1. Introduction
It has been reported that sympathetic and sensory nerves play a significant role in bone metabolism. Studies have shown that sympathetic nerves have an impact on both bone formation and resorption [1–5]. In this interaction, the sensory neuropeptide calcitonin gene-related peptide (CGRP) molecules regulate osteoclastogenesis [4–6]. Studies have reported that neural tissues play a role in regulating blood flow and bone metabolism. Therefore, they are involved in bone remodeling metabolism [7,8]. Regions with higher bone metabolism have more nerve endings, which can aid in bone reconstruction by influencing bone metabolism [9,10].
In studies, it has been reported that capsaicin is a neurotoxic agent and is effective through transient receptor potential vanilloid 1 (TRPV1) molecules on sensory neurons [11,12]. As a result, TRPV1 is activated, and neurotoxicity has been reported as a result of the accumulation of calcium and sodium cations [13]. It has been reported that low-dose capsaicin administration plays a role in pain sensation due to TRPV1 activation. It has been reported that the application of high doses of capsaicin has a neurotoxin effect as a result of affecting massive ion flow [13,14]. Studies have reported that dorsal root neurons have losses in the immunoreactivity of CGRP when high capsaicin is administered [14–16]. A long-term blocking effect on sensory receptors has been reported from systemic administration of capsaicin [17].
In the studies performed, nerve endings were evaluated on the implant surface, and as a result, neurofilament protein (NFP) was detected in the bone in an area of 200 mm. but the exact role of these structures has not been determined [18,19]. It has not been fully determined whether these structures have any function in implant osseointegration or whether they play a role as a regulator in bone metabolism [20].
In a rat study aiming to examine the effect of capsaicin on bone, bone loss was found to be higher in the capsaicin-free rat group compared to the capsaicin-treated group. In an article investigating the effect of capsaicin on bone, it was reported that bone reduction was higher in the capsaicin-administered group than in the non-administered group [21]. In another rat study, it was stated that the amount of bone loss decreased by 40% and the number of osteoclasts decreased in rats that received capsaicin [15]. In their study, Offley et al. stated that capsaicin caused an increase in the number of osteoclasts and therefore negatively affected trabecular integrity, and they explained this by using different doses in different bone structures in rats [16].
Studies have reported that implant success is determined by its immobility and osseointegration. Branemark et al. coined the term ‘implant osseointegration’, which refers to a direct functional and structural connection between an implant and bone [22]. Implants can prevent functional loss resulting from tooth loss. Implants are composed of a variety of materials, each with unique designs and surface properties [23].
Capsaicin is assumed to have antioxidant properties. It can prevent lung emphysema and the development of free radical-related injuries, such as autoimmune nephrosis and muscular dystrophy. Oxidative stress is considered a contributing factor in the development of these diseases. Reactive oxidants can trigger free-radical reactions that may have adverse effects on the human body. In a study on rats, it was suggested that capsaicin may have a therapeutic effect on carbohydrate and lipid metabolism, as well as oxidative stress. However, further research is needed to confirm these findings [24].
Titanium implants are a commonly used material in craniomaxillofacial surgery, as well as in human and veterinary orthopedics. In the literature, the effects of many biological substances on the application of titanium implants have been investigated. This study aimed to examine the effects of systemic administration of capsaicin at different doses on oxidative stress and bone-implant connection parameters after surgical integration of the implants in rat tibias.
2. Materials and Methods
2.1. Animals and Study Design
This study was approved by Harran University (2019-006-03) Local Ethics Committee of Animal Experiments. It was carried out at the Experimental Research Center of Harran University, and the Helsinki Declaration rules were strictly followed during the experiments. Wistar albino rats used in the experiments were obtained from the Experimental Research Center of Harran University.
The number of animals was determined by power analysis in the pilot study with an alpha error of 0.05 and a beta error of 0.20, and it was determined that a minimum of 21 rats were required for the study. In order to ensure accurate statistical analysis against the possibility of death of the rats during the experimental stages, the study started with 8 rats in each group, making a total of 24 rats.
This study aimed to evaluate the effect of capsaicin on the osteointegration of dental implants after titanium-manufactured implants were integrated into the tibia of rats and examined them histopathologically. For statistical significance, 8 in each study group; In this study, twenty-four male Wistar albino rats weighing 250-300 g were used. Rats were first divided into 3 groups.
Implant+control group the joint capsule was opened in the location where the right femur and tibia meet and titanium-manufactured machined surfaced implants with a 2.5 mm diameter and 4 mm length were integrated. The rats were systemically given saline (daily intraperitoneally during the experimental period).
The same surgical procedure was applied to the rats in the implant + capsaicin dosage 1 and implant capsaicin dosage 2 groups. Systemically, capsaicin was administered 25 mg/kg and 50 mg/kg intraperitoneally to rats daily during the experiment respectively.
Eight rats from each group were euthanized and sacrificed on the 28th day with a taking of blood. After the experimental procedures, the samples which were implants surrounded with bone tissues were fixed in buffered formaldehyde solution for 72 hours.
2.2. Surgical Procedures
Before the surgical procedures, Ketamine Hydrochloride (Ketamidor-Richte Pharma) 10 mg/kg and Xylazine (Rompun-Bayer, Germany) 50 mg/kg injection were administered intramuscularly to all subjects for anesthesia, following the rules of asepsis and antisepsis. After the operation area was cleaned with Povidone Iodine, it was covered with sterile drapes, leaving the operation area exposed. For local hemostasis, 0.5 cc, 4% articaine containing 0.006 mg/ml epinephrine was applied to the operation area. The joint capsules of the rats were exposed with a median made to the junction of the incision of the tibia and femur. And after the corticocancellous bone part of the femur bone where it articulates with the tibia was reached.
Implant beds were created with 2.5 mm diameter and 4 mm length and were opened with the help of a drill on the part of the corticocancellous bone where the right femur of each rat connects with the tibia with saline irrigation. Titanium-manufactured 2.5 mm diameter and 4 mm length machined surfaced implants were integrated into each cavity at the bone level. All subjects were given 50 mg/kg cefazolin sodium as an antibiotic intramuscularly for postoperative 3 days to prevent infection. As a pain reliever, 1mg/kg tramadol hydrochloride was given intramuscularly for 3 days postoperatively. All wound sites were primarily sutured.
2.3. Histopathological Analysis
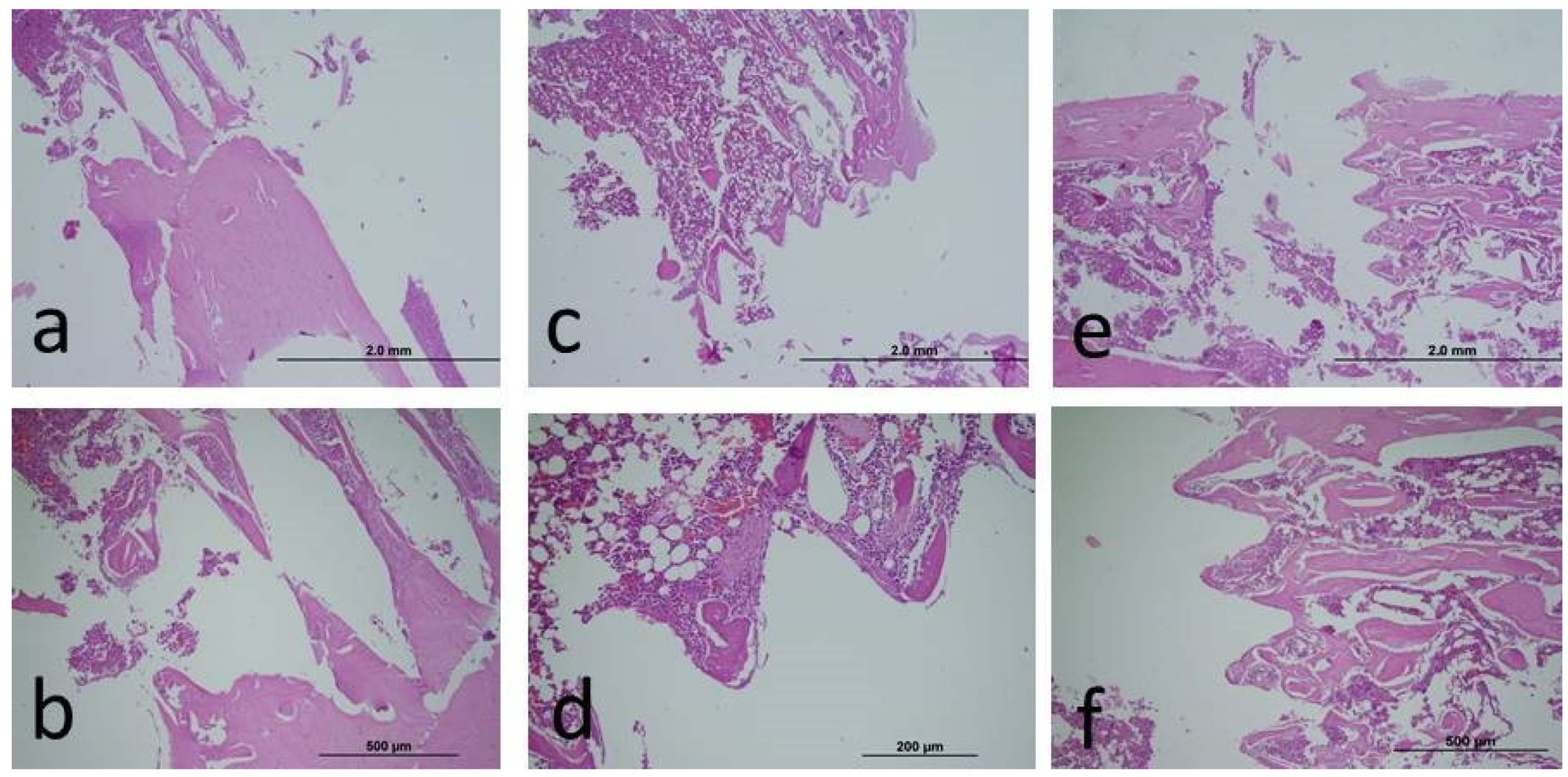
After the experiments, blood serum samples were collected from the heart for biochemical oxidative stress analysis. In addition, bone tissues were cut with the implant, and histopathological samples were obtained for bone-implant connection. Samples were fixed in 10% neutral formaldehyde before histological analysis. Before hard tissue analysis, decalcification was performed using an EDTA solution. In this way, the samples were made soft. The samples, which were passed through the alcohol series required for the analysis, were dehydrated, cleaned, and embedded in paraffin. Sagittal sections were obtained using a microtome blade (5-6 µm) for the areas to be analyzed. Afterwards, Hematoxylon-Eosin staining was performed and examined using a light microscope.
2.4. Biochemical Analysis
In biochemical analysis, the kit protocol of the manufacturer was applied. Then, a microplate reader was used for value reading (Cytation-1, Biotek). The oxidative stress index was obtained by the ratio of the total oxidant status value with the total antioxidant status value (25,26). Trolox was used as the calibrator material in the analysis. trolox is known as a water-soluble vitamin E analog. Blood samples taken for oxidative stress analyses were kept at -80 degrees. In the analysis, oxidative stress parameters; total oxidative status (TOS), total antioxidant status (TAS), and Oxidative stress index (OSI), were evaluated.
2.5. Semi-Quantitative Scoring of Histopathologic Parameters
A semi-quantitative scoring was determined by examining the cells in the bone tissue such as osteoblast, osteocyte, and osteoclast. In the evaluation of histological sections, fifteen different areas were scanned for each slide, and then the average value of ten randomly selected cells was determined. From these averages, ten points were obtained for each animal group, and then these values were analyzed statistically. The method we used has also been used in previous histochemical studies of bone tissue [27–29].
2.6. Statistical Analysis
While evaluating the data in the research, a statistical analysis program of IBM SPSS Statistics 22 was used. Kolmogorov-Smirnov and Shapiro-Wilks tests were used to test whether the data in the study were normally distributed. In the analysis of data that was found to be normally distributed, One Way Anova was used to identify the group that caused the difference, and Tukey HSD test was used for comparisons between groups. In the analysis of data that was not normally distributed, Kruskall Wallis test was used to identify the group that caused the difference, and Mann Whitney U test was used for comparisons between groups.
It was determined that the parameters did not show normal distribution, The Kruskal-Wallis test was used in the comparison of the data between groups, and the Mann-Whitney U test was used in the pairwise comparisons to determine the group that caused the difference. While interpreting the results, 0.05 was used as the significance level; If p<0.05, the variables do not come from a normal distribution, and if p>0.05, the variables come from a normal distribution.
3. Results
Three rats (1 animal from each group) in total died during and after the experiment, as a result of the analyses performed on 21 animals. In
Table 1, the TAS value was 1.19±0.15 in the only implant integrated group, 1.38±0.09 in the capsaicin dosage 1 group, and 1.45±0.08 in the capsaicin dosage 2 group. These differences were also statistically significant (p<0.05). In
Table 1, the TOS value was 15.21±1.35 in the control animals, 11.81±0.65 in the capsaicin dosage 1 group, and 11.11±0.88 in the capsaicin dosage 2 group. These differences were also statistically significant (p<0.05). In
Table 1, the OSI value was 1.3±0.2 in the controls, 0.87±0.05 in the capsaicin dosage 1 group, and 0.77±0.07 in the capsaicin dosage 2 group. After the analysis, significant differences were obtained between the control group and the capsaicin groups (p<0.05). However, no significant differences were detected among the capsaicin treatment groups (p>0.05).
As a result of the analysis, although the BIC was higher numerically in the capsaicin-applied groups, no significant difference could be obtained (p>0.05). In
Table 2, the ratio of osseointegration of capsaicin treatment groups was measured. In the statistical analysis between the groups; although numerical osseointegration values were higher in experimental groups compared to controls, statistical difference was not found in terms of capsaicin administrated groups’ percentage values when compared with the controls (p>0.05) (
Figure 1).
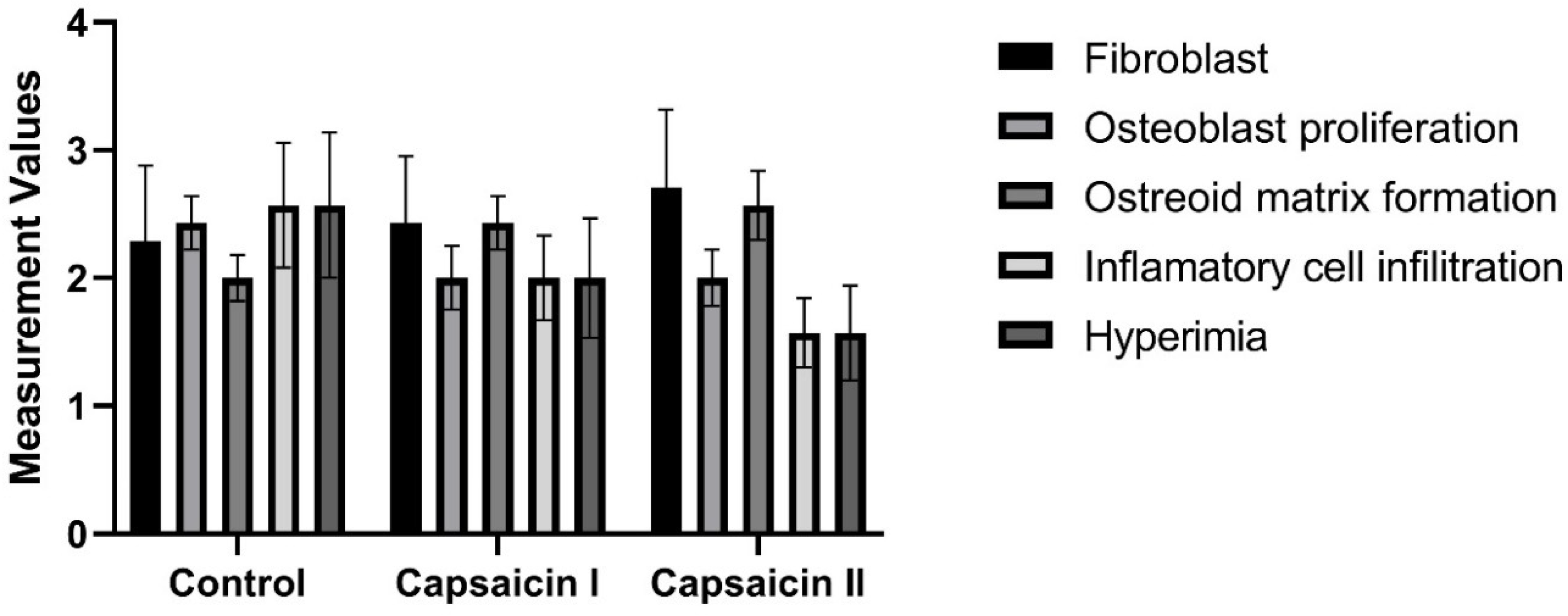
There is no statistically significant difference between groups in terms of fibroblast, osteoblast proliferation, osteoid matrix formation, inflammatory cell infiltration, and hyperemia measurement values (p>0.05) (
Table 3) (
Figure 2).
In
Table 2, the percentage values of Capsaicin administered to the rats were measured. In the statistical analyzes between the groups; No statistically significant difference was found in terms of capsaicin percentage values (p=0.134).
4. Discussion
Lucke et al. investigated the osteointegration of titanium implants in a study. Researchers conducted and evaluated days 7, 14, 21, 28, 35, and 42 of the study [30]. In this study, the osseointegration levels of titanium implants were evaluated on the 28th day to evaluate the early period osteointegration, which is important in implant-supported prosthetic treatment. Offley et al. found that sensory neurons function in the remodeling of the bone and that this event is regulated by transmitters released from peripheral nerve terminals [16].
In treatments using capsaicin, osteoclast count will increase, while bone volume, osteoblast count, and activity will decrease [16]. In another study, 37.5 mg/kg capsaicin was shown to not affect bone remodeling, while 150 mg/kg capsaicin was reported to increase TRAP 5b levels in plasma. However, since the osteocalcin concentration in the plasma is not affected, it has been claimed to contribute positively to sensory nerve innervation [16,17]. In this study, capsaicin with two doses (25 mg/kg and 50 mg/kg) was applied, and although not at a statistical level, the osseointegration level was determined to be higher at the numerical level in the capsaicin groups when compared to the controls. There are in vivo studies showing the effect of the sensory nervous system on bone tissue metabolism [5,17]. In a study where 150 mg/kg of capsaicin was applied, it was reported that the strength increased in the proximal tibia but the amount of trabecular bone decreased. In addition, high-dose capsaicin (150 mg/kg) has been claimed to increase bone fragility due to its support for osteoclast development [17]. Osseointegration means the adhesion of new bone to the implant surface without any fiber tissue [31]. Therefore, bone implant connection and bone metabolism in peri-implant tissues are closely related. 3 weeks after implantation, the areas of bone around the implant in the sensory denervated group were lower than in the sensory non-denervated group. It has also been reported that sensory denervations can reduce the activity of osteoblasts that support bone formation [16,17]. A study reported that capsaicin-induced small dorsal root ganglion cells, leading to the loss of unmyelinated sensory axons. Other studies have shown that CGRP inhibits osteoclastogenesis and bone resorption, while also stimulating osteoblast proliferation and bone formation [32–34].
Zhang et al. reported that capsaicin-sensitive sensory nerve fibers were CGRP-positive nerve fibers [17]. In addition, it has been reported that when regular capsaicin treatment is applied CGRP-positive nerve fibers are depleted, bone formation is inhibited and bone resorption is increased [35].
In the study by Zhang et al., a significant decrease in density in tibia cancellous bone mass was observed after capsaicin treatment. This shows that normal mechanical loading does not lead to a normal balance of bone remodeling when capsaicin is administered. It has also been suggested that hindlimb suspension (HLS) does not cause additional bone loss after capsaicin treatment depletes CGRP-positive nerve fibers [17].
In light of this information, in this study, 25 and 50 mg/kg capsaicin was administered systemically and no significant difference could be obtained between the groups. Capsaicin has a similar effect on bone metabolism in implant osseointegration. Apart from that, capsaicin appears to be a potent antioxidant in preventing in vivo free radical reactions. It was concluded in vivo that capsaicin consumed for several days may have a beneficial and potential therapeutic effect [24]. In this study, the oxidative stress values of the capsicin-applied groups were lower than the control group. A significant difference between the groups was reported. This shows us that systemic capsaicin has antioxidant properties. Sheppard et al. reported in 2022 that free oxygen radicals play an important role in the fracture healing process and showed that alpha-tocopherol, which has antioxidant properties, contributes to fracture healing by suppressing free oxygen radicals [36].
The study reported that capsaicin is a powerful antioxidant through different mechanisms [37]. In the study conducted by Chaudhary et al., statistically significant differences were obtained in the antioxidant levels in the plasma of rats administered capsaicin. As a result, it is reported that capsaicin functions to protect cells from oxidative stress damage [38]. A study was conducted on rats with hypercholesterolemia to examine their antioxidant levels. The study found no significant change in the levels of ascorbic acid and α-tocopherol after being fed a capsaicin diet [39].
On the first day after dental implants are applied, osteoblasts collapse on the implant surface covered by calcified fibrils and form collagen fibrils from osteoid tissue. Following the implantation process, reparative trabecular bone forms in the surrounding tissue of the implant [40]. Osteoblasts; in addition to being important for the osteogenesis process, induce the production of bone matrix proteins such as collagen type 1α1, osteocalcin, and alkaline phosphatase [41]. The dental implants themselves, their surface, tissue, and type of bone tissue are factors that affect osseointegration. In the osseointegration process, there are periods such as osteophilic, osteoinductive, osteoconductive, and osteoadaptive. The first and second of these periods require a good host bone bed and a maximum internal healing period [42]. In a rat study aiming to examine the effect of capsaicin on bone; bone loss was found to be higher in the capsaicin-free rat group compared to the capsaicin-treated group. Therefore, the current study was designed around the osseointegration process of capsaicin, which alters its healing potential [21]. In a rat study, the most common type of connective tissue formed during the healing phase in animals is fibroblasts, and these immature fibroblasts are extremely important for cell differentiation [43].
In the literature, a study in which capsaicin was given to rats at 125 mg/kg was claimed to cause bone loss and reduce bone formation. He explained this situation as being due to the difference in the age or species of the rats.
The literature suggests that the compensatory mechanism during the modeling phase of skeletal growth may interfere with bone formation inhibited by capsaicin treatment. Additionally, it has been reported that capsaicin neuronal ablation has a pro-resorptive effect that can lead to bone loss.
Inflammation is an extremely important mechanism for immunity, and a controlled inflammatory immune response is critical for bone formation, osseointegration, and successful regenerative capacity [44].
TRPV1 is directly related to oxidative stress in many studies including hippocampus, pain, ischemia-reperfusion, and brain damage [45,46]. We can explain the suppression of oxidative damage in capsaicin groups with TRPV1, which emerged in the study of TRPV1 of capsaicin.
Studies have reported that capsaicin has antioxidant properties. It has also been emphasized that capsaicin has a positive effect on the bone healing mechanism. In our study, different doses of capsaicin were administered. Bone cells and inflammation, which play a role in bone healing, were evaluated. As a result, an increase in bone cells was observed in the capsaicin groups. There was also a decrease in inflammation. In light of these results, there is a similarity between bone healing and implant osteointegration. Systemic applications of capsaicin may also positively affect this process.
5. Conclusions
In this study, systemic capsaicin was administered at different doses. Capsaicin has antioxidant activity, but it has not been successful in bone osteointegration. However, capsaicin was observed to affect osteoclasts. We think that this affects the oxidative stress mechanism. More studies are needed for more accurate and reliable results.
Author Contributions
Conceptualization, M.B.B and M.G.; methodology, S.D.; software, K.S..; validation, S.D.; formal analysis, G.A.; investigation, M.E.P.; resources, M.E.P.; data curation, M.G..; writing—original draft preparation, M.B.B.; writing—review and editing, M.G.; visualization, S.D.; supervision, M.G.; project administration, M.B.B.; funding acquisition, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
There is no funding.
Acknowledgments
The authors wish to thanks Implance Dental Implant System, AGS Medical Corporation, Istanbul,Turkey for providing the titanium implants.
Competing Interests
The authors declerate there is no competing interests. The authors do not have any financial interests, either directly or indirectly, in the products or information listed in the paper..
References
- Togari A. Adrenergic regulation of bone metabolism: possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc Res Tech. 2002 Jul 15;58(2):77-84. [CrossRef]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002 Nov 1;111(3):305-317. [CrossRef]
- Togari A, Arai M, Kondo A. The role of the sympathetic nervous system in controlling bone metabolism. Expert Opin Ther Targets. 2005;9(5):931-40. [CrossRef]
- Togari A, Arai M. Pharmacological topics of bone metabolism: the physiological function of the sympathetic nervous system in modulating bone resorption. J Pharmacol Sci. 2008;106(4):542-546. [CrossRef]
- Togari A, Arai M, Kondo H. The neuro-osteogenic network: The sympathetic regulation of bone resorption. Japanese Dental Science Review. 2012;48(2):61-70. [CrossRef]
- Ishizuka K, Hirukawa K, Nakamura H, Togari A. Inhibitory effect of CGRP on osteoclast formation by mouse bone marrow cells treated with isoproterenol. Neurosci Lett. 2005 Apr 29;379(1):47-51. [CrossRef]
- Yamashiro T, Fujiyama K, Fujiyoshi Y, Inaguma N, Takano-Yamamoto T. Inferior alveolar nerve transection inhibits the increase in osteoclast appearance during experimental tooth movement. Bone. 2000 Jun;26(6):663-669. [CrossRef]
- Hara-Irie F, Amizuka N, Ozawa H. Immunohistochemical and ultrastructural localization of CGRP-positive nerve fibers at the epiphyseal trabecules facing the growth plate of rat femurs. Bone. 1996 Jan;18(1):29-39. [CrossRef]
- Hukkanen M, Konttinen YT, Santavirta S, Paavolainen P, Gu XH, Terenghi G, Polak JM. Rapid proliferation of calcitonin gene-related peptide-immunoreactive nerves during healing of rat tibial fracture suggests neural involvement in bone growth and remodelling. Neuroscience. 1993;54(4):969-979. [CrossRef]
- Li J, Ahmad T, Spetea M, Ahmed M, Kreicbergs A. Bone reinnervation after fracture: a study in the rat. J Bone Miner Res. 2001 Aug;16(8):1505-1510. [CrossRef]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997 Oct 23;389(6653):816-824. [CrossRef]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487-517. [CrossRef]
- Newson PN, van den Buuse M, Martin S, Lynch-Frame A, Chahl LA. Effects of neonatal treatment with the TRPV1 agonist, capsaicin, on adult rat brain and behaviour. Behav Brain Res. 2014;272:55-65. [CrossRef]
- Hwang SJ, Oh JM, Valtschanoff JG. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005 Jun 21;1047(2):261-266. [CrossRef]
- Adam C, Llorens A, Baroukh B, Cherruau M, Saffar JL. Effects of capsaicin-induced sensory denervation on osteoclastic resorption in adult rats. Exp Physiol. 2000;85(1):62-66. [CrossRef]
- Offley SC, Guo TZ, Wei T, Clark JD, Vogel H, Lindsey DP, Jacobs CR, Yao W, Lane NE, Kingery WS. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J Bone Miner Res. 2005;20(2):257-267. [CrossRef]
- Zhang ZK, Guo X, Lao J, Qin YX. Effect of capsaicin-sensitive sensory neurons on bone architecture and mechanical properties in the rat hindlimb suspension model. J Orthop Translat. 2017;10:12-17. [CrossRef]
- Wada S, Kojo T, Wang YH, Ando H, Nakanishi E, Zhang M, Fukuyama H, Uchida Y. Effect of loading on the development of nerve fibers around oral implants in the dog mandible. Clin Oral Implants Res. 2001;12(3):219-224. [CrossRef]
- Huang B, Ye J, Zeng X, Gong P. Effects of capsaicin-induced sensory denervation on early implant osseointegration in adult rats. R Soc Open Sci. 2019;6(1):181082. [CrossRef]
- Yerit KC, Posch M, Seemann M, Hainich S, Dörtbudak O, Turhani D, Ozyuvaci H, Watzinger F, Ewers R. Implant survival in mandibles of irradiated oral cancer patients. Clin Oral Implants Res. 2006;17(3):337-344. [CrossRef]
- Hill EL, Turner R, Elde R. Effects of neonatal sympathectomy and capsaicin treatment on bone remodeling in rats. Neuroscience, 1991;44:747-55. [CrossRef]
- Fiorellini JP, Nevins ML. Dental Implant Considerations in the Diabetic Patient. Periodontology 2000. 2000;23(1):73-77. [CrossRef]
- Aljateeli M, Wang HL. Implant Microdesigns and Their Impact on Osseointegration. Implant Dentistry. 2013;22(2):127-132. [CrossRef]
- Lee CY, Kim M, Yoon SW, Lee CH. Short-term control of capsaicin on blood and oxidative stress of rats in vivo. Phytother Res. 2003 May;17(5):454-458. [CrossRef]
- Koyuncu I, Kocyigit A, Gonel A, Arslan E, Durgun M. The Protective Effect of Naringenin-Oxime on Cisplatin-Induced Toxicity in Rats. Biochem Res Int. 2017;2017:9478958. [CrossRef]
- Erel O. A new automated colorimetric method formeasuring total oxidant status. Clin Biochem. 2005;38(12):1103-1111. [CrossRef]
- Miron RJ, Zhang Q, Sculean A, et al. Osteoinductive potential of 4 commonly employed bone grafts. Clin Oral Investig. 2016;20:2259-65. [CrossRef]
- Lee MK, DeConde AS, Lee M, et al. Biomimetic scaffolds facilitate healing of critical-sized segmental mandibular defects. Am J Otolaryngol. 2015;36:1-6. [CrossRef]
- Erdmann N, Bondarenko A, Hewicker-Trautwein M, et al. Evaluation of the soft tissue biocompatibility of MgCa0.8 and surgical steel 316L in vivo: a comparative study in rabbits. Biomed Eng Online. 2010;9:63. [CrossRef]
- Lucke M, Schmidmaier G, Sadoni S, Wildemann B, Schiller R, Haas NP, Raschke M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone. 2003;32(5):521-531. [CrossRef]
- Franchi M, Fini M, Martini D, Orsini E, Leonardi L, Ruggeri A, Giavaresi G, Ottani V. Biological fixation of endosseous implants. Micron. 2005;36(7-8):665-671. [CrossRef]
- Takahashi N, Matsuda Y, Sato K, de Jong PR, Bertin S, Tabeta K, Yamazaki K. Neuronal TRPV1 activation regulates alveolar bone resorption by suppressing osteoclastogenesis via CGRP. Sci Rep. 2016;6:29294. [CrossRef]
- Granholm S, Henning P, Lerner UH. Comparisons between the effects of calcitonin receptor-stimulating peptide and intermedin and other peptides in the calcitonin family on bone resorption and osteoclastogenesis. J Cell Biochem. 2011;112(11):3300-3012. [CrossRef]
- Tian G, Zhang G, Tan YH. Calcitonin gene-related peptide stimulates BMP-2 expression and the differentiation of human osteoblast-like cells in vitro. Acta Pharmacol Sin. 2013;34(11):1467-1474. [CrossRef]
- Chen J, Ma G, Liu W, Liu Y, Ding Y. The influence of the sensory neurotransmitter calcitonin gene-related peptide on bone marrow mesenchymal stem cells from ovariectomized rats. J Bone Miner Metab. 2017;35(5):473-484. [CrossRef]
- Sheppard, A.J., Barfield, M.A., Barton, S., Dong, Y. 2022. Understanding Reactive Oxygen Species in Bone Regeneration: A Glance at Potential Therapeutics and Bioengineering Applications. Frontiers in Bioengineering and Biotechnology, 10. [CrossRef]
- Ertekin F, Keçeci T. The effect of capsaicin on TBARS and TAS in rats with hypothyroidism. Journal of Istanbul Veterinary Sciences,2022;6(2):98-104. [CrossRef]
- Chaudhary, A., Gour, J. K., & Rizvi, S. I. (2022). Capsaicin has potent anti-oxidative effects in vivo through a mechanism which is non-receptor mediated. Archives of physiology and biochemistry, 128(1), 141-147. [CrossRef]
- Manjunatha, H., & Srinivasan, K. (2007). Hypolipidemic and antioxidant effects of curcumin and capsaicin in high-fat-fed rats. Canadian Journal of Physiology and Pharmacology, 85(6), 588–596. [CrossRef]
- Marco, F.; Milena, F.; Gianluca, G.; Vittoria, O. Peri-implant osteogenesis in health and osteoporosis. Micron 2005, 36, 630–644. [CrossRef]
- Jensen, E.D.; Gopalakrishnan, R.; Westendorf, J.J. Regulation of gene expression in osteoblasts. Biofactors 2010, 36, 25–32. [CrossRef]
- Nielsen JJ, Low SA, Ramseier NT, Hadap RV, Young NA, Wang M, et al. Analysis of the bone fracture targeting properties of osteotropic ligands. J Control Release 2021;329:570-84. [CrossRef]
- Ramanathan V, Venugopalan S, Ganapathy D, Ramadoss R, Kumar SM, Kannan RK, Jayakumar A, Duraisamy R. Effect of Dietary Amino Acids L-Arginine and Lysine on Implant Osseointegration. J Pharm Bioallied Sci. 2022 Jul;14(Suppl 1): S106-S109. Epub 2022 Jul 13. [CrossRef] [PubMed] [PubMed Central]
- Amengual-Peñafiel, L., Brañes-Aroca, M., Marchesani-Carrasco, F., Jara-Sepúlveda, M. C., Parada-Pozas, L., & Cartes-Velásquez, R. (2019). Coupling between Osseointegration and Mechanotransduction to Maintain Foreign Body Equilibrium in the Long-Term: A Comprehensive Overview. Journal of Clinical Medicine, 8(2), 139. [CrossRef]
- Huang T, Lin Y, Pang Q, Shen W, Chen X, Tu F. The Synergistic Effect of TRPV1 on Oxidative Stress-Induced Autophagy and Apoptosis in Microglia. Anal Cell Pathol (Amst). 2021 Jul 15;2021:7955791. [CrossRef] [PubMed] [PubMed Central]
- Yazğan , Nazıroğlu M. Ovariectomy-Induced Mitochondrial Oxidative Stress, Apoptosis, and Calcium Ion Influx Through TRPA1, TRPM2, and TRPV1 Are Prevented by 17β-Estradiol, Tamoxifen, and Raloxifene in the Hippocampus and Dorsal Root Ganglion of Rats. Mol Neurobiol 54, 7620–7638 (2017). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).