Submitted:
30 May 2024
Posted:
31 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
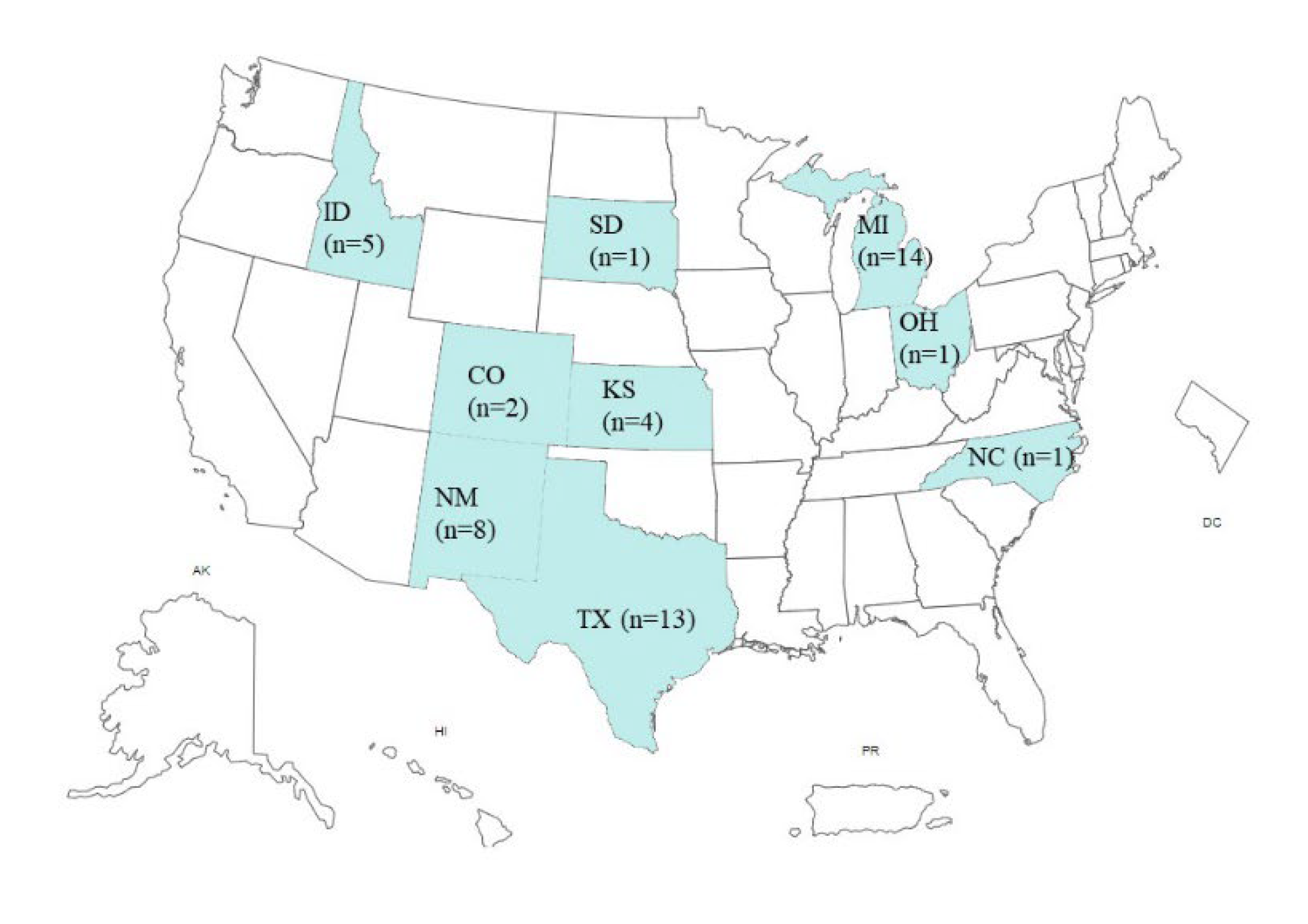
2. Recent Bovine Influenza A Virus Outbreak in Domestic Ruminants in the U.S.
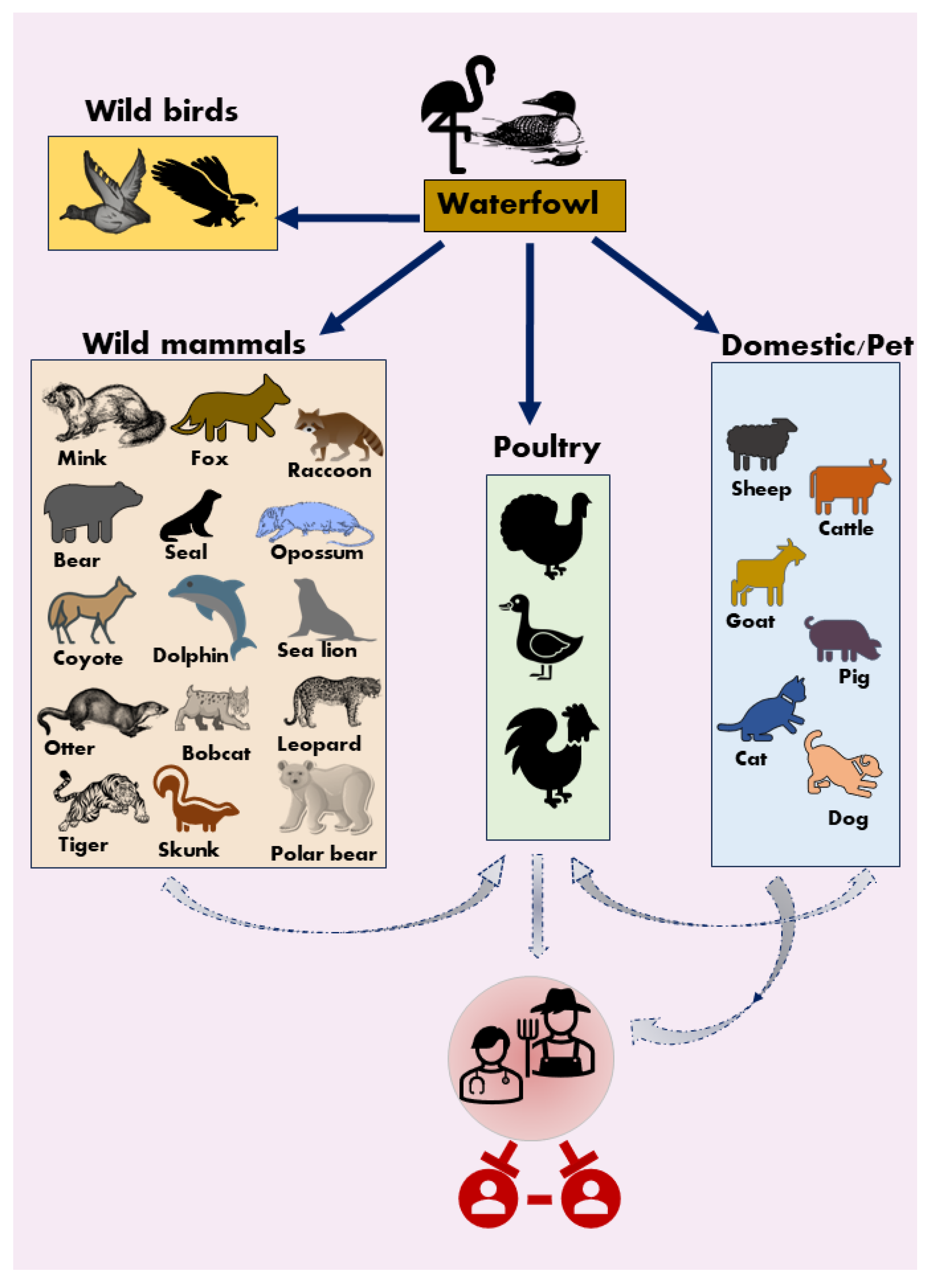
3. HPAI H5N1 Virus Infection in Mammals
4. HPAI H5N1 Virus Infection in Humans
5. HA, NA, and NS also Determine Virus Tropism and Host Range
6. Vaccines against HPAI H5N1
7. Conclusion and Future Directions
Author Contributions
Funding
Acknowledgement
Conflicts of Interest
References
- Yoon, S.W.; Webby, R.J.; Webster, R.G. Evolution and ecology of influenza A viruses. Curr Top Microbiol Immunol 2014, 385, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y. Pathogenicity and virulence of influenza. Virulence 2023, 14, 2223057. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Katsushima, N.; Nagai, Y.; Shoji, M.; Itagaki, T.; Sakamoto, M.; Kitaoka, S.; Mizuta, K.; Nishimura, H. Clinical features of influenza C virus infection in children. J Infect Dis 2006, 193, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sheng, Z.; Lin, T.; Sreenivasan, C.; Gao, R.; Thomas, M.; Druce, J.; Hause, B.M.; Kaushik, R.S.; Li, F.; et al. Genetic and antigenic characteristics of a human influenza C virus clinical isolate. J Med Virol 2020, 92, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sheng, Z.; Huang, C.; Wang, D.; Li, F. Influenza D virus. Curr Opin Virol 2020, 44, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, R.; Zhou, B.; Chou, T.W.; Ghedin, E.; Sheng, Z.; Gao, R.; Zhai, S.L.; Wang, D.; Li, F. Development and Characterization of a Reverse-Genetics System for Influenza D Virus. J Virol 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, C.C.; Liu, R.; Gao, R.; Guo, Y.; Hause, B.M.; Thomas, M.; Naveed, A.; Clement, T.; Rausch, D.; Christopher-Hennings, J.; et al. Influenza C and D Viruses Demonstrated a Differential Respiratory Tissue Tropism in a Comparative Pathogenesis Study in Guinea Pigs. J Virol 2023, 97, e0035623. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, C.C.; Thomas, M.; Kaushik, R.S.; Wang, D.; Li, F. Influenza A in Bovine Species: A Narrative Literature Review. Viruses 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Suarez, D.L.; Schultz-Cherry, S. Immunology of avian influenza virus: a review. Dev Comp Immunol 2000, 24, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Chen, H.; Gao, G.F.; Shu, Y.; Kawaoka, Y. H5N1 influenza viruses: outbreaks and biological properties. Cell Res 2010, 20, 51–61. [Google Scholar] [CrossRef] [PubMed]
- H5N1 Bird Flu Detections across the United States (Backyard and Commercial). Availabe online: https://www.cdc.gov/flu/avianflu/data-map-commercial.html (accessed on April, 23,2024).
- Avian Influenza in Birds. Availabe online: https://www.cdc.gov/flu/avianflu/avian-in-birds.htm (accessed on April,23, 2024).
- Goat in Minnesota tests positive for HPAI. Availabe online: https://www.avma.org/news/goat-minnesota-tests-positive-hpai (accessed on April,24,2024).
- United States of America - Influenza A viruses of high pathogenicity (Inf. with) (non-poultry including wild birds) (2017-) - Follow up report 43. Availabe online: https://wahis.woah.org/#/in-review/4451?reportId=166488&fromPage=event-dashboard-url (accessed on April, 24,2024).
- United States of America - Influenza A viruses of high pathogenicity (Inf. with) (non-poultry including wild birds) (2017-) - Follow up report 44. Availabe online: https://wahis.woah.org/#/in-review/4451?reportId=166639&fromPage=event-dashboard-url (accessed on April 24,2024).
- Highly Pathogenic Avian Influenza (HPAI) Detections in Livestock. Availabe online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/livestock (accessed on May,2nd, 2024).
- Kristensen, C.; Larsen, L.E.; Trebbien, R.; Jensen, H.E. The avian influenza A virus receptor SA-alpha2,3-Gal is expressed in the porcine nasal mucosa sustaining the pig as a mixing vessel for new influenza viruses. Virus Res 2024, 340, 199304. [Google Scholar] [CrossRef] [PubMed]
- Gunning, R.F.; Brown, I.H.; Crawshaw, T.R. Evidence of influenza A virus infection in dairy cows with sporadic milk drop syndrome. Vet Rec 1999, 145, 556–557. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.H.; Crawshaw, T.R.; Harris, P.A.; Alexander, D.J. Detection of antibodies to influenza A virus in cattle in association with respiratory disease and reduced milk yield. Vet Rec 1998, 143, 637–638. [Google Scholar] [PubMed]
- Crawshaw, T.R.; Brown, I.H.; Essen, S.C.; Young, S.C. Significant rising antibody titres to influenza A are associated with an acute reduction in milk yield in cattle. Vet J 2008, 178, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, M.; Fernandez, A.; Ariyama, N.; Colom-Rivero, A.; Rivera, C.; Nunez, P.; Sanhueza, P.; Johow, M.; Araya, H.; Torres, J.C.; et al. Mass mortality event in South American sea lions (Otaria flavescens) correlated to highly pathogenic avian influenza (HPAI) H5N1 outbreak in Chile. Vet Q 2023, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Murawski, A.; Fabrizio, T.; Ossiboff, R.; Kackos, C.; Jeevan, T.; Jones, J.C.; Kandeil, A.; Walker, D.; Turner, J.C.M.; Patton, C.; et al. Highly pathogenic avian influenza A(H5N1) virus in a common bottlenose dolphin (Tursiops truncatus) in Florida. Commun Biol 2024, 7, 476. [Google Scholar] [CrossRef] [PubMed]
- Keawcharoen, J.; Oraveerakul, K.; Kuiken, T.; Fouchier, R.A.; Amonsin, A.; Payungporn, S.; Noppornpanth, S.; Wattanodorn, S.; Theambooniers, A.; Tantilertcharoen, R.; et al. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis 2004, 10, 2189–2191. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, B.T.; Berhane, Y.; Nadeau, M.S.; Embury-Hyatt, C.; Lung, O.; Xu, W.; Lair, S. Influenza A(H5N1) Virus Infections in 2 Free-Ranging Black Bears (Ursus americanus), Quebec, Canada. Emerg Infect Dis 2023, 29, 2145–2149. [Google Scholar] [CrossRef]
- Puryear, W.; Sawatzki, K.; Hill, N.; Foss, A.; Stone, J.J.; Doughty, L.; Walk, D.; Gilbert, K.; Murray, M.; Cox, E.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Outbreak in New England Seals, United States. Emerg Infect Dis 2023, 29, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Elsmo, E.J.; Wunschmann, A.; Beckmen, K.B.; Broughton-Neiswanger, L.E.; Buckles, E.L.; Ellis, J.; Fitzgerald, S.D.; Gerlach, R.; Hawkins, S.; Ip, H.S.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Clade 2.3.4.4b Infections in Wild Terrestrial Mammals, United States, 2022. Emerg Infect Dis 2023, 29, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Plaza, P.I.; Gamarra-Toledo, V.; Rodriguez Eugui, J.; Rosciano, N.; Lambertucci, S.A. Pacific and Atlantic sea lion mortality caused by highly pathogenic Avian Influenza A(H5N1) in South America. Travel Med Infect Dis 2024, 59, 102712. [Google Scholar] [CrossRef] [PubMed]
- Thanawongnuwech, R.; Amonsin, A.; Tantilertcharoen, R.; Damrongwatanapokin, S.; Theamboonlers, A.; Payungporn, S.; Nanthapornphiphat, K.; Ratanamungklanon, S.; Tunak, E.; Songserm, T.; et al. Probable tiger-to-tiger transmission of avian influenza H5N1. Emerg Infect Dis 2005, 11, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Aguero, M.; Monne, I.; Sanchez, A.; Zecchin, B.; Fusaro, A.; Ruano, M.J.; Del Valle Arrojo, M.; Fernandez-Antonio, R.; Souto, A.M.; Tordable, P.; et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill 2023, 28. [Google Scholar] [CrossRef]
- Bordes, L.; Vreman, S.; Heutink, R.; Roose, M.; Venema, S.; Pritz-Verschuren, S.B.E.; Rijks, J.M.; Gonzales, J.L.; Germeraad, E.A.; Engelsma, M.; et al. Highly Pathogenic Avian Influenza H5N1 Virus Infections in Wild Red Foxes (Vulpes vulpes) Show Neurotropism and Adaptive Virus Mutations. Microbiol Spectr 2023, 11, e0286722. [Google Scholar] [CrossRef] [PubMed]
- Reperant, L.A.; van Amerongen, G.; van de Bildt, M.W.; Rimmelzwaan, G.F.; Dobson, A.P.; Osterhaus, A.D.; Kuiken, T. Highly pathogenic avian influenza virus (H5N1) infection in red foxes fed infected bird carcasses. Emerg Infect Dis 2008, 14, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Leguia, M.; Garcia-Glaessner, A.; Munoz-Saavedra, B.; Juarez, D.; Barrera, P.; Calvo-Mac, C.; Jara, J.; Silva, W.; Ploog, K.; Amaro, L.; et al. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat Commun 2023, 14, 5489. [Google Scholar] [CrossRef] [PubMed]
- Siegers, J.Y.; Ferreri, L.; Eggink, D.; Veldhuis Kroeze, E.J.B.; Te Velthuis, A.J.W.; van de Bildt, M.; Leijten, L.; van Run, P.; de Meulder, D.; Bestebroer, T.; et al. Evolution of highly pathogenic H5N1 influenza A virus in the central nervous system of ferrets. PLoS Pathog 2023, 19, e1011214. [Google Scholar] [CrossRef] [PubMed]
- Ly, H. Highly pathogenic avian influenza H5N1 virus infection of companion animals. Virulence 2024, 15, 2289780. [Google Scholar] [CrossRef] [PubMed]
- Thiry, E.; Zicola, A.; Addie, D.; Egberink, H.; Hartmann, K.; Lutz, H.; Poulet, H.; Horzinek, M.C. Highly pathogenic avian influenza H5N1 virus in cats and other carnivores. Vet Microbiol 2007, 122, 25–31. [Google Scholar] [CrossRef]
- Szalus-Jordanow, O.; Golke, A.; Dzieciatkowski, T.; Czopowicz, M.; Kardas, M.; Mickiewicz, M.; Moroz-Fik, A.; Lobaczewski, A.; Markowska-Daniel, I.; Frymus, T. Upper Respiratory Tract Disease in a Dog Infected by a Highly Pathogenic Avian A/H5N1 Virus. Microorganisms 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Rabalski, L.; Milewska, A.; Pohlmann, A.; Gackowska, K.; Lepionka, T.; Szczepaniak, K.; Swiatalska, A.; Sieminska, I.; Arent, Z.; Beer, M.; et al. Emergence and potential transmission route of avian influenza A (H5N1) virus in domestic cats in Poland, June 2023. Euro Surveill 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Wolf, P.U.; Uhl, W.; Gerst, S.; Harder, T.; Starick, E.; Vahlenkamp, T.W.; Mettenleiter, T.C.; Teifke, J.P. Distribution of lesions and antigen of highly pathogenic avian influenza virus A/Swan/Germany/R65/06 (H5N1) in domestic cats after presumptive infection by wild birds. Vet Pathol 2007, 44, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yeom, M.; Vu, T.T.H.; Do, H.Q.; Na, W.; Lee, M.; Jeong, D.G.; Cheon, D.S.; Song, D. Characterization of highly pathogenic avian influenza A (H5N1) viruses isolated from cats in South Korea, 2023. Emerg Microbes Infect 2024, 13, 2290835. [Google Scholar] [CrossRef]
- Sillman, S.J.; Drozd, M.; Loy, D.; Harris, S.P. Naturally occurring highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b infection in three domestic cats in North America during 2023. J Comp Pathol 2023, 205, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Burrough, E.; Magstadt, D.; Petersen, B.; Timmermans, S.; Gauger, P.; Zhang, J.; Siepker, C.; Mainenti, M.; Li, G.; Thompson, A.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerging Infectious Disease journal 2024, 30. [Google Scholar] [CrossRef]
- Kuiken, T.; Rimmelzwaan, G.; van Riel, D.; van Amerongen, G.; Baars, M.; Fouchier, R.; Osterhaus, A. Avian H5N1 influenza in cats. Science 2004, 306, 241. [Google Scholar] [CrossRef] [PubMed]
- Domestic dog tests positive for avian influenza in Canada Availabe online: https://www.canada.ca/en/food-inspection-agency/news/2023/04/domestic-dog-tests-positive-for-avian-influenza-in-canada.html (accessed on April 25,2024).
- Human Infection caused by Avian Influenza A (H5N1) - Chile. Availabe online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON461 (accessed on April 25, 2024).
- de Jong, M.D.; Bach, V.C.; Phan, T.Q.; Vo, M.H.; Tran, T.T.; Nguyen, B.H.; Beld, M.; Le, T.P.; Truong, H.K.; Nguyen, V.V.; et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med 2005, 352, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Milton, S.; Abdul Hamid, C.; Reinoso Webb, C.; Presley, S.M.; Shetty, V.; Rollo, S.N.; Martinez, D.L.; Rai, S.; Gonzales, E.R.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection in a Dairy Farm Worker. N Engl J Med, 2024. [Google Scholar] [CrossRef]
- State health officials investigate a detection of H5 influenza virus in a human in Colorado. Availabe online: https://cdphe.colorado.gov/press-release/state-health-officials-investigate-a-detection-of-h5-influenza-virus-in-a-human (accessed on April 25, 2024).
- Fouchier, R.A.; Schneeberger, P.M.; Rozendaal, F.W.; Broekman, J.M.; Kemink, S.A.; Munster, V.; Kuiken, T.; Rimmelzwaan, G.F.; Schutten, M.; Van Doornum, G.J.; et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A 2004, 101, 1356–1361. [Google Scholar] [CrossRef]
- Belser, J.A.; Lash, R.R.; Garg, S.; Tumpey, T.M.; Maines, T.R. The eyes have it: influenza virus infection beyond the respiratory tract. Lancet Infect Dis 2018, 18, e220–e227. [Google Scholar] [CrossRef] [PubMed]
- Taft, A.S.; Ozawa, M.; Fitch, A.; Depasse, J.V.; Halfmann, P.J.; Hill-Batorski, L.; Hatta, M.; Friedrich, T.C.; Lopes, T.J.; Maher, E.A.; et al. Identification of mammalian-adapting mutations in the polymerase complex of an avian H5N1 influenza virus. Nat Commun 2015, 6, 7491. [Google Scholar] [CrossRef] [PubMed]
- Hatta, M.; Gao, P.; Halfmann, P.; Kawaoka, Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 2001, 293, 1840–1842. [Google Scholar] [CrossRef] [PubMed]
- Hatta, M.; Hatta, Y.; Kim, J.H.; Watanabe, S.; Shinya, K.; Nguyen, T.; Lien, P.S.; Le, Q.M.; Kawaoka, Y. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog 2007, 3, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Tammiranta, N.; Isomursu, M.; Fusaro, A.; Nylund, M.; Nokireki, T.; Giussani, E.; Zecchin, B.; Terregino, C.; Gadd, T. Highly pathogenic avian influenza A (H5N1) virus infections in wild carnivores connected to mass mortalities of pheasants in Finland. Infect Genet Evol 2023, 111, 105423. [Google Scholar] [CrossRef] [PubMed]
- Human Infection with highly pathogenic avian influenza A(H5N1) virus in Chile. Availabe online: https://www.cdc.gov/flu/avianflu/spotlights/2022-2023/chile-first-case-h5n1-addendum.htm (accessed on April 25, 2024).
- Gabriel, G.; Dauber, B.; Wolff, T.; Planz, O.; Klenk, H.D.; Stech, J. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc Natl Acad Sci U S A 2005, 102, 18590–18595. [Google Scholar] [CrossRef]
- Bussey, K.A.; Bousse, T.L.; Desmet, E.A.; Kim, B.; Takimoto, T. PB2 residue 271 plays a key role in enhanced polymerase activity of influenza A viruses in mammalian host cells. J Virol 2010, 84, 4395–4406. [Google Scholar] [CrossRef]
- Bean, W.J.; Schell, M.; Katz, J.; Kawaoka, Y.; Naeve, C.; Gorman, O.; Webster, R.G. Evolution of the H3 influenza virus hemagglutinin from human and nonhuman hosts. J Virol 1992, 66, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Gu, M.; Liu, K.; Li, Q.; Li, J.; Shi, L.; Li, X.; Wang, X.; Hu, J.; Liu, X.; et al. T160A mutation-induced deglycosylation at site 158 in hemagglutinin is a critical determinant of the dual receptor binding properties of clade 2.3.4.4 H5NX subtype avian influenza viruses. Vet Microbiol 2018, 217, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.G.; Kim, Y.I.; Casel, M.A.B.; Choi, J.H.; Gil, J.R.; Rollon, R.; Kim, E.H.; Kim, S.M.; Ji, H.Y.; Park, D.B.; et al. HA N193D substitution in the HPAI H5N1 virus alters receptor binding affinity and enhances virulence in mammalian hosts. Emerg Microbes Infect 2024, 13, 2302854. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; de Vries, E.; van Kuppeveld, F.J.M.; Matrosovich, M.; de Haan, C.A.M. Second sialic acid-binding site of influenza A virus neuraminidase: binding receptors for efficient release. FEBS J 2021, 288, 5598–5612. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.; de Haan, C.A. Letter to the editor: Highly pathogenic influenza A(H5N1) viruses in farmed mink outbreak contain a disrupted second sialic acid binding site in neuraminidase, similar to human influenza A viruses. Euro Surveill 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Brenner, D.; Wang, Z.; Dauber, B.; Ehrhardt, C.; Hogner, K.; Herold, S.; Ludwig, S.; Wolff, T.; Yu, K.; et al. The NS segment of an H5N1 highly pathogenic avian influenza virus (HPAIV) is sufficient to alter replication efficiency, cell tropism, and host range of an H7N1 HPAIV. J Virol 2010, 84, 2122–2133. [Google Scholar] [CrossRef] [PubMed]
- Hale, B.G.; Randall, R.E.; Ortin, J.; Jackson, D. The multifunctional NS1 protein of influenza A viruses. J Gen Virol 2008, 89, 2359–2376. [Google Scholar] [CrossRef]
- Garcia-Sastre, A.; Egorov, A.; Matassov, D.; Brandt, S.; Levy, D.E.; Durbin, J.E.; Palese, P.; Muster, T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 1998, 252, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Hoffmann, E.; Webster, R.G. The NS1 gene of H5N1 influenza viruses circumvents the host anti-viral cytokine responses. Virus Res 2004, 103, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Jiao, P.; Wang, A.; Zhao, F.; Tian, G.; Wang, X.; Yu, K.; Bu, Z.; Chen, H. The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. J Virol 2006, 80, 11115–11123. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wang, Z.; Uprety, T.; Sreenivasan, C.C.; Sheng, Z.; Hause, B.M.; Brunick, C.; Wu, H.; Luke, T.; Bausch, C.L.; et al. A fully human monoclonal antibody possesses antibody-dependent cellular cytotoxicity (ADCC) activity against the H1 subtype of influenza A virus by targeting a conserved epitope at the HA1 protomer interface. J Med Virol 2023, 95, e28901. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Pascua, P.N.Q.; Nguyen, H.T.; Chesnokov, A.; Champion, C.; Mishin, V.P.; Wentworth, D.E.; Gubareva, L.V. New insights into the neuraminidase-mediated hemagglutination activity of influenza A(H3N2) viruses. Antiviral Res 2023, 218, 105719. [Google Scholar] [CrossRef]
- Yu, J.; Sreenivasan, C.; Sheng, Z.; Zhai, S.L.; Wollman, J.W.; Luo, S.; Huang, C.; Gao, R.; Wang, Z.; Kaushik, R.S.; et al. A recombinant chimeric influenza virus vaccine expressing the consensus H3 hemagglutinin elicits broad hemagglutination inhibition antibodies against divergent swine H3N2 influenza viruses. Vaccine 2023, 41, 6318–6326. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Sun, C.; Gao, R.; Wang, H.; Liu, W.; Yu, K.; Zhou, G.; Zhao, B.; Yu, L. A Temperature-Dependent Translation Defect Caused by Internal Ribosome Entry Site Mutation Attenuates Foot-and-Mouth Disease Virus: Implications for Rational Vaccine Design. J Virol 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yang, D.; Gao, R.; Liang, T.; Wang, H.; Zhou, G.; Yu, L. Modification of the internal ribosome entry site element impairs the growth of foot-and-mouth disease virus in porcine-derived cells. J Gen Virol 2016, 97, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Sreenivasan, C.C.; Sheng, Z.; Hause, B.M.; Zhou, B.; Wentworth, D.E.; Clement, T.; Rausch, D.; Brunick, C.; Christopher-Hennings, J.; et al. Human Monoclonal Antibody Derived from Transchromosomic Cattle Neutralizes Multiple H1 Clades of Influenza A Virus by Recognizing a Novel Conformational Epitope in the Hemagglutinin Head Domain. J Virol 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Nogales, A.; Martinez-Sobrido, L. Reverse Genetics Approaches for the Development of Influenza Vaccines. Int J Mol Sci 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Govorkova, E.A.; Webby, R.J.; Humberd, J.; Seiler, J.P.; Webster, R.G. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J Infect Dis 2006, 194, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Fujii, K.; Kino, Y.; Kawaoka, Y. An improved reverse genetics system for influenza A virus generation and its implications for vaccine production. Proc Natl Acad Sci U S A 2005, 102, 16825–16829. [Google Scholar] [CrossRef] [PubMed]
- Webby, R.J.; Perez, D.R.; Coleman, J.S.; Guan, Y.; Knight, J.H.; Govorkova, E.A.; McClain-Moss, L.R.; Peiris, J.S.; Rehg, J.E.; Tuomanen, E.I.; et al. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet 2004, 363, 1099–1103. [Google Scholar] [CrossRef]
- Panickan, S.; Bhatia, S.; Bhat, S.; Bhandari, N.; Pateriya, A.K.; Kalaiyarasu, S.; Sood, R.; Tripathi, M. Reverse genetics based H5N2 vaccine provides clinical protection against H5N1, H5N8 and H9N2 avian influenza infection in chickens. Vaccine 2022, 40, 6998–7008. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G. Influenza Reverse Genetics-Historical Perspective. Cold Spring Harb Perspect Med 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Zeng, X.; Li, Y.; Shi, J.; Chen, H. Protective efficacy of the H5 inactivated vaccine against different highly pathogenic H5N1 avian influenza viruses isolated in China and Vietnam. Avian Dis 2010, 54, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Zhang, Q.; Gu, C.; Shi, J.; Deng, G.; Ma, S.; Liu, J.; Chen, P.; Guan, Y.; Jiang, Y.; et al. A live attenuated vaccine prevents replication and transmission of H7N9 virus in mammals. Sci Rep 2015, 5, 11233. [Google Scholar] [CrossRef]
- Kozlov, M. US will vaccinate birds against avian flu for first time - what researchers think. Nature 2023, 618, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E.; Spackman, E.; Pantin-Jackwood, M. Success factors for avian influenza vaccine use in poultry and potential impact at the wild bird-agricultural interface. Ecohealth 2014, 11, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Abdelwhab, E.M.; Hafez, H.M. An overview of the epidemic of highly pathogenic H5N1 avian influenza virus in Egypt: epidemiology and control challenges. Epidemiol Infect 2011, 139, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Desvaux, S.; Grosbois, V.; Pham, T.T.; Dao, D.T.; Nguyen, T.D.; Fenwick, S.; Roger, F.; Ellis, T.; Peyre, M. Evaluation of the vaccination efficacy against H5N1 in domestic poultry in the Red River Delta in Vietnam. Epidemiol Infect 2013, 141, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zeng, X.; Cui, P.; Yan, C.; Chen, H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg Microbes Infect 2023, 12, 2155072. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Bai, X.; Li, M.; Zeng, X.; Xu, J.; Li, P.; Wang, M.; Song, X.; Zhao, Z.; Tian, G.; et al. Highly Pathogenic Avian Influenza Virus (H5N1) Clade 2.3.4.4b Introduced by Wild Birds, China, 2021. Emerg Infect Dis 2023, 29, 1367–1375. [Google Scholar] [CrossRef]
- Swayne, D.E. Principles for vaccine protection in chickens and domestic waterfowl against avian influenza: emphasis on Asian H5N1 high pathogenicity avian influenza. Ann N Y Acad Sci 2006, 1081, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Fact Sheet: USDA Continues Partner Engagement to Mitigate Highly Pathogenic Avian Influenza for 2023 Season. Availabe online: https://www.usda.gov/media/press-releases/2023/04/14/fact-sheet-usda-continues-partner-engagement-mitigate-highly (accessed on April 27, 2024).
- Prevention and Antiviral Treatment of Bird Flu Viruses in People. Availabe online: https://www.cdc.gov/flu/avianflu/prevention.htm#anchor_1647619820462 (accessed on April 27, 2024).
- Wan, X.F.; Ferguson, L.; Oliva, J.; Rubrum, A.; Eckard, L.; Zhang, X.; Woolums, A.R.; Lion, A.; Meyer, G.; Murakami, S.; et al. Limited Cross-Protection Provided by Prior Infection Contributes to High Prevalence of Influenza D Viruses in Cattle. J Virol 2020, 94. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




