Submitted:
31 May 2024
Posted:
31 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Strains of Acetic Acid Bacteria (AAB)
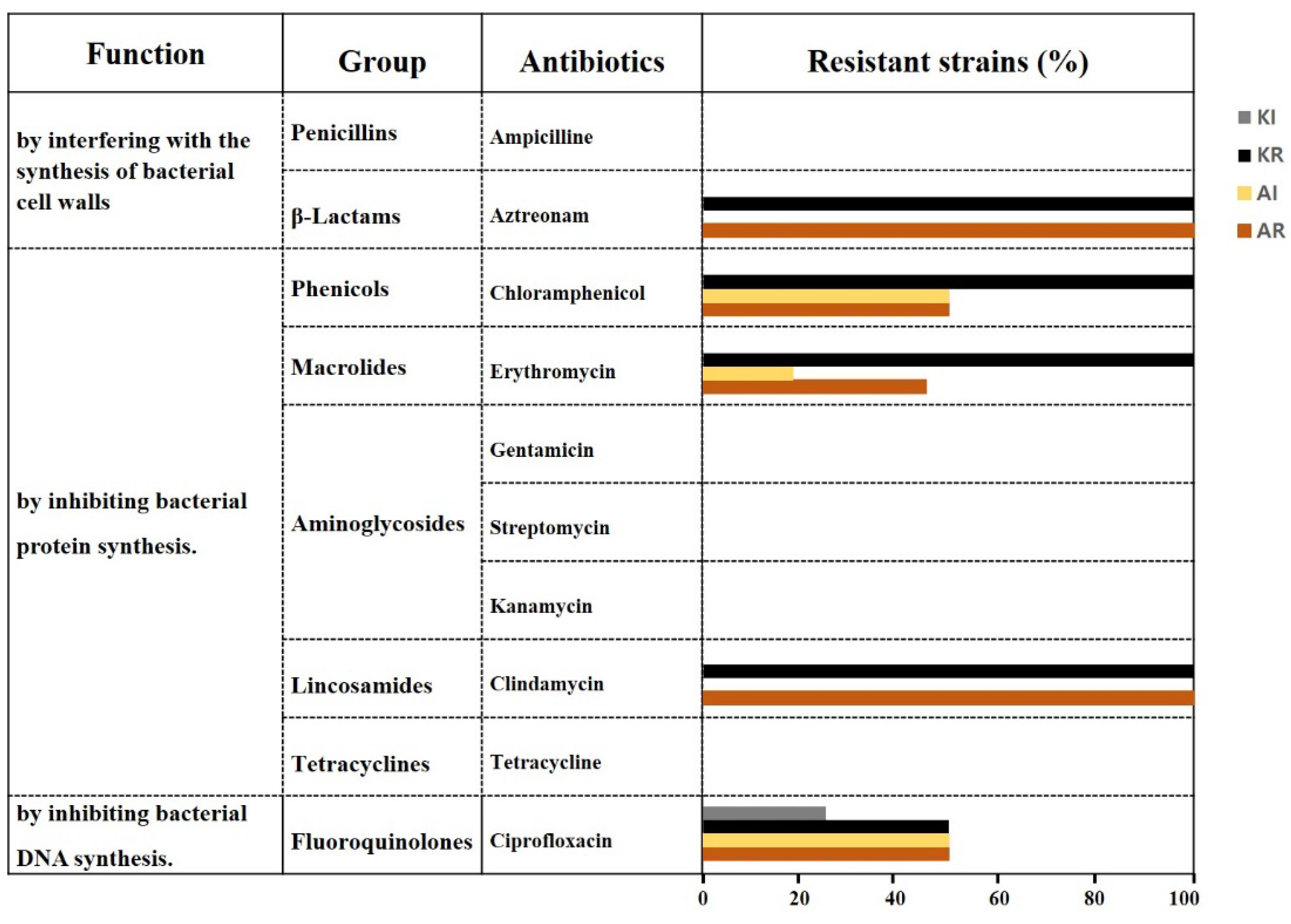
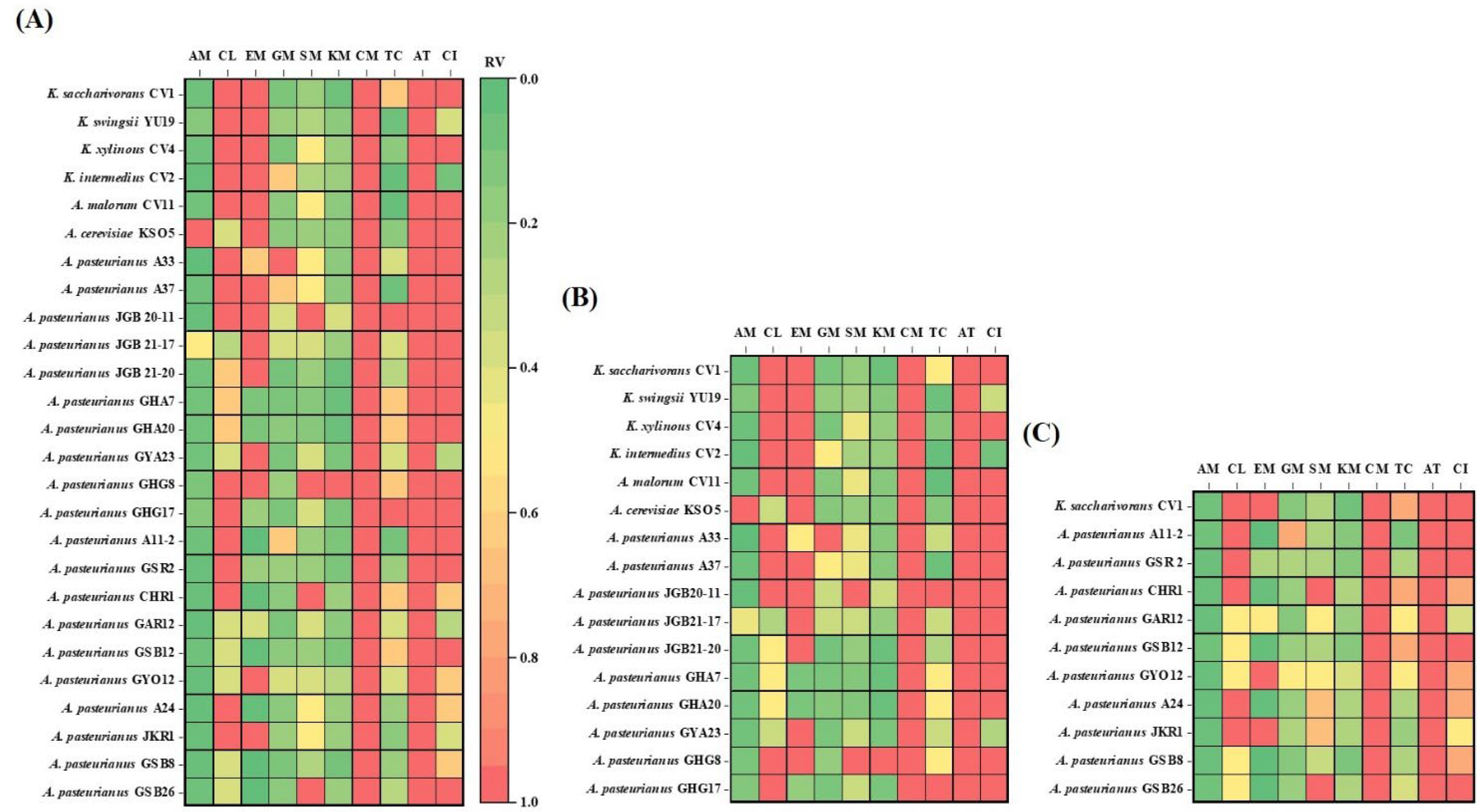
2.2. Antibiotic Resistance of Acetic Acid Bacteria
2.3. Resistance and Sensitivity of AAB to Aztreonam and Ampicilline Antibiotics
2.4. Sensitivity of AAB to Aminoglycosides (GM, SM, and KM) and Tetracycline Antibiotics
2.5. Resistance of AAB to Erythromycin and Clindamycin Antibiotics
2.6. Resistance of AAB to Chloramphenicol and Ciprofloxacin Antibiotics
2.7. Putative Proteins Related to Antibiotic Resistance Mechanisms
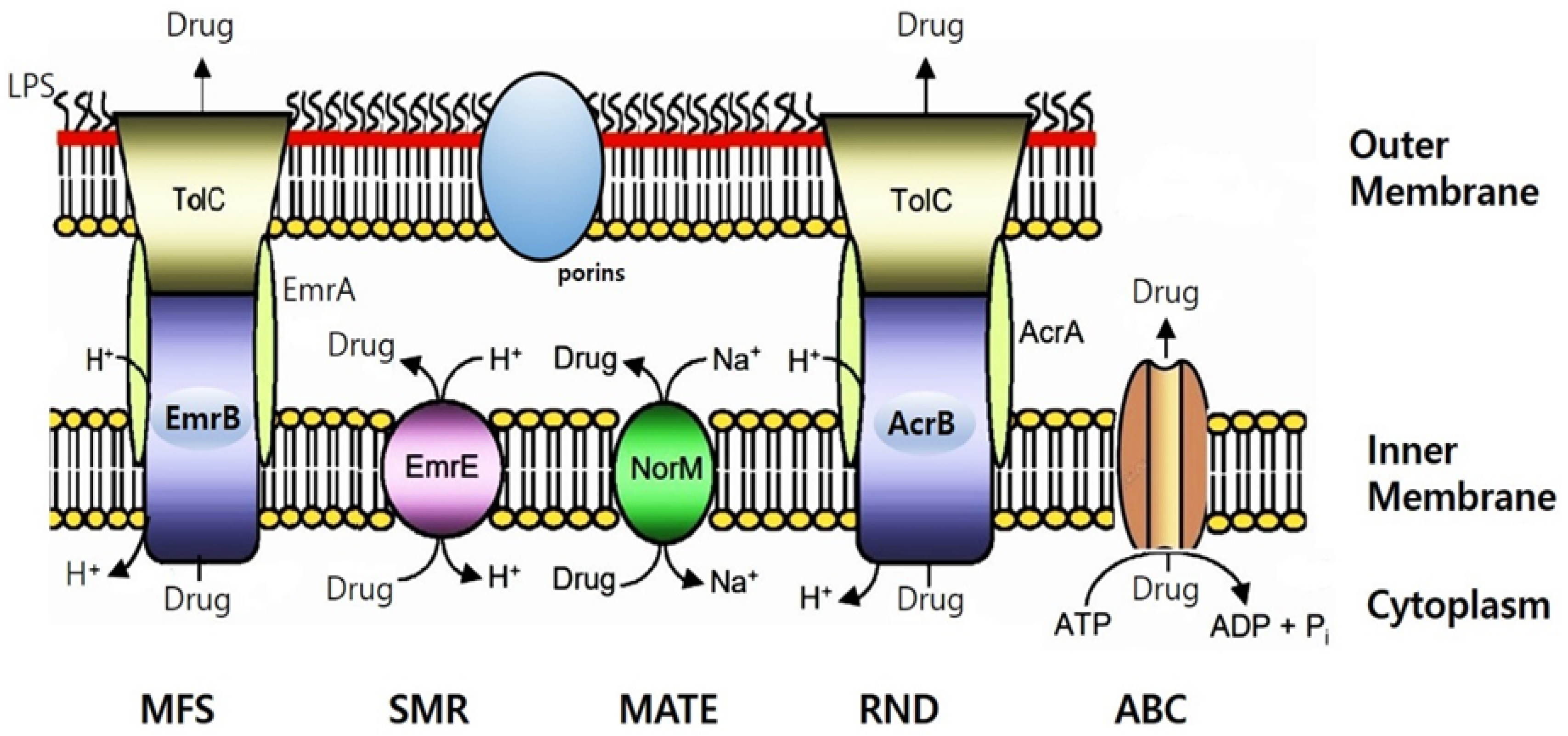
3. Discussion
4. Materials and Methods
4.1. Preparation of Acetic Acid Bacteria (AAB)
4.2. Assesment of Antibiotic Resistance for AAB
4.3. Bioinformatics
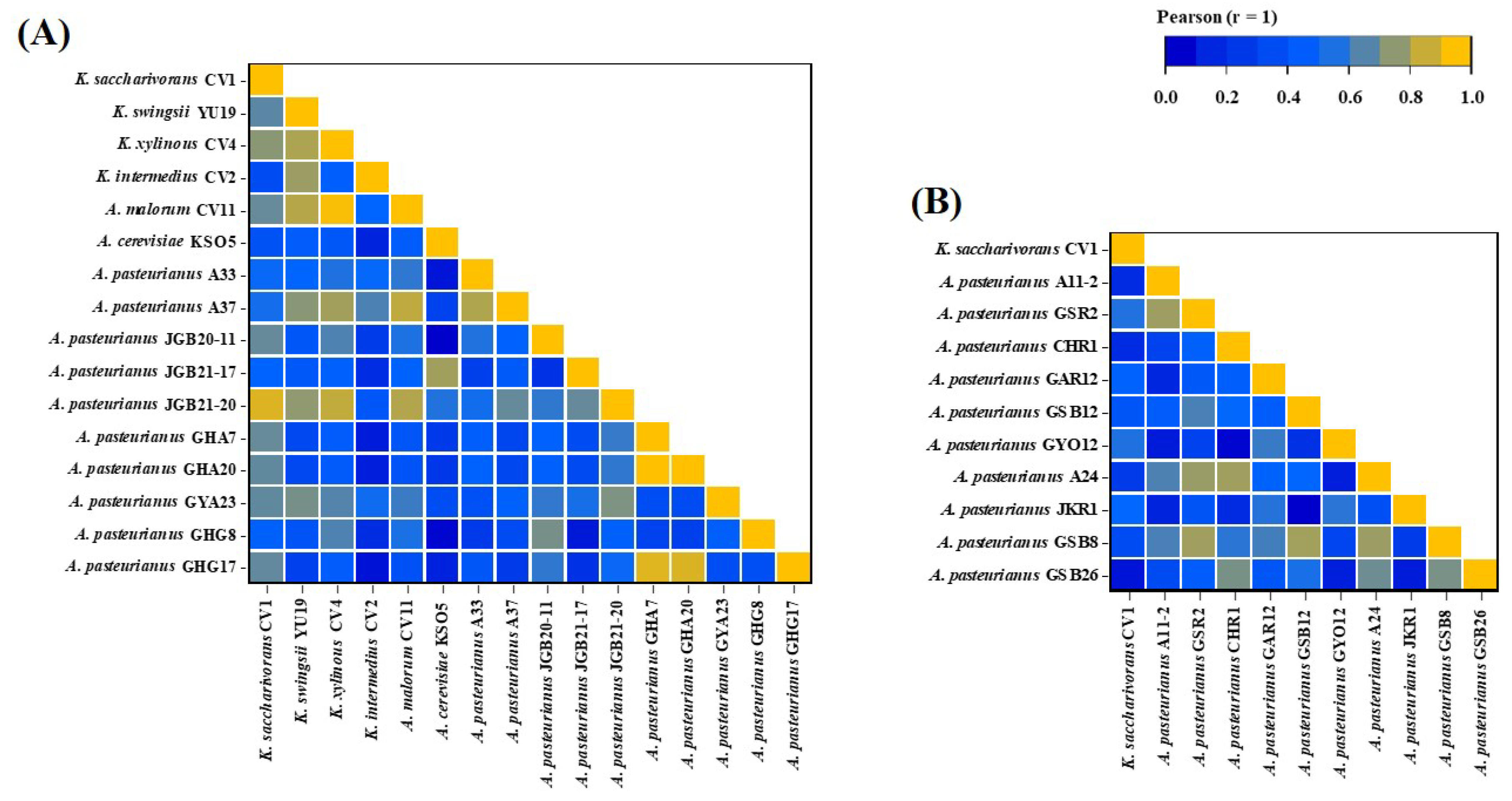
4.4. Correlation Heatmap
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomes, R.J.; Borges, M.D.F.; Rosa, M.D.F.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic acid bacteria in the food industry: Systematics, characteristics and applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef] [PubMed]
- De Roos, J.; De Vuyst, L. Acetic acid bacteria in fermented foods and beverages. Curr. Opin. Biotechnol. 2018, 49, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, G.; Zhang, J.; Bao, J. Fermentative production of high titer gluconic and xylonic acids from corn stover feedstock by Gluconobacter oxydans and techno-economic analysis. Bioresour. Technol. 2016, 219, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chang, Y.; Xie, S.; Song, J.; Wang, M. Impacts of bioprocess engineering on product formation by Acetobacter pasteurianus. Appl. Microbiol. Biotechnol. 2018, 102, 2535–2541. [Google Scholar] [CrossRef]
- Chouaia, B.; Gaiarsa, S.; Crotti, E.; Comandatore, F.; Degli Esposti, M.; Ricci, I.; Alma, A.; Favia, G.; Bandi, C.; Dafonchio, D. Acetic acid bacteria genomes reveal functional traits for adaptation to life in insect guts. Genome Biol. Evol. 2014, 6, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Kersters, K.; Lisdiyanti, P.; Komagata, K.; Swings, J. The family Acetobacteraceae: The genera Acetobacter, Acidomonas, Asaia, Gluconacetobacter, Gluconobacter, and Kozakia. The Prokaryotes, 2006, 5, 163–200. [Google Scholar] [CrossRef] [PubMed]
- Soemphol, W.; Deeraksa, A.; Matsutani, M.; Yakushi, T.; Toyama, H.; Adachi, O.; Yamada, M.; Matsushita, K. Global analysis of the genes involved in the thermotolerance mechanism of thermotolerant Acetobacter tropicalis SKU1100. Biosci. Biotech. Biochem. 2011, 75, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, J.Y.; Gwon, H.M.; Kim, S.Y.; Yeo, S.H. Determination of quality characteristics by the reproduction of grain vinegars reported in ancient literature. Korean J. Food Preserv. 2020, 27, 859–871. [Google Scholar] [CrossRef]
- El-Askri, T.; Yatim, M.; Sehli, Y.; Rahou, A.; Belhaj, A.; Castro, R.; Durán-Guerrero, E.; Hafidi, M.; Zouhair, R. Screening and characterization of new Acetobacter fabarum and Acetobacter pasteurianus strains with high ethanol–thermo tolerance and the optimization of acetic acid production. Microorganisms, 2022, 10, 1741. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.Y.; Jeong, W.S.; Gwon, H.; Kim, S.Y.; Yeo, S.H. Culture and function-related characteristics of six acetic acid bacterial strains isolated from farm-made fermented vinegars. Korean J. Food Preserv. 2022, 29, 142–156. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jeong, W.-S.; Kim, S.-Y.; Yeo, S.-H. Quality and functional characterization of acetic acid bacteria isolated from farm-produced fruit vinegars. Fermentation, 2023, 9, 447. [Google Scholar] [CrossRef]
- Kohlmann, K.; Barenberg, K.; Anders, A.; Gatermann, S.G. Acetobacter indonesiensis bacteremia in child with metachromatic leukodystrophy. Emerg. Infect. Dis. 2016, 22, 1681–1683. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xie, X.; Li, Y.; Liang, T.; Zhong, H.; Ma, J.; Yang, L.; Yang, J.; Li, L.; Xi, Y.; et al. Metagenomics-based analysis of the age-related cumulative effect of antibiotic resistance genes in gut microbiota. Antibiotics, 2021, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Cepec, E.; Trček, J. Antimicrobial resistance of Acetobacter and Komagataeibacter species originating from vinegars. Int. J. Environ. Res. Public Health 2022, 19, 463. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Fukaya, M. Analysis of proteins responsive to acetic acid in Acetobacter: Molecular mechanisms conferring acetic acid resistance in acetic acid bacteria. Int. J. Food Microbiol. 2008, 125, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, Y.; Hong, H. Classification of acetic acid bacteria and their acid resistant mechanism. AMB Expr. 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Martins, A.; Spengler, G.; Molnar, J. Efflux pumps of Gram-negative bacteria: what they do, how they do it, with and how to deal with them. Front. Pharmacol. 2013, 4, 168. [Google Scholar] [CrossRef] [PubMed]
- Auda, I.G.; Ali Salman, I.M.; Odah, J.G. Efflux pumps of Gram-negative bacteria in brief. Gene Rep. 2020, 20, 100666. [Google Scholar] [CrossRef]
- Putman, M.; van Veen, H.W.; Konings, W.N. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 2000, 64, 672–693. [Google Scholar] [CrossRef] [PubMed]
- Nanda, K. : Taniguchi, M.; Ujike, S.; Ishihara, N.; Mori, H.; Ono, H.; Murooka, Y. Characterization of acetic acid bacteria in traditional acetic acid fermentation of rice vinegar (Komesu) and unpolished rice vinegar (Kurosu) produced in Japan. Appl. Environ. Microbiol. 2001, 67, 986–990. [Google Scholar] [CrossRef]
- Song, N.E.; Cho, S.H.; Baik, S.H. Microbial community, and biochemical and physiological properties of Korean traditional black raspberry (Robus coreanus Miquel) vinegar. J. Sci. Food. Agric. 2016, 96, 3723–3730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zang, N.; Shi, J.; Feng, W.; Liu, Y.; Liang, X. Comparative proteome of Acetobacter pasteurianus Ab3 during the high acidity rice vinegar fermentation. Appl. Biochem. Biotechnol. 2016, 177, 1573–1588. [Google Scholar] [CrossRef]
- Baek, C.-H.; Baek, S.-Y.; Lee, S.H.; Kang, J.-E.; Choi, H.-S.; Kim, J.-H.; Yeo, S.-H. Characterization of Acetobacter sp. Strain CV1 isolated from a fermented vinegar. Microbiol. Biotechnol. Lett. 2015, 43, 126–133. [Google Scholar] [CrossRef]
- https://en.wikipedia.org/wiki/Aztreonam#cite_ref-10, "Aztreonam spectrum of bacterial susceptibility and Resistance" (PDF).
- Hancock, R.E.W.; Brinkman, F.S. Function of Pseudomonas porins in uptake and efflux. Annu. Rev. Microbiol. 2002, 56, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed]
- Taber, H.W.; Mueller, J.P.; Miller, P.F.; Arrow, A.S. Bacterial uptake of aminoglycoside antibiotics. Microbiol. Rev. 1987, 51, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.M.; Shepherd, M. A mini-review: environmental and metabolic factors affecting aminoglycoside efficacy. World J. Microbiol. Biotechnol. 2023, 39, 7. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R. Mechanisms of resistance to macrolides and lincosamides: Nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 2002, 34, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, A.A.; Said, H.S.; Elfeky, S.M.; Shaaban, M.I. Inhibition of erythromycin and erythromycin-induced resistance among Staphylococcus aureus clinical isolates. Antibiotics 2023, 12, 503. [Google Scholar] [CrossRef]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef]
- Elder, F.C.T.; Feil, E.J.; Snape, J.; Gaze, W.H.; Kasprzyk-Hordern, B. The role of stereochemistry of antibiotic agents in the development of antibiotic resistance in the environment. Environ. Int. 2020, 139, 105681. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.L.; Hedi, L.A.; Lien, D.M. Chloramphenicol resistance in Pseudomonas cepacia because of decreased permeability. Antimicrob. Agents Chemother. 1989, 33, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Molina-Henares, A.J.; de la Torre, J.; Duque, E.; Ramos, J.L. Characterization of the RND family of multidrug efflux pumps: in silico to in vivo confirmation of four functionally distinct subgroups. Microb. Biotechnol. 2010, 3, 691–700. [Google Scholar] [CrossRef]
- Poole, K. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob Agents Chemother. 2000, 44, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.K.; Baker, M.E.; Rouch, D.A.; Page, M.G.P.; Skurray, R.A.; Paulsen, I.T.; Chater, K.F.; Baldwin, S.A.; Henderson, P.J.F. Membrane transport proteins: implications of sequence comparisons. Curr. Opin. Cell Biol. 1992, 4, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Pao, S.S.; Paulsen, I.T.; Saier, M.H. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998, 62, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.P.; Kumar, S.; Varela, M.F. Identification, cloning, and functional characterization of EmrD-3, a putative multidrug efflux pump of the major facilitator superfamily from Vibrio cholera O395. Arch. Microbiol. 2009, 191, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Kodama, K.; Shiota, S.; Mine, T.; Kataoka, A.; Mizushima, T.; Tsuchiya, T. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob. Agents Chemother. 1998, 42, 1778–1782. [Google Scholar] [CrossRef] [PubMed]
- Marger, M.D.; Saier Jr., M. H. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem. Sci. 1993, 18, 13–20. [Google Scholar] [CrossRef]
- Saier Jr., M. H.; Tam, R.; Reizer, A.; Reizer, J. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol. Microbiol. 1994, 11, 841–847. [Google Scholar] [CrossRef]
- van Veen, H.W.; Konings, W.N. The ABC family of multidrug transporters in microorganisms. Biochim. Biophys. Acta 1998, 1365, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, P.A. MDR pumps as crossroads of resistance: antibiotics and bacteriophages. Antibiotics 2022, 11, 734. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Schweizer, H.P. Bacterial resistance to antibiotics: Active efflux and reduced uptake. Adv. Drug Deliv. Rev. 2005, 57, 1486–1513. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Plésiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in gram negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; Verzelloni, E.; Canonico, M. Aerobic submerged fermentation by acetic acid bacteria for vinegar production: Process and biotechnological aspects. Process. Biochem. 2014, 49, 1571–1579. [Google Scholar] [CrossRef]
- Saichana, N.; Matsushita, K.; Adachi, O.; Frébort, I.; Frebortova, J. Acetic acid bacteria: a group of bacteria with versatile biotechnological applications. Biotechnol. Adv. 2015, 33, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shao, Y.; Chen, F. Overview on mechanisms of acetic acid resistance in acetic acid bacteria. World J. Microbiol. Biotechnol. 2015, 31, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Barrao, C.; Saad, M.M.; Cabello, F.E.; Bravo, D.; Chappuis, M.-L.; Ortega Pérez, R.; Junier, P.; Perret, X.; Barja, F. Metaproteomics and ultrastructure characterization of Komagataeibacter spp. involved in high-acid spirit vinegar production. Food Microbiol. 2016, 55, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Pagès, J.-M.; Chloë, J.; Winterhalter, M. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 1994, 6, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Okusu, H.; Ma, D.; Nikaido, H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple antibiotic resistance (Mar) mutants. J. Bacteriol. 1996, 178, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [PubMed]
| No. | Strains | KACC no. | NCBI aceession no. | Origin (vinegar) | Region |
|---|---|---|---|---|---|
| Komagataeibacter spp.(4) | |||||
| 1 | K. swingsii YU19 | KACC 92275P | PP504479 | Apple | Santa barbara, USA |
| 2 | K. xylinous CV4 | KACC 17012 | PP474345 | Apple | Mungyeong, Korea |
| 3 | K. intermedius CV2 | KACC 17072 | PP474454 | Loquat | Jindo, Korea |
| 4 | K. saccharivorans CV1 | KACC 17057 | AB759966 | Rice | Jindo, Korea |
| Acetobacter spp. (22) | |||||
| 5 | A. malorum CV11 | KACC 92076P | PP504490 | Apple | Yecheon, Korea |
| 6 | A. cerevisiae KSO5 | KACC 92352P | PP478110 | Magnolia berry | Seongnam, Korea |
| 7 | A. pasteurianus A33 | KACC 92250P | PP479652 | Peach | Sejong, Korea |
| 8 | A. pasteurianus A37 | KACC 92206P | PP479653 | Plum | Hongcheon, Korea |
| 9 | A. pasteurianus JGB20-11 | KACC 92382P | PP478112 | Korean blackberry | Gochang, Korea |
| 10 | A. pasteurianus JGB21-17 | KACC 92383P | PP478120 | Korean blackberry | Gochang, Korea |
| 11 | A. pasteurianus JGB21-20 | KACC 92350P | PP478123 | Korean blackberry | Gochang, Korea |
| 12 | A. pasteurianus GHA7 | KACC 92351P | PP478158 | Apple | Hongcheon, Korea |
| 13 | A. pasteurianus GHA20 | KACC 92384P | PP478164 | Apple | Hongcheon, Korea |
| 14 | A. pasteurianus GYA23 | KACC 92385P | PP478167 | Apple | Yecheon, Korea |
| 15 | A. pasteurianus GHG8 | KACC 92534P | PP478186 | Grape | Hamyang, Korea |
| 16 | A. pasteurianus GHG17 | KACC 92535P | PP479654 | Grape | Hamyang, Korea |
| 17 | A. pasteurianus A11-2 | KACC 92203P | PP477813 | Brown rice | Hongcheon, Korea |
| 18 | A. pasteurianus GSR2 | KACC 92424P | PP478168 | Brown rice | Sancheong, Korea |
| 19 | A. pasteurianus CHR1 | KACC 92423P | PP478175 | Brown rice | Hongseong, Korea |
| 20 | A. pasteurianus GAR12 | KACC 92445P | PP479656 | Brown rice | Andong, Korea |
| 21 | A. pasteurianus GSB12 | KACC 92446P | PP478170 | Black rice | Sancheong, Korea |
| 22 | A. pasteurianus GYO12 | KACC 92447P | PP478173 | Five grains | Yecheon, Korea |
| 23 | A. pasteurianus A24 | KACC 92204P | PP477814 | Rice | Seoul, Korea |
| 24 | A. pasteurianus JKR1 | KACC 92533P | PP478188 | Rice | Gimje, Korea |
| 25 | A. pasteurianus GSB8 | KACC 92531P | PP478181 | Barley | Seongnam, Korea |
| 26 | A. pasteurianus GSB26 | KACC 92532P | PP478182 | Barley | Seongnam, Korea |
| No. | Strains | AM | CL | EM | GM | SM | KM | CM | TC | AT | CI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | K. swingsii YU19 | R | R | R | R | I | |||||
| 2 | K. xylinous CV4 | R | R | R | R | R | |||||
| 3 | K. intermedius CV2 | R | R | R | R | ||||||
| 4 | K. saccharivorans CV1 | R | R | R | R | R | |||||
| 5 | A. malorum CV11 | R | R | R | R | R | |||||
| 6 | A. cerevisiae KSO5 | I | R | R | R | R | |||||
| 7 | A. pasteurianus A33 | R | I | R | R | R | |||||
| 8 | A. pasteurianus A37 | R | R | R | R | R | |||||
| 9 | A. pasteurianus JGB20-11 | R | R | R | R | R | |||||
| 10 | A. pasteurianus JGB21-17 | I | R | R | R | R | |||||
| 11 | A. pasteurianus JGB21-20 | I | R | R | R | R | |||||
| 12 | A. pasteurianus GHA7 | I | R | R | R | ||||||
| 13 | A. pasteurianus GHA20 | I | R | R | I | ||||||
| 14 | A. pasteurianus GYA23 | I | R | R | R | I | |||||
| 15 | A. pasteurianus GHG8 | R | R | R | R | I | |||||
| 16 | A. pasteurianus GHG17 | R | I | R | R | R | |||||
| 17 | A. pasteurianus A11-2 | R | R | R | R | ||||||
| 18 | A.pasteurianus GSR2 | R | I | R | R | I | |||||
| 19 | A. pasteurianus CHR1 | R | R | R | I | ||||||
| 20 | A. pasteurianus GAR12 | I | I | R | R | I | |||||
| 21 | A. pasteurianus GSB12 | I | R | R | R | ||||||
| 22 | A. pasteurianus GYO12 | I | R | R | R | I | |||||
| 23 | A. pasteurianus A24 | R | R | R | I | ||||||
| 24 | A. pasteurianus JKR1 | R | R | R | R | I | |||||
| `25 | A. pasteurianus GSB8 | I | R | R | I | ||||||
| 26 | A. pasteurianus GSB26 | I | R | R | I |
| Species | Multidrug resistance transporters | References |
|---|---|---|
| K. saccharivorans LMG 1582T 1) | AcrA, MdtA, MdtB, MexB, OprM, MuxB, OpmB, Bcr-1, EmrE, QacE | Cepec and Trček et al. [14] |
| K. saccharivorans CV1 | AcrA, AcrB, EmrA, EmrE, NorM, Bcr/CflA, ABC transporter, | This study |
| K. swingsii LMG 22125T | AcrA, MdtA, MdtB, MexB, OprM, Bcr-1, EmrE, QacE | Cepec and Trček et al. [14] |
| A. pasteurianus LMG 1262T | AcrA, MuxB, OpmB, OprM, Bcr-1 | Cepec and Trček et al. [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





