Submitted:
30 May 2024
Posted:
31 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Biological Material
2.3. Genetic Testing
2.4. Chemical Biopsy (Solid-Phase Microextraction) Protocol and LC-HRMS Analysis
2.5. Data Processing and Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Nowosielski, M.; Galldiks, N.; Iglseder, S.; Kickingereder, P.; Von Deimling, A.; Bendszus, M.; Wick, W.; Sahm, F. Diagnostic challenges in meningioma. Neuro. Oncol. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bi, W.L.; Dunn, I.F. Medical management of meningioma in the era of precision medicine. Neurosurg. Focus 2018, 44. [Google Scholar] [CrossRef]
- Ghalavand, M.A.; Asghari, A.; Farhadi, M.; Taghizadeh-Hesary, F.; Garshasbi, M.; Falah, M. The genetic landscape and possible therapeutics of neurofibromatosis type 2. Cancer Cell Int. 2023, 23. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, D.S.; Semenova, G.; Kuo, Y.M.; Andrews, A.J.; Ammoun, S.; Hanemann, C.O.; Chernoff, J. An essential role for the tumor-suppressor merlin in regulating fatty acid synthesis. Cancer Res. 2017, 77. [Google Scholar] [CrossRef]
- Melone, M.A.B.; Valentino, A.; Margarucci, S.; Galderisi, U.; Giordano, A.; Peluso, G. The carnitine system and cancer metabolic plasticity review-article. Cell Death Dis. 2018, 9, 228. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Adams, S.H. Acylcarnitines-old actors auditioning for new roles in metabolic physiology. Nat. Rev. Endocrinol. 2015, 11. [Google Scholar] [CrossRef]
- Bogusiewicz, J.; Burlikowska, K.; Jaroch, K.; Gorynska, P.Z.; Gorynski, K.; Birski, M.; Furtak, J.; Paczkowski, D.; Harat, M.; Bojko, B. Profiling of carnitine shuttle system intermediates in gliomas using solid-phase microextraction (SPME). Molecules 2021, 26, 6112. [Google Scholar] [CrossRef]
- Kant, S.; Kesarwani, P.; Prabhu, A.; Graham, S.F.; Buelow, K.L.; Nakano, I.; Chinnaiyan, P. Enhanced fatty acid oxidation provides glioblastoma cells metabolic plasticity to accommodate to its dynamic nutrient microenvironment. Cell Death Dis. 2020, 11. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X.; Zhang, Y.; Zhang, K.; Zhan, C.; Shi, X.; Li, Y.; Zhao, J.; Bai, Y.; Wang, Y.; et al. Metabolic profiling analysis upon acylcarnitines in tissues of hepatocellular carcinoma revealed the inhibited carnitine shuttle system caused by the downregulated carnitine palmitoyltransferase 2. Mol. Carcinog. 2019, 58, 749–759. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacı, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef] [PubMed]
- Bogusiewicz, J.; Kupcewicz, B.; Goryńska, P.Z.; Jaroch, K.; Goryński, K.; Birski, M.; Furtak, J.; Paczkowski, D.; Harat, M.; Bojko, B. Investigating the Potential Use of Chemical Biopsy Devices to Characterize Brain Tumor Lipidomes. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Płotka-Wasylka, J.; Tobiszewski, M. How to evaluate methods used in chemical laboratories in terms of the total chemical risk? – a ChlorTox Scale. Green Anal. Chem. 2023, 5. [Google Scholar] [CrossRef]
- Petrilli, A.M.; Fernández-Valle, C. Role of merlin/NF2 inactivation in tumor biology. Oncogene 2016, 35. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Karas, P.J.; Hadley, C.C.; Bayley, V.J.C.; Basit Khan, A.; Jalali, A.; Sweeney, A.D.; Klisch, T.J.; Patel, A.J. The role of merlin/NF2 loss in meningioma biology. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, X.; Wu, Y. Effects of Glucose Metabolism, Lipid Metabolism, and Glutamine Metabolism on Tumor Microenvironment and Clinical Implications. Biomolecules 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Zoni, E.; Minoli, M.; Bovet, C.; Wehrhan, A.; Piscuoglio, S.; Ng, C.K.Y.; Gray, P.C.; Spahn, M.; Thalmann, G.N.; Kruithof-De Julio, M. Preoperative plasma fatty acid metabolites inform risk of prostate cancer progression and may be used for personalized patient stratification. BMC Cancer 2019, 19, 1–18. [Google Scholar] [CrossRef]
- Yaligar, J.; Teoh, W.W.; Othman, R.; Verma, S.K.; Phang, B.H.; Lee, S.S.; Wang, W.W.; Toh, H.C.; Gopalan, V.; Sabapathy, K.; et al. Longitudinal metabolic imaging of hepatocellular carcinoma in transgenic mouse models identifies acylcarnitine as a potential biomarker for early detection. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Yu, D.; Xuan, Q.; Zhang, C.; Hu, C.; Li, Y.; Zhao, X.; Liu, S.; Ren, F.; Zhang, Y.; Zhou, L.; et al. Metabolic Alterations Related to Glioma Grading Based on Metabolomics and Lipidomics Analyses. Metabolites 2020, 10, 478. [Google Scholar] [CrossRef]
- Nam, S.L.; Paulina de la Mata, A.; Dias, R.P.; Harynuk, J.J. Towards standardization of data normalization strategies to improve urinary metabolomics studies by gc×gc-tofms. Metabolites 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xia, Y. Pretreating and normalizing metabolomics data for statistical analysis. Genes Dis. 2024, 11. [Google Scholar] [CrossRef]
- Nam, S.L.; Giebelhaus, R.T.; Tarazona Carrillo, K.S.; de la Mata, A.P.; Harynuk, J.J. Evaluation of normalization strategies for GC-based metabolomics. Metabolomics 2024, 20. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zheng, X.; Yang, M.; Zhao, A.; Li, M.; Chen, T.; Panee, J.; Jia, W.; Ji, G. Serum lipid alterations identified in chronic hepatitis B, hepatitis B virus-associated cirrhosis and carcinoma patients. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Liu, S.; Li, J.; Wu, L.; Lv, X.; Xu, J.; Chen, B.; Zhao, S.; Yang, H. CPT2 down-regulation promotes tumor growth and metastasis through inducing ROS/NFκB pathway in ovarian cancer. Transl. Oncol. 2021, 14. [Google Scholar] [CrossRef]
- Zeng, K.; Li, Q.; Song, G.; Chen, B.; Luo, M.; Miao, J.; Liu, B. CPT2-mediated fatty acid oxidation inhibits tumorigenesis and enhances sorafenib sensitivity via the ROS/PPARγ/NF-κB pathway in clear cell renal cell carcinoma. Cell. Signal. 2023, 110. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, X.; Yan, H.; Wu, J.; Yang, Y.; He, J.; Chen, J.; Jiang, Z.; Wu, F.; Jiang, Z. Downregulation of CPT2 promotes proliferation and inhibits apoptosis through p53 pathway in colorectal cancer. Cell. Signal. 2022, 92. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Lv, D.; Zheng, Y.; Wu, M.; Xu, C.; Zhang, Q.; Wu, L. Downregulation of CPT2 promotes tumorigenesis and chemoresistance to cisplatin in hepatocellular carcinoma. Onco. Targets. Ther. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Nakagawa, H.; Enooku, K.; Kudo, Y.; Hayata, Y.; Nakatsuka, T.; Tanaka, Y.; Tateishi, R.; Hikiba, Y.; Misumi, K.; et al. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut 2018, 67. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Bogusiewicz, J.; Bojko, B. Insight into new opportunities in intra-surgical diagnostics of brain tumors. TrAC Trends Anal. Chem. 2023, 162, 117043. [Google Scholar] [CrossRef]
- Bogusiewicz, J.; Gaca-Tabaszewska, M.; Olszówka, D.; Jaroch, K.; Furtak, J.; Harat, M.; Pawliszyn, J.; Bojko, B. Coated Blade Spray-Mass Spectrometry as a New Approach for the Rapid Characterization of Brain Tumors. Molecules 2022, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tascon, M.; Alam, M.N.; Gómez-Ríos, G.A.; Pawliszyn, J. Development of a Microfluidic Open Interface with Flow Isolated Desorption Volume for the Direct Coupling of SPME Devices to Mass Spectrometry. Anal. Chem. 2018, 90. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Bis, A.; Zima, A. ChlorTox Base – a useful source of information on popular reagents in terms of chemical hazards and greenness assessment. Green Anal. Chem. 2023, 6. [Google Scholar] [CrossRef]
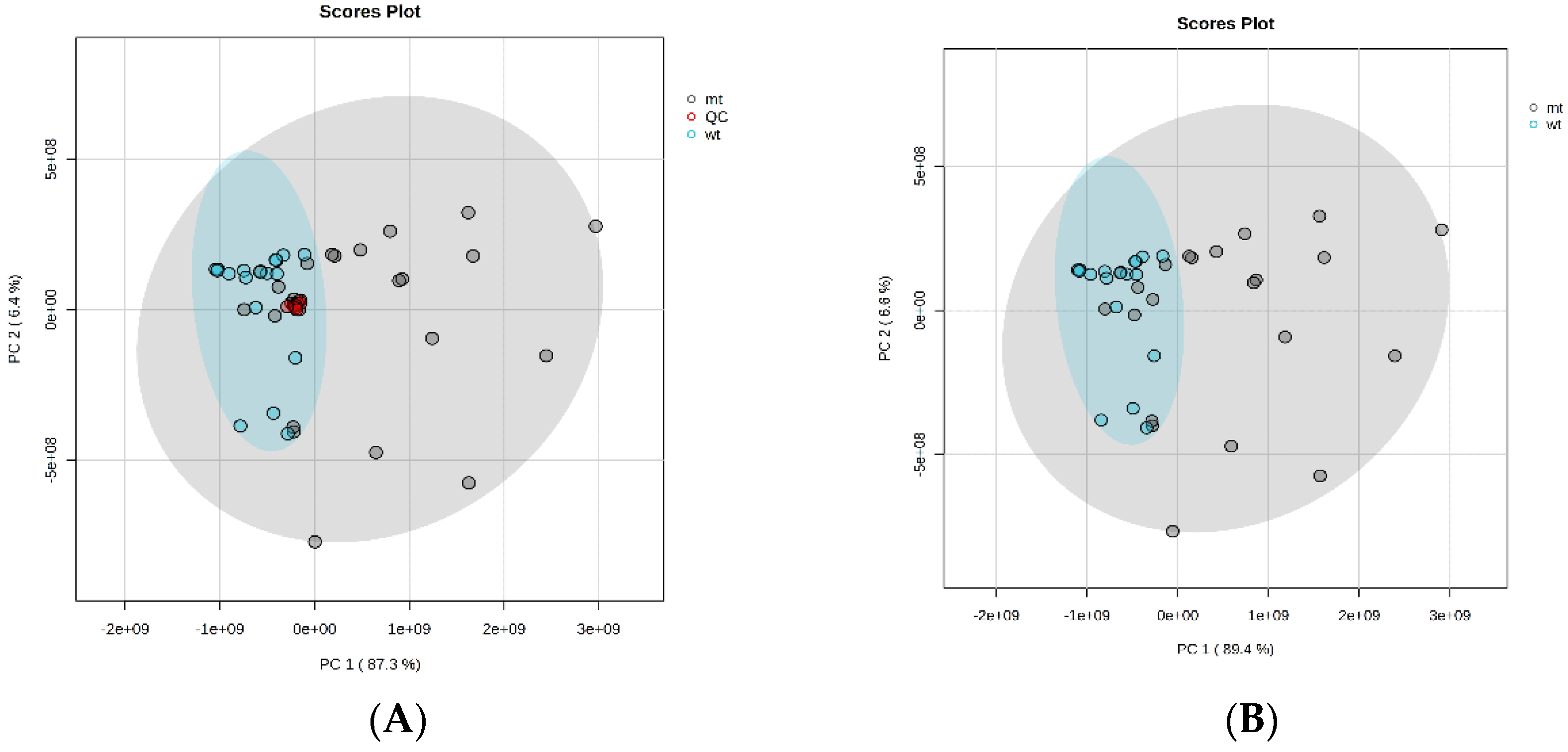
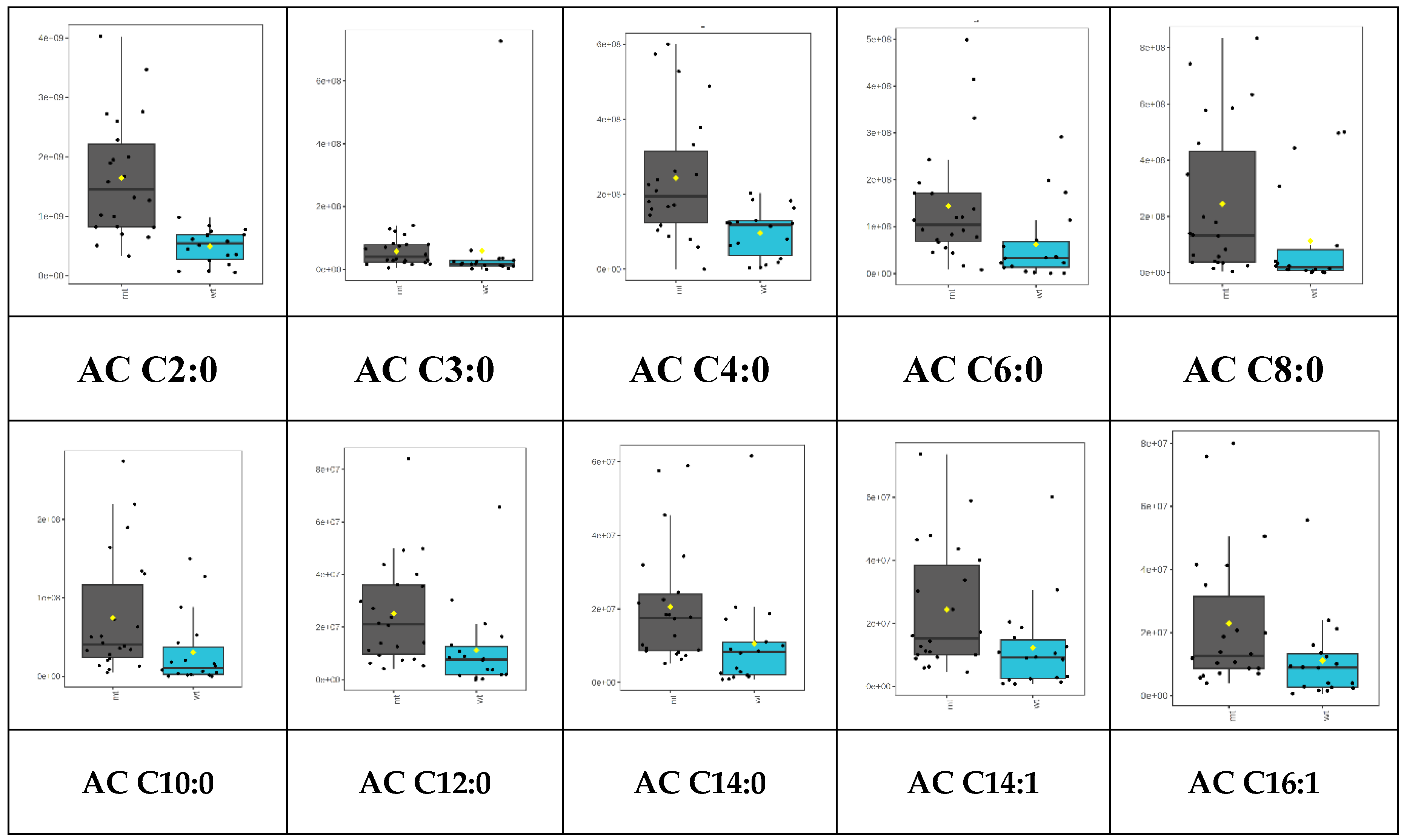
| Acylcarnitine | m/z | RT | Raw data | Normalized data | |||||
|---|---|---|---|---|---|---|---|---|---|
| NF2mt/ NF2wt ratio | p-value | FDR | NF2mt/ NF2wt ratio | p-value | FDR | ||||
| SCAC | AC C2:0 | 204.1230 | 13.49 | 3.33 | <0.05 | <0.05 | 1.19 | 0.095 | 0.300 |
| AC C3:0 | 218.1387 | 11.94 | 2.50 | <0.05 | <0.05 | 0.51 | 0.737 | 0.789 | |
| AC C4:0 | 232.1543 | 10.70 | 2.50 | <0.05 | <0.05 | 0.93 | 0.527 | 0.719 | |
| AC C5:0 | 246.1700 | 9.92 | 2.28 | 0.206 | 0.219 | 0.77 | 0.100 | 0.300 | |
| MCAC | AC C6:0 | 260.1856 | 9.28 | 2.31 | <0.05 | <0.05 | 1.15 | 0.251 | 0.470 |
| AC C8:0 | 288.2169 | 8.60 | 2.16 | <0.05 | <0.05 | 1.22 | 0.155 | 0.388 | |
| AC C10:0 | 316.2484 | 8.24 | 2.41 | <0.05 | <0.05 | 1.29 | 0.219 | 0.468 | |
| AC C10:1 | 314.2326 | 8.29 | 1.77 | 0.066 | 0.066 | 0.69 | 0.946 | 0.989 | |
| AC C12:0 | 344.2796 | 7.95 | 2.21 | <0.05 | <0.05 | 1.21 | 0.657 | 0.758 | |
| LCAC | AC C14:0 | 372.3108 | 7.75 | 1.94 | <0.05 | <0.05 | 1.02 | 0.657 | 0.758 |
| AC C14:1 | 370.2952 | 7.73 | 2.00 | <0.05 | <0.05 | 1.07 | 0.459 | 0.688 | |
| AC C16:0 | 400.3423 | 7.63 | 1.63 | 0.055 | 0.074 | 0.60 | <0.05 | 0.196 | |
| AC C16:1 | 398.3266 | 7.65 | 2.06 | <0.05 | <0.05 | 1.08 | 0.367 | 0.611 | |
| AC C18:0 | 428.3734 | 7.63 | 1.11 | 0.219 | 0.219 | 0.42 | <0.05 | 0.176 | |
| AC C18:1 | 426.3579 | 7.49 | 1.68 | 0.119 | 0.137 | 0.69 | <0.05 | 0.178 | |
| Analysis step | Reagents | CAS | CHsub | ChlorTox [g] | Total ChlorTox [g] |
|---|---|---|---|---|---|
| SPME | Methanol | 67-56-1 | 4.81 | 0.08 | 0.13 |
| Isopropanol | 67-63-0 | 3.13 | 0.05 | ||
| Instrumental Analysis | Ammonium acetate | 631-61-8 | 0.00 | 0.00 | 3.87 |
| Acetonitrile | 75-05-8 | 2.25 | 3.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





