1. Introduction
Functionalization of nanoparticles (NP) through covalent bonding of various molecules has become a versatile technique in nanochemistry [
1,
2,
3,
4]. Such functionalization can have various intentions ranging from stabilizing the surface of NP to controlled drug-release [
5,
6,
7,
8,
9,
10,
11]. Covalent bonding of surface ligands, drug molecules, luminescent probes or other surface functionalities to NP is clearly superior to adsorption through non-covalent forces such as hydrogen bonding, dipolar forces, or van der Waals forces. Covalent bonding allows far more stable and selective binding of the surface functionalities, defined chemical moieties, and controlled release through specific reactions reversing the covalent binding [
12,
13,
14,
15,
16]. This also includes coordinative bonds [
17,
18]. The functional molecules defining the application can be either directly anchored on the NP surface through functions like carboxylates, phosphonates, or alkylsilanes ([molecule–anchor] conjugates) or by using [molecule–end group] conjugates combining the functional molecule and a reactive end group for covalent binding to functionalize NP surfaces. For both cases, multifunctional molecules allowing broad variation and good chemical stability are required [
19,
20].
Thiosemicarbazones (TSC) have continuously received attention from their interesting biological properties and anti-bacterial, anti-malarial, anti-parasitic, and anti-proliferative activities have been reported [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30]. This includes TSCs covalently bound/conjugated to other biologically relevant molecules such as glucose or coumarin to enforce their biological activities [
31,
32,
33]. Also, the binding of metals to TSCs or such molecular conjugates to generate anti-proliferative properties has previously been studied [
34,
35,
36,
37,
38,
39,
40].
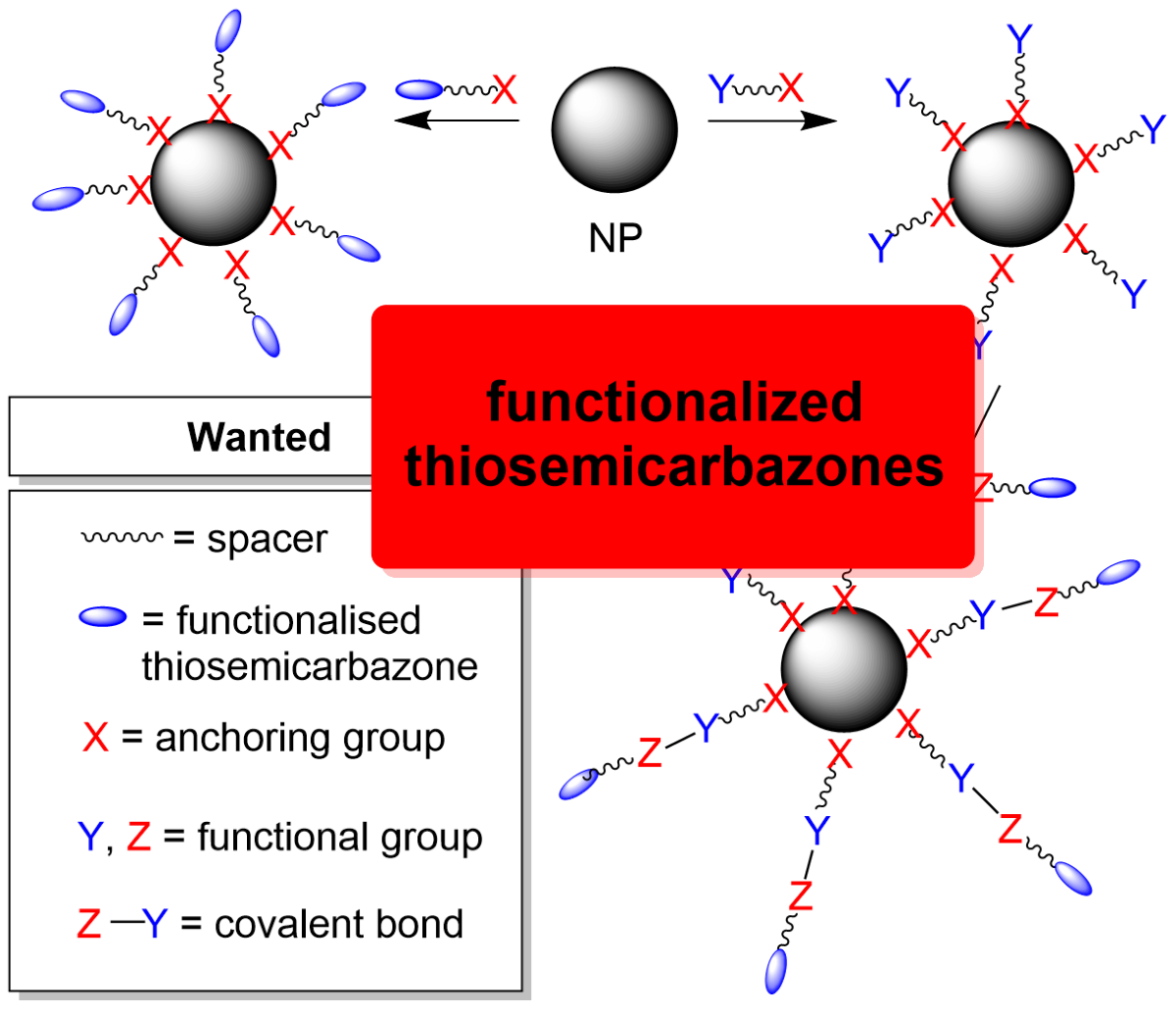
In contrast to this, the idea of binding metal-TSC complexes or [drug–TSC] conjugates to surfaces of NP is new [
41,
42,
43]. The potential suitability of TSCs to serve as ligands for metal coordination covalently anchored on NP surfaces and conjugating them to further functional units at the same time, is obvious from the up to four possible substituents on both the
N1 and
N4 functions of the general TSC R
1R
2C=N(1)–NH–C(S)–N(4)R
3R
4 backbone (
Scheme 1A and B, marked in green) and from the modular synthesis from the R
3- and R
4-substituted thiosemicarbazides and any R
1- and R
2-substituted carbonyl (
Scheme 1D, blue and red marking, respectively).
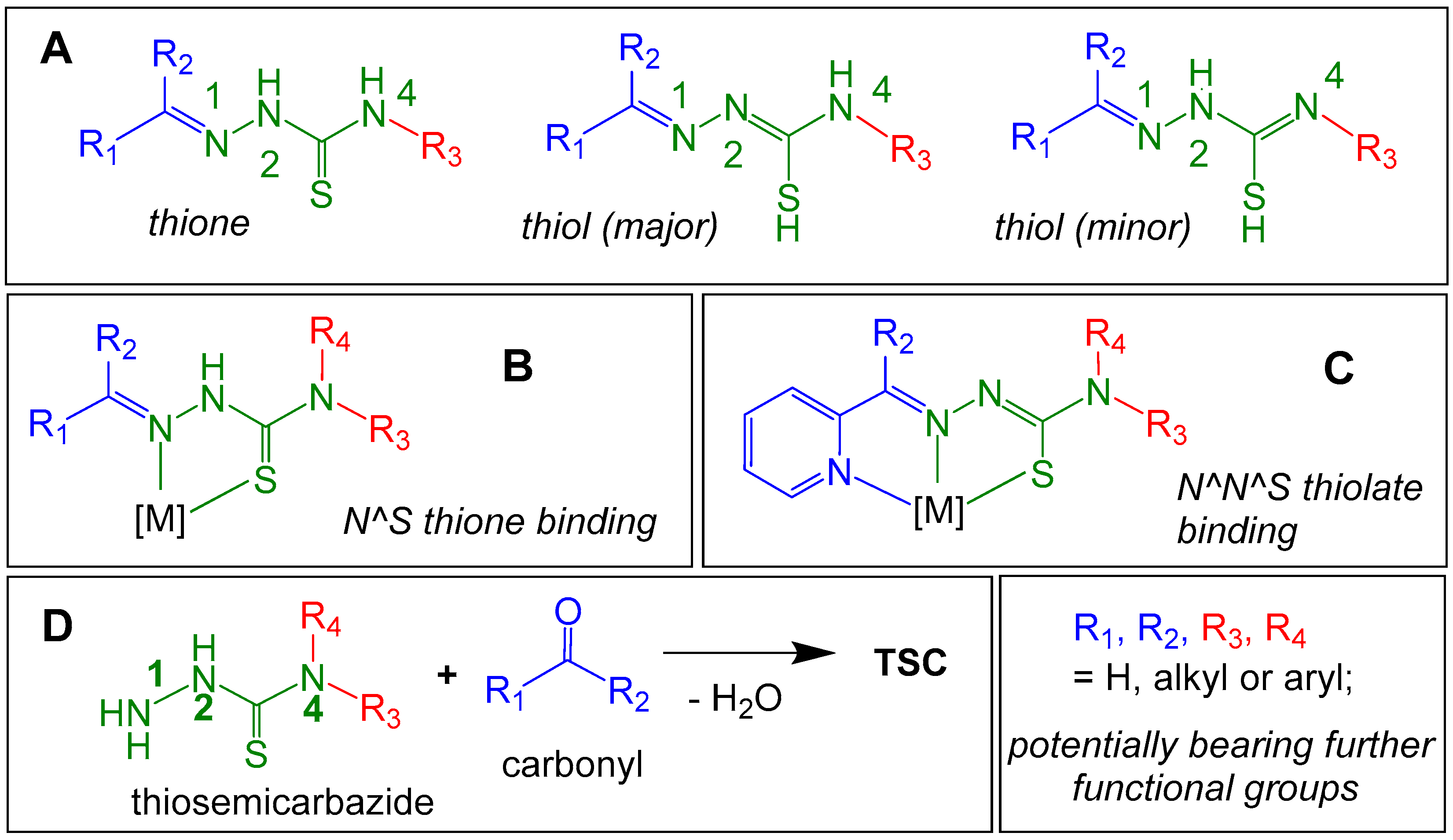
TSCs have several donor atoms in their NNC(S)N backbone that allow metal coordination, a five-ring chelate N1^S coordination is the most prominent [
27,
28,
29,
30]. This coordination sphere can be extended using further coordination units such as pyridyl (
Scheme 1C). The TSC immanent thione-thiole tautomerism (
Scheme 1A) contributes to selective metal ion binding through the charge of the ligands with the neutral thione vs. the anionic thiolate forms. Thus, even if R
1 is part of the metal coordination site, R
2, R
3, and R
4 are still available to carry additional functional groups allowing to conjugate the TSC to other functional molecules (fluorescent or bioactive) AND conjugate the entire molecular entity to the surface of a NP thus forming trilateral [functional-molecule–TSCcomplex–NP] conjugates (
Scheme 2 and
Scheme 3).
TSCs have previously been used for the functionalization of NP using various non-covalent approaches, including nano formulation/encapsulation [
44,
38]. In contrast to this, approaches for covalent binding of TSC onto NP are scarce. Till recently, only the condensation of citrate-functionalized Au NP with
p-methyl-carbohydrazonethioamide [
41] was reported, while the binding of a phenolate-acridine-TSC molecules on an Ag NP surface is depending on the coordination of this molecule on the surface and not on covalent bonds [
42]. In a very recent approach, Fe
3O
4@SiO
2 core-shell NP were reacted subsequently with (3-chloropropyl)trimethoxysilane (CPTMS), 4-hydroxyacetophenone, thiosemicarbazide, and CuSO
4.5H
2O to yield a fully covalently linked [NP–TSC–Cu] conjugate with the Cu(II) ions bound by two different anchored 4-oxoacetophenone-TSC moieties [
43]. In further recent reports, the surface of NPs was functionalized using materials such as dextran or chitosan before attaching TSCs or TSC complexes to them, but the character of the bonding between the TSCs and NP surface is not clear [
49,
50,
51,
52,
53,
54].
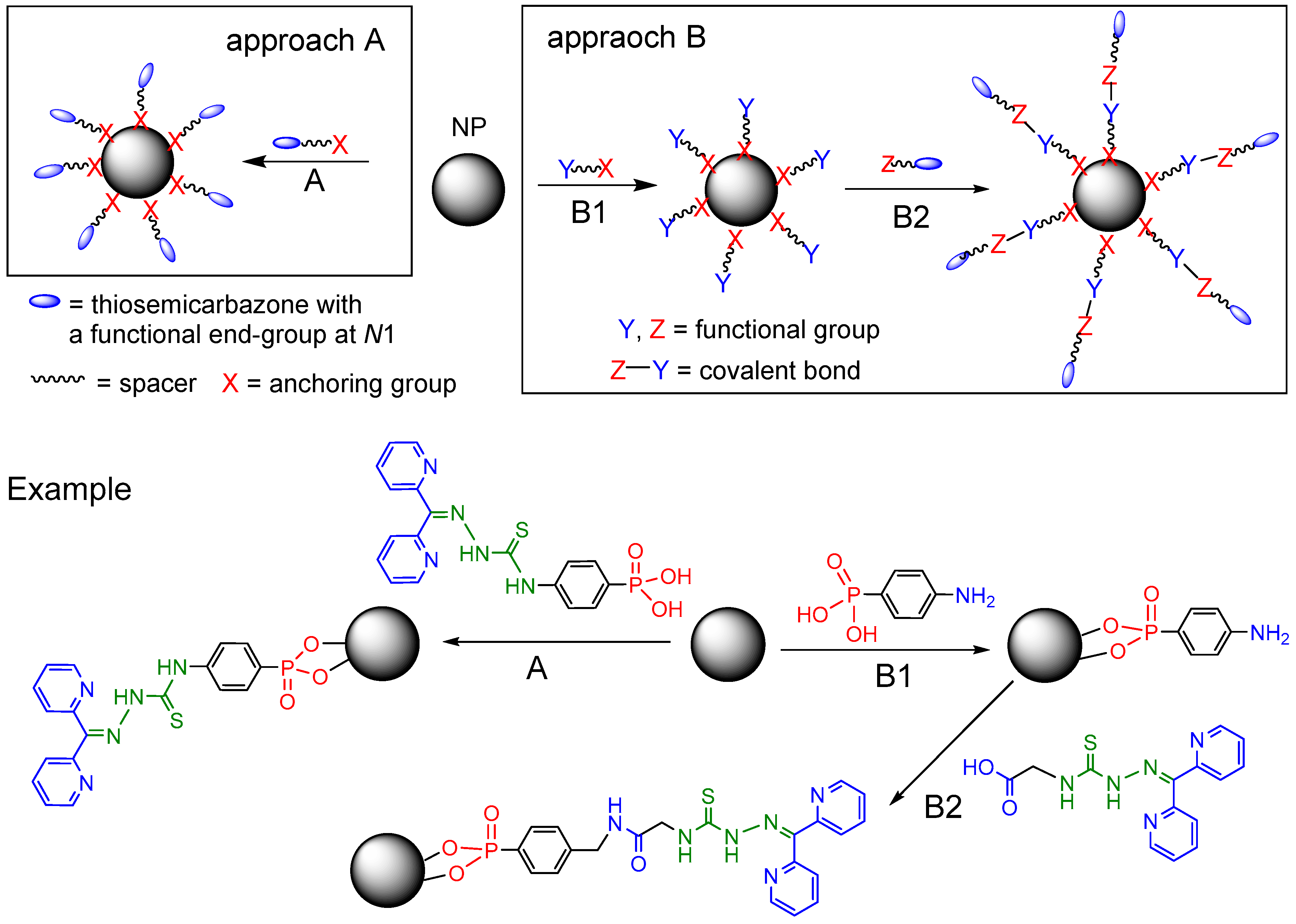
We thus embarked to explore possibilities to bind TSC molecules covalently to NP surfaces or use them as covalent connections between NP-anchoring functions and biologically relevant molecular entities (trilateral conjugates). Herein we report on our first attempts to synthesize functionalized TSC derivatives for direct covalent/coordinative anchoring of TSC molecules on NP surfaces ([TSC–X–NP] conjugates),
Scheme 2, approach A) or TSC derivatives (TSC–Z) which can be bound to NP surfaces using additional anchoring units (Y–X) for covalent connection in [TSC–Z–Y–X–NP] conjugates (
Scheme 2, approach B).
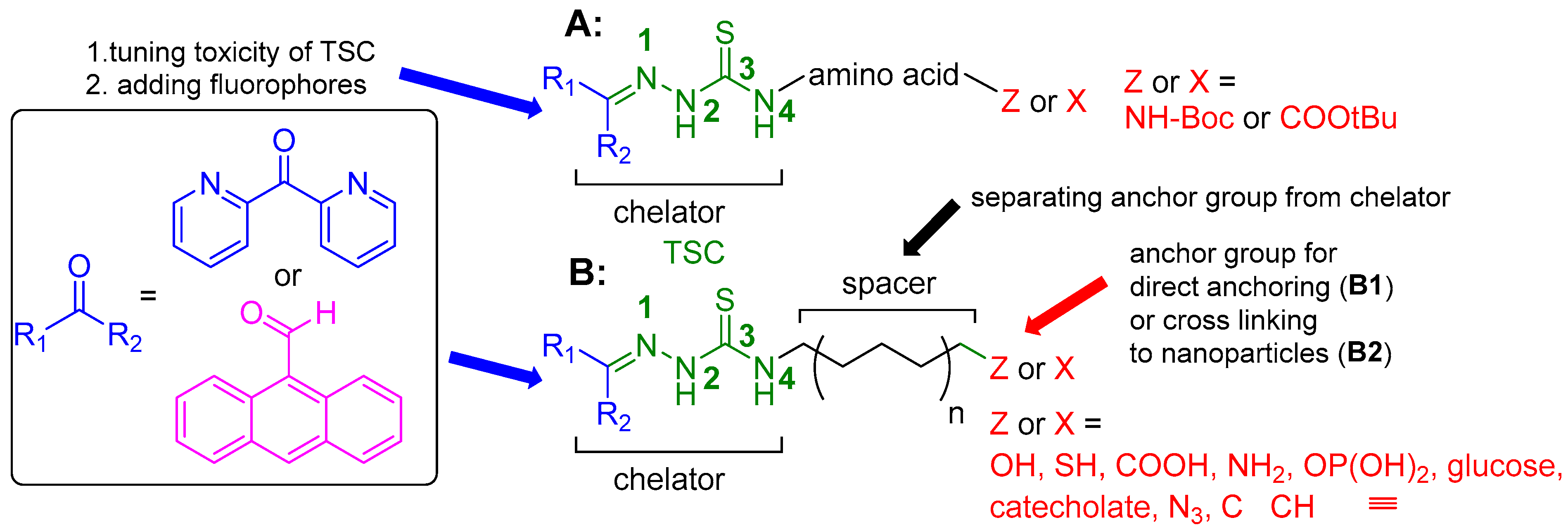
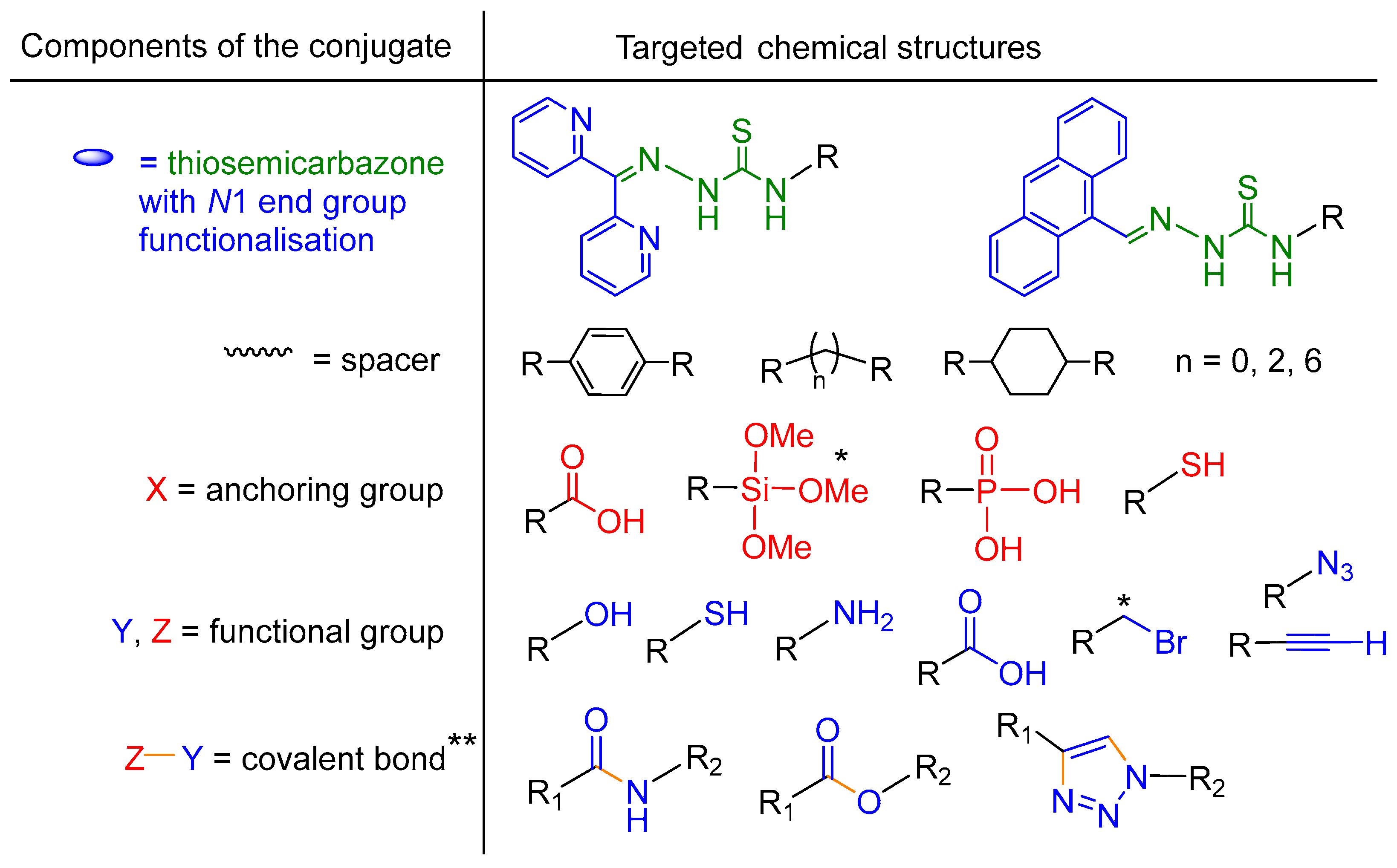
We functionalized the TSCs in this study at the
N1 position with di-2-pyridyl ketone (
Scheme 3), as the resulting di-2-pyridyl-imine group usually leads to an increased toxicity of TSCs [
22,
55,
56,
57,
58]. Also, we placed 9-anthraldehyde (anthracene-9-carbaldehyde) in this position to form the fluorescent 9-anthracenylmethyl tag (
Scheme 3)[
59].
In the
N4 position we placed various chemical functionalities. They were either protected amino acids with their NHBoc protected functions terminating the conjugate, or hydrocarbon spacers to functional groups such as phosphonic acid (OP(OH)
2), glucose,
o-hydroquinone, and thiol (SH), or OH for anchoring on NPs or OH, NH
2, COOH, N
3 or C≡CH (
Scheme 3) for further connection via simple reactions such as amide or ester formation or click chemistry.
With the molecular TSC conjugates shown in
Scheme 3 in hands will allow us to generate trilateral [functional-molecule–TSCcomplex–NP] conjugates in future work. In this contribution we will exclusively report on these molecular [TSC–spacer–anchor] and [TSC–spacer–end group] conjugates.
2. Results and Discussion
2.1. General Approaches and Targets
In the focus of the first group (group A in
Scheme 2) of target structures TSC–X, were TSCs containing potentially coordinating amine, carboxylate, hydroxy, phosphonate, silane, and thiol group (
Scheme 4, anchoring group). TSCs with a thiol group can be used for the direct attachment to gold or other NP with a soft character in view of the
hard and
soft
acids-and
bases (HSAB) principle [
60]. Hard NP surfaces such as metal oxides can be coordinated using amine, carboxylate, hydroxy, phosphonate, or silane groups.
Amine, carboxylate, and hydroxy groups together with alkyne and azide functionalities can also be used to covalently link the TSCs to pre-functionalized nanoparticles by “click” chemistry or the formation of esters and amides (TSC–Z + Y–X–NP) (group B in
Scheme 2 and
Scheme 4, for details, see
Section 2.2). TSCs with bromide functions are interesting precursors for the introduction of other anchoring groups by the substitution of the bromide function or for a three component click reactions with bromides as precursors for azides, as will be outlined later.
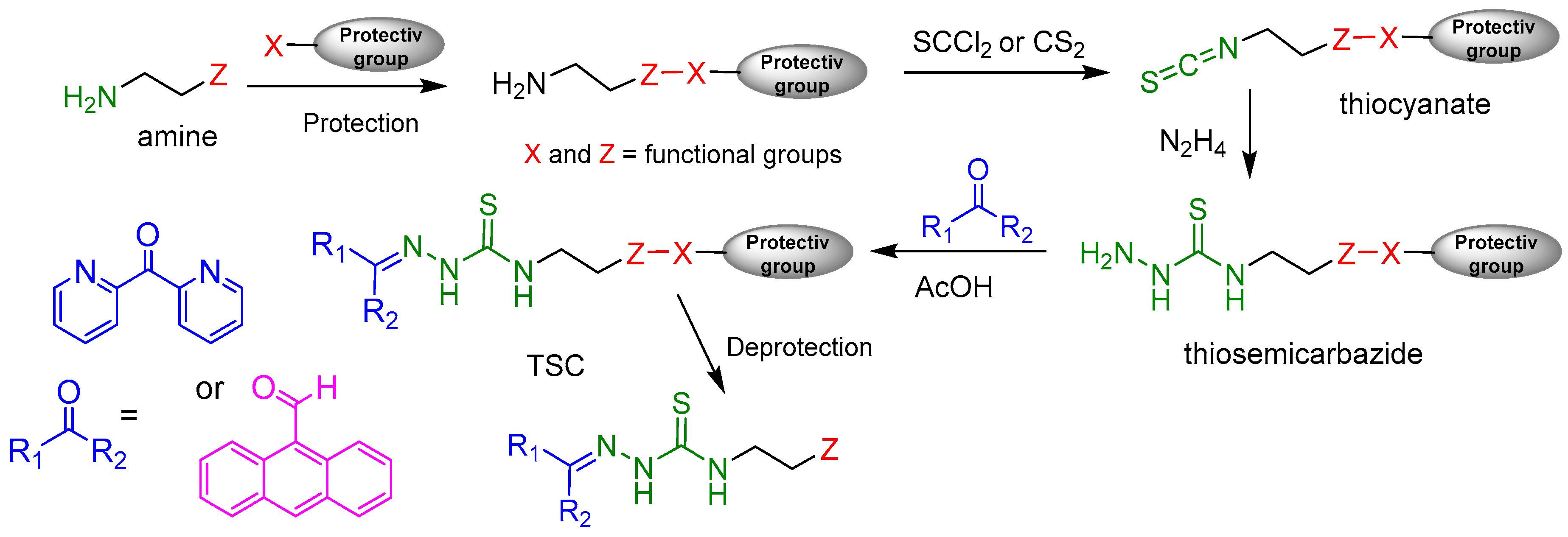
The synthesis of the
N4 functionalized TSCs started from bifunctional, primary amines with the general synthesis shown in
Scheme 5 (details in
Section 2.2 and the
Supplementary Materials). Reaction of the amines with thiophosgene (SCCl
2) or carbon disulfide (CS
2) lead to thiocyanates. Reaction with hydrazine (N
2H
2) gave the thiosemicarbazides. These were reacted with the two carbonyl compounds di-2-pyridyl ketone and 9-anthraldehyde to give the protected TSCs. In some cases, we deprotected them, in other cases, the protected TSCs are the final products.
The amine and the anchoring group should be spatially separated in these starting materials to reduce the influence of the anchor group on the properties of the TSC later on (
Scheme 3). The nature of this spacer function will probably also affect the biological properties of the TSC and we chose the rigid 1,4-phenylene, the slightly more flexible 1,4-cyclohexane, and the fully flexible alkyl spacers ethylene or hexylene (
Scheme 4). Depending on the reactivity of the anchoring group, the intermediate protection of amine, thiol and carboxylic acid functions is necessary due to the high reactivity of thiophosgene SCCl
2 and hydrazine used for the TSC synthesis.
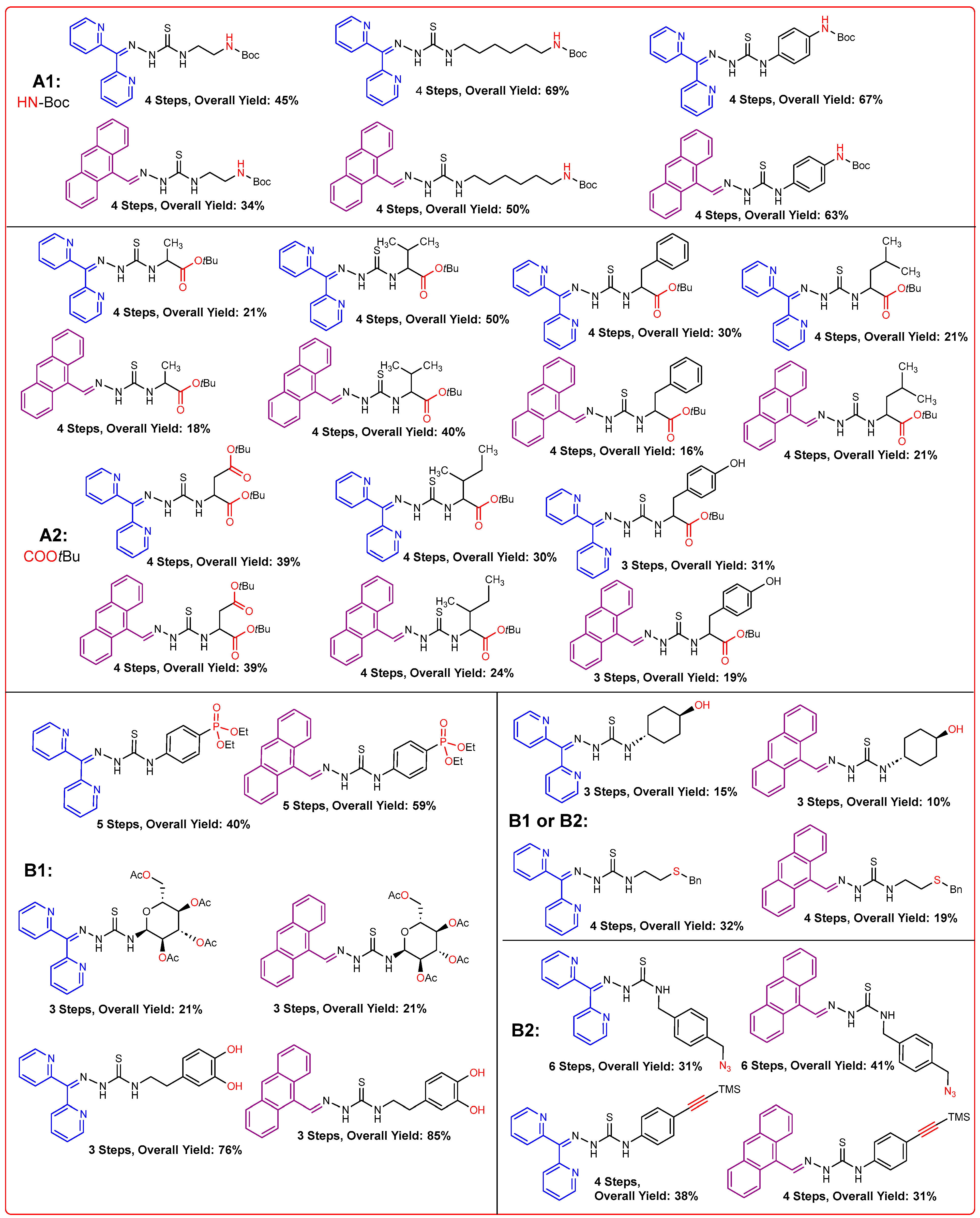
In total we synthesized 34 TSC derivatives with di-pyridyl ketone (
Scheme 6, marked in blue) or 9-anthraldehyde (
Scheme 6, marked in purple) in the
N1 position and various functional groups (marked in red) and spacers in the
N4 position (for details, see
Section 2.2).
Details of the syntheses of the thiosemicarbazides and the thiosemicarbazones can be found in the
Supplementary Materials. The reported products are generally air- and hydrolysis stable. The anthracenyl derivatives seem to be light-sensitive and should be stored under the exclusion of light. All products are soluble in MeCN and dimethyl sulfoxide (DMSO) and were characterized using
1H and
13C nuclear magnetic resonance (NMR) spectroscopy, electron-spray-ionization mass spectrometry (ESI-MS) and UV-vis absorption and in part, UV-vis absorption and photoluminescence spectroscopy (spectra in the
Supplementary Materials).
2.2. Syntheses of TSCs
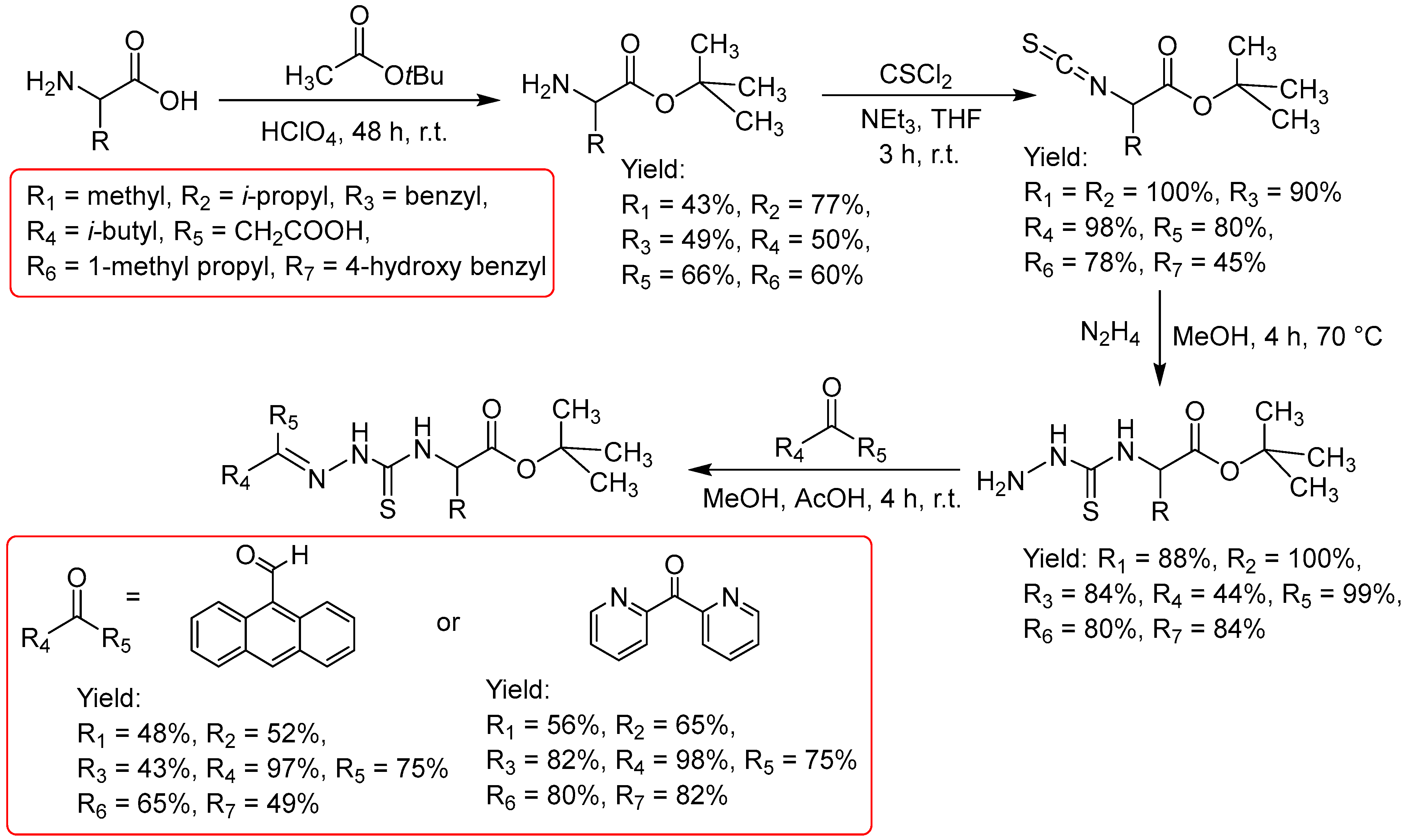
2.2.1. Thiosemicarbazones Derived from Amino Acids
The purpose of introducing amino acids at the TSC-
N4 group is motivated by our wish to use these building blocks for the attachment of peptides for further functionalization [
19]. We have recently reported
tert-butyloxycarbonyl (Boc)-protected
L-lysine-TSC derivatives for the addition of the cell-penetrating peptide sc18 [
38].
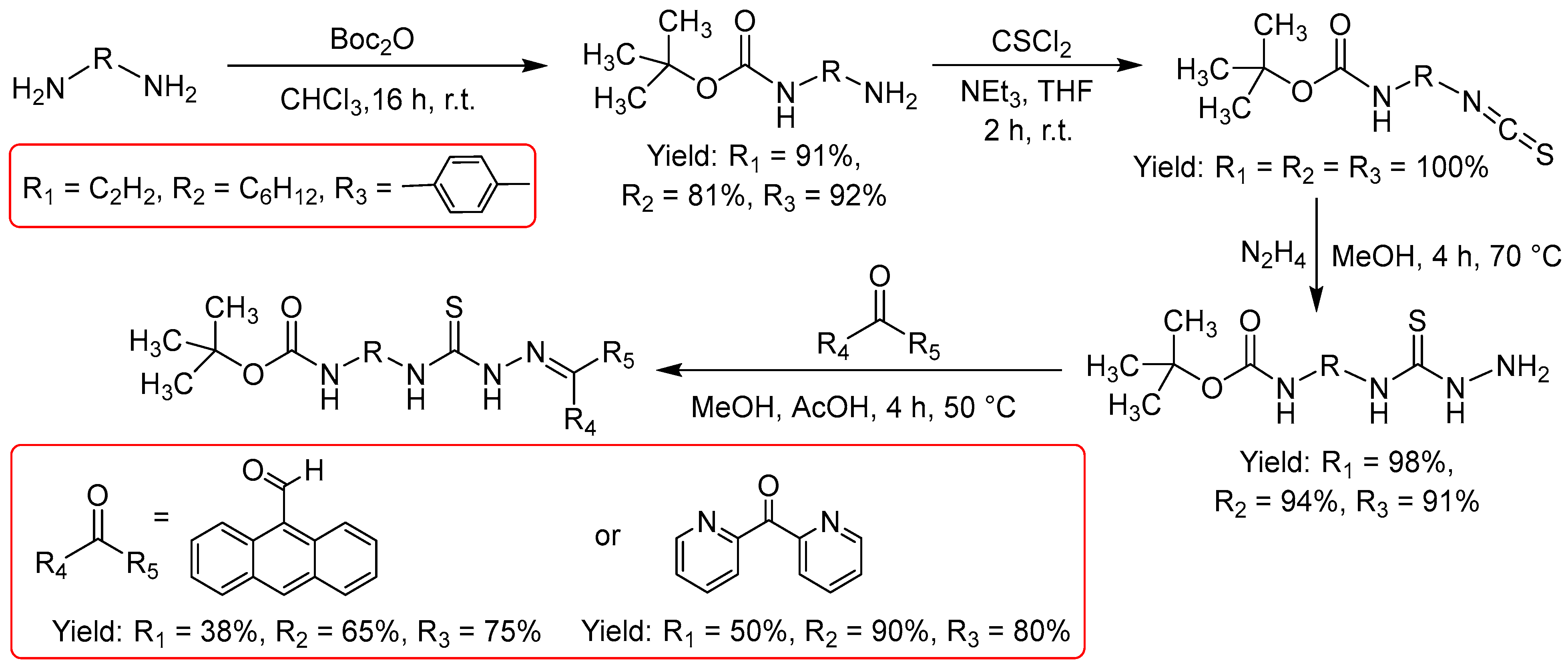
Starting with the Boc-protection of the carboxylic acid function, the final products, including both the anthraldehyde and the dipyridylketone, were obtained as Boc-protected derivatives in a four-step procedure in reasonable to good overall yields ranging from 18 to 50%. The yields for the formation of the thiocyanates from the Boc-protected amines varied massively with the lowest values for R = 4-hydroxymethyl benzyl. Maybe the additional hydroxymethyl function is not well tolerated. The yields for the thiosemicarbazide formation reaction (
Figure 1, bottom right) are generally excellent, while the Boc-protection and the condensation with the ketones requires optimization as some yields are only around 50% or even below.
Since the NH2 function of the amino acid is functionalized and not the C-α atom, the stereochemistry at C-α is retained.
2.2.2. Thiosemicarbazones Derived from Diamines
The same motivation, to final conjugates for further functionalization using peptides motivated to start from diamines (
Figure 2). The four-step reaction sequence in overall yields ranging from 50 to 68%. The Boc-protection of one amine function was generally very successful with excellent yields (81 to 92%). The thiocyanates were formed quantitatively. For the thiosemicarbazide formation from the thiocyanates we obtained excellent yields, while some condensations require optimization as yields were as low as 38 or 50%.
2.2.3. Thiosemicarbazones Derived from Acetobromo-α-D-Glucose
Glucose has been reported as an interesting functionality to selectively address cancer cells [
61,
62,
63,
64,
65]. Therefore, it is not surprising, that TSCs with glucose moiety at the
N4 position and their biological activities have previously been reported [
64,
65,
66,
67,
68,
69]. However, the
N1 position was not functionalized with dipyridyl ketone and 9-anthraldehyde yet. Also, the possibility to use glucose-tagged TSC in polysaccharide nanoconjugates was not studied yet. Finally, the glucose moiety might also anchor efficiently on metaloxide NP surfaces.
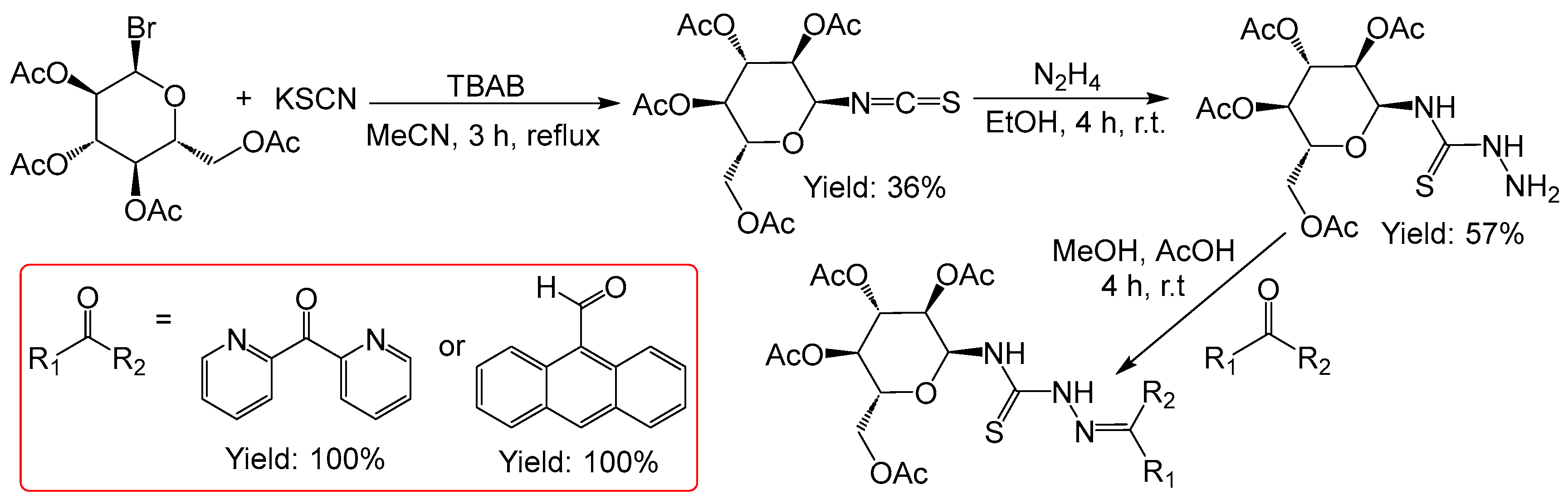
We started the synthesis from acetobromo-α-D-glucose and received the two target conjugates in a three-step process in overall yields of 20% (
Figure 3). Both, the formation of the thiocyanate and the reaction with hydrazine require optimization.
2.2.4. Thiosemicarbazones with a Hydroxy-Alkyl Function
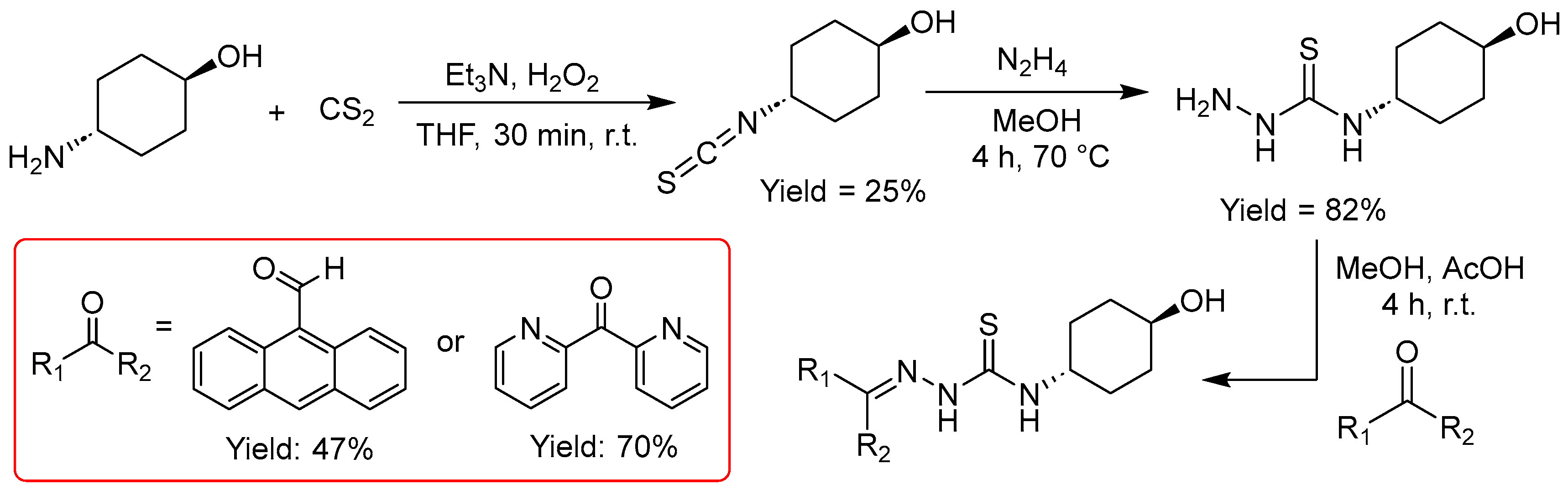
Hydroxy functions are very versatile and the target HO–CnHm spacer moiety could potentially be used for the formation of esters for further conjugation or for direct anchoring to NPs.
In a three-step reaction procedure, starting from trans-4-amino-cyclohexanol we obtained the N4-4-hydroxy-cyclohexanol TSCs in overall yields of 10 and 14%, which definitely calls for optimization, especially for the reaction of the amine with carbon disulfide (CS2) to the thiocyanate. The stereochemistry of the 4-hydroxy-cyclohexanol was retained.
2.2.5. Thiosemicarbazones with a o-Hydroquinone Function
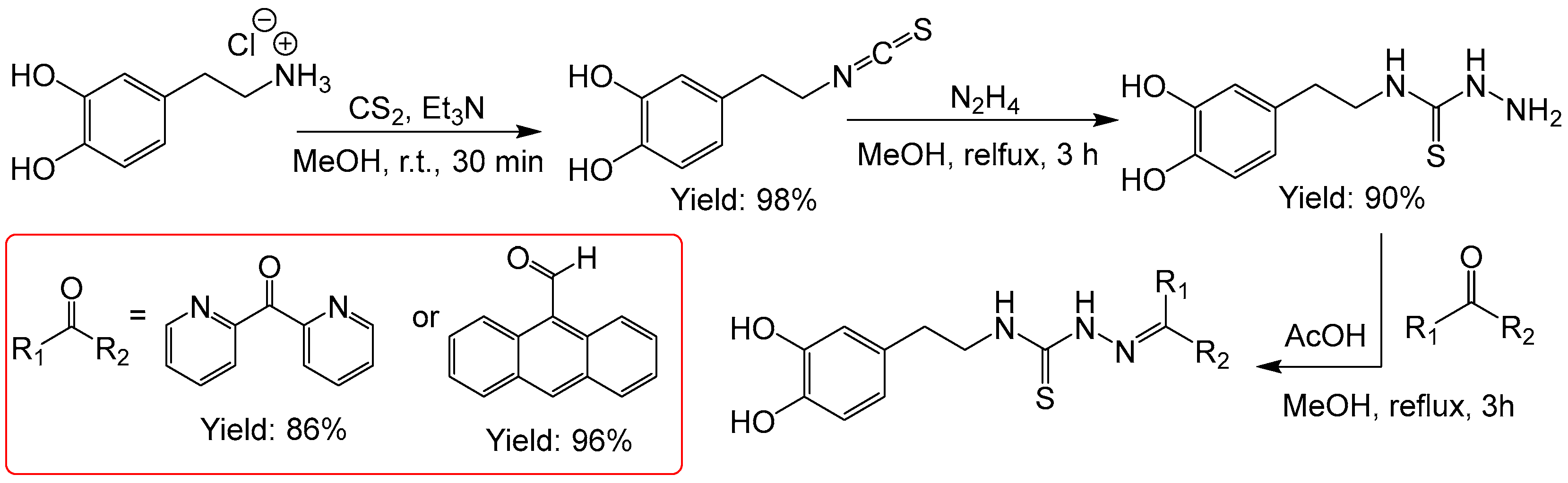
Benzene-1,2-diol (
o-hydroquinone) represents a very suitable anchoring function for iron oxides in the form of catecholate [
70,
71]. We introduced this moiety starting from 4-(1,2-benzene-1,2-diol)ethylammonium chloride (dopamine hydrochloride) and received the two TSCs in very good overall yields of 76 and 85% yields in three steps (
Figure 5). The 1,2-diol functionality is obviously fully tolerated.
2.2.6. Thiosemicarbazones Derived from Amino Thiols
A benzyl-protected thiol was the target of the reaction starting from 2-amino-1-thiol and benzyl chloride (
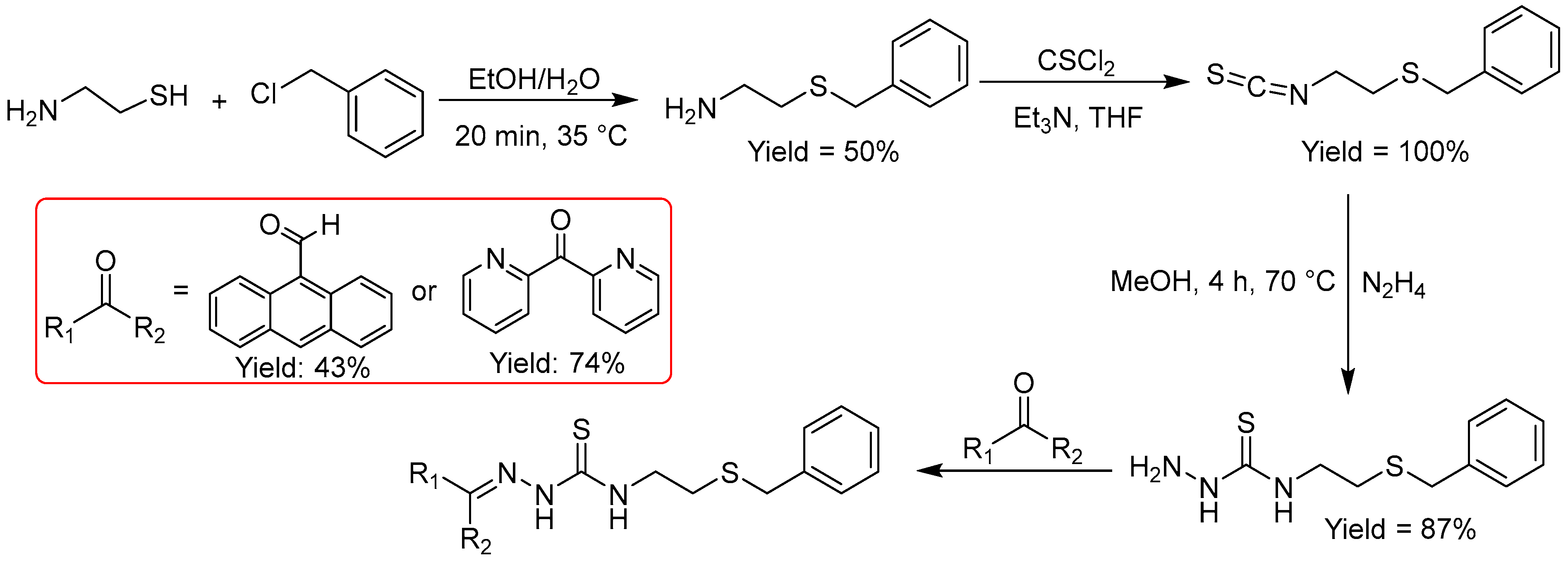
Figure 6). However, overall yields were moderate (32%) to low (19%). Both the formation of the thio-benzyl unit and the condensation reactions require optimization, while the formation of the thiocyanate using thiophosgene was quantitative.
The benzyl protecting group can be removed by using strong acids or bases, or reductive conditions using alkali metals or stannyl hydrides and the product used for anchoring to thiophilic metal NP such as Au or Ag [
72].
2.2.7. Thiosemicarbazones with an Alkyne Function
Terminal alkynes are versatile groups for azide-alkyne click reactions (AAC), which has been frequently used for bioconjugation [
73,
74,
75].
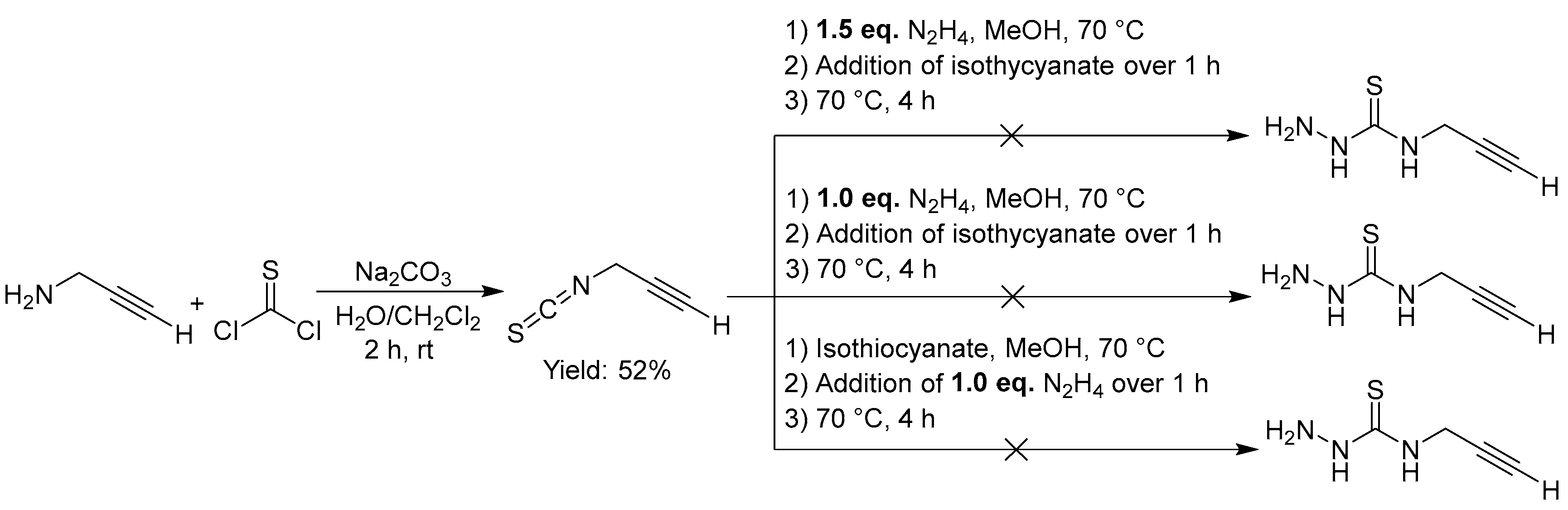
Unfortunately, the synthesis of thiosemicarbazides derived from propargyl amine (
Figure 7) was not successful. While the reaction of propargyl amine with thiophosgene gave the thiocyanate in 52% yield, the following reaction of the isothiocyanate with hydrazine hydrate failed to give the target thiosemicarbazide. Our failure is in line with a previous report using hydroxy-isothiocyanates [
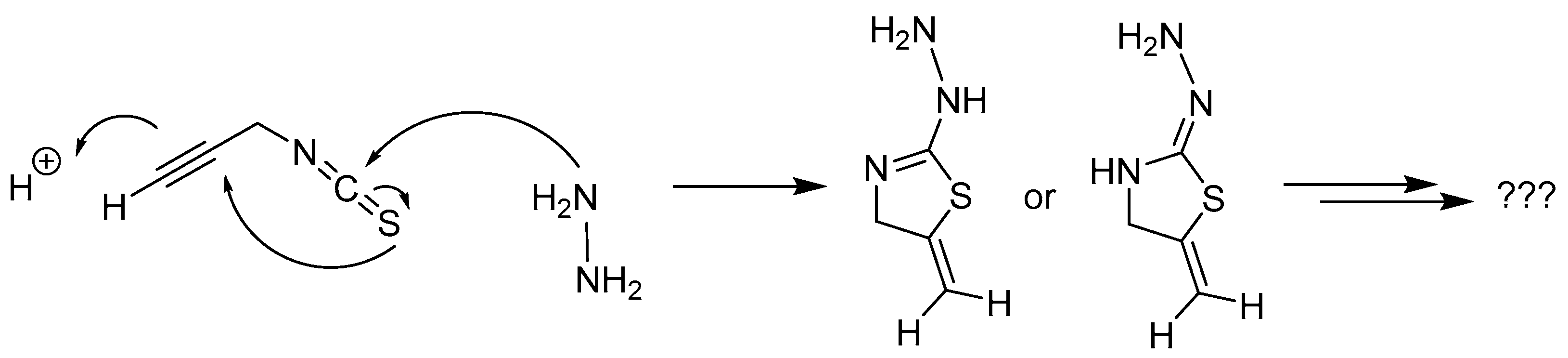
76] and we assume, that a rapid cyclisation reaction occurs, preventing the product formation (
Scheme 7) [
77,
78].
Consequently, we introduced a more rigid spacer function between the thiosemicarbazide and alkyne function (
Figure 8) and obtained
N4-alkyne-TMS functionalized TSC in four steps in good to reasonable yields.
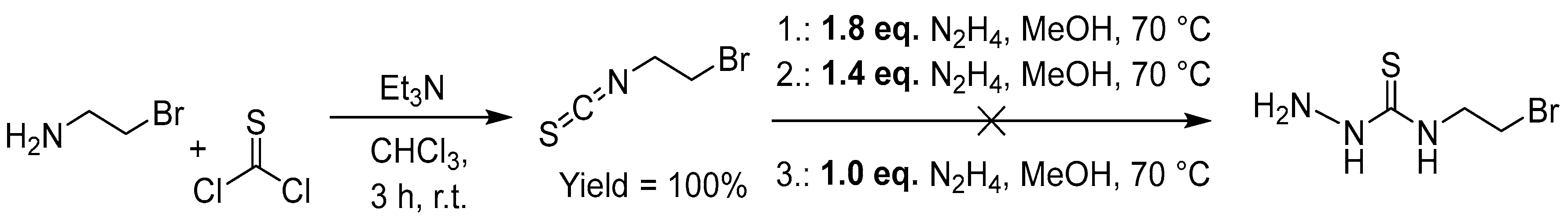
2.2.8. Attempted Synthesis of Thiosemicarbazones with a Bromide Function
Bromide is a very versatile functional group that can either be converted into other functional groups for conjugation, such as amines, alcohols or cyanides. At the same time it can be directly used in a three component azide-alkyne click (AAC) reaction as azide precursor (bromide + NaN
3 + alkyne) [
73,
74,
75,
79]. Starting from 1,2-amino-bromo-ethane, we received the 1-bromo-2-thiocyanate in quantitative yield (
Figure 9) through reactions with thiophosgene. The subsequent attempts to achieve the bromo-ethylene functionalized thiosemicarbazide through reaction with hydrazine failed (
Figure 9).
In a similar way as supposed for our attempts of introducing a terminal alkyne function (
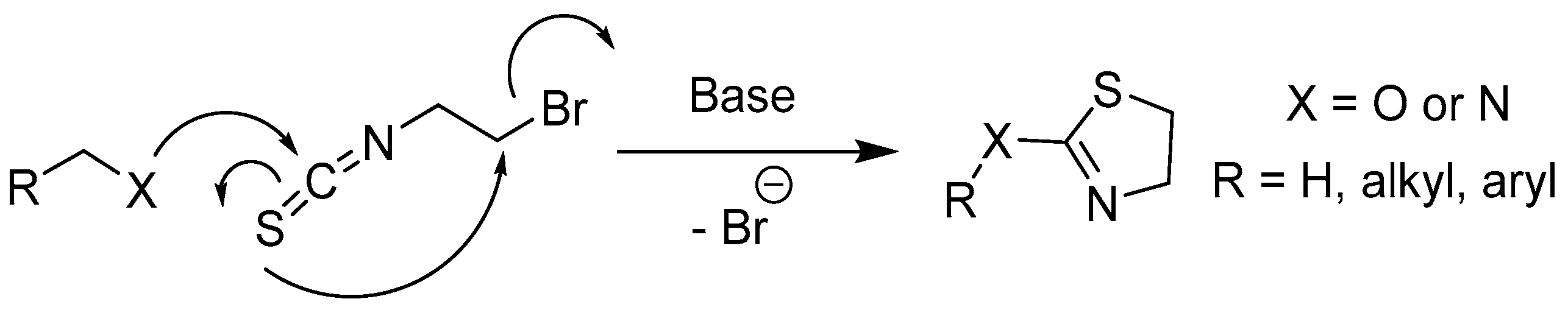
Figure 7), we suspected that a flexible spacer function between the bromide and isothiocyanate function probably allows a detrimental cyclisation reaction (
Scheme 8) [
63,
64,
65]. Consequently, we introduced a more rigid spacer function between the thiosemicarbazide and alkyne function (
Figure 10).
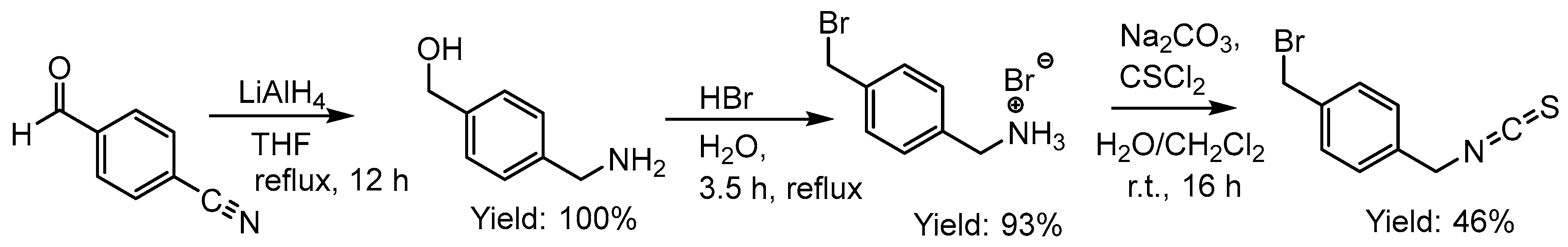
We therefore converted 4-cyano-benzaldehyde into the 4-amine derivative in quantitative yield, reacted it with HBr to form the hydrobromide (93% yield) and converted this to the thiocyanate in 46% yield using thiophosgene (
Figure 10). Unfortunately, also from this more rigid thiocyanate, the reaction with N
2H
4 (same conditions as in
Figure 9) to form the thiosemicarbazides failed. In future work we will explore the late-stage introduction of bromide into the TSC.
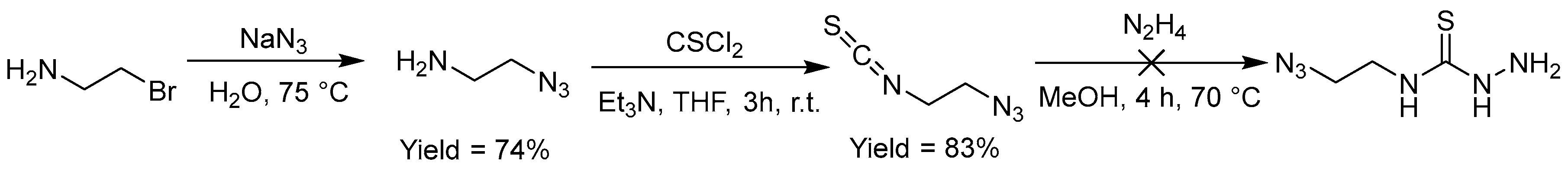
2.2.9. Thiosemicarbazones with an Azide Function
Azide-substituted TSC derivatives are excellent candidates for covalent conjugation using the azide-alkyne click (AAC) reaction [
73,
74,
75,
79]. Thus, 2-amino-1-bromoethane was reacted with NaN
3 to yield 2-azidoethylamine in 74% (
Figure 11). The conversion to the thiocyanate using thiophosgene was also successful (83% yield). When reacting the thiocyanate with hydrazine under our established conditions, we failed to receive the azido derivative. Very probably, the flexible spacer function between the azide and isothiocyanate function probably led to cyclisation reactions, confirming our assumptions for the attempts for alkyne and Br substituted TSCs with flexible spacers (
Figure 7 and
Figure 9).
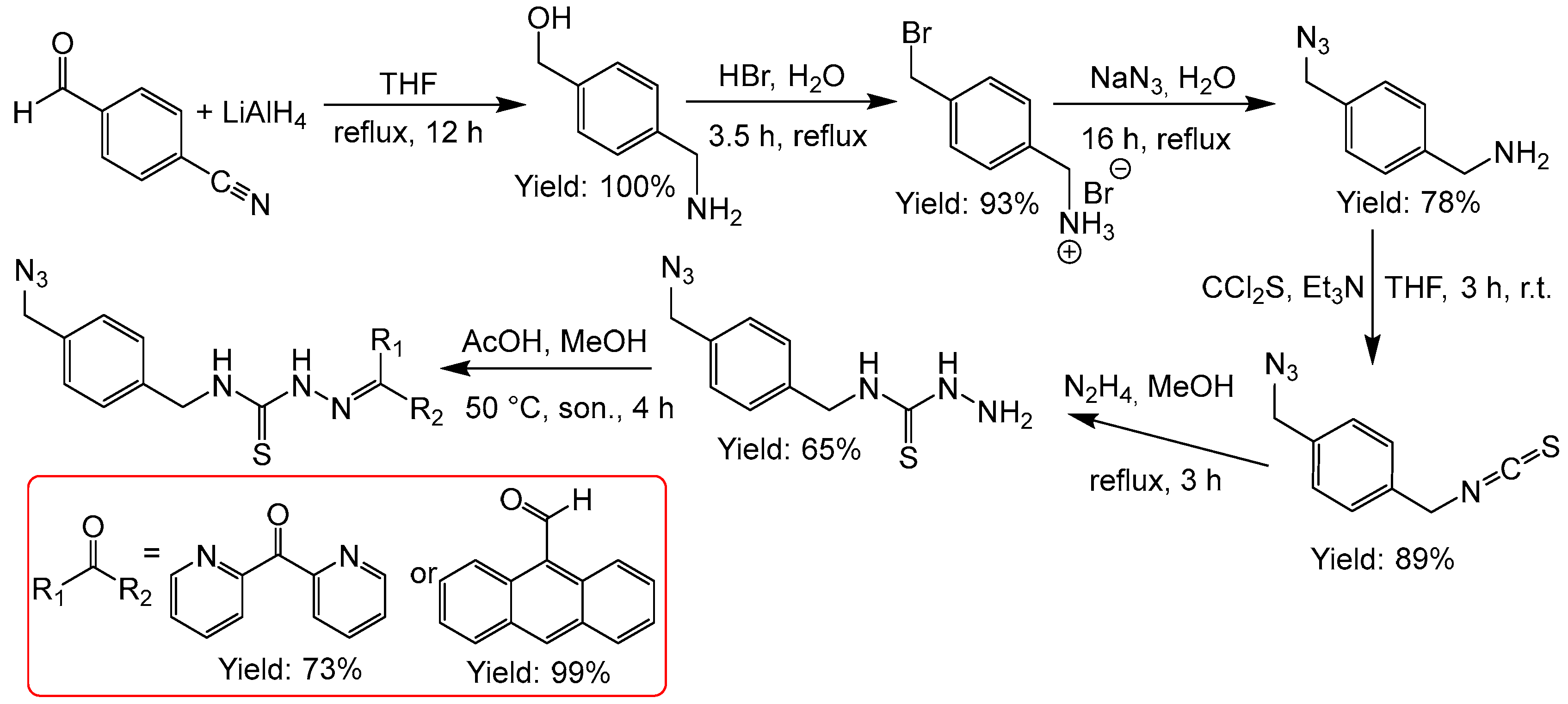
When introducing the rigid phenylene spacer function between the thiosemicarbazide and azide function, we obtained the two azide functionalized TSCs in a six-step reaction sequence in remarkable 36 and 42% overall yields, starting from 4-cyano-benzaldehyde (
Figure 12).
2.2.10. Thiosemicarbazones with a Phosphonate Group
Phosphonates are suitable groups for anchoring on iron oxide NPs [
80,
81,
82]. Phosphonylation of 4-iodo-nitrobenzene allowed to introduce a diethylphosphonato-phenyl unit as end-group at the
N4 terminus of the TSC (
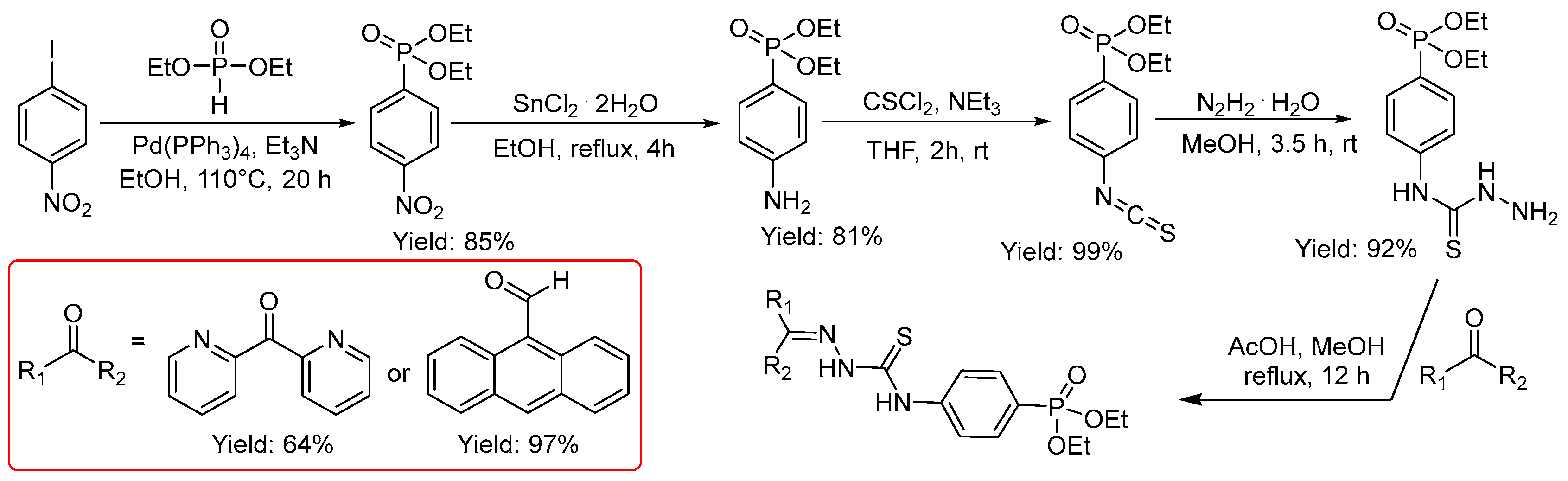
Figure 13) in a five-step reaction sequence in 61% and 40% overall yield, respectively.
2.2.11. Attempted Synthesis of Thiosemicarbazones with a Silane Group
Silanes are versatile groups for covalent bonding to surfaces of SiO
2 NP or core-shell NP with a SiO
2 shell [
83]. Starting from amino-propyl-trimethoxysilane we received the 4-
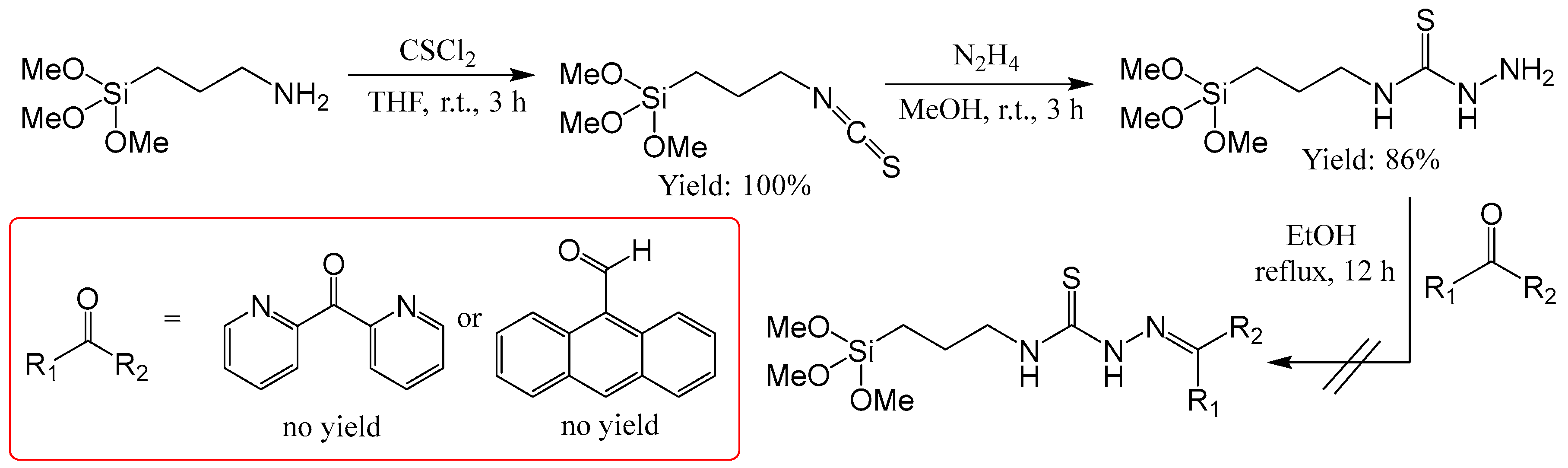
N-trimethoxysilane-propylene thiosemicarbazone in an excellent yield of 86% (
Figure 14). However, the subsequent condensation reactions failed in both cases. The reason is very probably hydrolysis as water cannot completely be avoided in the reaction. The condensation step (thiosemicarbazide + ketone or aldehyde) requires acidic or basic catalysts and produces an equivalent of water.
At present we are thinking about refinement of the condensation method to avoid even traces of water by efficient trapping of water from the condensation and using alternative catalysts.
3. Materials and Methods
3.1. Materials and Synthesis
The supplied material and all details on the synthesis of the compounds are provided in the
Supplementary Materials.
3.2. Instrumentation
1H, 13C, and correlation spectra were recorded on a Bruker Avance II 300 MHz (1H: 300 MHz, 13C: 75 MHz), equipped with a double resonance (BBFO) 5 mm observe probe head with a z-gradient coil or on a Bruker Avance III 499 (1H: 499 MHz, 13C: 125 MHz) using a TCI Prodigy 5 mm probe head with z-gradient coil (Bruker, Rheinhausen, Germany). Chemical shifts were reported relative to TMS (1H, 13C). HR-ESI-MS spectra in the positive mode were measured using a Thermo scientific LTW Orbitrap XL mass spectrometer at 70 eV (ThermoFisher Scientific, Waltham, MA, USA). UV-vis absorption spectra were recorded on a Varian Cary 60 spectrophotometer (Varian Medical Systems, Darmstadt, Germany). UV-vis photoluminescence spectra were obtained using a FLS100 spectrometer with a 450 W xenon light source (Edinburgh Instrument, Livingston, Scotland). The spectrometer was equipped with a PMT-900 detector and a double grating Czerny-Turner monochromator. All measurements were performed at room temperature in MeCN.
4. Conclusions and Outlook
In this work we have derivatized thiosemicarbazones (TSCs) for the functionalization of nanoparticles (NPs) using the modular character of TSCs allowing to react N4-functionalized thiosemicarbazides with carbonyl compounds (R1/R2) to form a plethora of TSCs R1R2C=N(1)–N(2)H–C(S)–N(4)R3R4 structures.
We have followed the approach of introducing the functional groups di-2-pyridyl imine for metal ion coordination and 9-anthracenyl-methyl as luminescent probe as CR1/R2 functions on the N1 atom through their carbonyl compounds. On the other hand, functional groups for direct anchoring on the NPs or as reactive groups for the formation of conjugates, were introduced modifying the N4-R3/R4 site on the precursor thiosemicarbazides. This allowed us to extensively use amines as starting materials, reacting them with SCCl2 or CS2 to form the corresponding thiocyanates and reacting these with hydrazine to form the thiosemicarbazides. This method allowed to synthesize a large number of TSC with Boc-protected amino acids and Boc-NH terminated diamines in N4 position. It also allowed to introduce phenyl-(ethyl)phosphonate, 4-hydroxy cyclohexyl, o-hydroquinone, S-benzyl, dimethyl-phenylene-N3 and phenyl-C≡C-TMS end groups.
For the potentially directly NP anchoring end groups, the remarkably high overall yields for the phosphonate, received as ethyl esters in 40 and 61% overall yields after five steps and the very successful synthesis of the hydroquinone derivatives in overall yields of 76 and 85% in three steps opens right away the door to their future use as anchoring groups for NPs. In contrast to this, the introduction of the S-benzyl group for NP anchoring (as thiolate) requires further optimization as overall yields are moderate to low (32 and 19%). The same is true for the synthesis of the two N4-glucose substituted TSCs with 20% overall yields. Unfortunately, we failed to introduce trimethoxysilane group for potential direct anchoring on SiO2 or other metal oxides. However, it is only the last step, the condensation with the carbonyl compounds, that requires further optimization.
Within the series of end groups for potential covalent conjugation, the NH-boc and C-Boc terminated diamines and amino acid represent a huge potential. Some of them were synthesized in excellent overall yields of up to 70%, while others require optimization (yields down to 16%). The very versatile terminal N3 group for the important AAC (alkyne-azide click) reaction was very successfully introduced with overall yields of 36 and 42% after six steps. Here we encountered the aspect that a rigid spacer is needed between the thiocyanate and the azide group for the reaction with hydrazine. The same is true for this reaction with thiocyanates with alkyne (C≡CH) or bromide. For the alkynes (C≡C-TMS) and the N3 rigid spacers between the two functional groups allowed the successful conversion to the thiosemicarbazones, while for Br, this move did not help. For the less rigid alkyl spacers we suppose cyclization reactions circumventing the product formation. For Br the overall high reactivity of the thiocyanate opens even more destructive pathways and we seek for a late-stage introduction of Br in N4-substituted TSCs in future work.
Future work will include the optimization of some of the reaction protocols as described above, but more important, having very versatile functionalized TSCs carrying either a metal-coordinating di-2-pyridyl function or a luminescent 9-anthranyl tag in hands, we will study the NP anchoring of these TSCs either by direct coordination to the NP surface or through conjugation of the TSCs e.g. by click reactions with surface-functionalized NPs in depths.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Materials contains the Experimental Section with details on Materials and Syntheses as well as Supplementary Figures S1 to S302 showing NMR spectra. Further figures are: Figure S303. UV-vis absorption spectra of dipyridyl ketone TSCs measured in MeCN. Figure S304. UV-vis absorption spectroscopy of 9-anthraldehyde TSCs measured in MeCN. Figure S305. Normalized emission spectra of the 9-antraldehyde TSCs in MeCN upon irradiation at 365 nm.
Author Contributions
Conceptualization A.K.; methodology, J.H. and A.K.; validation, J.H. and A.K.; formal analysis, J.H.; investigation, J.H., L.R., D.O, and S.J.; resources, A.K.; data curation, J.H., L.R., D.O., and S.J.; writing—original draft preparation, A.K.; writing—review and editing, J.H. and A.K.; visualization, J.H.; supervision, A.K.; project administration, A.K.; Both authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the University of Cologne, Faculty of Mathematics and Natural Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available from the authors on request.
Acknowledgments
Dr. Daniel Friedrich, Dr. P. Hegemann, and Daniela Naumann, NMR platform, Department of Chemistry, University of Cologne for NMR measurements, Michael Neihs, MS platform, Department of Chemistry, University of Cologne for HR-MS measurements, and Joshua Friedel for UV-vis photoluminescence measurements.
Conflicts of Interest
The authors declare no conflict of interest:.
Sample Availability
Samples of the compounds are available from the authors on request.
References
- McNamara, W.R.; Snoeberger, R.C.; Li, G.; Schleicher, J.M.; Cady, C.W.; Poyatos, M.; Schmuttenmaer, C.A.; Crabtree, R.H.; Brudvig, G.W.; Batista, V.S. Acetylacetonate Anchors for Robust Functionalization of TiO2 Nanoparticles with Mn(II)−Terpyridine Complexes. J. Am. Chem. Soc. 2008, 130, 14329–14338. [CrossRef]
- Neouze, M.; Schubert, U. Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands. Monatsh. Chem. 2008, 139, 183–195. [CrossRef]
- Vigderman, L.; Zubarev, E.R. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules. Adv. Drug Deliv. Rev. 2013, 65 (5), 663–676. [CrossRef]
- Kumar, R.; Roy, I.; Ohulchanskyy, T.Y.; Goswami, L.N.; Bonoiu, A.C.; Bergey, E.J.; Tramposch, K.M.; Maitra, A.; Prasad, P.N. Covalently Dye-Linked, Surface-Controlled, and Bioconjugated Organically Modified Silica Nanoparticles as Targeted Probes for Optical Imaging. ACS Nano 2008, 2, 449–456. [CrossRef]
- Verma, G.; Shetake, N.G.; Barick, K.C.; Pandey, B.N.; Hassan, P.A.; Priyadarsini, K.I. Covalent immobilization of doxorubicin in glycine functionalized hydroxyapatite nanoparticles for pH-responsive release. New J. Chem. 2018, 42, 6283–6292. [CrossRef]
- Latorre, A.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Somoza, A. Multifunctionalization of magnetic nanoparticles for controlled drug release: A general approach. Eur. J. Med. Chem. 2014, 82, 355–362. [CrossRef]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. Multifunctionalization of magnetic nanoparticles for controlled drug release: A general approach pH-Responsive Mesoporous Silica and Carbon Nanoparticles for Drug Delivery. Bioengineering 2017, 4 (1), 3. [CrossRef]
- Choy, C.J.; Geruntho, J.J.; Davis, A.L.; Berkman, C.E. Tunable pH-Sensitive Linker for Controlled Release. Bioconjugate Chem. 2016, 27, 824−830. [CrossRef]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [CrossRef]
- Gambinossi, F.; Mylon, S.E.; Ferri, J.K. Aggregation kinetics and colloidal stability of functionalized nanoparticles. Adv. Colloid Interface Sci. 2015, 222, 332-349. [CrossRef]
- Burger, A.; Srikantharajah, R.; Peukert, W.; Hirsch, A. Individualization and Stabilization of Zinc Oxide Nanorods by Covalent Functionalization with Positively Charged Catechol Derivatives. Chem.–Eur. J. 2017, 23, 17257–17268. [CrossRef]
- Wen, J.; Yang, K.; Liu, F.; Li, H.; Xu, Y.; Sun, S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem. Soc. Rev. 2017, 46, 6024-6045. [CrossRef]
- Xue, Y.; Bai, H.; Peng, B.; Fang, B.; Baell, J.; Li, L.; Huang, W.; Voelcker, N.H. Stimulus-cleavable chemistry in the field of controlled drug delivery. Chem. Soc. Rev. 2021, 50, 4872-4931. [CrossRef]
- Dal Corso, A.; Pignataro, L.; Belvisi, L.; Gennari, C. Innovative Linker Strategies for Tumor-Targeted Drug Conjugates. Chem.–Eur. J. 2019, 25, 14740–14757. [CrossRef]
- Yang, X.; Pan, Z.; Choudhury, M.R.; Yuan, Z.; Anifowose, A.; Yu, B.; Wang, W.; Wang, B. Making smart drugs smarter: The importance of linker chemistry in targeted drug delivery. Med. Res. Rev. 2020, 40, 2682–2713. [CrossRef]
- Yan, Y.; Ding, H. pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials 2020, 10, 1613. [CrossRef]
- Zheng, H.; Xing, L.; Cao, Y.; Che, S. Coordination bonding based pH-responsive drug delivery systems. Coord. Chem. Rev. 2013, 257, 1933–1944. [CrossRef]
- Novio, F.; Simmchen, J.; Vázquez-Mera, N.; Amorín-Ferré, L.; Ruiz-Molina, D. Coordination polymer nanoparticles in medicine. Coord. Chem. Rev. 2013, 257, 2839–2847. [CrossRef]
- Gessner, I.; Neundorf, I. Nanoparticles Modified with Cell-Penetrating Peptides: Conjugation Mechanisms, Physicochemical Properties, and Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2536. [CrossRef]
- Jeong, W.-j.; Bu, J.; Kubiatowicz, L.J.; Chen, S.S.; Kim, Y.S.; Hong. S. Peptide–nanoparticle conjugates: a next generation of diagnostic and therapeutic platforms? Nano Converg. 2018, 5 (38), 1–18. [CrossRef]
- Summers, K.L. A Structural Chemistry Perspective on the Antimalarial Properties of Thiosemicarbazone Metal Complexes. Mini-Rev. Med. Chem. 2019, 19, 569–590. [CrossRef]
- Kalinowski, D.S.; Quach, P.; Richardson, D.R. Thiosemicarbazones: the new wave in cancer treatment. Fut. Med. Chem. 2009, 1, 689-703. [CrossRef]
- Kang, I.-J.; Wang, L.-W.; Hsu, T.-A.; Yueh, Lee, C.C.; Lee, Y.-C.; Lee, C.-Y.; Chao, Y.-S.; Shih, S.-R.; Chern, J.-H. Isatin-β-thiosemicarbazones as potent herpes simplex virus inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 1948–1952. [CrossRef]
- Fonteh, P.N.; Keter, F.K.; Meyer, D. New bis(thiosemicarbazonate) gold(III) complexes inhibit HIV replication at cytostatic concentrations: Potential for incorporation into virostatic cocktails. J. Inorg. Biochem. 2011, 105, 1173–1180. [CrossRef]
- Bisceglie, F.; Bacci, C.; Vismarra, A.; Barilli, E.; Pioli, M.; Orsoni, N.; Pelosi, G. Antibacterial activity of metal complexes based on cinnamaldehyde thiosemicarbazone analogues. J. Inorg. Biochem. 2020, 203, 110888. [CrossRef]
- Machado, J.F.; Marques, F.; Pinheiro, T.; Brito, M.J.V.; Scalese, G.; Pérez-Díaz, L.; Otero, L.; António, J.P.M.; Gambino, D.; Morais, T.S. Copper(I)-Thiosemicarbazone Complexes with Dual Anticancer and Antiparasitic Activity. ChemMedChem 2023, 18, e202300074. [CrossRef]
- Dilworth, J.R.; Hueting, R. Metal complexes of thiosemicarbazones for imaging and therapy. Inorg. Chim. Acta 2012, 389, 3–15. [CrossRef]
- Jain, P.; Sharma, S.; Kuma, N.; Misra, N. Ni(II) and Cu(II) complexes of bidentate thiosemicarbazone ligand: Synthesis, structural, theoretical, biological studies and molecular modeling. Appl. Organomet. Chem. 2020, 34, e5736. [CrossRef]
- Mathuber, M.; Hager, S.; Keppler, B.K.; Heffeter, P.; Kowol, C.R. Liposomal formulations of anticancer copper(II) thiosemicarbazone complexes. Dalton Trans. 2021, 50, 16053–16066. [CrossRef]
- Fischer, B.; Kryeziu, K.; Kallus, S.; Heffeter, P.; Berger, W.; Kowol, C.R.; Keppler, B.K. Nanoformulations of anticancer thiosemicarbazones to reduce methemoglobin formation and improve anticancer activity. RSC Adv. 2016, 6, 55848–55859. [CrossRef]
- Toan, V.N.; Thanh, N.D.; Tri, N.M.; Huong, N.T.T. Synthesis and biological screening of thiosemicarbazones of substituted 3-acetylcoumarins having d-glucose moiety. Bioorg. Med. Chem. Lett. 2020, 30, 127664. [CrossRef]
- Thanh, N.D.; Duc, H.T.; Duyen, V.T.; Tuong, P.M.; Quoc, N.V. Synthesis and antibacterial and antifungal activities of N-(tetra-O-acetyl-β-d-glucopyranosyl)thiosemicarbazones of substituted 4-formylsydnones. Chem. Cent. J. 2015, 9, 60. [CrossRef]
- Thanh, N.D.; Giang, N.T.K.; Quyen, T.H.; Huong, D.T.; Toan, V.N. Synthesis and evaluation of in vivo antioxidant, in vitro antibacterial, MRSA and antifungal activity of novel substituted isatin N-(2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl)thiosemicarbazones. Eur. J. Med. Chem. 2016, 123, 532-543. [CrossRef]
- Dong, X.; Wang, H.; Zhang, H.; Li, M.; Huang, Z.; Wang, Q.; Li, X. Copper-thiosemicarbazone complexes conjugated-cellulose fibers: Biodegradable materials with antibacterial capacity. Carbohydr. Polym. 2022, 294, 119839. [CrossRef]
- Akam, E.A.; Tomat, E. Targeting Iron in Colon Cancer via Glycoconjugation of Thiosemicarbazone Prochelators. Bioconjugate Chem. 2016, 27 (8), 1807–1812. [CrossRef]
- Milunovic, M.N.M.; Enyedy, É.A.; Nagy, N.V.; Kiss, T.; Trondl, R.; Jakupec, M.A.; Keppler, B.K.; Krachler, R.; Novitchi, G.; Arion, V.B. l- and d-Proline Thiosemicarbazone Conjugates: Coordination Behavior in Solution and the Effect of Copper(II) Coordination on Their Antiproliferative Activity. Inorg. Chem. 2012, 51 (17), 9309–9321. [CrossRef]
- Paterson, B.M.; Karas, J.A.; Scanlon, D.B.; White, J.M.; Donnelly P.S. Versatile New Bis(thiosemicarbazone) Bifunctional Chelators: Synthesis, Conjugation to Bombesin(7−14)-NH2, and Copper-64 Radiolabeling. Inorg. Chem. 2010, 49, 4, 1884–1893. [CrossRef]
- Haseloer, A.; Lützenburg, T.; Strache, J.P.; Neudörfl, J.; Neundorf, I.; Klein, A. Building up PtII−Thiosemicarbazone−Lysine−sC18 Conjugates. ChemBioChem 2021, 22, 694–704. [CrossRef]
- Kallus, S.; Uhlik, L.; Schoonhoven, S.; Pelivan, K.; Berger, W.; Enyedy, E.A.; Hofmann, T.; Heffeter, P.; Kowol, C.R.; Keppler, B.K. Synthesis and biological evaluation of biotin-conjugated anticancer thiosemicarbazones and their iron(III) and copper(II) complexes. J. Inorg. Biochem. 2019, 190, 85–97. [CrossRef]
- Sung, Y.S.; Wu, W.; Ewbank, M.A.; Utterback, R.D.; Marty, M.T.; Tomat, E. Albumin Conjugates of Thiosemicarbazone and Imidazole-2-thione Prochelators: Iron Coordination and Antiproliferative Activity. ChemMedChem 2021, 16, 2764– 2768. [CrossRef]
- Franciscato, D.S.; Matias, T.A.; Shinohara, J.; Gonçalves, J.M.; Coelho, N.P.; Fernandes, C.S.; Basso, E.A.; Nakatani, H.S.; Araki, K.; Toma, H.E.; de Souza, V.R. Thiosemicarbazone@Gold nanoparticle hybrid as selective SERS substrate for Hg2+ ions. Spectrochim. Acta A Mol. Biomol. 2018, 204, 174–179. [CrossRef]
- Ali, I.; Isaac, I.O.; Ahmed, F.; Aslam, F.; Ali, S.; Imran, M.; Alharthy, R.D.; Shah, M.R.; Malik, M.I.; Hameed, A. Acridine-Thiosemicarbazones-Stabilized Silver Nanoparticles as a Selective Sensor for Copper(II)-Ion in Tap Water. ChemistrySelect 2019, 4, 8757–8763. [CrossRef]
- Kaviani, N.; Behrouz, S.; Jafari, A.A.; Rad, M.N.S. Functionalization of Fe3O4@SiO2 nanoparticles with Cu(I)-thiosemicarbazone complex as a robust and efficient heterogeneous nanocatalyst for N-arylation of N-heterocycles with aryl halides. RSC Adv. 2023, 13, 30293–30305. [CrossRef]
- Britta, E.A.; da Silva, C.C.; Rubira, A.F.; Nakamura, C.V.; Borsali, R. Generating nanoparticles containing a new 4-nitrobenzaldehyde thiosemicarbazone compound with antileishmanial activity. Mater. Sci. Eng. C 2016, 69, 1159–1166. [CrossRef]
- Khan, A.A.; Alanazi, A.M.; Alsaif, N.; Algrain, N.; Wani, T.A.; Bhat, M.A. Enhanced Efficacy of Thiosemicarbazone Derivative-Encapsulated Fibrin Liposomes against Candidiasis in Murine Model. Pharmaceutics 2021, 13, 333. [CrossRef]
- Holley, C.K.; Sinquefield, B.; Majd, S. Optimization of the Single Emulsion Method for Encapsulation of a Cancer Drug in Nanoparticles. 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 2019, 1078–1081. [CrossRef]
- Oliveira, C.G.; Dalmolin, L.F.; Silva, R.T.C.; Lopez, R.F.V.; Maia, P.I.S.; Moreto, J.A. PLGA-nanoparticles loaded with a thiosemicarbazone derived palladium(II) complex as a potential agent to new formulations for human ovarian carcinoma treatment. New J. Chem. 2020, 44, 14928–14935. [CrossRef]
- Spadola, G.; Sanna, V.; Bartoli, J.; Carcelli, M.; Pelosi, G.; Bisceglie, F.; Restivo, F.M.; Degola, D. Rogolino, D. Thiosemicarbazone nano-formulation for the control of Aspergillus flavus. Environ. Sci. Pollut. Res. 2020, 27, 20125–20135. [CrossRef]
- Hernández, W.; Vaisberg, A.J.; Tobar, M.; Álvarez, M.; Manzur, J.; Echevarría, Y.; Spodine, E. In vitro antiproliferative activity of palladium(II) thiosemicarbazone complexes and the corresponding functionalized chitosan coated magnetite nanoparticles. New J. Chem. 2016, 40, 1853–1860. [CrossRef]
- Gholipour, N.; Akhlaghi, M.; Kheirabadi, A.M.; Geramifar, P.; Beiki, D. Development of Ga-68 labeled, biotinylated thiosemicarbazone dextran-coated iron oxide nanoparticles as multimodal PET/MRI probe. Int. J. Biol. Macromol. 2020, 148, 932–941. [CrossRef]
- Shafiei, I.; Tavassoli, S.P.; Rahmatollahi, H.R.; Ghasemian, R.; Salehzadeh, A. A Novel Copper Oxide Nanoparticle Conjugated by Thiosemicarbazone Promote Apoptosis in Human Breast Cancer Cell Line. J. Clust. Sci. 2022, 33, 2697–2706. [CrossRef]
- Badrooh, M.; Shokrollahi, F.; Javan, S.; Ghasemipour, T.; Mojdehi, S.R.; Farahnak, H.; Noveiri, M.J.S.; Hedayati, M.; Salehzadeh, A. Trigger of apoptosis in adenocarcinoma gastric cell line (AGS) by a complex of thiosemicarbazone and copper nanoparticles. Mol. Biol. Rep. 2022, 49, 2217–2226. [CrossRef]
- Habibi, A.; Ataollah, S.; Shandi, S.; Salehzadeh, A.; Moradi-Shoeili, Z. Novel pyridinecarboxaldehyde thiosemicarbazone conjugated magnetite nanoparticulates (MNPs) promote apoptosis in human lung cancer A549 cells. Biol. Inorg. Chem. 2020, 25, 13–22. [CrossRef]
- Izadpanah, M.R.; Salehzadeh, A.; Zaefizadeh, M.; Nikpasand, M. Functionalisation of Fe3O4 nanoparticles by 2-((pyrazol-4-yl)methylene)hydrazinecarbothioamide enhances the apoptosis of human breast cancer MCF-7 cells. IET Nanobiotechnol. 2020, 14, 508–518. [CrossRef]
- Shakya, B.; Yadav, P.N. Thiosemicarbazones as Potent Anticancer Agents and their Modes of Action. Mini-Rev. Med. Chem. 2020, 20, 638–661. [CrossRef]
- Richardson, D.R.; Sharpe, P.C.; Lovejoy, D.B.; Senaratne, D.; Kalinowski, D.S.; Islam, M.; Bernhardt, P.V. Dipyridyl Thiosemicarbazone Chelators with Potent and Selective Antitumor Activity Form Iron Complexes with Redox Activity. J. Med. Chem. 2006, 49 (22), 6510–6521. [CrossRef]
- Yu, Y.; Kalinowski, D.S.; Kovacevic, Z.; Siafakas, A.R.; Jansson, P.J.; Stefani, C.; Lovejoy, D.B.; Sharpe, P.C.; Bernhardt, P.V.; Richardson, D.R. Thiosemicarbazones from the Old to New: Iron Chelators That Are More Than Just Ribonucleotide Reductase Inhibitors. J. Med. Chem. 2009, 52, 5271–5294. [CrossRef]
- Jansson, P.J.; Sharpe, P.C.; Bernhardt, P.V.; Richardson, D.R. Novel Thiosemicarbazones of the ApT and DpT Series and Their Copper Complexes: Identification of Pronounced Redox Activity and Characterization of Their Antitumor Activity. J. Med. Chem. 2010, 53, 5759–5769. [CrossRef]
- Kate, A.N.; Kumbhar, A.A.; Khan, A.A.; Joshi, P.V.; Puranik, V.G. Monitoring Cellular Uptake and Cytotoxicity of Copper(II) Complex Using a Fluorescent Anthracene Thiosemicarbazone Ligand. Bioconjugate Chem. 2014, 25 (1), 102–114. [CrossRef]
- Reimers, J.R.; Ford, M.J.; Marcuccio, S.M.; Ulstrup, J.; Hush, N.S. Competition of van der Waals and chemical forces on gold–sulfur surfaces and nanoparticles. Nat. Rev. Chem. 2017, 1, 0017. [CrossRef]
- Patra, M.; Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. A Potent Glucose–Platinum Conjugate Exploits Glucose Transporters and Preferentially Accumulates in Cancer Cells. Angew. Chem., Int. Ed. 2016, 55, 2550–2554. [CrossRef]
- Tekade, R.K.; Sun, X. The Warburg effect and glucose-derived cancer theranostics. Drug Discov. Today 2017, 22, 1637–1653. [CrossRef]
- Fadaka, A.; Ajiboye, B.; Ojo, O.; Adewale, O.; Olayide, I.; Emuowhochere, R. Biology of glucose metabolization in cancer cells. J. Oncol. Sci. 2017, 3, 45–51. [CrossRef]
- Toan, V.N.; Thanh, N.D.; Tri, N.M.; Huong, N.T.T. Synthesis and biological screening of thiosemicarbazones of substituted 3-acetylcoumarins having D-glucose moiety. Bioorg. Med. Chem. Lett. 2020, 30, 127664. [CrossRef]
- Zhang, Q.; Shao, J.; Wang, J.; Gong, X.-J.; Liu, W.-X.; Wang, S.; Zhang, Y.; Yang, S.; Zhang, Q.-S.; Wie, J.-X.; Tian, J.-L. Antitumor effects of new glycoconjugated PtII agents dual-targeting GLUT1 and Pgp proteins. Dalton Trans. 2022, 51, 16082. [CrossRef]
- Bisceglie, F.; Tavone, M.; Mussi, F.; Azzoni, S.; Montalbano, S.; Franzoni, S.; Tarasconi, P.; Buschini, A.; Pelosi, G. Effects of polar substituents on the biological activity of thiosemicarbazone metal complexes. J. Inorg. Biochem. 2018, 79, 60–70. [CrossRef]
- Thanh, N.D.; Giang, N.T.K.; Quyen, T.H.; Huong, D.T.; Toan, V.N. Synthesis and evaluation of in vivo antioxidant, in vitro antibacterial, MRSA and antifungal activity of novel substituted isatin N-(2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl)thiosemicarbazones. Eur. J. Med. Chem. 2016, 123, 532–543. [CrossRef]
- Ghosh, S.; Misra, A.K.; Bhatia, G.; Khan, M.M.; Khanna, A.K. Syntheses and evaluation of glucosyl aryl thiosemicarbazide and glucosyl thiosemicarbazone derivatives as antioxidant and anti-dyslipidemic agents. Bioorg. Med. Chem. Lett. 2009, 19, 386–389. [CrossRef]
- Tenchiu, A.-C.; Kostas, I.D.; Kovala-Demertzi, D.; Terzis, A. Synthesis and characterization of new aromatic aldehyde/ketone 4-(β-d-glucopyranosyl)thiosemicarbazones. Carbohydr. Res. 2009, 344 (11), 1352–1364. [CrossRef]
- Shultz, M.D.; Reveles, J.U.; Khanna, S.N.; Carpenter, E.E. Reactive Nature of Dopamine as a Surface Functionalization Agent in Iron Oxide Nanoparticles. J. Am. Chem. Soc. 2007, 129 (9), 2482–2487. [CrossRef]
- Ilyas, S.; Ullah, N.K.; Ilyas, M.; Wennhold, K.; Iqbal, M.; Schlößer, H.A.; Hussain, M.S.; Mathur, S. Mediating the Fate of Cancer Cell Uptake: Dual-Targeted Magnetic Nanovectors with Biotin and Folate Surface Ligands. ACS Biomater. Sci. Eng. 2020, 6 (11), 6138–6147. [CrossRef]
- Akao, A.; Nonoyama, N.; Yasuda, N. A mild and practical deprotection method for benzyl thioethers. Tetrahedron Lett. 2006, 47, 5337–5340. [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108 (8), 2952–3015. [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A Strain-Promoted [3 + 2] Azide−Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc. 2004, 126 (46), 15046–15047. [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [CrossRef]
- Tajima, H.; Li, G. Synthesis of Hydroxyalkyl Isothiocyanates. Synlett 1997, 7, 773–774. [CrossRef]
- Viart, H.M.; Larsen, T.S.; Tassone, C.; Andresen, T.L.; Clausen, M.H. Propargylamine–isothiocyanate reaction: efficient conjugation chemistry in aqueous media. Chem. Commun. 2014, 50, 7800–7802. [CrossRef]
- Arshadi, S.; Vessally, E.; Edjlali, L.; Hosseinzadeh-Khanmiri, R.; Ghorbani-Kalhor, E. N-Propargylamines: versatile building blocks in the construction of thiazole cores. Beilstein J. Org. Chem. 2017, 13, 625–638. [CrossRef]
- Mohammadnezhad, G.; Amirian, A. M.; Görls, H.; Plass, W.; Sandleben, A.; Schäfer, S.; Klein, A. Redox Instability of Copper(II) Complexes of a Triazine-Based PNP Pincer. Eur. J. Inorg. Chem. 2021, 2021, 1140–1151. [CrossRef]
- Das, M.; Mishra, D.; Dhak, P.; Gupta, S.; Maiti, T.K.; Basak, A.; Pramanik, P. Biofunctionalized, Phosphonate-Grafted, Ultrasmall Iron Oxide Nanoparticles for Combined Targeted Cancer Therapy and Multimodal Imaging. Small 2009, 5 (24), 2883–2893. [CrossRef]
- De Rosales, R.T.M.; Tavaré, R.; Glaria, A.; Varma, G.; Protti, A.; Blower, P.J. 99mTc-Bisphosphonate-Iron Oxide Nanoparticle Conjugates for Dual-Modality Biomedical Imaging. Bioconjugate Chem. 2011, 22 (3), 455–465. [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397. [CrossRef]
- Lee, C. H.; Park, S. H.; Chung, W.; Kim, J. Y.; Kim, S. H. Preparation and characterization of surface modified silica nanoparticles with organo-silane compounds. Colloids Surf. A: Physicochem. Eng. Asp., 2011, 384 (1-3), 218–322. [CrossRef]
Scheme 1.
A: Schematic representation of thiosemicarbazones (TSC) in their thione and thiol forms (R, R’, R’’ = H, alkyl, or aryl) with numbering of the TSC backbone (in green. B: N^S thione coordination of a TSC to metal fragments [M]. C: 2-acetylpyridine-thiosemicarbazones (R2 = CH3) showing tridentate N^N^S thiolate binding. D: Formation of a TSC from a thiosemicarbazone and a carbonyl.
Scheme 1.
A: Schematic representation of thiosemicarbazones (TSC) in their thione and thiol forms (R, R’, R’’ = H, alkyl, or aryl) with numbering of the TSC backbone (in green. B: N^S thione coordination of a TSC to metal fragments [M]. C: 2-acetylpyridine-thiosemicarbazones (R2 = CH3) showing tridentate N^N^S thiolate binding. D: Formation of a TSC from a thiosemicarbazone and a carbonyl.
Scheme 2.
Schematic approaches to [TSC–X anchor] (A) and [TSC–Z–Y–X anchor] (B) conjugates with an example.
Scheme 2.
Schematic approaches to [TSC–X anchor] (A) and [TSC–Z–Y–X anchor] (B) conjugates with an example.
Scheme 3.
Targeted functionalization of TSC in this work. A: [R1R2TSC–spacer–amino acid] conjugates B: [R1R2TSC–spacer–anchor] (B1) and [R1R2TSC–spacer–end group] conjugates (B2).
Scheme 3.
Targeted functionalization of TSC in this work. A: [R1R2TSC–spacer–amino acid] conjugates B: [R1R2TSC–spacer–anchor] (B1) and [R1R2TSC–spacer–end group] conjugates (B2).
Scheme 4.
Components of the TSC–X anchor (A) and TSC–Z–Y–X anchor (B) conjugates attempted in this work and their chemical structures. * Targeted but not accomplished. ** For future work.
Scheme 4.
Components of the TSC–X anchor (A) and TSC–Z–Y–X anchor (B) conjugates attempted in this work and their chemical structures. * Targeted but not accomplished. ** For future work.
Scheme 5.
General procedure for the synthesis of TSCs in this work.
Scheme 5.
General procedure for the synthesis of TSCs in this work.
Scheme 6.
Summary of functionalized TSCs reported in this work (the labels
A1,
A2,
B1, and
B2 refer to
Scheme 3).
Scheme 6.
Summary of functionalized TSCs reported in this work (the labels
A1,
A2,
B1, and
B2 refer to
Scheme 3).
Figure 1.
Synthesis of TSCs derived from amino acids.
Figure 1.
Synthesis of TSCs derived from amino acids.
Figure 2.
Synthesis of TSCs starting with diamines introducing hydrocarbon spacers.
Figure 2.
Synthesis of TSCs starting with diamines introducing hydrocarbon spacers.
Figure 3.
Synthesis of TSCs from acetobromo-α-D-glucose.
Figure 3.
Synthesis of TSCs from acetobromo-α-D-glucose.
Figure 4.
Synthesis of TSCs starting from trans-4-hydroxy-cyclohexanol.
Figure 4.
Synthesis of TSCs starting from trans-4-hydroxy-cyclohexanol.
Figure 5.
Synthesis of TSCs starting from dopamine.
Figure 5.
Synthesis of TSCs starting from dopamine.
Figure 6.
Synthesis of TSCs derived from amino thiols.
Figure 6.
Synthesis of TSCs derived from amino thiols.
Figure 7.
Attempted synthesis of thiosemicarbazones with an alkyne function starting from propargyl amine.
Figure 7.
Attempted synthesis of thiosemicarbazones with an alkyne function starting from propargyl amine.
Scheme 7.
Proposed cyclisation reaction preventing the formation of propargyl amine thiosemicarbazide.
Scheme 7.
Proposed cyclisation reaction preventing the formation of propargyl amine thiosemicarbazide.
Figure 8.
Synthesis of synthesis of TSCs with alkyne functions on rigid phenylene spacers.
Figure 8.
Synthesis of synthesis of TSCs with alkyne functions on rigid phenylene spacers.
Figure 9.
Attempted synthesis of the 1-bromo-2-N4-thiosemicarbazide.
Figure 9.
Attempted synthesis of the 1-bromo-2-N4-thiosemicarbazide.
Scheme 8.
Proposed cyclisation reaction preventing the formation of 2-thiazoline derivatives from 1-Br-2-N-thiocyanate and alcohols or amines.
Scheme 8.
Proposed cyclisation reaction preventing the formation of 2-thiazoline derivatives from 1-Br-2-N-thiocyanate and alcohols or amines.
Figure 10.
Synthesis of TSCs with Br functions on rigid spacers.
Figure 10.
Synthesis of TSCs with Br functions on rigid spacers.
Figure 11.
Attempted synthesis of thiosemicarbazones with an azide function.
Figure 11.
Attempted synthesis of thiosemicarbazones with an azide function.
Figure 12.
Synthesis of TSCs with azide functions using the rigid dimethyl-phenylene spacer.
Figure 12.
Synthesis of TSCs with azide functions using the rigid dimethyl-phenylene spacer.
Figure 13.
Synthesis of TSCs with a phosphonate group.
Figure 13.
Synthesis of TSCs with a phosphonate group.
Figure 14.
Synthesis of TSCs with a silane group.
Figure 14.
Synthesis of TSCs with a silane group.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).