1. Introduction
One of the most significant challenges in contemporary pig production is to improve efficiency and reduce housing costs without compromising animal welfare and farm sustainability [
1] . Pigs are a social species and they stablish hierarchies for a better use of resources and to reduce conflicts. This is not different in commercial conditions, where it is common for pigs to form hierarchical groups where some animals hold a dominant position over others [
2]. Although to be a dominant or subordinate animal depends on several factors, such as temperament and genetics; one of the most important is the body weight and age, as older and larger pigs have more opportunities to win a fight. Consequently, although there are exceptions, there is a high correlation between the size of the pigs and the probability of being a dominant animal in the pen. Larger pigs have the privilege of eating in their preferred manner, while smaller pigs face difficulty accessing feeders, which are often occupied by high-ranking animals [
3] in the preferred hours of the day. If the animals are mixed with unknown individuals after the group has been created, they will establish new hierarchies, leading to aggressive behavior and fighting [
4].
In any case, cohorts of pigs will be formed by dominant and subordinate animals and the fact that these hierarchies will be more or less important will depend on the management done by the farmer. For instance, if the animals are mixed regularly, the formation of hierarchies will be very visible in the form of fights and injuries produced by the animals to each other and if there is a high competition for resources the difference between the dominant and subordinate animals will be also more evident. In this respect, one of the most important factors of competition in a pig farm is the space, so stocking density will be a key factor to give importance to the hierarchy. In fact, a high population density of pigs in a pen has a negative impact on their comfort, whereas lower pen density results in improvements in animal welfare indicators [
5] . Therefore, both the hierarchy formation after mixing unknown animals and stocking density are factors that affect pig production, especially in terms of welfare and zootechnical parameters[
6,
7].
The present study assumes then that dominant and subordinate pigs reared under two scenarios, one with limited mixing of animals and low stocking density in comparison to one with two mixing procedures during the growing period and higher stocking density, will have different patterns of feeding when registered with automatic feeders. However, in addition to this, although all pigs will be fed in the same environment and group size and with the same diet, the present study hypothesises that the gut microbiota present in both types of animals (dominant and subordinate) will differ. It is known that there is a link between gut microbiota and growth rates [
8] , that there is a shift in the gut microbial communities as pigs age [
8], and that intestinal health is an important factor for maintaining animal health and performance [
9]. However, research assessing the intestinal microbiota and its relationship with pig hierarchies and behavior is in its early stages. Therefore, studies that interweave the gut microbiota with hierarchical positions and different stocking densities, and the possibility to identify microbial biomarkers of such conditions are of relevance at this moment.
To determine the arrangement of hierarchies it is necessary to observe the interactions between the animals[
4,
10]. Therefore, in the present study, a dominance
versus subordinate Test will be subjected to growing pigs, including a control treatment (no mixing and low stocking densities) and a stress treatment (mixed twice during the growing period and with higher stocking density). The aim is to investigate how hierarchy affects feeding behavior, growth, and fecal microbiota.
2. Materials and Methods
2.1. Animals and Treatments
This study was carried out on an experimental farm for growing pigs in Monells, Girona, Spain, from June to October 2022. Eight pens were used for this study, with eight piglets of Duroc breed in each pen, four castrated males and four females (64 animals in total), weighing 18kg at arrival (2 months old) and maintained during the whole growing period, until 140kg (7 months old). Four of those pens were considered the stress treatment, which consisted of a higher density (1 m2 per animal) and mixing animals twice during the growing period. The other 4 pens were considered the control treatment, which consisted of a lower density (1.5 m2 per animal) and maintenance of stable groups during the growing period. The first mixing of animals for the stress treatment, which consisted of moving just the females among pens, was performed 61 days after the start of the study, and the second one, which consisted of moving just the males among pens, was performed 22 days later. In both cases, for males and females, three animals were changed to the other three pens (one per pen) and the fourth, the smallest female and the smallest male, remained in the original pen. This study was divided into three phases. Phase 1 was defined as the period previous to any mixture, phase 2 as the period after the first mixture (female pigs) and before the second mixture (male pigs), and period 3 as the period after the second mixture until slaughter.
2.2. Dominance versus Subordinate Test
To study the relationship between animals in terms of dominance and subordination all the possible combinations of two individuals in each pen of 8 pigs were tested, amounting to a total of 28 distinct combinations per pen. The test was based on an adaptation of Parent et al.(2012) [
11] and consisted of different consecutive sessions over 7 days in a way that each animal did the test only once per day (4 pairing pigs per pen and day). The full process was repeated in three instances during the growing period: just before the first mixing of the stress treatment animals, just after the first mixing, and just after the second mixing. The procedure entailed placing two pigs at the entrance of an outdoor testing pen (4 meters long and 4 meters wide) that contained a trough with sliced apples just at the opposite side (around 100 g was offered each time). The pigs underwent prior training, during which the group was acclimated to the testing pen, and they were accustomed to consuming apples from the trough. The initial assessment aimed to ascertain which animal initiated the consumption of apples and whether any territorial displacements occurred during the test. In cases where no clear displacement occurred, the animal that first ate the apple was deemed the dominant one (“Dominant”). In the event of displacement or confrontation, dominance status was set to the winner. Subsequently, following no more than 30 seconds of apple consumption, the dominant animal was gently removed from the feeding area and relocated to the pig's entrance to facilitate the evaluation of the second pig. This second pig was then granted one minute of solitary access to the apples, after which the dominant pig was reintroduced. A draw was recorded if the subordinate pig consumed the apples without intervention by the dominant counterpart. The dominance status was confirmed if the dominant pig successfully displaced the subordinate during this phase (“Dominant Confirmed”). This second phase allotted one minute for the dominant pig to assert its dominance. After this interval, the test was concluded. The test was deemed inconclusive and non-evaluable in instances where neither of the two pigs consumed apples within a two-minute window or if an animal considered dominant the first time was not able to displace the other pig the second time.
To quantify the dominance test results, two measurements were used: “Dominant” (first animal eating in the first assessment or winner of a fight), and “Dominant Confirmed” (first animal eating in the first assessment and animal displacing the other pig in the second one). If a Dominant or a confirmed Dominant were annotated, it was quantified as a positive qualification in their hierarchy (+1), while the other animal that intervened was quantified negatively (-0.5). In the case of a draw, the contribution to their hierarchy was neutral (0). For a specific time range (before or after a mix), the mean for both Dominant and Confirmed Dominant was calculated for each animal. After that, a ranking score was built as follows: . A higher-ranking score indicated a higher position in the dominance hierarchy. Thus, we established the two highest-ranking animals per pen as dominant, the two lowest as submissive, and the rest (4) as intermediate. As already mentioned, we repeated three times the test along the study, just before the stress treatment pigs were mixed, just after the first/female mixture and just after the second/male one.
2.3. Feeding Behaviour
The animals were identified utilizing ear electronic tags (e-tags), which provided information on the daily consumption of feed in terms of quantity (expressed as the total mass in grams) and duration. This data was acquired using an electronic feeding system. Several measurements were derived from this dataset:
Feed Intake: This metric quantified the total mass, in grams, consumed by each animal per day.
Number of Feeder Visits per Day: This measurement indicated how frequently each animal visited the electronic feeder daily.
Total Feeding Duration per Day: This metric represented the cumulative time during which each e-tag was in contact with the electronic feeder within a single day.
Median Duration of Feeding Session: This measurement reflected the median duration of all feeding sessions from each day for each animal, from which the median of medians was taken.
Time Slots: This category included the total feeding duration within a 4-hour time window, measured both in absolute terms (seconds) and as a percentage of the total feeding time in a day.
Additionally, these measurements were normalized by dividing the value obtained by each pig individually by the median value of all the pigs in their respective pen. This standardization allowed for the assessment of changes relative to other animals sharing the same pen, independent of the passage of time. As food consumption depends on the size and age of the animals, this effect was controlled throughout the study thanks to the standardization. In addition, weight measurements were recorded for each animal on five occasions: at 0 (beginning), 21, 77, 103, and 134 days from the beginning of the study. The final weight measurement was also conducted at 141 or 148 days, depending on the sacrifice date, when animals were 7 months old, as 50% of the animals were slaughtered on day 141 and the other 50% on day 148 of the study.
2.4. Microbial DNA Extraction, Sequencing, and Bioinformatics Analysis
Three stool samplings were obtained from the animals directly from the anus to avoid contamination of the faeces on 58, 100, and 133 days after the start of the study to investigate the microbiota population. Microbial DNA was extracted with the DNeasy PowerSoil Kit (QIAGEN, Hilden, Germany) following the manufacturer’s recommendations. Extracted DNA was sent to the Centro de Regulación Genómica (CRG) for paired-end (2 × 300 bp) sequencing on an Illumina MiSeq (Illumina, San Diego, CA, USA). The 16S rRNA gene fragment was amplified using the primers V3_F357_N: 5'-CCTACGGGNGGCWGCAG-3' and V4_R805: 5'-GACTACHVGGGTATCTAATCC-3'. Sequences were analyzed with QIIME2 [
12] ; barcode sequences, primers, and low-quality reads (Phred score < 30) were removed. The quality control process also trimmed sequences based on the expected amplicon length and removed chimeras. Afterward, sequences were clustered into Amplicon Sequence Variants (ASVs) at 99% of identity. ASVs were classified to the lowest possible taxonomic level based on a primer-specific trained version of the GreenGenes2 Database (released 2022-10) [
13]. Before estimating the diversity indices, samples were rarefied at 31,731 reads to correct for the sequencing depth. Diversity metrics were estimated with the vegan R package v2.6-2 [
14] . The α-diversity was evaluated with the Shannon index [
15] , and the β-diversity was assessed using the Whittaker index [
16] .
2.5. Statistics
All the analyses were run in R (R version 4.3.1 (2023-06-16 ucrt)) [
17] and the p-value threshold was fixed at 0.05.
2.5.1. Weight versus Hierarchy
The weight measurements of each animal were collected and integrated with their respective hierarchical status within the designated experimental phase. When only a single weight measurement was available for a given phase, a linear regression model was constructed. In this model, the dependent variable was the weight of the animal, and the independent variable was the hierarchical status, treated as a categorical factor. Additionally, treatment and age were introduced as covariates in recognition of their known influence on weight. The reference category for hierarchy was “Dominant” (in contrast with “Intermediate” and “Submissive”), while the reference category for treatment was “Control” (in contrast with “Stress”).
Conversely, in instances where multiple weight measurements were obtained, a mixed-effects linear model was employed. This mixed-effects model retained the same structure as the simple linear model, encompassing the weight as the dependent variable, hierarchical status as a categorical factor, and the covariates of treatment and age. However, it introduced a random effect component attributed to each animal's unique identification (ID).
2.5.2. Feeding Behavior versus Hierarchy
In the context of the analysis of feeding behavior measurements, for each experimental phase and animal, median values were computed (except for time slots, where means were used). Subsequently, linear models were constructed, utilizing these medians as the dependent variable, with hierarchical ranks (dominant and submissive) as independent categorical factors, and treatment and pen as covariate factors. Pen was excluded as a covariate when the dependent variable consisted of relative measurements (normalized by the median value within the respective pen).
Changes in hierarchical classes across the experiment were classified as “Better” if the change implied a hierarchical improvement (submissive to either intermediate or dominant, intermediate to dominant), and “Worse” if the change worsened their hierarchical position (dominant to either intermediate or submissive, intermediate to submissive). The linear mixed model was composed of the feeding behavior as the dependent variable, the fixed effect was the interaction between the phase factor (reference being “phase 2”) and the hierarchical change factor (reference being “Better”), and the random effect was the animal’s ID.
In Phase 1, weight measurements were conducted on two occasions. In the subsequent phase, weight assessments were limited to a single instance. During the third and final phase, most of the animals underwent tripartite weight measurements. An evaluation of hierarchical distinctions was carried out through the construction of linear (mixed) models. The t-statistic was used to assess the significance of the coefficient estimates for non-reference levels in a linear regression model with categorical predictors. If the t-statistic was sufficiently large (relative to its standard error), it corresponded to a small p-value, indicating a significant difference between the groups.
In the analysis of the relationship between feeding behavior and hierarchy (or its change), two animals were excluded from the study due to incomplete feed data, although their hierarchy status was recorded for all instances (except for one of them in phase 3, and therefore it was used for the standalone phase 3 analysis). The primary consequence of this exclusion was a statistical comparison of 16 dominant animals to 15 submissive animals in the third phase of the study, in addition to the exclusion when their hierarchical classes changed: two animals less in the worsened group from phase 2 to phase 3 (19 versus 17), and an animal less in the worsened group from phase 1 to phase 3 (15 versus 14). In order to evaluate whether the frequency of specific hierarchical changes was statistically different across treatments or compared to at random, Pearson's Chi-squared test for count data was used.
2.5.3. Microbial Biomarker Identification
Biomarker identification was done with NetMoss2 [
18], an R-based tool developed for integrating large-scale datasets to identify microbial biomarkers. NetMoss2 is based on a Network-based differential abundance approach to identify driver bacteria associated with state transitions. Therefore, allowing the identification of significant biomarkers in the transition between two conditions like in our case, dominant vs submissive pigs. A microbial co-occurrence network was constructed using SparCC [
19] based on the raw genus abundance matrix of each condition. Network graphical representation was done with CytoScape [
20] . The topological parameters of the network and nodes' centralities values were calculated using the CentiScaPe plugin. The Module division was calculated using WGCNA [
21] , and the NetMoss score that is used to measure the driving force of every node in the transition of the network structure was estimated as described in [
18] using the following model:
where A and B represent the dominant and subordinate networks, respectively. NeighborsA represents all neighboring modules in the dominant network, and NeighborsB represents all neighboring modules in the subordinate network. The FDR method was employed to correct for multiple testing, and the classification performance of the microbial biomarkers was evaluated after 5-fold cross-validation as implement the ´netROC´ function of NetMoss2.
3. Results
3.1. Effect of Hierarchy on Weight
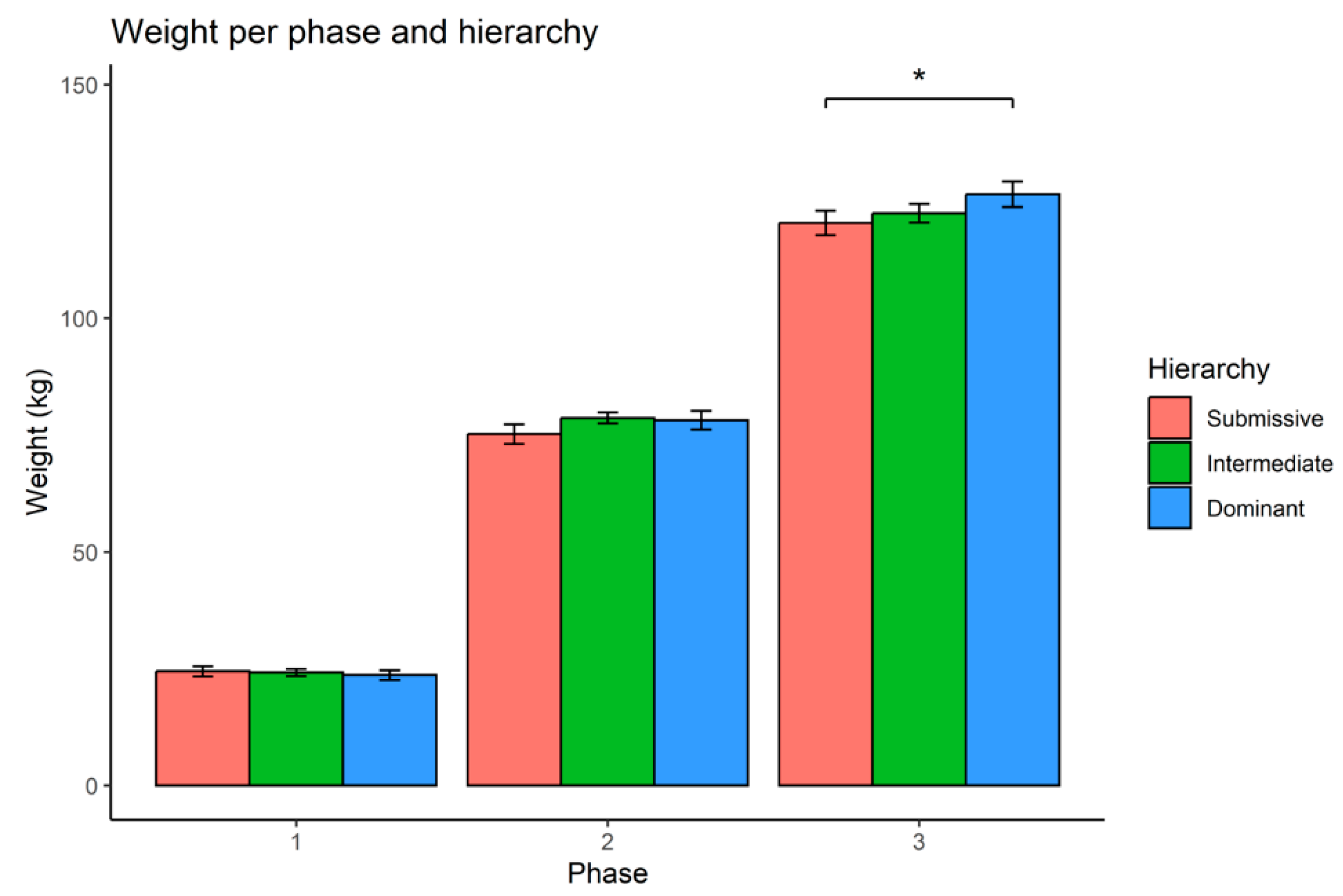
The results showed that differences between the weights of dominant and submissive animals increased across the experimental phases. In the third phase, a statistically significant difference between these two hierarchical categories emerged (p-value = 0.018) (
Figure 1), with dominant animals exhibiting greater body weight than their submissive counterparts.
3.2. Hierarchy and Feeding Behavior
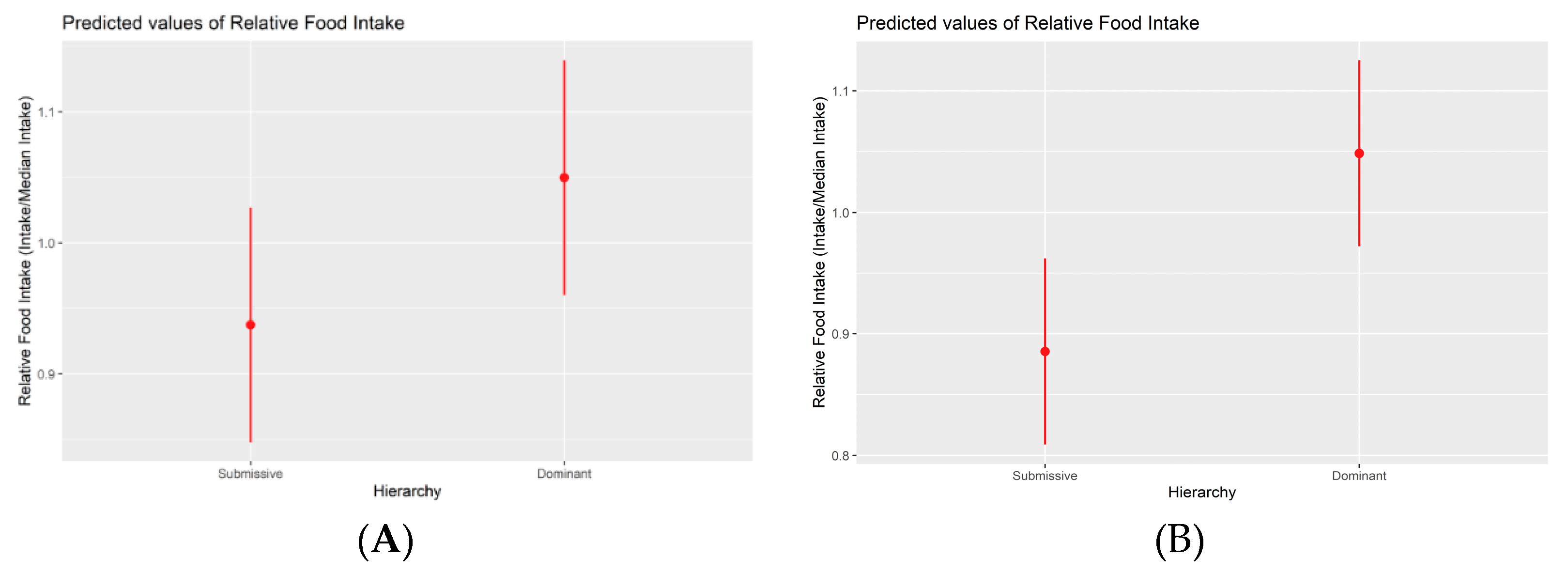
Upon thorough examination of various feeding parameters, including total intake, feed rate, daily time spent eating, median time spent per visit, the number of visits, and time spent patterns across different periods, no statistically significant differences were discernible among the three hierarchical classes during the initial experimental phase. During the second phase, discernible alterations in feeding behavior materialized for the first time between submissive and dominant animals. Submissive pigs exhibited a notably lower level of food consumption (mean of 2476 +/- 97.7 grams) compared to their dominant counterparts (median of 2745 +/- 118 grams, p-value = 0.049). Similar patterns emerged when employing relative measures based on the pen's median. Submissive animals exhibited an 11% lower food consumption compared to dominants (p-value = 0.034), as illustrated in
Figure 2. In the third and final phase, submissive pigs continued exhibiting a significantly lower food consumption level (mean of 2870 +/- 104 grams) compared to their dominant counterparts (mean of 3387 grams +/- 93.6, p-value = 0.002). This trend persisted when employing relative measures based on the pen's median, with submissive animals displaying an 18% lower food consumption than dominants (p-value = 0.001;
Figure 2).
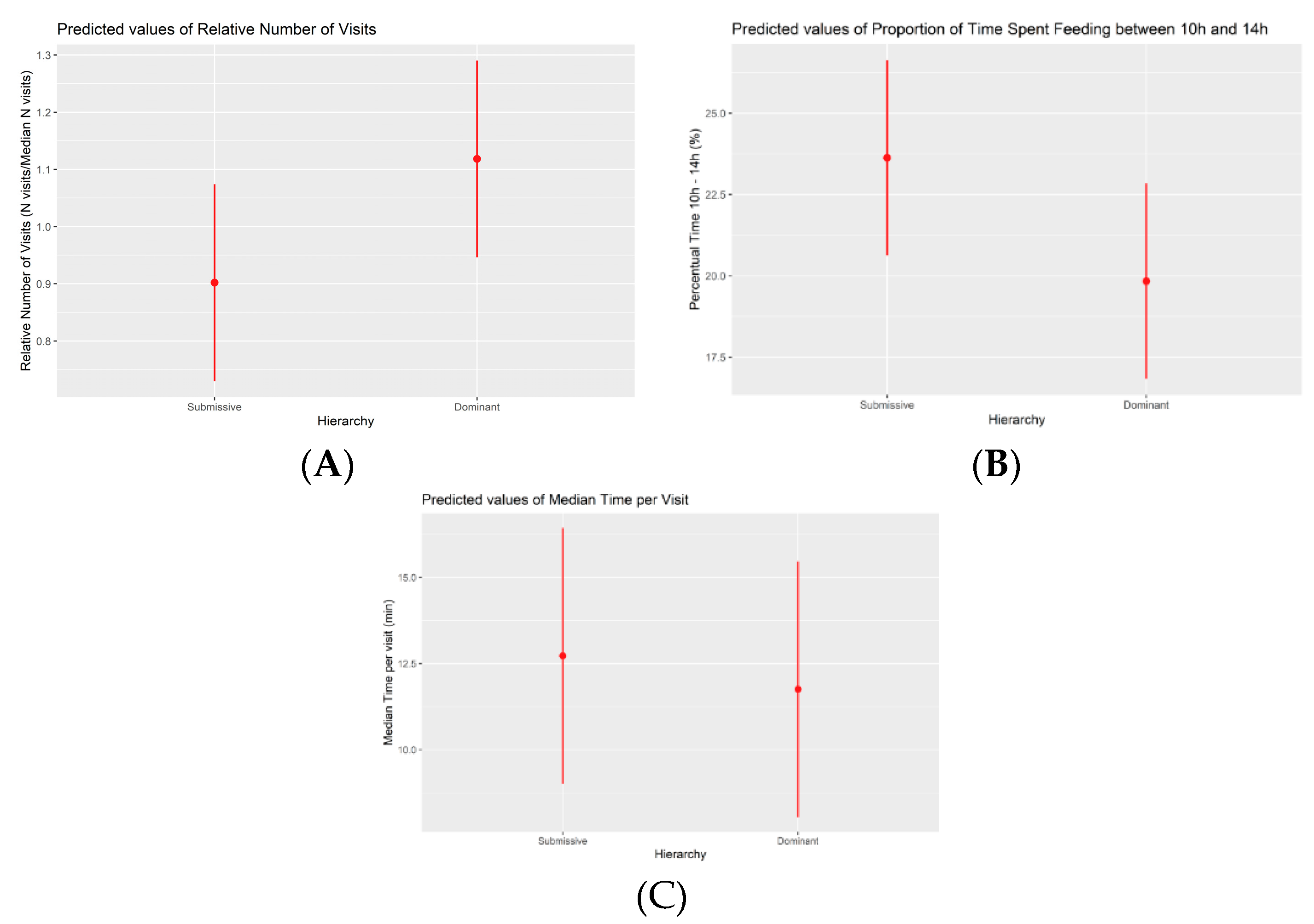
The frequency of visits to the electronic feeder exhibited variations among hierarchical classes. Specifically, submissives displayed a 22% reduction in visits (
Figure 3) relative to the pen's median, with dominants serving as the reference category (p-value = 0.034). Another significant alteration observed in phase 3 pertained to the daily total duration of feeding visits. In relative terms, submissive animals exhibited a 12% decrease (p-value = 0.041) in daily feeding duration compared to dominants. For the last significant change between both hierarchical classes, submissives spent proportionally more time feeding between 10h and 13:59h than dominants, both across all pens (3.80% increase, p-value = 0.033,
Figure 3), and relative to each pen (17% increase, p-value = 0.030). Finally, a non-significant difference (p = 0.427) of 58 seconds more in median time per meal was observed in submissive pigs when compared to dominant subjects.
3.3. Changes in Hierarchical Status
Across the duration of the experiment, a substantial proportion of the animals experienced alterations in their hierarchical classes, and these changes were not limited to the pigs that were relocated to a different pen (stress group). In global, most animals did change their hierarchical class at some point throughout the experiment: 16 out of the 64 animals remained in their class for the whole study. The proportion of these 16 stable animals differed for each class: 37.5% for Dominants (37.5% for both Control and Stress groups), 25% for Intermediate (18.8% for Control and 31.3% for Stress), and 12.5% for Submissives (25% for Control and 0% for Stress). No significant differences (p. value > 0.05) were found between any of the groups (hierarchy) or subgroups (hierarchy-treatment).
From phase 1 to phase 2, the following changes were observed: out of the 16 dominant animals in phase 1, five of them changed to intermediate (three from the Control group, and two from the Stress group), and one to submissive (Stress); out of the 32 intermediate animals in phase 1, five became dominant (three Control, two Stressed), and eight became submissive (five from Control, three from Stress); and out of the 16 submissive animals, one became dominant (Stress group), and eight became intermediate (five Control, three Stressed). Interestingly, all changes were exactly balanced out by their opposite transition. No statistically significant changes were found in any behavioral indicators between animals that climbed (14, eight Control & six Stress) or descended (14, eight Control & six Stressed) the hierarchical ladder.
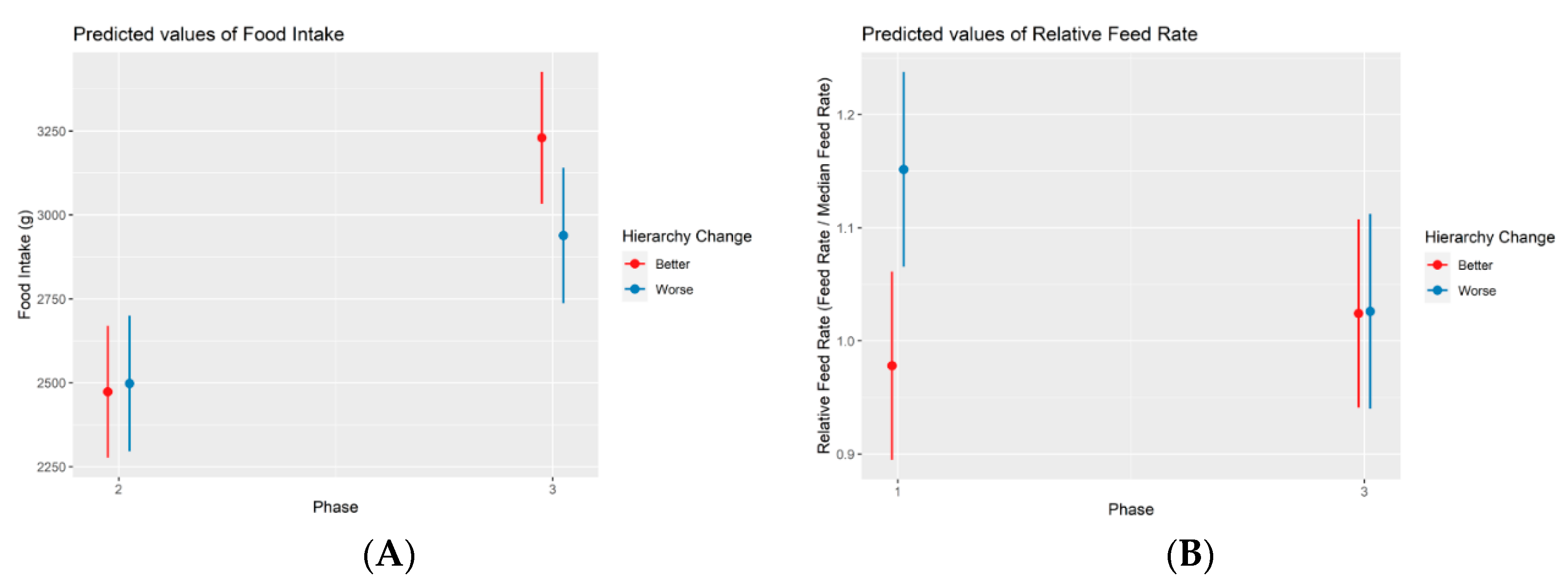
From phase 2 to phase 3, the following changes were observed: out of the 16 dominant animals in phase 2, six of them changed to intermediate (four Control & two Stressed), and one changed to submissive (Stress); out of the 31 intermediate animals in phase 2, seven became dominant (four Control & three Stressed), and 10 became submissive (four Control & six Stressed); and out of the 16 submissive animals, 12 became intermediate (five Control & seven Stressed). When comparing statistically the animals whose hierarchical status improved (19, nine Control & 10 Stressed) and worsened (17, eight Control & nine Stressed), the total daily intake was found to be decreased (-315.78 grams on average) for worsened animals (
Figure 4) in phase 3 (p-value = 0.034).
From phase 1 to phase 3, the following changes were observed: out of the 16 dominant animals in phase 1, seven of them changed to intermediate (four Control & three Stressed), and one to submissive (Stressed); out of the 31 intermediate animals in phase 1, seven became dominant (four Control and three Stressed), and six became submissive (two Control & four Stressed); and out of the 16 submissive animals, one became dominant (Stressed), and seven became submissive (three Control & four Stressed). When comparing statistically the animals that improved (15, seven Control & eight Stressed) and worsened (14, six Control & eight Stressed), the relative feed rate was found to be decreased (-17% on average) for worsened animals (
Figure 4) in phase 3 (p-value = 0.047).
3.3.1. Extreme Examples
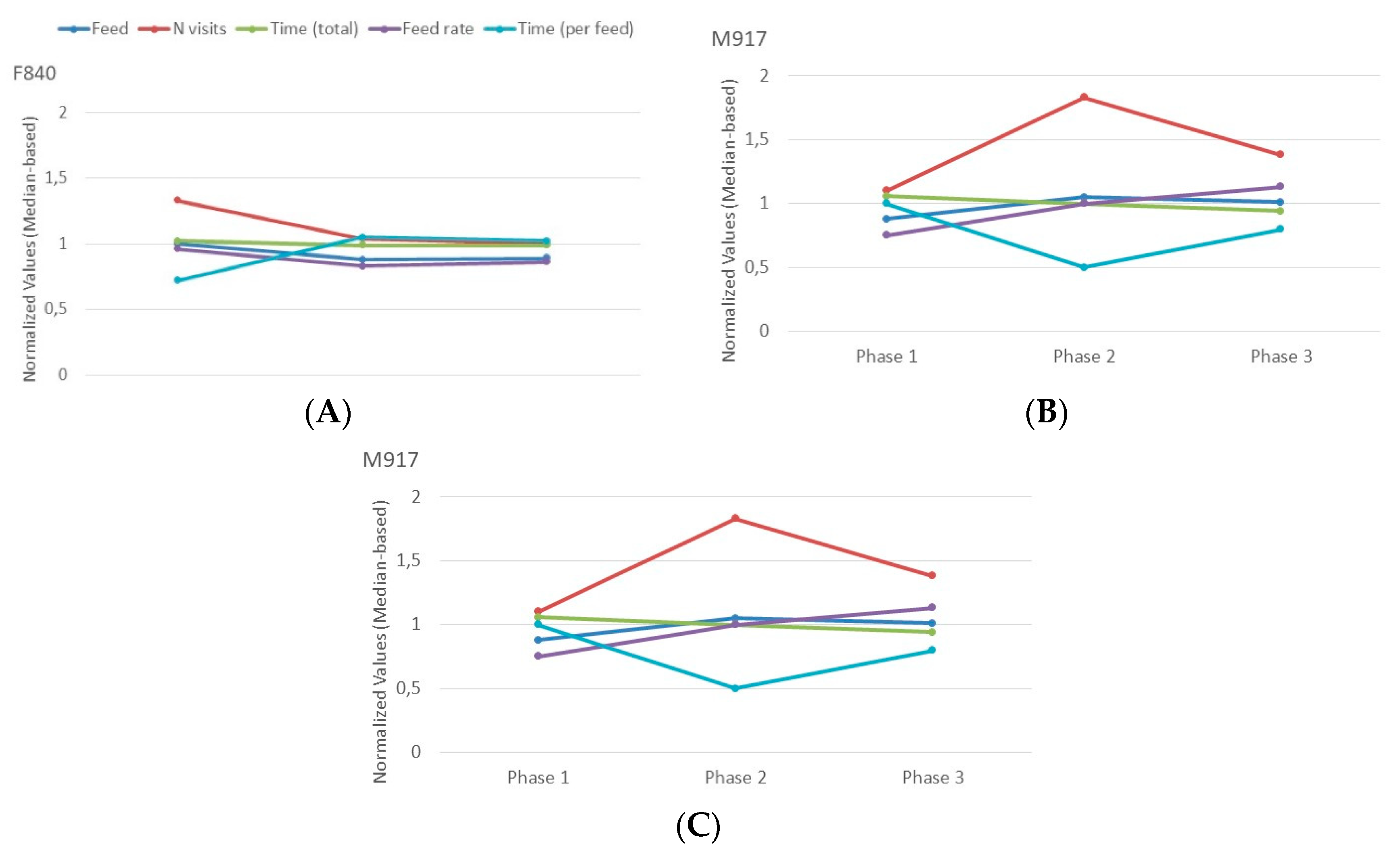
Among all the hierarchical changes, only two animals, F840 and M917, exhibited an extreme transition that persisted over time. At the beginning of the experiment, F840 held a dominant status, while M917 occupied a submissive role. Initially, both animals belonged to the same pen. During the initial mixing (from phase 1 to phase 2), F840 was transferred to a different pen, where it transitioned from a dominant to a submissive position (remaining submissive in phase 3). Conversely, M917, which remained in its original pen, transitioned from submissive to dominant (from phase 1 to 2) and maintained this status in phase 3. Given these extreme changes, these two cases were individually scrutinized to determine whether the observed shifts in hierarchy were mirrored by alterations in feeding behavior.
For F840, intake, feed rate, and especially the number of visits decreased along with its hierarchical status compared to its (Pen) peers (
Figure 5A). On the other hand, the median duration of its visits increased (similar to the non-significant difference found at group level in
Figure 3C). The total time, though, did not change. Globally, the altered levels in Phase 2 were kept in phase 3. In the case of M917, an inverse effect was observed. Feed, feed rate, and especially the number of visits increased along with its hierarchical status compared to its (Pen) peers (
Figure 5B). On the other hand, the median duration of its visits decreased. As with F840, the total time did not change substantially, although a slight decrease was observed over time. The changes in Phase 2 were in part balanced out in Phase 3, but the changes were nonetheless still present.
3.4. Microbiota Characterization
Having fecal samples for all animals in phase 3, their microbiota was sequenced and quantified. From there, the analysis was focused on contrasting the diversity indices and genus abundance between the dominant and submissive hierarchical groups of samples.
3.4.1. Diversity
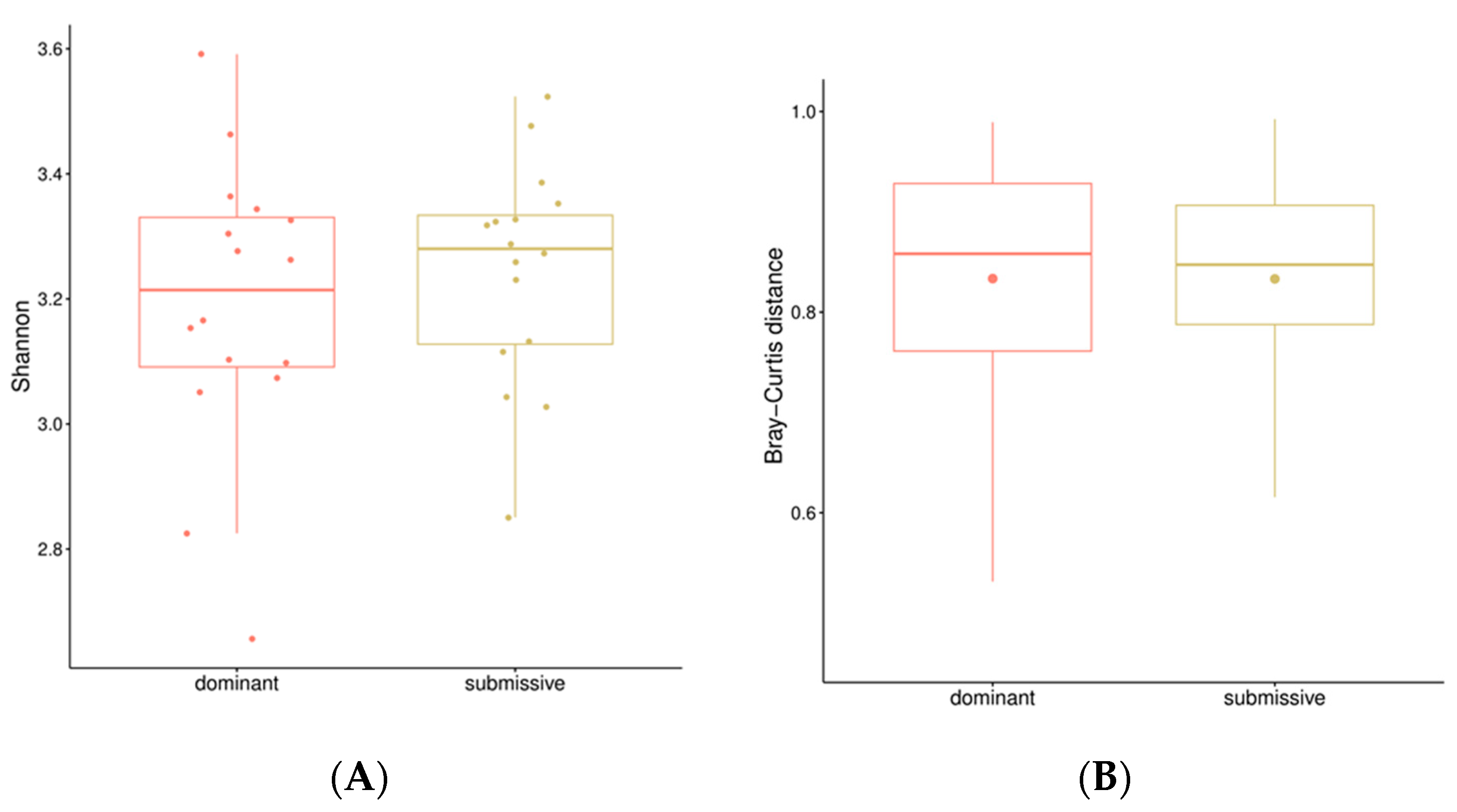
Shannon-alpha diversity was calculated for each sample within both extreme hierarchical classes: dominant and submissive. Alpha diversity helps understand the number of species present within samples. As shown in
Figure 6, no significant difference was found between both groups. Similarly, the Beta diversity based on the Bray-Curtis dissimilarity index, which takes into account differences between samples, did not show any significant differences between the dominant and submissive pigs.
3.4.2. Hierarchical-Associated Microbial Biomarker
Shifts of microbial network modules were assessed to identify microbial biomarkers associated with hierarchical classes. For this reason, NetMoss2 was employed.
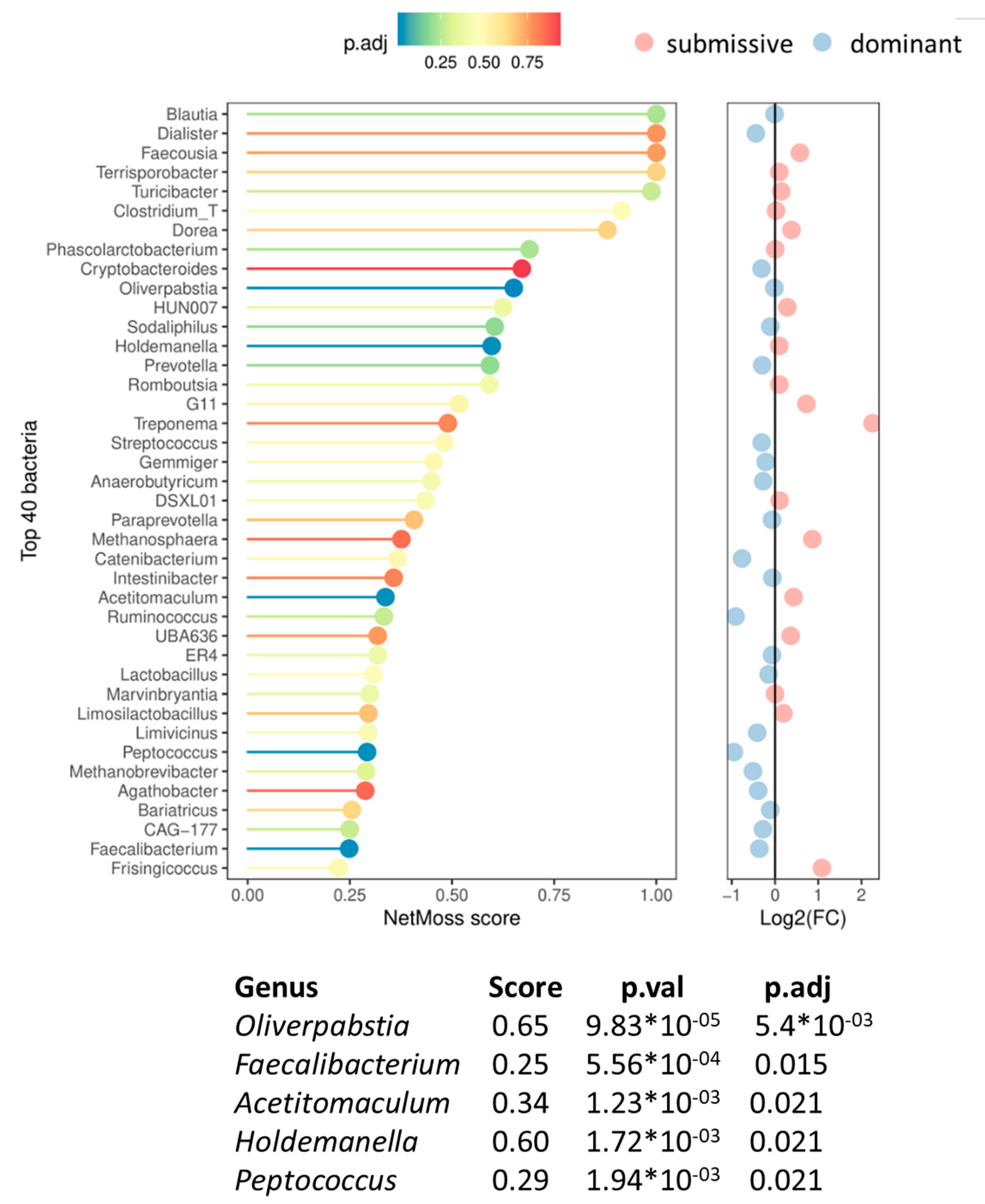
Figure 7 represents the genus distribution between class network inference and scoring (Score threshold >= 0.25). Differential abundance patterns were observed in five genera (p. adj. < 0.05). Among them,
Oliverpabstia, Peptococcus, and
Faecalibacterium were significantly more abundant in dominant animals, while
Holdemanella and
Acetitomaculum were overrepresented in submissive ones. To be noted, the prosed microbial biomarkers showed a high ability to distinguish between the two experimental conditions (AUC = 0.92).
4. Discussion
The present study provides useful data for understanding the feeding behavior within the hierarchy of a group of pigs and its relationship with faecal microbiota. The results primarily revealed differences between submissive and dominant animals, with those considered dominant showing greater weight gains at the end of the study. The heavier pigs acting as dominants have already been addressed in the literature [
22] . This approach is mainly founded on the premise that larger animals occupy feeders most of the time, resulting in greater consumption and growth [
3] . In fact, social hierarchy in pigs can impact growth performance through various mechanisms. A previous study [
10] suggested that social ranking influences feeding behavior, coping styles, and physiological responses, which can indirectly affect growth. Concretely, they found that pigs with dominant status visited the feeder more frequently but spent less time there compared to subordinate and intermediary pigs, which is in accordance with the results found in this study, where more visits were found in dominant than submissive pigs. In this sense, submissive pigs are more affected by competition than dominants, which resembles the results found in previous studies where group-housed pigs were reported to have fewer meals and lower daily feed intake but higher meal size, and longer meal duration [
23,
24] which can restrict growth rate under commercial-like conditions due this feeder competition, aggression, and overall social stress [
23,
24] . What notably attracts attention and reinforces this rationale are the extreme examples encountered in the observed hierarchical shifts. As the experiment progressed, one animal transitioned from dominance to submissiveness, while another shifted from submissiveness to dominance. These pigs serve as representatives of the behavioral profile of these groups since, as they changed positions in the hierarchy, their feeding behaviors also changed according to what was observed in the hierarchical classes. The connection between hierarchy and weight has also been recently studied through the observation of queue formation at feeders. In non-preferred feeder access locations, the presence of light pigs led researchers to infer that these were submissive animals, while those in better positions in the queue would be dominant [
25] . On the other hand, the observed patterns suggest that our proposed dominance test would allow us to identify those animals that would outperform their submissive pen peers in terms of growth, or conversely, those that would underperform in similar circumstances compared to their dominant peers. Therefore, the test is useful for determining the hierarchical position of a pig within a group, particularly if we consider the relationship already discussed between dominance and growth in pigs as a validation source for the model.
Another highly interesting result of the present work is that variance across hierarchy classes was fairly common (only 25% of all animals remained in their original hierarchical class). In addition, no particular hierarchical movement (or lack thereof) was significantly more frequent in any treatment. The only two differences observed were that the most extreme hierarchical changes (dominant to submissive or vice versa) only occurred within the stressed group, and that no stressed submissive animal remained submissive for the whole experiment. These results have two consequences. Firstly, in general, the hierarchy in a group of pigs seems to be not very stable along the time, with few differences in animals that are mixed during the growing period in relation to those not mixed. This trend particularly affects intermediate pigs, and to a lesser extent, the dominant ones, who tend to maintain their status more consistently compared to the others. It is important to note that in the present study, the mixing of animals was performed twice—first with females and then with males. The mixing was done randomly between pens for both dominant and intermediate pigs. However, this was not the case for submissive pigs, as the criterion was to always retain the smallest female and male in their original pen. This approach allowed these potentially last-ranked animals to improve their positions when new animals arrived. This can explain why, in the control group, 25% of the submissive animals remained submissive throughout the study, whereas in the stress group (mixed), none of them remained submissive for the entire study. Therefore, in this case, the model used in the study was helping the submissive animals to change their social status.
Another interesting result of the paper was to see how submissive pigs were eating in the central part of the day during the study, carried out mainly in summer in a warm country such as Spain. In fact, in this case, submissive animals could access food during a high-temperature range probably because dominants were resting. However, it is important to note that the effect was not visible in the warmest phase of the study (phase 2), although the size of the animals, being the maximum at the latest phase of the study, could explain why the effect was seen at the end of the growing period and not in previous ones. In general, during summer, pigs prefer to feed early in the morning and early in the evening when the temperature is not as high [
26] . In another study looking at the behavioural plasticity of subordinate penmates under limited feeder access (single feeder), the smallest pigs (i.e., outcompeted) ate most of their daily feed intake during nighttime [
3,
27] . In general, the literature suggests that pigs can adjust their feeding behaviour in response to constraints within their environment such as limited access to feeder or social dynamics to meet their metabolic needs [
24] .
The fecal microbiota analysis suggests microbial signatures with strong classification capacity (AUC=0.92) between dominant and submissive pigs characterized by a higher abundance of beneficial genera
Faecalibacterium and
Peptococcus, but a lower prevalence of
Holdemanella in the microbial ecosystems of dominant pigs.
Faecalibacterium is a strictly anaerobic butyrate producer bacteria positively associated with human health [
28] . Like in humans, the relative abundance of
Faecalibacterium in pigs increases with age [
29] . Moreover,
Faecalibacterium has been reported to be linked to feeding efficiency in pigs [
30] , depleted in a pig model of social stress [
31] , and negatively linked with incidence of diarrhea, while increasing weight gain in pre-weaned dairy heifers [
32] .
Peptococcus, another indicator of dominance has been reported as positively associated with body weight, average daily gain, and immunocompetence in pigs [
33,
34] . Meanwhile,
Holdemanella, a member of the Erysipelotrichaceae family that was most abundant in submissive pigs, has been recently suggested as an indicator of prolongated stress in pigs [
citation will be available in June] and also associated with neurologic disorders [
35] .
5. Conclusions
The present study tested a hierarchical test in pigs that demonstrated to have a high correlation with the body weight of animals, as it is described in the literature for the relationship between weight and hierarchy in pigs. According to the results, the dominant animals had higher gains during the growing period than the submissive. In addition, they were performing more visits to the feeder throughout the day. In contrast, the submissive pigs performed more visits to the feeder at the midday, when in a summer environment, as it was the case in the study, it is expected a reduction in the number of visits to the feeder. By means of a repetition of the hierarchy test during the growing period, it was observed how most pigs changed their category (dominant, submissive or intermediate), although the dominant animals were more stable during the study. When the faecal microbiota was assessed, differential abundance patterns were observed in five genera showing strong classification capacity, Oliverpabstia, Peptococcus, and Faecalibacterium were more abundant in dominant animals, while Holdemanella and Acetitomaculum were overrepresented in submissive ones at the end of the study. Therefore, dominant and subordinate animals not only show differences in performance and feeding behaviour, but also in some specific microbial biomarkers that can be found in faeces and that can be used to assess the social status of an animal and other aspects related with pig welfare in the future.
Author Contributions
“Conceptualization, A.D., J.O.A and Y.R.C.; methodology, G.Z.P., R.R. and A.C..; formal analysis, J.O.A and L.B.C.; resources, A.D. and Y.R.C.; data curation, J.O.A. and Y.R.C.; writing—original draft preparation, J.O.A and G.Z.P.; writing—review and editing, A.D, L.B.C and Y.R.C.. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research was funded by Ministerio de Ciencia e Innovación de España, grant number PDC2021-121269-100.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of IRTA (protocol code 10329 with date of approval 17-april 2019).
Data Availability Statement
Data will be available under request to the corresponding author.
Acknowledgments
The caretakers of the pigs from Monells, IRTA, are acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Camp Montoro, J. et al. Effect of space allowance and mixing on growth performance and body lesions of grower-finisher pigs in pens with a single wet-dry feeder. Porc. Health Manag. 7, 7 (2021).
- Meese, G. B. & Ewbank, R. The establishment and nature of the dominance hierarchy in the domesticated pig. Anim. Behav. 21, 326–334 (1973).
- Georgsson, L. & Svendsen, J. Degree of competition at feeding differentially affects behavior and performance of group-housed growing-finishing pigs of different relative weights1. J. Anim. Sci. 80, 376–383 (2002).
- Tong, X. et al. Reestablishment of Social Hierarchies in Weaned Pigs after Mixing. Animals 10, 36 (2019).
- Fu, L. et al. Stocking density affects welfare indicators of growing pigs of different group sizes after regrouping. Appl. Anim. Behav. Sci. 174, 42–50 (2016).
- Da Fonseca De Oliveira, A. C., Costa, L. B., Weber, S. H., Ramayo-Caldas, Y. & Dalmau, A. Mixed management in growing and finishing pigs: Differences between gender and their impacts on behavior, growth performance, and physiological parameters. PLOS ONE 18, e0284481 (2023).
- Zhang, Z. F., Li, J., Park, J. C. & Kim, I. H. Effect of Vitamin Levels and Different Stocking Densities on Performance, Nutrient Digestibility, and Blood Characteristics of Growing Pigs. Asian-Australas. J. Anim. Sci. 26, 241–246 (2013).
- Lee, J. H. et al. Comparative analysis of the pig gut microbiome associated with the pig growth performance. J. Anim. Sci. Technol. 65, 856–864 (2023).
- Celi, P. et al. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci. Technol. 234, 88–100 (2017).
- Da Fonseca De Oliveira, A. C., Webber, S. H., Ramayo-Caldas, Y., Dalmau, A. & Costa, L. B. Hierarchy Establishment in Growing Finishing Pigs: Impacts on Behavior, Growth Performance, and Physiological Parameters. Animals 13, 292 (2023).
- Parent, J. P., Meunier-Salaün, M. C., Vasseur, E. & Bergeron, R. Stability of social hierarchy in growing female pigs and pregnant sows. Appl. Anim. Behav. Sci. 142, (2012).
- Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
- McDonald, D. et al. Greengenes2 unifies microbial data in a single reference tree. Nat. Biotechnol. 1–4 (2023). [CrossRef]
- Oksanen, J. et al. vegan: Community Ecology Package. (2022).
- Shannon, C. E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 27, 379–423 (1948).
- Whittaker, R. H. Evolution and Measurement of Species Diversity. TAXON 21, 213–251 (1972).
- R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, Austria, 2024).
- Xiao, L., Zhang, F. & Zhao, F. Large-scale microbiome data integration enables robust biomarker identification. Nat. Comput. Sci. 2, (2022).
- Friedman, J. & Alm, E. J. Inferring Correlation Networks from Genomic Survey Data. PLoS Comput. Biol. 8, (2012).
- Shannon, P. et al. Cytoscape: A software Environment for integrated models of biomolecular interaction networks. Genome Res. 13, (2003).
- Zhang, B. & Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 4, (2005).
- Rasmussen, D. K., Weber, R. & Wechsler, B. Effects of animal/feeding-place ratio on the behaviour and performance of fattening pigs fed via sensor-controlled liquid feeding. Appl. Anim. Behav. Sci. 98, 45–53 (2006).
- De Haer, L. C. M. & De Vries, A. G. Feed intake patterns of and feed digestibility in growing pigs housed individually or in groups. Livest. Prod. Sci. 33, 277–292 (1993).
- Bornett, H. L. I., Morgan, C. A., Lawrence, A. B. & Mann, J. The effect of group housing on feeding patterns and social behaviour of previously individually housed growing pigs. Appl. Anim. Behav. Sci. 70, 127–141 (2000).
- Romero-Aguirregomezcorta, J. et al. Differences in Weight, Hierarchy, and Incidence of Lameness between Two Groups of Adult Pigs Derived from Assisted Reproductive Technologies. Animals 12, 3578 (2022).
- Cross, A. J., Brown-Brandl, T. M., Keel, B. N., Cassady, J. P. & Rohrer, G. A. Feeding behavior of grow-finish swine and the impacts of heat stress. Transl. Anim. Sci. 4, 986–992 (2020).
- Chen, C. Y. et al. Influence of heritable social status on daily gain and feeding pattern in pigs. J. Anim. Breed. Genet. 127, 107–112 (2010).
- Martín, R. et al. Faecalibacterium : a bacterial genus with promising human health applications. FEMS Microbiol. Rev. 47, fuad039 (2023).
- Gaukroger, C. H. et al. Changes in Faecal Microbiota Profiles Associated With Performance and Birthweight of Piglets. Front. Microbiol. 11, 917 (2020).
- Kubasova, T. et al. Effects of host genetics and environmental conditions on fecal microbiota composition of pigs. PLOS ONE 13, e0201901 (2018).
- Nguyen, T. Q. et al. Identification of intestinal and fecal microbial biomarkers using a porcine social stress model. Front. Microbiol. 14, 1197371 (2023).
- Foditsch, C. et al. Oral Administration of Faecalibacterium prausnitzii Decreased the Incidence of Severe Diarrhea and Related Mortality Rate and Increased Weight Gain in Preweaned Dairy Heifers. PLOS ONE 10, e0145485 (2015).
- Oh, J. K. et al. Association between the body weight of growing pigs and the functional capacity of their gut microbiota. Anim. Sci. J. 91, e13418 (2020).
- Ramayo-Caldas, Y. et al. Leveraging host-genetics and gut microbiota to determine immunocompetence in pigs. Anim. Microbiome 3, 74 (2021).
- Geng, S. et al. Gut Microbiota Are Associated With Psychological Stress-Induced Defections in Intestinal and Blood–Brain Barriers. Front. Microbiol. 10, 3067 (2020).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).