Submitted:
30 May 2024
Posted:
31 May 2024
You are already at the latest version
Abstract
Keywords:
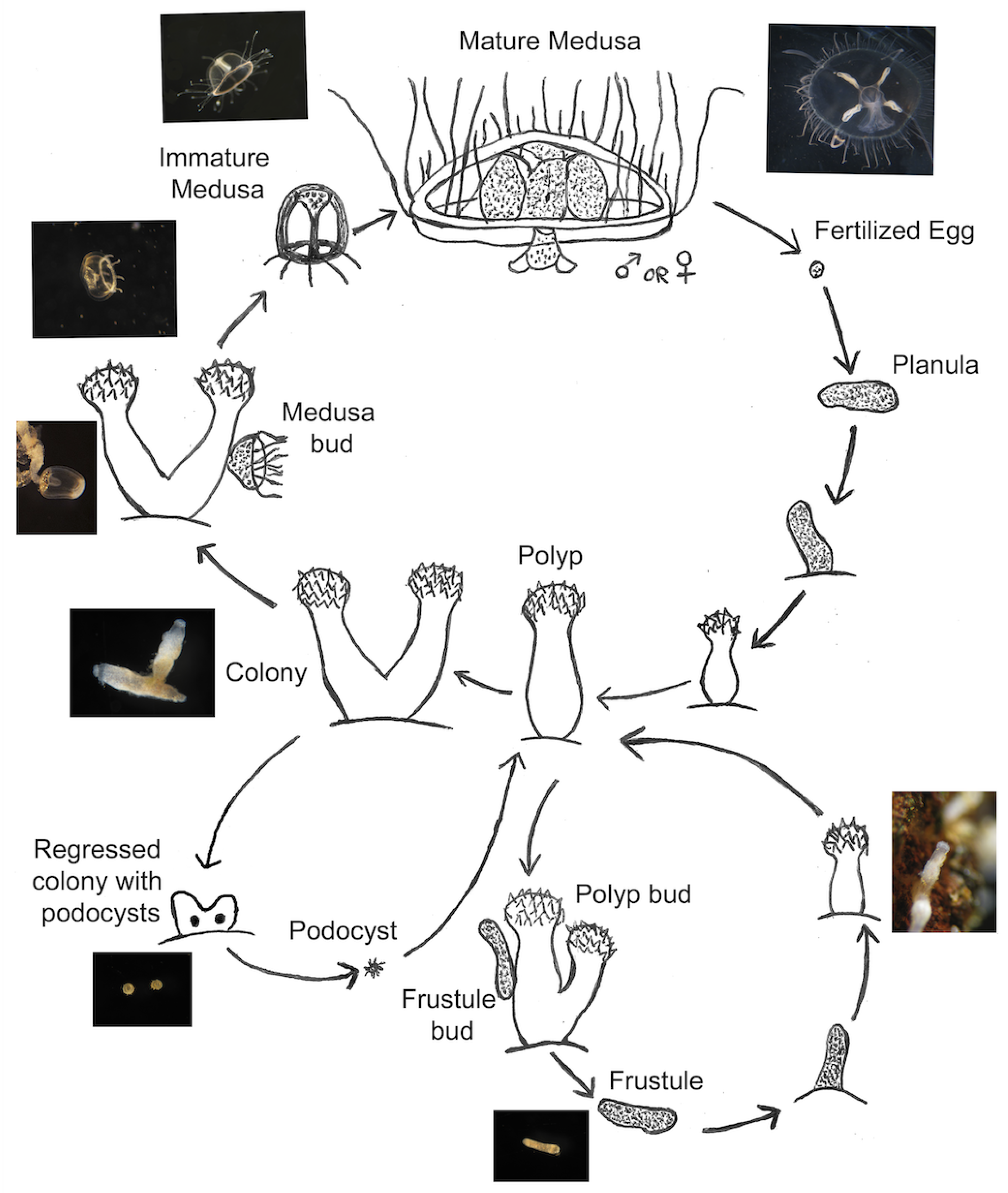
1. Introduction
2. Materials and Methods
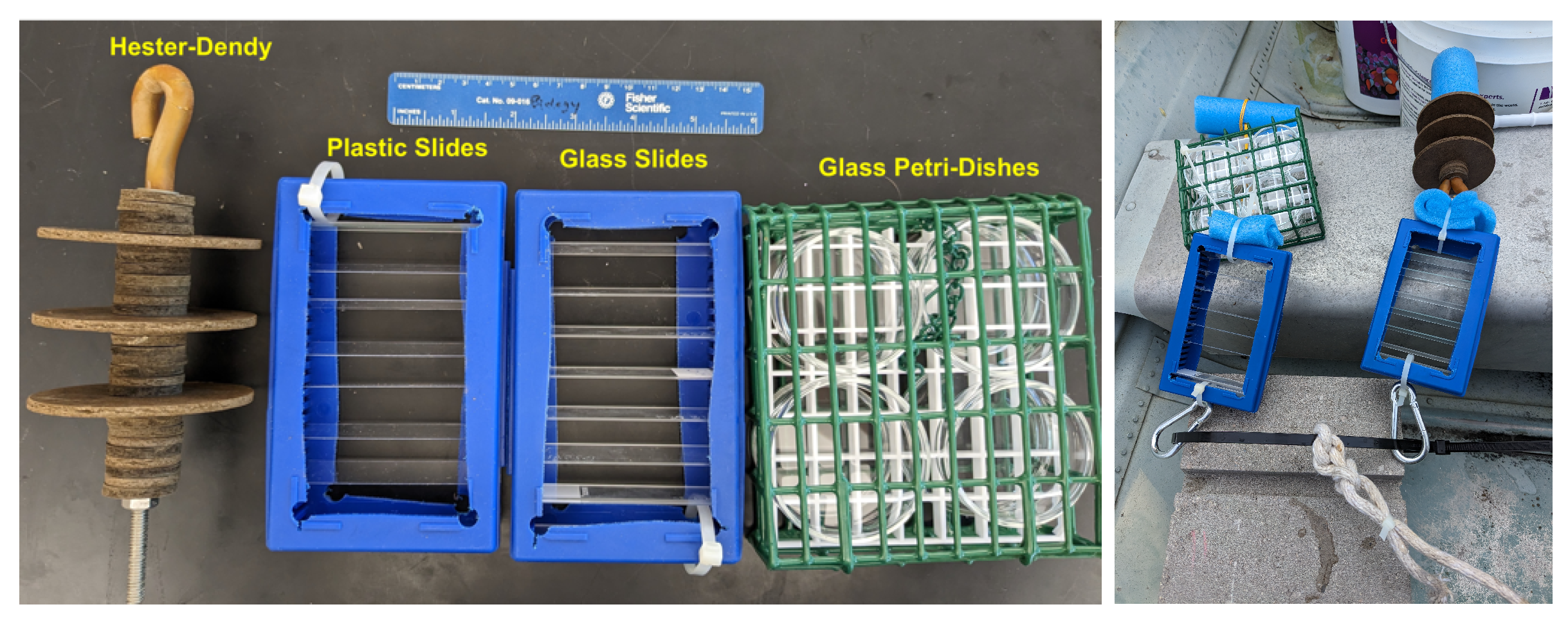
2.1. Laboratory Pilot Substrate Study
2.2. Construction of Sampling Apparatus
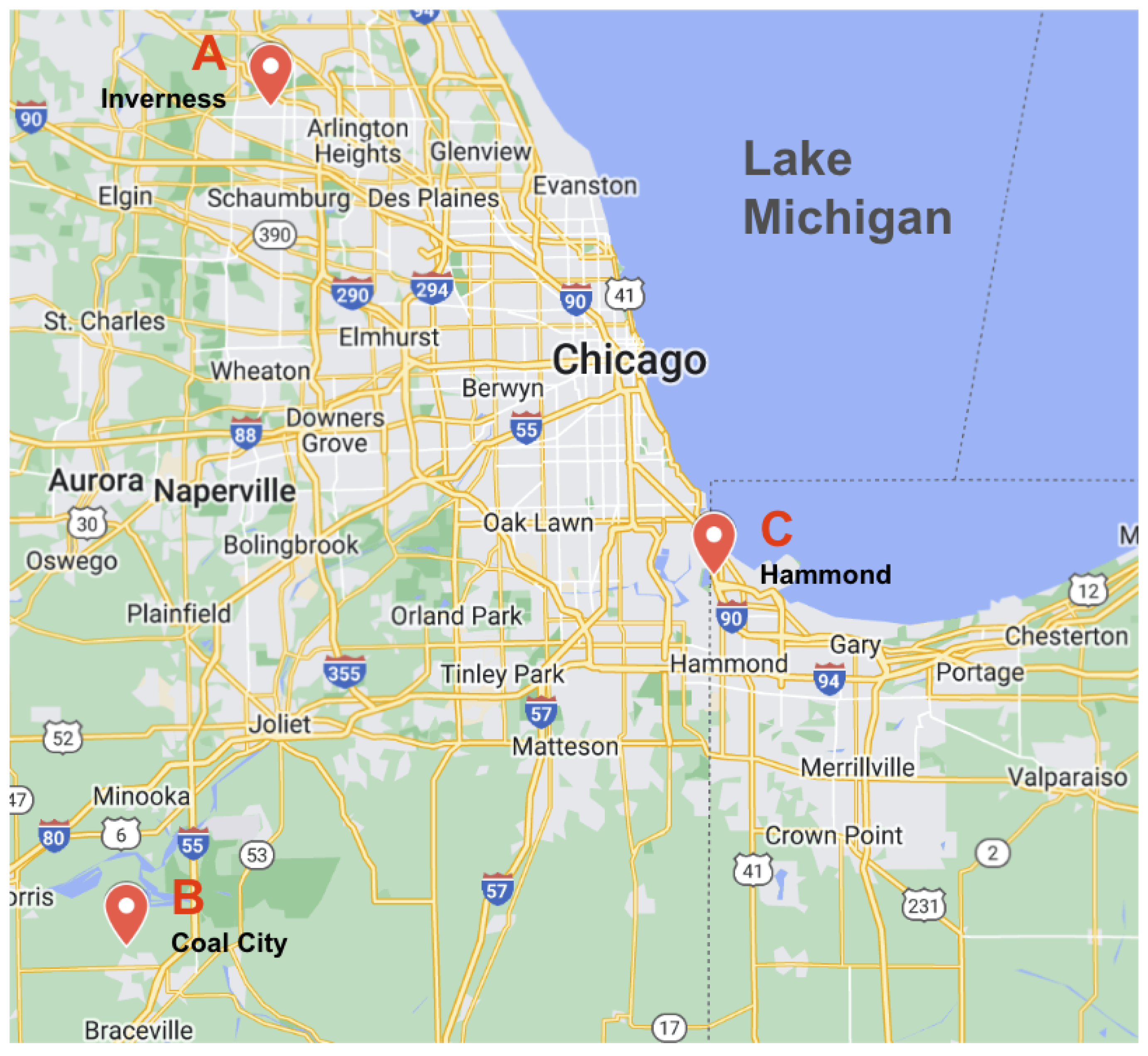
2.3. Field Site Descriptions
2.4. Setup Deployments and Counting
2.5. Follow-Up Laboratory Experiments
3. Results
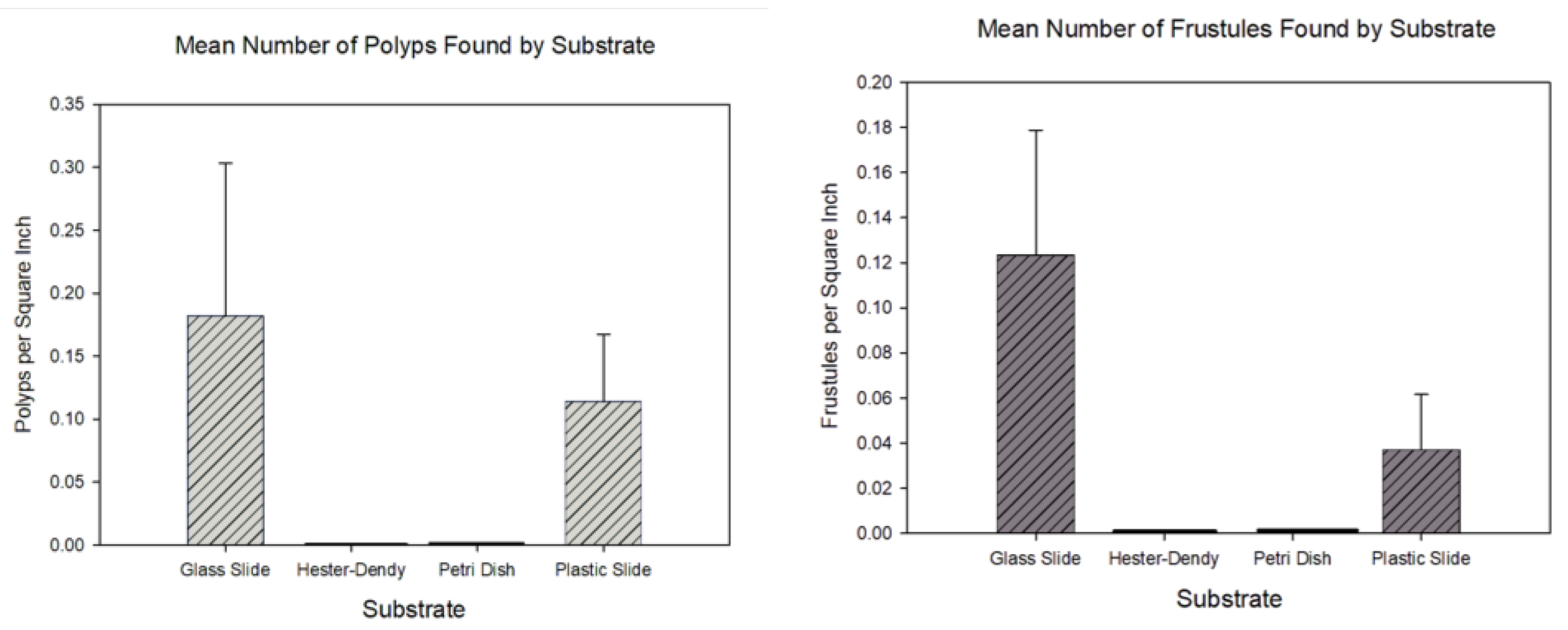
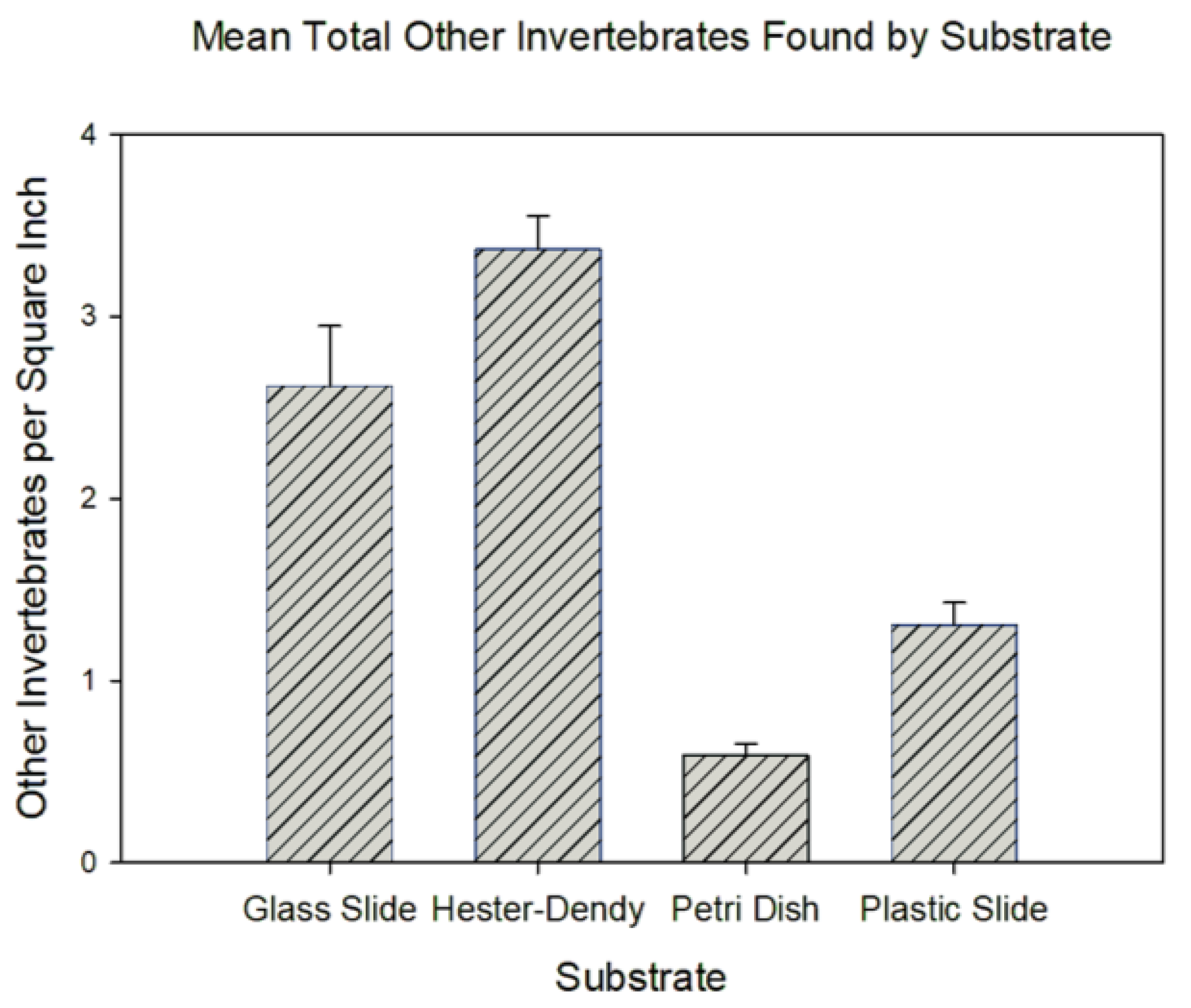
3.1. Pilot Laboratory Substrates
3.2. Field Results
3.3. Follow-Up Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchessaux, G.; Lüskow, F.; Bejean, M.; Pakhomov, E.A. Increasing temperature facilitates polyp spreading and medusa appearance of the invasive hydrozoan Craspedacusta sowerbii. Biology 2022, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.A.; Westerman, E.L.; Harris, L.G. Elevated seasonal temperatures eliminate thermal barriers of reproduction of a dominant invasive species: A community state change for northern communities? Diversity and Distributions 2017, 23, 1182–1192. [Google Scholar] [CrossRef]
- Walsh, J.R.; Hansen, G.J.; Read, J.S.; Vander Zanden, M.J. Comparing models using air and water temperature to forecast an aquatic invasive species response to climate change. Ecosphere 2020, 11, e03137. [Google Scholar] [CrossRef]
- Beric, B.; MacIsaac, H.J. Determinants of rapid response success for alien invasive species in aquatic ecosystems. Biological Invasions 2015, 17, 3327–3335. [Google Scholar] [CrossRef]
- Coughlan, N.E.; Lyne, L.; Cuthbert, R.N.; Cunningham, E.M.; Lucy, F.E.; Davis, E.; Caffrey, J.M.; Dick, J.T. In the black: Information harmonisation and educational potential amongst international databases for invasive alien species designated as of union concern. Global Ecology and Conservation 2020, 24, e01332. [Google Scholar] [CrossRef]
- Simberloff, D. Maintenance management and eradication of established aquatic invaders. Hydrobiologia 2021, 848, 2399–2420. [Google Scholar] [CrossRef]
- Soto, I.; Cuthbert, R.N.; Ahmed, D.A.; Kouba, A.; Domisch, S.; Marquez, J.R.; Beidas, A.; Amatulli, G.; Kiesel, J.; Shen, L.Q.; et al. Tracking a killer shrimp: Dikerogammarus villosus invasion dynamics across Europe. Diversity and Distributions 2023, 29, 157–172. [Google Scholar] [CrossRef]
- Linders, T.E.W.; Schaffner, U.; Eschen, R.; Abebe, A.; Choge, S.K.; Nigatu, L.; Mbaabu, P.R.; Shiferaw, H.; Allan, E. Direct and indirect effects of invasive species: Biodiversity loss is a major mechanism by which an invasive tree affects ecosystem functioning. Journal of Ecology 2019, 107, 2660–2672. [Google Scholar] [CrossRef]
- Britton, J.R.; Lynch, A.J.; Bardal, H.; Bradbeer, S.J.; Coetzee, J.A.; Coughlan, N.E.; Dalu, T.; Tricarico, E.; Gallardo, B.; Lintermans, M.; et al. Preventing and controlling nonnative species invasions to bend the curve of global freshwater biodiversity loss. Environmental Reviews 2023, 31, 310–326. [Google Scholar] [CrossRef]
- Scalici, M.; Chiesa, S.; Mancinelli, G.; Rontani, P.M.; Voccia, A.; Nonnis Marzano, F. Euryhaline aliens invading Italian inland waters: The case of the Atlantic blue crab Callinectes sapidus Rathbun, 1896. Applied Sciences 2022, 12, 4666. [Google Scholar] [CrossRef]
- Lord, J.P. Impact of seawater temperature on growth and recruitment of invasive fouling species at the global scale. Marine ecology 2017, 38, e12404. [Google Scholar] [CrossRef]
- Emery-Butcher, H.E.; Beatty, S.J.; Robson, B.J. The impacts of invasive ecosystem engineers in freshwaters: A review. Freshwater Biology 2020, 65, 999–1015. [Google Scholar] [CrossRef]
- Ricciardi, A.; Iacarella, J.C.; Aldridge, D.C.; Blackburn, T.M.; Carlton, J.T.; Catford, J.A.; Dick, J.T.; Hulme, P.E.; Jeschke, J.M.; Liebhold, A.M.; et al. Four priority areas to advance invasion science in the face of rapid environmental change. Environmental Reviews 2021, 29, 119–141. [Google Scholar] [CrossRef]
- Angel, D.L.; Edelist, D.; Freeman, S. Local perspectives on regional challenges: Jellyfish proliferation and fish stock management along the Israeli Mediterranean coast. Regional Environmental Change 2016, 16, 315–323. [Google Scholar] [CrossRef]
- González-Duarte, M.M.; Megina, C.; López-González, P.J.; Galil, B. Cnidarian alien species in expansion. The Cnidaria, Past, Present and Future: The world of Medusa and her sisters 2016, pp. 139–160.
- Purcell, J.E. Successes and challenges in jellyfish ecology: Examples from Aequorea spp. Marine Ecology Progress Series 2018, 591, 7–27. [Google Scholar] [CrossRef]
- Rodrigues, T.; Domínguez-Pérez, D.; Almeida, D.; Matos, A.; Antunes, A. Medusozoans reported in Portugal and its ecological and economical relevance. Regional studies in marine science 2020, 35, 101230. [Google Scholar] [CrossRef]
- Smith, A.S.; Alexander Jr, J.E. Potential effects of the freshwater jellyfish Craspedacusta sowerbii on zooplankton community abundance. Journal of plankton research 2008, 30, 1323–1327. [Google Scholar] [CrossRef]
- Minchin, D.; Caffrey, J.M.; Haberlin, D.; Germaine, D.; Walsh, C.; Boelens, R.; Doyle, T.K. First observations of the freshwater jellyfish Craspedacusta sowerbii Lankester, 1880 in Ireland coincides with unusually high water temperatures 2016.
- Parent, G. Une page d’histoire des sciences contemporaines: Un siècle d’observations sur la méduse d’eau douce, Craspedacusta sowerbii Lank. Publications de la Société Linnéenne de Lyon 1982, 51, 47–63. [Google Scholar] [CrossRef]
- Didžiulis, V. Fact Sheet–Craspedacusta sowerbyi. Online database of the North European and Baltic Network on Invasive Alien Species–NOBANIS. Invasive Alien Species 2006, pp. 1–7.
- Lewis, C.; Migita, M.; Hashimoto, H.; Collins, A.G. On the occurrence of freshwater jellyfish in Japan 1928–2011: eighty-three years of records of mamizu kurage (Limnomedusae, Olindiidae). Proceedings of the Biological Society of Washington 2012, 125, 165–179. [Google Scholar] [CrossRef]
- Caputo, L.; Fuentes, R.; Woelfl, S.; Castañeda, L.E.; Cárdenas, L. Phenotypic plasticity of clonal populations of the freshwater jellyfish Craspedacusta sowerbii (Lankester, 1880) in Southern Hemisphere lakes (Chile) and the potential role of the zooplankton diet. Austral Ecology 2021, 46, 1192–1197. [Google Scholar] [CrossRef]
- Pérez-Bote, J.L.; Muñoz, A.; Morán, R.; Roso, R.; Romero, A.J. First record of Craspedacusta sowerbyi Lankester, 1880 (Cnidaria: Limnomedusae: Olindiidae) in the Proserpina Reservoir (Extremadura, SW Spain) with notes on their feeding habits. Belgian Journal of Zoology 2006, 136, 163. [Google Scholar]
- Fish, G.R. Craspedacusta sowerbyi Lankester (Coelenterata: Limnomedusae) in New Zealand lakes. New Zealand Journal of Marine and Freshwater Research 1971, 5, 66–69. [Google Scholar] [CrossRef]
- Rayner, N.A. First record of Craspedacusta sowerbyi Lankester (Cnidaria: Limnomedusae) from Africa. Hydrobiologia 1988, 162, 73–77. [Google Scholar] [CrossRef]
- McKercher, E.; O; Connell, D.; Fuller, P.; Liebig, J.; Larson, J.; Makled, T.; Fusaro, A.; .; Daniel, W. Craspedacusta sowerbii Lankester, 1880. https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=1068, 2004. Accessed: 2024-05-09.
- Marchessaux, G.; Bejean, M. From frustules to medusae: A new culture system for the study of the invasive hydrozoan Craspedacusta sowerbii in the laboratory. Invertebrate biology 2020, 139, e12308. [Google Scholar] [CrossRef]
- Folino-Rorem, N.C.; Reid, M.; Peard, T. Culturing the freshwater hydromedusa, Craspedacusta sowerbii under controlled laboratory conditions. Invertebrate Reproduction & Development 2016, 60, 17–27. [Google Scholar] [CrossRef]
- Acker, T.S.; Muscat, A.M. The ecology of Craspedacusta sowerbii Lankester, a freshwater hydrozoan. American Midland Naturalist 1976, pp. 323–336.
- Payne, F. Further studies on the life history of Craspedacusta ryderi, a fresh-water hydromedusan. The Biological Bulletin 1926, 50, 433–443. [Google Scholar] [CrossRef]
- Matthews, D.C. A comparative study of Craspedacusta sowerbyi and Calpasoma dactyloptera life cycles 1966.
- Payne, F. A study of the fresh-water medusa, Craspedacusta ryderi. Journal of Morphology 1924, 38, 387–429. [Google Scholar] [CrossRef]
- Davis, C.C. Notes on the food of Craspedacusta sowerbii in Crystal Lake, Ravenna, Ohio. Ecology 1955, 36, 364–366. [Google Scholar] [CrossRef]
- Lucas, K.; Colin, S.P.; Costello, J.H.; Katija, K.; Klos, E. Fluid interactions that enable stealth predation by the upstream-foraging hydromedusa Craspedacusta sowerbyi. The Biological Bulletin 2013, 225, 60–70. [Google Scholar] [CrossRef]
- Dodson, S.I.; Cooper, S.D. Trophic relationships of the freshwater jellyfish Craspedacusta sowerbyi Lankester 1880. Limnology and Oceanography 1983, 28, 345–351. [Google Scholar] [CrossRef]
- Stefani, F.; Leoni, B.; Marieni, A.; Garibaldi, L. A new record of Craspedacusta sowerbii, Lankester 1880 (Cnidaria, Limnomedusae) in northern Italy. Journal of Limnology 2010, 69, 189. [Google Scholar] [CrossRef]
- DeVries, D.R. The freshwater jellyfish Craspedacusta sowerbyi: A summary of its life history, ecology, and distribution. Journal of Freshwater Ecology 1992, 7, 7–16. [Google Scholar] [CrossRef]
- Gophen, M.; Shealtiel, L.; et al. Record of the alien species Craspedacusta sowerbyi Lankester, 1880 (Cnidaria: Limnomedusae) in Lake Kinneret catchment area. BioInvasions Records 2012, 1, 29–31. [Google Scholar] [CrossRef]
- Akçaalan, R.; Isinibilir, M.; Gürevin, C.; Sümer, A. A new contribution of biodiversity of Sapanca lake: Craspedacusta sowerbyi Lankester, 1880 (Cnidaria: Hydrozoa). Journal of FisheriesSciences. com 2011, 5, 43. [Google Scholar] [CrossRef]
- Jakovčev-Todorović, D.; Đikanović, V.; Skorić, S.; Cakić, P. Slatkovodna meduza Craspedacusta sowerbyi Lankester, 1880 (Hydrozoa, Olindiidae)-50 godina istraživanja u Srbiji. Archives of Biological Sciences 2010, 62, 123–127. [Google Scholar] [CrossRef]
- Jankowski, T.; Strauss, T.; Ratte, H.T. Trophic interactions of the freshwater jellyfish Craspedacusta sowerbii. Journal of plankton research 2005, 27, 811–823. [Google Scholar] [CrossRef]
- Duggan, I.C.; Eastwood, K.R. Detection and distribution of Craspedacusta sowerbii: observations of medusae are not enough 2012.
- Klotz, R.U. Hidden neozoans in macrozoobenthos: The polyp stage of the freshwater jellyfish Craspedacusta sowerbii. PhD thesis, Dissertation, München, Ludwig-Maximilians-Universität, 2022, 2022.
- McClary, A. The effect of temperature on growth and reproduction in Craspedacusta sowerbii. Ecology 1959, 40, 158–162. [Google Scholar] [CrossRef]
- Siquier, M.F.; Alanis, W.S.; Debat, C.M. First record of Craspedacusta sowerbii Lankester, 1880 (Hydrozoa, Limnomedusae) in a natural freshwater lagoon of Uruguay, with notes on polyp stage in captivity. Brazilian Journal of Biology 2017, 77, 665–672. [Google Scholar] [CrossRef]
- Wang, Y. Genetic population structure and environmental impact on Craspedacusta at the medusa and polyp stages. PhD thesis, Ludwig Maximilian University of Munich, 2022.
- Folino-Rorem, N.C.; Renken, C.J. Effects of salinity on the growth and morphology of the invasive, euryhaline hydroid Cordylophora (Phylum Cnidaria, Class Hydrozoa). Invertebrate biology 2018, 137, 78–90. [Google Scholar] [CrossRef]
- Dinno, A.; Dinno, M.A. Package ‘dunn. test’. CRAN Repos 2017, 10, 1–7. [Google Scholar]
- Amemiya, I. Fresh-water Medusa found in the Tank of my Laboratory. Japanese Journal of Zoology 1929, 3, 3. [Google Scholar]
- Gasith, A.; Gafny, S.; Hershkovitz, Y.; Goldstein, H.; Galil, B.S.; et al. The invasive freshwater medusa Craspedacusta sowerbii Lankester, 1880 (Hydrozoa: Olindiidae) in Israel. Aquatic Invasions 2011, 6, S147–S152. [Google Scholar] [CrossRef]
- Bushnell Jr, J.H.; Porter, T.W. The occurrence, habitat, and prey of Craspedacusta sowerbyi (particularly polyp stage) in Michigan. Transactions of the American Microscopical Society 1967, pp. 22–27.
- Galarce, L.C.; Riquelme, K.V.; Osman, D.Y.; Fuentes, R.A.; et al. A new record of the non indigenous freshwater jellyfish Craspedacusta sowerbii Lankester, 1880 (Cnidaria) in Northern Patagonia (40 S, Chile). BioInvasions Records 2013, 2, 263–270. [Google Scholar] [CrossRef]
- Lytle, C. Patterns of budding in the freshwater hydroid Craspedacusta. The Biology of Hydra and Some Other Coelenterates; University of Miami Press: Miami, FL, USA, 1961; pp. 317–336. [Google Scholar]
- Stanković, I.; Ternjej, I. New ecological insight on two invasive species: Craspedacusta sowerbii (Coelenterata: Limnomedusae) and Dreissenia polymorpha (Bivalvia: Dreissenidae). Journal of Natural History 2010, 44, 2707–2713. [Google Scholar] [CrossRef]
- van Walraven, L.; van Bleijswijk, J.; van der Veer, H.W. Here are the polyps: In situ observations of jellyfish polyps and podocysts on bivalve shells. PeerJ 2020, 8, e9260. [Google Scholar] [CrossRef]
- Jeunen, G.J.; Lipinskaya, T.; Gajduchenko, H.; Golovenchik, V.; Moroz, M.; Rizevsky, V.; Semenchenko, V.; Gemmell, N.J. Environmental DNA (eDNA) metabarcoding surveys show evidence of non-indigenous freshwater species invasion to new parts of Eastern Europe. Metabarcoding and Metagenomics 2022, 6, e68575. [Google Scholar] [CrossRef]
- Blackman, R.C.; Carraro, L.; Keck, F.; Altermatt, F. Measuring the state of aquatic environments using eDNA—upscaling spatial resolution of biotic indices. Philosophical Transactions of the Royal Society B 2024, 379, 20230121. [Google Scholar] [CrossRef]
- Dumont, H.J. The distribution and ecology of the fresh-and brackish-water medusae of the world. Studies on the ecology of tropical zooplankton 1994, pp. 1–12.
- Gießler, S.; Strauss, T.; Schachtl, K.; Jankowski, T.; Klotz, R.; Stibor, H. Trophic Positions of Polyp and Medusa Stages of the Freshwater Jellyfish Craspedacusta sowerbii Based on Stable Isotope Analysis. Biology 2023, 12, 814. [Google Scholar] [CrossRef]
- Oualid, J.A.; Iazza, B.; Tamsouri, N.M.; El Aamri, F.; Moukrim, A.; López-González, P.J. Hidden diversity under morphology–based identifications of widespread invasive species: The case of the ‘well–known’hydromedusa Craspedacusta sowerbii Lankester 1880. Animal Biodiversity and Conservation 2019, 42, 301–316. [Google Scholar] [CrossRef]
Short Biography of Authors
 |
Jonathan A. Zhu (B.S., Biology, Mathematics with Statistics, Wheaton College, 2023) is a Master’s student studying computational biology at Carnegie Mellon University. He has worked as a research assistant, laboratory assistant, and teaching assistant at Wheaton College. Jonathan has a wide range of research interests but is particularly interested in mathematical modeling and computational inference regarding invasive species spread and marine/freshwater invertebrate zoology. |
 |
Nadine C. Folino-Rorem (PhD, University of New Hampshire, 1989) is a professor in the Biological and Health Sciences Department at Wheaton College, IL, where she has served on faculty since 1993. She received both her doctorate and Master’s degree from the University of New Hampshire, Durham, N.H. Nadine’s teaching responsibilities range from introductory biology, research methods, invertebrate biology, to environmental ethics. Her research students and colleagues focus on the taxonomic and ecological aspects of invasive, freshwater cnidarians. |
| Location | Secchi Depth (m) | Conductivity (S/cm) | Temperature | pH | Setup Site | Latitude | Longitude |
|---|---|---|---|---|---|---|---|
| Inverness (IL) | 0.75 | 1055 | 25.8ºC | 8.26 | 1 | 42.116540°N | 88.122080°W |
| 2 | 42.116540°N | 88.122060°W | |||||
| Coal City (IL) | 2 | 228.6 | 28.3ºC | 7.93 | 1 | 41.316213°N | 88.271704°W |
| 2 | 41.316053°N | 88.271673°W | |||||
| Hammond (IN) | 1 | 927.0 | 25.2ºC | 8.60 | 1 | 41.671930°N | 87.511810°W |
| 2 | 41.672540°N | 87.512360°W |
| Substrate | Polyps and/or Colonies per slide | Frustules per slide | Polyps per colony |
|---|---|---|---|
| Glass | 28.29 | 24.71 | 3.237 |
| Plastic | 17.93 | 19.00 | 2.933 |
| p-value | 0.3734 | 0.7414 | 0.1759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




