021, 13, 4556. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Ouyang, Y.F.; Zhang, X.F.; Su, C.; Bai, J.; Zhang, B.; Hong, Z.X.; Du, S.F.; Wang, H.J. Waist circumference of the elderly over 65 years old in China increased gradually from 1993 to 2015: A cohort study. Biomed Environ Sci 2022, 35, 604–612. [Google Scholar]
- Obesity Treatment Guidelines 2016. Available online: https://www.jstage.jst.go.jp/article/naika/107/2/107_262/_pdf/-char/ja. (accessed on 18 March 2023).
- Stevens, J.; Katz, E.G.; Huxley, R.R. Associations between gender, age and waist circumference. Eur J Clin Nutr 2010, 64, 6–15. [Google Scholar] [CrossRef]
- Abreu, S.; Santos, R.; Moreira, C.; Santos, P.C.; Mota, J.; Moreira, P. Food consumption, physical activity and socio-economic status related to BMI, waist circumference and waist-to-height ratio in adolescents. Public Health Nutr 2014, 17, 1834–1849. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).