1. Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are the key drugs in treatment of non-small cell lung cancer (NSCLC) patients with
EGFR mutation [
1]. Currently, 1
st/2
nd-generation (1/2G) EGFR-TKIs and a 3rd-generation (3G) TKI, osimertinib are available in daily clinical practice. Additionally, several novel 3G EGFR-TKIs are now in clinical development [
2,
3,
4]. However, secondary mutations such as T790M and C797S result in the acquired resistance to 1/2G EGFR-TKIs and 3G EGFR-TKIs, respectively [
5,
6,
7,
8]. Therefore, a novel type of EGFR-TKIs, the 4th-generation (4G) EGFR-TKIs, that can overcome both the T790M and C797S mutations, even if they are present
in cis, are now under early clinical development [
9,
10].
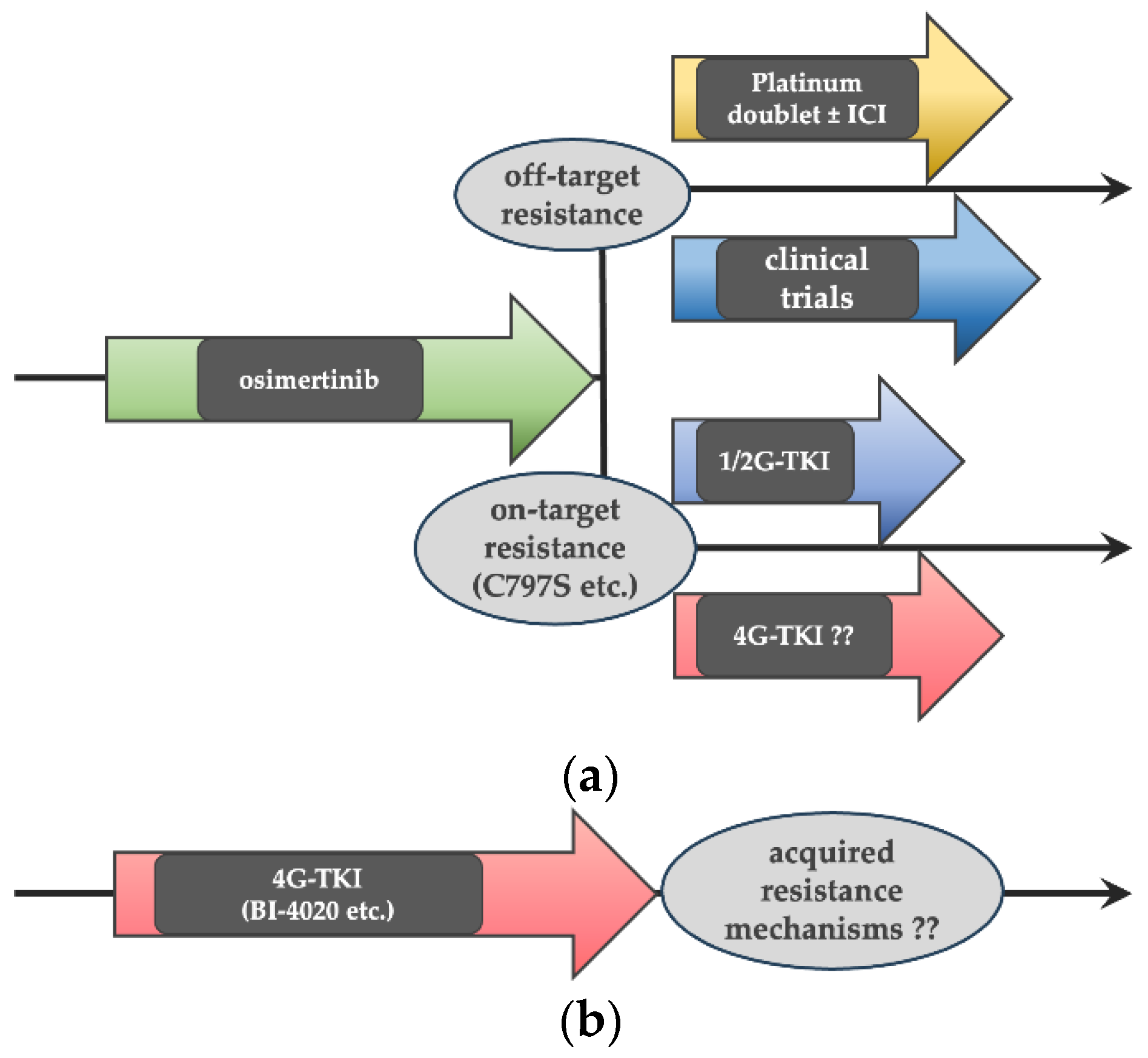
Many of the current guidelines recommend the 3G TKI osimertinib as a front-line treatment for
EGFR-mutated NSCLC patients, as a result of the significant improvement in prolonged progression-free and overall survival with osimertinib compared with 1G EGFR-TKIs in the FLAURA Phase III trial [
8]. Therefore, treatment strategies involving 4G EGFR-TKIs would be either (a) as a second-line treatment after front-line osimertinib failure or (b) as a front-line therapy for
EGFR-mutated NSCLC (
Figure 1).
In this study, we compared the efficacy of a 4G EGFR-TKI with a 1G EGFR-TKI (erlotinib) and several 3G EGFR-TKIs against in vitro cell models harboring secondary mutations that may cause acquired resistance to front-line osimertinib. We also explored acquired resistance mechanisms to the 4G EGFR-TKI using in vitro cell models with acquired resistance to the 4G EGFR-TKI after chronic drug exposure.
2. Materials and Methods
2.1. Exploration of Acquired Resistance Mechanisms to Front-Line Osimertinib
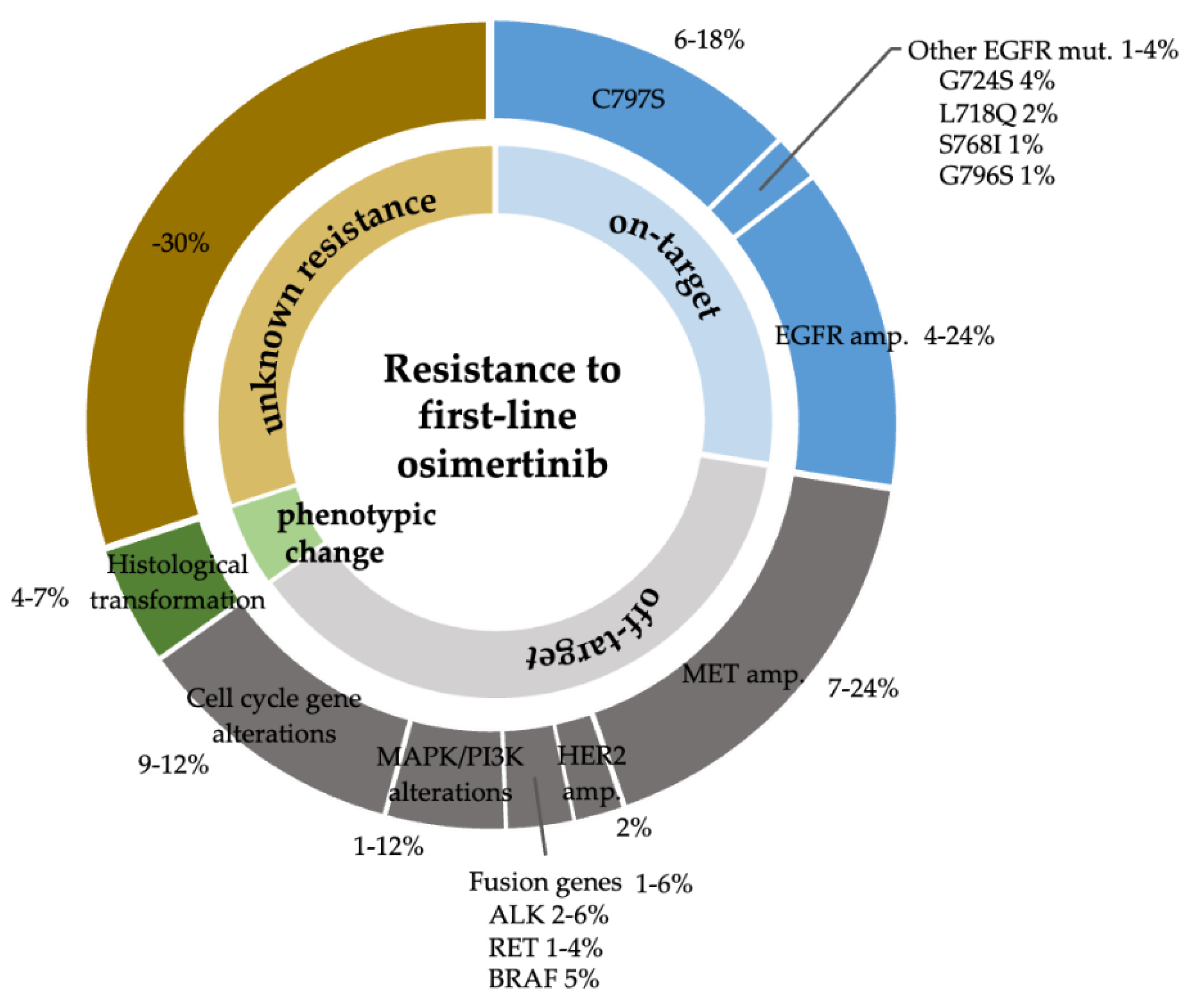
Acquired resistance mechanisms to EGFR-TKIs are classified as on-target mechanisms (mainly from secondary EGFR mutations), off-target mechanisms (activation of a bypass signaling pathway), and histological transformation [
11]. Acquired resistance mechanisms to front-line osimertinib were searched using PubMed in December 2022. Acquired resistance mutations that were reported only at second-line osimertinib failure were excluded. In the seven publications that met the inclusion criteria [
12,
13,
14,
15,
16,
17,
18], we identified four secondary mutations (C797S, G724S, L718Q, and S768I) as secondary mutations that may cause acquired resistance to front-line osimertinib. Data of acquired resistance mechanisms to front-line osimertinib were updated at the time of writing by adding four publications that were reported after December 2022 [
8,
19,
20,
21] (
Figure 2).
2.2. Cell Lines and Reagents
Ba/F3 cell lines harboring one of C797S, G724S, or L718Q secondary mutations were established in our previous study[
22]. In this study, we planned to establish Ba/F3 cells harboring S768I as the secondary mutation as previously described [
22,
23,
24,
25].
EGFR-mutated lung cancer cell lines (HCC827, HCC4006, PC9, and H1975) were cultured in RPMI 1640 medium (Wako, Osaka, Japan) with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA). Cell authentication was confirmed using STR profiling as described in our previous studies [
26,
27,
28]. BI4020-resistant lung cancer cells were established by chronic exposure to BI4020 at increasing concentrations from 1 nM to 2000 nM as previously described [
26,
27].
We purchased the following compounds from Selleck Chemicals (Houston, TX, USA): 1G EGFR-TKI (erlotinib; S7786), 3G EGFR-TKIs (osimertinib; S7297, alflutinib; S6868, lazertinib; S8724, almonertinib; S8817), and 4G EGFR-TKI (BI4020; S8921). The 3G EGFR-TKIs rezivertinib (HY-109189) and befotertinib (HY-137433) were purchased from MedChemExpress (Monmouth Junction, NJ, USA). Each compound was dissolved in DMSO (Sigma-Aldrich, St. Louis, MO, USA).
2.3. Growth Inhibition Assay
Growth inhibition assay was performed as described previously [
24,
26,
29]. Briefly, Ba/F3 cells or lung cancer cells were seeded at a density of 2–3 × 10
3/well in 96-well plates in RPMI 1640 medium supplemented with 10% FBS and cultured for 24 h. Then, DMSO or a designated drug(s) at the indicated concentration was added. After 72 h, the Cell Counting Kit-8 reagent (Dojindo Laboratories, Kumamoto, Japan) was used to evaluate growth following the manufacturer’s protocol, and then, the IC
50 values were calculated. The efficacy of each drug was also assessed using the sensitivity index (SI) as previously described [
25]. The SI was defined as the IC
50 value divided by the trough concentration of a given drug (IC
50/Ctrough max 100) at the recommended dose in clinical trials.
2.4. Western Blot Analysis
Cells were treated with indicated concentrations of drug for 24 h. The cells were then washed twice with phosphate-buffered saline and lysed in lysis buffer. Protein content was determined using a bicinchoninic acid protein assay (Bio-Rad, Hercules, CA, USA); samples were electrophoresed and transferred to polyvinylidene difluoride membranes (Bio-Rad). Immunoblotting was performed using the Transblot Turbo Transfer System (Bio-Rad) following the manufacturer’s instructions. Blocking buffer and antibody solutions were obtained from Takara (Kusatsu, Japan). Antibodies against phosphor-MET (#3126), total-MET (#8198), pErk (#4370), E-cadherin (#3195), Vimentin (#5741), β-actin (#4970), and horseradish peroxidase–conjugated secondary anti-rabbit immunoglobulin G (#7074) were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.5. Human Phospho-RTK Array
The relative phosphorylation levels of 43 receptor-tyrosine kinases (RTK) were screened using the Human Phospho-RTK Array Kit (R&D Systems, Minneapolis, MN, USA). Briefly, PC9 parental and resistant cells were cultured in RPMI-1640 containing 10% FBS and 4000 nM of BI4020 for 72 h. Cells were lysed in array buffer prior to reaching confluence. The arrays were blocked with blocking buffer and incubated with 200 µg cell lysate overnight at 4 °C. The arrays were washed and incubated with a horseradish peroxidase (HRP)-conjugated phospho-kinase antibody, and the chemiluminescence was detected.
2.6. Mutation Analysis and Gene Copy Number Analysis
Total RNA was prepared using a RNeasy® Plus Mini kit (250) (Qiagen, Hilden, Germany), following the manufacturer’s protocol. Random-primed, first-strand cDNA was synthesized from 10 µg of total RNA using the Rever Tra Ace® qPCR・RT kit (TOYOBO, Osaka, Japan) following the manufacturer’s instructions. Mutation analysis of exons 18 to 21 of the
EGFR gene was done by direct sequencing using primer sets as previously described [
26].
Genomic DNA was extracted using a DNeasy® Blood & Tissue Kit (250) (Qiagen) following the manufacturer’s protocol. The copy number of the MET gene relative to a LINE-1 repetitive element was measured by quantitative real-time PCR using the SYBR Green Method (Power SYBR Green PCR Master Mix; Qiagen) as described previously [
26]. PCR was done in triplicate for each primer set. Normal genomic DNA was used as a standard sample.
3. Results
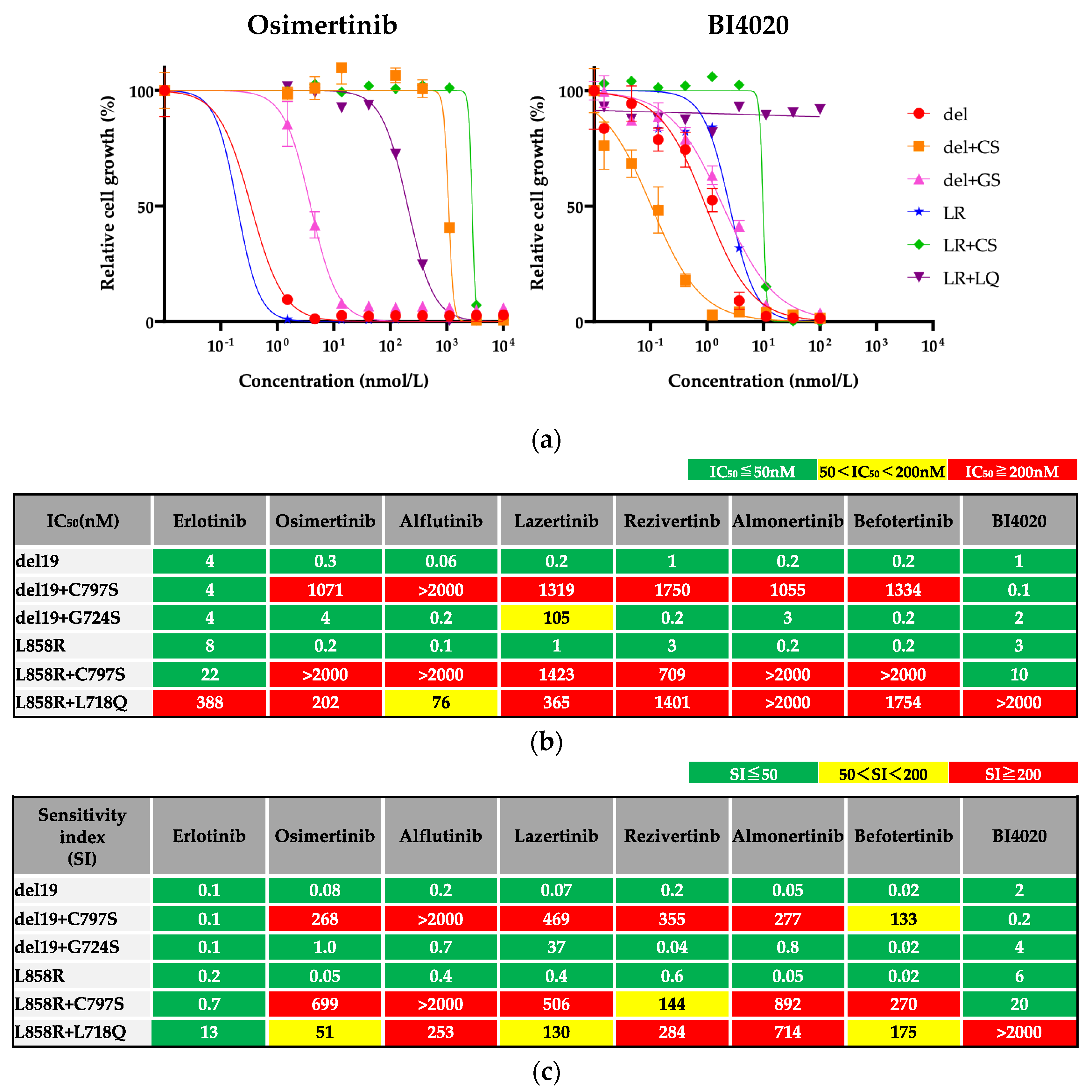
3.1. Efficacy of BI4020 and Other TKIs against Osimertinib Resistant Secondary Mutations
Because we could not establish IL-3 independent Ba/F3 cells with S768I secondary mutation, we evaluated drug efficacies using the other Ba/F3 cells. The efficacy of a 1G EGFR-TKI (erlotinib), novel 3G EGFR-TKIs (alflutinib, lazertinib, rezivertinib, almonertinib, befotertinib) and a 4G EGFR-TKI (BI4020) against Ba/F3 cells harboring one of the osimertinib resistant secondary mutations, as well as those with activating mutation alone, was evaluated. The secondary mutations conferred insensitivity to osimertinib (
Figure 3a). In contrast, many of the parental and resistant cell lines responded similarly to BI4020 (similar IC
50 values and SI), except for Ba/F3 cells with L858R+L718Q mutations (
Figure 3b). The IC
50 values of each drug are summarized in
Figure 3c.
In the evaluation of SI (
Figure 3d), we found that all 3G TKIs could not overcome the C797S secondary mutation. Additionally, Ba/F3 cells with the L718Q secondary mutation were insensitive to all 3G TKIs and BI4020. In contrast, erlotinib was effective in all Ba/F3 cells tested. These results suggest that erlotinib may be the most useful TKI after front-line osimertinib failure from on-target resistance mechanisms.
3.2. Exploration of Acquired Resistance Mechanisms to Front-Line BI4020
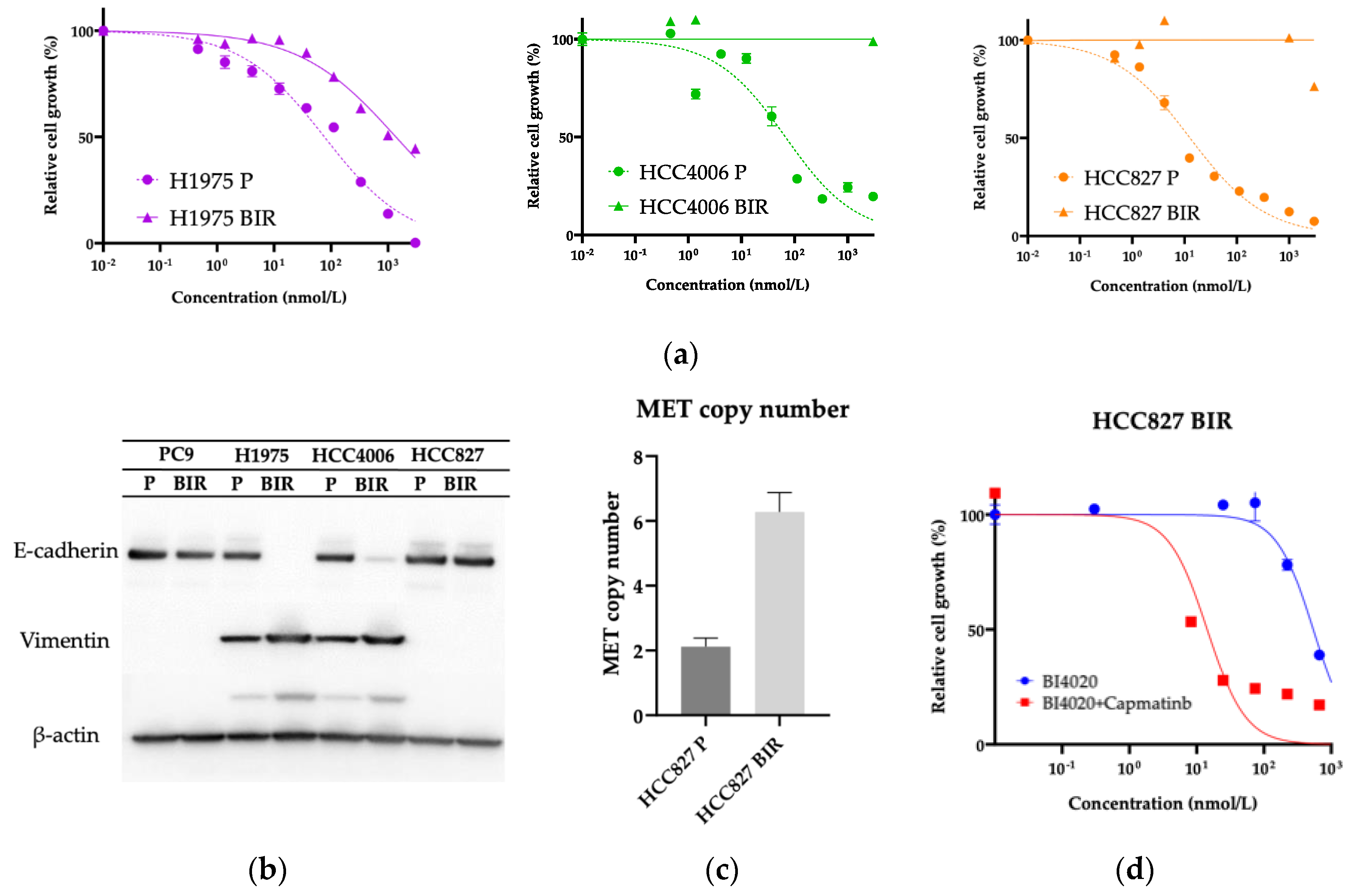
We successfully established acquired BI4020 resistant (BIR) cells from PC9, H1975, HCC4006, and HCC827 lung cancer cell lines by chronic exposure of cells to BI4020 at increasing concentrations (
Figure 4a and
Figure 5a). We first examined any potential secondary mutations of the
EGFR kinase domain (exons 18–21) in the four resistant lines; no secondary mutation was detected (data not shown). We and other groups have reported that HCC4006 and H1975 cells often acquire TKI resistance via epithelial-mesenchymal transition (EMT) [
11,
27,
28,
30,
31,
32,
33,
34]. Because the morphological changes of these resistant cells were similar to those previously reported, we further evaluated E-cadherin and vimentin expression. As shown in
Figure 4b, both H1975BIR and HCC4006BIR cells, but not HCC827BIR cells, exhibited EMT.
On the other hand,
MET gene amplification has been reported as an acquired resistant mechanism to EGFR-TKIs in HCC827 cells [
11,
26,
35,
36,
37,
38]. Therefore, we examined
MET gene copy number in HCC827BIR cells and HCC827 parental cells using real-time PCR. As shown in
Figure 4c,
MET gene copy number was increased in HCC827BIR cells. The combination treatment with capmatinib, a MET-TKI, plus BI4020 was effective in inhibiting the growth of HCC827BIR cells compared with BI4020 alone (
Figure 4d).
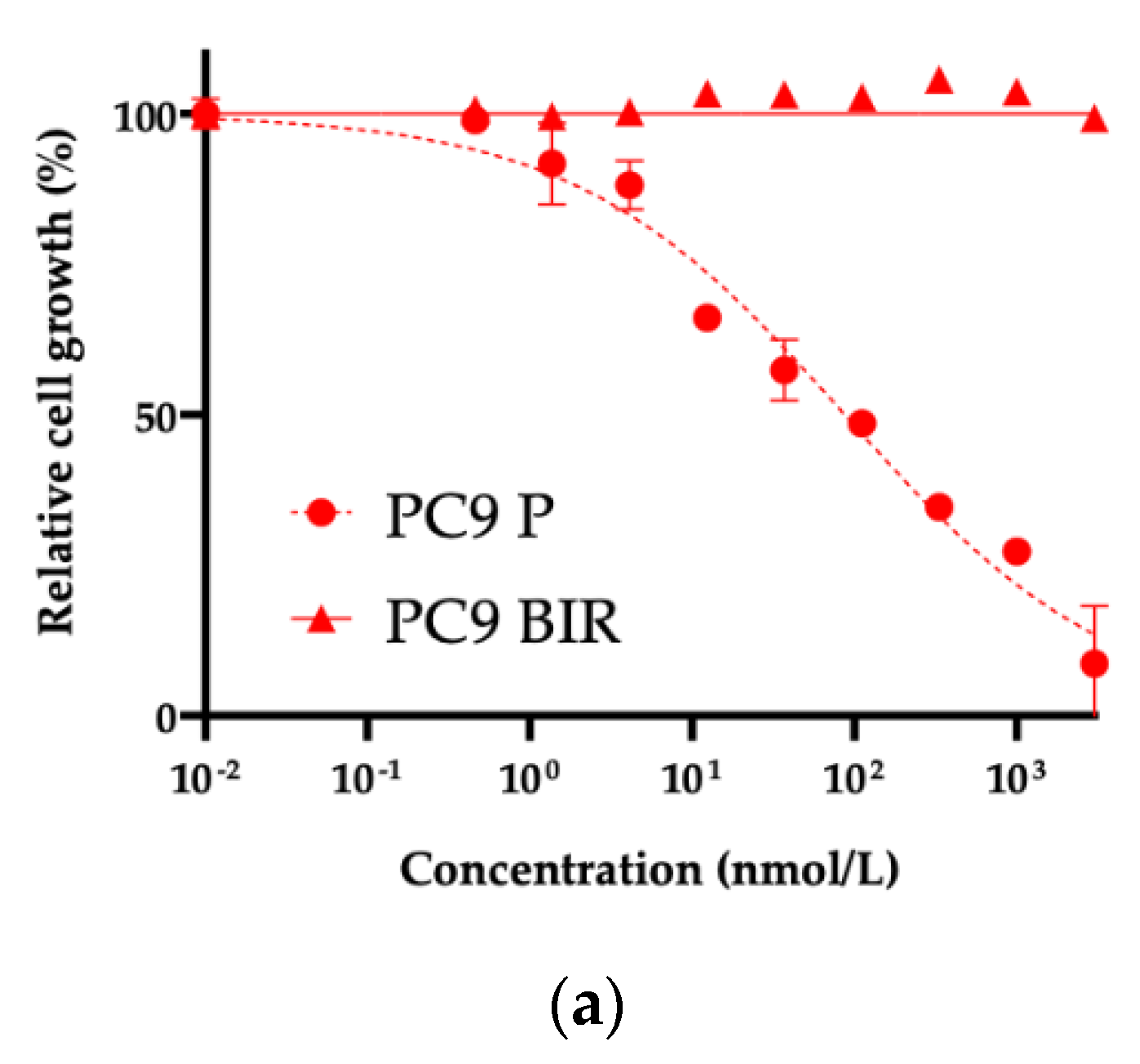
Various resistance mechanisms to 1–3G TKIs have been reported in PC9 cells. PC9 BIR cells did not harbor a secondary
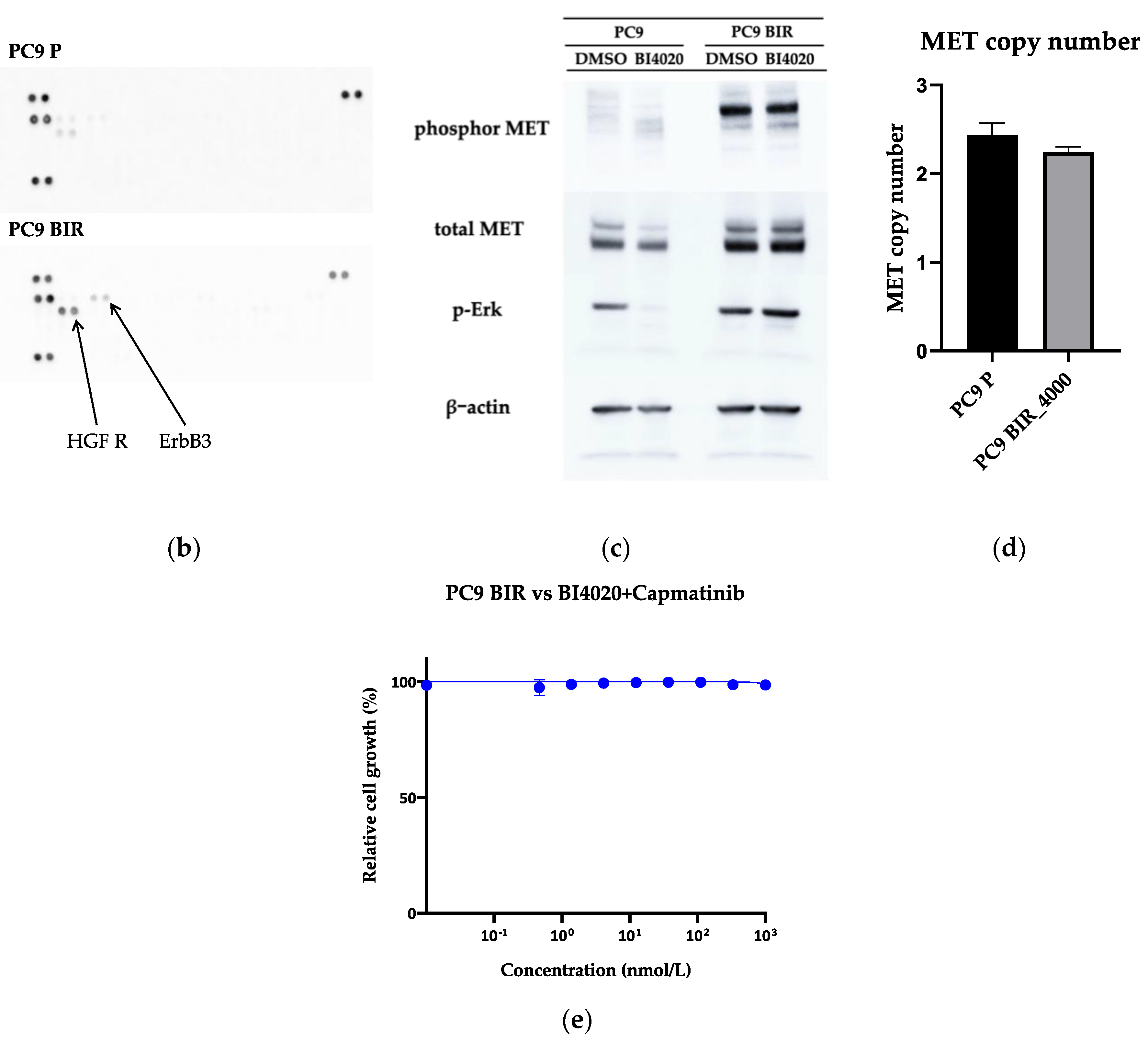
EGFR mutation, and thus we used a human phosphor-RTK assay. As shown in
Figure 5b, HGF-R (MET) phosphorylation was increased in PC9 BIR cells compared with PC9 parental cells. This result was confirmed by western blot (
Figure 5c). However, we did not observe
MET gene copy number gain in PC9 BIR cells; furthermore, the MET-TKI capmatinib did not restore BI4020 sensitivity in PC9 BIR cells. This result suggests that increased MET protein expression (either total or phosphorylated MET) does not necessarily mean that MET activation is the mechanism of acquired resistance to EGFR-TKIs.
4. Discussion
The T790M mutation in the
EGFR gene is the most frequent mechanism of acquired resistance to 1G or 2G EGFR-TKIs, present in ~50% of all NSCLC cases. Initially, 3G EGFR-TKIs that can overcome T790M-mediated resistance have been developed for use in second-line setting after treatment failure of 1G EGFR-TKIs [
39,
40]. Several studies have reported that a tertiary C797S mutation (mainly
in cis), which impairs the covalent binding between the cysteine residue at position 797 of EGFR and 3G EGFR-TKI, induces acquisition of resistance to second-line 3G EGFR-TKIs [
12,
41,
42]. Therefore, to inhibit the growth of
EGFR-mutated NSCLC cells with triple
EGFR mutations, 4G EGFR-TKIs have been developed [
9,
10]. In addition to BI-4020 that was used in our study, various 4G EGFR-TKIs are now being studied. Yun et al. reported that JIN-02 inhibited cell growth to a greater degree than osimertinib in an EGFR 19Del/T790M/C797S
in cis model (IC
50 values: 92.1 nM vs > 4,000 nM) [
43]. Sun et al. reported that the IC
50 values of BBT-176 against EGFR 19Del/C797S, EGFR 19Del/T790M/C797S, and EGFR L858R/C797S were 4.36, 1.79, and 5.35 nmol/L, respectively, while the values were 304.39, 124.82, and 573.72 nmol/L for osimertinib [
10]. Based on these promising
in vitro data, several clinical trials using 4G EGFR-TKI are ongoing, such as the SYMPHONY phase 1/2 trial (NCT04862780) of a 4G EGFR-TKI, BLU-945, as monotherapy or combination therapy of BLU-945 plus osimertinib. This study enrolled
EGFR-mutated NSCLC patients who had received ≥1 EGFR TKI(s), and 48% of patients experienced tumor regression at doses of 400 mg/day or higher with BLU-945 monotherapy [
44].
The great success of osimertinib, a 3G EGFR-TKI, in the FLAURA study [
8] led to the recommendation of the use of osimertinib in front-line setting in many countries. Several recent studies that explored the efficacy and safety of novel front-line combination treatments, such as the FLAURA2 study and MARIPOSA study, also involve a 3G EGFR-TKI as one of combined agents [
45,
46,
47]. Therefore, there may not be many chances to use 4G EGFR-TKIs as originally expected (i.e., after treatment failures of 1G/2G TKI, and then 3G TKI).
In this study, we evaluated the potential utility of a 4G EGFR-TKI for the treatment of NSCLC with common
EGFR mutations. However, in our
in vitro model reflecting the use of 4G EGFR-TKIs after treatment failure of front-line osimertinib, we observed that erlotinib, a 1G EGFR-TKI, showed wider efficacy than the 4G EGFR-TKI (or other novel 3G EGFR-TKIs) against secondary mutations that emerge after front-line osimertinib treatment. These results suggest that 1G EGFR-TKI would be a suitable EGFR-TKI following osimertinib treatment failure as previously reported [
48,
49,
50]. However, in clinical practice, cytotoxic agents with/without an immune checkpoint inhibitor are used as second-line treatment after front-line osimertinib treatment failure, because only 25% of patients have on-target resistance mechanisms (
Figure 2). Because osimertinib re-challenge is sometimes effective after cytotoxic chemotherapies [
51], the use of osimertinib or 1G/2G EGFR-TKI as a re-challenging EGFR-TKI should be discussed [
52].
We also explored acquired resistance mechanisms to front-line 4G EGFR-TKI exposure using lung cancer cell lines with activating
EGFR mutation. While we did not find any secondary mutations in the three established resistant cell lines, we observed
MET gene amplification (HCC827) and EMT phenotypic change (HCC4006 and H1975) as the mechanisms of resistance to a 4G EGFR-TKI. Because 4G EGFR-TKIs are active against two major secondary mutations, T790M and C797S, it is reasonable that each lung cancer cell line acquired a “preferred” off-target resistance mechanism [
11]. Moreover, we observed increased phosphorylation of MET in PC9 BIR cells; however, the resistant cells did not acquire
MET gene copy number gain and, more importantly, the combination of a MET-TKI plus BI4020 did not show efficacy in this resistant cell line. This result may suggest that gene copy number, but not increased phosphor-MET (or total MET) expression, would be a useful biomarker indicating MET-mediated acquired resistance to EGFR-TKI.
5. Conclusions
Our results suggest that erlotinib, but not a 4G EGFR-TKI, may be the most suitable second-line TKI for NSCLC after acquisition of resistance to front-line osimertinib. Additionally, we observed that lung cancer cells acquire resistance to 4G EGFR-TKI using their “favorite” off-target resistance mechanisms after acquisition of resistance to 1G–3G EGFR-TKIs.
Author Contributions
Conceptualization, K.S.; methodology, S.F., K.S., A.H., H.O., and M.I.; validation, S.F. and K.S.; formal analysis, S.F.; investigation, S.F., K.S., S.O., H.O., and J.S.; resources, K.S. and T.M.; writing—original draft preparation, S.F. and K.S.; writing—review and editing, all authors; supervision, K.S., T.M., and Y.T.; project administration, K.S.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science [grant number 22K07291 to K. Suda].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All information will be available from the corresponding author upon reasonable requests.
Acknowledgments
We thank Ms. Keiko Obata for her technical assistance related to this study. We thank Gabrielle White Wolf, PhD, from Edanz (
https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflicts of Interest
Dr. Suda has received research funding from AstraZeneca and Guardant and has received honoraria from Chugai Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Amgen, Daiichi Sankyo, and Taiho. Dr. Hamada has received honoraria from AstraZeneca, Chugai Pharmaceuticals, and Ono Pharmaceuticals. Dr. Ohara has received honoraria from AstraZeneca. Dr. Mitsudomi has received research funding from AstraZeneca, Ono Pharmaceuticals, Merck Sharp & Dohme, and Chugai Pharmaceuticals; has received honoraria from AstraZeneca, Chugai Pharmaceuticals, Bristol-Myers Squibb, Merck Sharp & Dohme, and Ono Pharmaceuticals; and has been on the advisory board of AstraZeneca and Merck Sharp & Dohme. Dr. Tsutani has received research funding from AstraZeneca, Boehringer-Ingelheim, Chugai Pharmaceuticals, Daiichi Sankyo, Japan Blood Products Organization, Medtronic, Otsuka Pharmaceuticals, and Taiho and received honoraria from AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceuticals, Covidien Japan, CSL Behring, Eli Lilly, Japan Blood Products Organization, Johnson and Johnson, MiRTeL, MSD, Nihon Medi-Physics, Novartis, Ono Pharmaceuticals, Phase 1, Roche, Taiho, and Takeda Pharmaceuticals; he has been on the advisory boad of AstraZeneca, Chugai Pharmaceuticals, and Ono Pharmaceuticals. All other authors report no conflicts of interest.
References
- Hirsch FR, Suda K, Wiens J, Bunn PA, Jr.: New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016, 388(10048):1012-1024.
- Cho BC, Ahn MJ, Kang JH, Soo RA, Reungwetwattana T, Yang JC, Cicin I, Kim DW, Wu YL, Lu S et al: Lazertinib Versus Gefitinib as First-Line Treatment in Patients With EGFR-Mutated Advanced Non-Small-Cell Lung Cancer: Results From LASER301. J Clin Oncol 2023, 41(26):4208-4217.
- Passaro A, Wang J, Wang Y, Lee SH, Melosky B, Shih JY, Wang J, Azuma K, Juan-Vidal O, Cobo M et al: Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann Oncol 2024, 35(1):77-90.
- Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y, Liu C, Zhu S, Zhang X, Li Y et al: Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med 2022, 10(11):1019-1028.
- Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B: EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005, 352(8):786-792.
- Suda K, Murakami I, Obata K, Sakai K, Fujino T, Koga T, Ohara S, Hamada A, Soh J, Nishio K et al: Spatial heterogeneity of acquired resistance mechanisms to 1st/2nd generation EGFR tyrosine kinase inhibitors in lung cancer. Lung Cancer 2020, 148:100-104.
- Kim Y, Ko J, Cui Z, Abolhoda A, Ahn JS, Ou SH, Ahn MJ, Park K: The EGFR T790M mutation in acquired resistance to an irreversible second-generation EGFR inhibitor. Mol Cancer Ther 2012, 11(3):784-791.
- Chmielecki J, Gray JE, Cheng Y, Ohe Y, Imamura F, Cho BC, Lin MC, Majem M, Shah R, Rukazenkov Y et al: Candidate mechanisms of acquired resistance to first-line osimertinib in EGFR-mutated advanced non-small cell lung cancer. Nat Commun 2023, 14(1):1070.
- Lee EJ, Oh SY, Lee YW, Kim JY, Kim MJ, Kim TH, Lee JB, Hong MH, Lim SM, Baum A et al: Discovery of a novel potent EGFR inhibitor against EGFR activating mutations and on-target resistance in NSCLC. Clin Cancer Res 2024.
- Lim SM, Fujino T, Kim C, Lee G, Lee YH, Kim DW, Ahn JS, Mitsudomi T, Jin T, Lee SY: BBT-176, a Novel Fourth-Generation Tyrosine Kinase Inhibitor for Osimertinib-Resistant EGFR Mutations in Non-Small Cell Lung Cancer. Clin Cancer Res 2023, 29(16):3004-3016.
- Suda K, Mizuuchi H, Maehara Y, Mitsudomi T: Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation--diversity, ductility, and destiny. Cancer Metastasis Rev 2012, 31(3-4):807-814.
- Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, Lai Z, Markovets A, Vivancos A, Kuang Y et al: Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015, 21(6):560-562.
- Bersanelli M, Minari R, Bordi P, Gnetti L, Bozzetti C, Squadrilli A, Lagrasta CA, Bottarelli L, Osipova G, Capelletto E et al: L718Q Mutation as New Mechanism of Acquired Resistance to AZD9291 in EGFR-Mutated NSCLC. J Thorac Oncol 2016, 11(10):e121-123.
- Fassunke J, Müller F, Keul M, Michels S, Dammert MA, Schmitt A, Plenker D, Lategahn J, Heydt C, Brägelmann J et al: Overcoming EGFR(G724S)-mediated osimertinib resistance through unique binding characteristics of second-generation EGFR inhibitors. Nat Commun 2018, 9(1):4655.
- Ramalingam SS, Cheng Y, Zhou C, Ohe Y, Imamura F, Cho BC, Lin MC, Majem M, Shah R, Rukazenkov Y et al: Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Annals of Oncology 2018, 29:viii740.
- Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M: Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. British Journal of Cancer 2019, 121(9):725-737.
- Schmid S, Li JJN, Leighl NB: Mechanisms of osimertinib resistance and emerging treatment options. Lung Cancer 2020, 147:123-129.
- Schoenfeld AJ, Chan JM, Kubota D, Sato H, Rizvi H, Daneshbod Y, Chang JC, Paik PK, Offin M, Arcila ME et al: Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin Cancer Res 2020, 26(11):2654-2663.
- Hartmaier RJ, Markovets A, Cho BC, de Langen AJ, Goldberg SB, Goldman J, Le X, Okamoto I, Riess JW, Cosaert J et al: Abstract LB078: Tumor genomics in patients (pts) with advanced epidermal growth factor receptor mutant (EGFRm) non-small cell lung cancer (NSCLC) whose disease has progressed on first-line (1L) osimertinib therapy in the Phase II ORCHARD study. Cancer Research 2022, 82(12_Supplement):LB078-LB078.
- Piotrowska Z, Ahn MJ, Pang YK, How SH, Kim SW, Voon PJ, Cortinovis DL, De Castro Carpeno J, Tiseo M, Abreu DR et al: LBA53 ELIOS: A multicentre, molecular profiling study of patients (pts) with epidermal growth factor receptor-mutated (EGFRm) advanced NSCLC treated with first-line (1L) osimertinib. Annals of Oncology 2022, 33:S1420-S1421.
- Leonetti A, Verzè M, Minari R, Perrone F, Gnetti L, Bordi P, Pluchino M, Nizzoli R, Azzoni C, Bottarelli L et al: Resistance to osimertinib in advanced EGFR-mutated NSCLC: a prospective study of molecular genotyping on tissue and liquid biopsies. Br J Cancer 2024, 130(1):135-142.
- Nishino M, Suda K, Kobayashi Y, Ohara S, Fujino T, Koga T, Chiba M, Shimoji M, Tomizawa K, Takemoto T et al: Effects of secondary EGFR mutations on resistance against upfront osimertinib in cells with EGFR-activating mutations in vitro. Lung Cancer 2018, 126:149-155.
- Hamada A, Suda K, Koga T, Fujino T, Nishino M, Ohara S, Chiba M, Shimoji M, Takemoto T, Soh J et al: In vitro validation study of HER2 and HER4 mutations identified in an ad hoc secondary analysis of the LUX-Lung 8 randomized clinical trial. Lung Cancer 2021, 162:79-85.
- Koga T, Suda K, Nishino M, Fujino T, Ohara S, Hamada A, Soh J, Tirunagaru V, Vellanki A, Doebele RC et al: Activity and mechanism of acquired resistance to tarloxotinib in HER2 mutant lung cancer: an in vitro study. Transl Lung Cancer Res 2021, 10(8):3659-3670.
- Hamada A, Suda K, Nishino M, Obata K, Oiki H, Fukami T, Fukuda S, Fujino T, Ohara S, Koga T et al: Secondary Mutations of the EGFR Gene That Confer Resistance to Mobocertinib in EGFR Exon 20 Insertion. J Thorac Oncol 2024, 19(1):71-79.
- Suda K, Murakami I, Katayama T, Tomizawa K, Osada H, Sekido Y, Maehara Y, Yatabe Y, Mitsudomi T: Reciprocal and complementary role of MET amplification and EGFR T790M mutation in acquired resistance to kinase inhibitors in lung cancer. Clin Cancer Res 2010, 16(22):5489-5498.
- Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, Yatabe Y, Sekido Y, Mitsudomi T: Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol 2011, 6(7):1152-1161.
- Suda K, Murakami I, Yu H, Kim J, Tan AC, Mizuuchi H, Rozeboom L, Ellison K, Rivard CJ, Mitsudomi T et al: CD44 Facilitates Epithelial-to-Mesenchymal Transition Phenotypic Change at Acquisition of Resistance to EGFR Kinase Inhibitors in Lung Cancer. Mol Cancer Ther 2018, 17(10):2257-2265.
- Nishino M, Suda K, Koga T, Ohara S, Fujino T, Soh J, Tirunagaru V, Vellanki A, Doebele RC, Mitsudomi T: Activity of tarloxotinib-E in cells with EGFR exon-20 insertion mutations and mechanisms of acquired resistance. Thorac Cancer 2021, 12(10):1511-1516.
- Soucheray M, Capelletti M, Pulido I, Kuang Y, Paweletz CP, Becker JH, Kikuchi E, Xu C, Patel TB, Al-Shahrour F et al: Intratumoral Heterogeneity in EGFR-Mutant NSCLC Results in Divergent Resistance Mechanisms in Response to EGFR Tyrosine Kinase Inhibition. Cancer Res 2015, 75(20):4372-4383.
- Yoshida T, Song L, Bai Y, Kinose F, Li J, Ohaegbulam KC, Muñoz-Antonia T, Qu X, Eschrich S, Uramoto H et al: ZEB1 Mediates Acquired Resistance to the Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. PLoS One 2016, 11(1):e0147344.
- Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK et al: Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011, 3(75):75ra26.
- Weng CH, Chen LY, Lin YC, Shih JY, Lin YC, Tseng RY, Chiu AC, Yeh YH, Liu C, Lin YT et al: Epithelial-mesenchymal transition (EMT) beyond EGFR mutations per se is a common mechanism for acquired resistance to EGFR TKI. Oncogene 2019, 38(4):455-468.
- Ji W, Choi YJ, Kang MH, Sung KJ, Kim DH, Jung S, Choi CM, Lee JC, Rho JK: Efficacy of the CDK7 Inhibitor on EMT-Associated Resistance to 3rd Generation EGFR-TKIs in Non-Small Cell Lung Cancer Cell Lines. Cells 2020, 9(12).
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J et al: MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316(5827):1039-1043.
- Cappuzzo F, Jänne PA, Skokan M, Finocchiaro G, Rossi E, Ligorio C, Zucali PA, Terracciano L, Toschi L, Roncalli M et al: MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol 2009, 20(2):298-304.
- Yoshida T, Okamoto I, Okamoto W, Hatashita E, Yamada Y, Kuwata K, Nishio K, Fukuoka M, Jänne PA, Nakagawa K: Effects of Src inhibitors on cell growth and epidermal growth factor receptor and MET signaling in gefitinib-resistant non-small cell lung cancer cells with acquired MET amplification. Cancer Sci 2010, 101(1):167-172.
- Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, Toschi L, Rogers A, Mok T, Sequist L et al: Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell 2010, 17(1):77-88.
- Goss G, Tsai CM, Shepherd FA, Bazhenova L, Lee JS, Chang GC, Crino L, Satouchi M, Chu Q, Hida T et al: Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016, 17(12):1643-1652.
- Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WSME et al: Osimertinib or Platinum–Pemetrexed in <i>EGFR</i> T790M–Positive Lung Cancer. New England Journal of Medicine 2017, 376(7):629-640.
- Uchibori K, Inase N, Araki M, Kamada M, Sato S, Okuno Y, Fujita N, Katayama R: Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nature Communications 2017, 8(1):14768.
- Yu HA, Tian SK, Drilon AE, Borsu L, Riely GJ, Arcila ME, Ladanyi M: Acquired Resistance of EGFR-Mutant Lung Cancer to a T790M-Specific EGFR Inhibitor: Emergence of a Third Mutation (C797S) in the EGFR Tyrosine Kinase Domain. JAMA Oncol 2015, 1(7):982-984.
- Yun MR, Yu MR, Duggirala KB, Lee K, Lim SM, Jo A, Seah E, Kim C, Cho BC: 999P JIN-A02, a fourth-generation, highly effective tyrosine kinase inhibitor with intracranial activity, targeting EGFR C797S mutations in NSCLC. Annals of Oncology 2022, 33:S1010-S1011.
- Elamin YY, Nagasaka M, Shum E, Bazhenova L, Camidge DR, Cho BC, Felip E, Goto K, Lin C-C, Piotrowska Z et al: BLU-945 monotherapy and in combination with osimertinib (OSI) in previously treated patients with advanced EGFR-mutant (EGFRm) NSCLC in the phase 1/2 SYMPHONY study. Journal of Clinical Oncology 2023, 41(16_suppl):9011-9011.
- Planchard D, Jänne PA, Cheng Y, Yang JC, Yanagitani N, Kim SW, Sugawara S, Yu Y, Fan Y, Geater SL et al: Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N Engl J Med 2023, 389(21):1935-1948.
- Jänne PA, Planchard D, Kobayashi K, Cheng Y, Lee CK, Valdiviezo N, Laktionov K, Yang TY, Yu Y, Kato T et al: CNS Efficacy of Osimertinib With or Without Chemotherapy in Epidermal Growth Factor Receptor-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2024, 42(7):808-820.
- Cho BC, Felip E, Spira AI, Girard N, Lee JS, Lee SH, Ostapenko YV, Danchaivijitr P, Liu B, Alip A et al: LBA14 Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced non-small cell lung cancer (NSCLC): Primary results from MARIPOSA, a phase III, global, randomized, controlled trial. Annals of Oncology 2023, 34:S1306.
- Ercan D, Choi HG, Yun CH, Capelletti M, Xie T, Eck MJ, Gray NS, Jänne PA: EGFR Mutations and Resistance to Irreversible Pyrimidine-Based EGFR Inhibitors. Clin Cancer Res 2015, 21(17):3913-3923.
- Uchibori K, Inase N, Nishio M, Fujita N, Katayama R: Identification of Mutation Accumulation as Resistance Mechanism Emerging in First-Line Osimertinib Treatment. Journal of Thoracic Oncology 2018, 13(7):915-925.
- Rangachari D, To C, Shpilsky JE, VanderLaan PA, Kobayashi SS, Mushajiang M, Lau CJ, Paweletz CP, Oxnard GR, Jänne PA et al: EGFR-Mutated Lung Cancers Resistant to Osimertinib through EGFR C797S Respond to First-Generation Reversible EGFR Inhibitors but Eventually Acquire EGFR T790M/C797S in Preclinical Models and Clinical Samples. J Thorac Oncol 2019, 14(11):1995-2002.
- Araki T, Kanda S, Obara M, Agatsuma T, Kakizaki Y, Hama M, Yamamoto H, Takada M, Yamamoto M, Matsuo A et al: EGFR-TKI rechallenge in patients with EGFR-mutated non-small-cell lung cancer who progressed after first-line osimertinib treatment: A multicenter retrospective observational study. Respir Investig 2024, 62(2):262-268.
- Araki T, Kanda S, Komatsu M, Sonehara K, Tateishi K, Takada M, Kato A, Yamamoto M, Nishie K, Hama M et al: Rechallenge of afatinib for EGFR-mutated non-small cell lung cancer previously treated with osimertinib: a multicenter phase II trial protocol (REAL study). Transl Lung Cancer Res 2023, 12(6):1320-1327.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).