Submitted:
31 May 2024
Posted:
03 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
3. Recent RCTs
4. Discussion
5. Conclusion
6. Future Directions
Authors contributions
Funding
Conflicts of Interest
List of Abbreviations
| ABI | abiraterone |
| Act | Actinium |
| ADT | androgen deprivation therapy |
| ARPI | androgen receptor pathway inhibitor |
| BCR | Biochemical recurrence |
| CABA | cabazitaxel |
| CT | computed tomography |
| DOC | docetaxel |
| ENZA | enzalutamide |
| ENZA-P | randomized controlled phase 2 trial of the combination of enzalutamide and PSMA-based radioligand therapy |
| ESMO | European Society of Medical Oncology |
| LNM | lymph node metastases |
| Lu | lutetium |
| mCRPC | metastatic castration-resistant prostate cancer |
| mHSPC | metastatic hormone-sensitive prostate cancer |
| nmCRPC | nonmetastatic prostate cancer |
| OS | overall survival |
| PCa | prostate cancer |
| PCWG3 | Prostate cancer clinical trial working group 3 |
| PET | positron emission tomography |
| PSA | prostate specific antigen |
| PSAR | prostate specific antigen relapse |
| PSMA | prostate specific membrane antigen |
| Ra | Radium |
| RCT | randomized controlled trial |
| RLT | radioligand therapy |
| rPFS | radiological progression-free survival |
| Tb | Terbium |
References
- Montgomery, B.; Tretiakova, M.S.; Joshua, A.M.; Gleave, M.E.; Fleshner, N.; Bubley, G.J.; Mostaghel, E.A.; Chi, K.N.; Lin, D.W.; Sanda, M.; et al. Neoadjuvant Enzalutamide Prior to Prostatectomy. Clin. Cancer Res. 2017, 23, 2169–2176. [Google Scholar] [CrossRef]
- McKay, R.R.; Ye, H.; Xie, W.; Lis, R.; Calagua, C.; Zhang, Z.; Trinh, Q.D.; Chang, S.L.; Harshman, L.C.; Ross, A.E.; et al. Evaluation of Intense Androgen Deprivation Before Prostatectomy: A Randomized Phase II Trial of Enzalutamide and Leuprolide With or Without Abiraterone. J. Clin. Oncol. 2019, 37, 923–931. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef]
- Attard, G.; Murphy, L.; Clarke, N.W.; Cross, W.; Jones, R.J.; Parker, C.C.; Gillessen, S.; Cook, A.; Brawley, C.; Amos, C.L.; et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet 2022, 399, 447–460. [Google Scholar] [CrossRef]
- Small, E.J.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Ann. Oncol. 2019, 30, 1813–1820. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Fizazi, K.; Saad, F.; Shore, N.D.; De Giorgi, U.; Penson, D.F.; Ferreira, U.; Efstathiou, E.; Madziarska, K.; Kolinsky, M.P.; et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2197–2206. [Google Scholar] [CrossRef]
- Anton, A.; Pillai, S.; Semira, M.C.; Wong, S.; Shapiro, J.; Weickhardt, A.; Azad, A.; Kwan, E.M.; Spain, L.; Gunjur, A.; et al. Real-world first-line systemic therapy patterns in metastatic castration-resistant prostate cancer. BJUI Compass. 2022, 3, 205–213. [Google Scholar] [CrossRef]
- George, D.J.; Sartor, O.; Miller, K.; Saad, F.; Tombal, B.; Kalinovsky, J.; Jiao, X.; Tangirala, K.; Sternberg, C.N.; Higano, C.S. Treatment Patterns and Outcomes in Patients With Metastatic Castration-resistant Prostate Cancer in a Real-world Clinical Practice Setting in the United States. Clin. Genitourin. Cancer 2020, 18, 284–294. [Google Scholar] [CrossRef]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wulfing, C.; Kramer, G.; Eymard, J.C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef]
- Freedland, S.J.; Davis, M.; Epstein, A.J.; Arondekar, B.; Ivanova, J.I. Real-world treatment patterns and overall survival among men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US Medicare population. Prostate Cancer Prostatic Dis. 2023. [Google Scholar] [CrossRef]
- de Wit, R.; Freedland, S.J.; Oudard, S.; Marinov, G.; Capart, P.; Combest, A.J.; Peterson, R.; Ozatilgan, A.; Morgans, A.K. Real-world evidence of patients with metastatic castration-resistant prostate cancer treated with cabazitaxel: comparison with the randomized clinical study CARD. Prostate Cancer Prostatic Dis. 2023, 26, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; de Almeida Luz, M.; De Giorgi, U.; Gleave, M.; Gotto, G.T.; Pieczonka, C.M.; Haas, G.P.; Kim, C.S.; Ramirez-Backhaus, M.; Rannikko, A.; et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N. Engl. J. Med. 2023, 389, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Buteau, J.P.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; et al. Overall survival with [(177)Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): secondary outcomes of a randomised, open-label, phase 2 trial. Lancet Oncol 2024, 25, 99–107. [Google Scholar] [CrossRef] [PubMed]
- von Eyben, F.E.; Bauman, G.; von Eyben, R.; Rahbar, K.; Soydal, C.; Haug, A.R.; Virgolini, I.; Kulkarni, H.; Baum, R.; Paganelli, G. Optimizing PSMA radioligand therapy for patients with metastatic castration-resistant prostate cancer. A systematic review and meta-analysis. Int. J. Mol. Sci. 2020, 21, 9054. [Google Scholar] [CrossRef] [PubMed]
- Parida, G.K.; Panda, R.A.; Bishnoi, K.; Agrawal, K. Efficacy and Safety of Actinium-225 Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Prostate Cancer: A Systematic Review and Metanalysis. Med. Princ. Pract. 2023, 32, 178–191. [Google Scholar] [CrossRef]
- Sathekge, M.M.; Lawal, I.O.; Bal, C.; Bruchertseifer, F.; Ballal, S.; Cardaci, G.; Davis, C.; Eiber, M.; Hekimsoy, T.; Knoesen, O.; et al. Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): a multicentre, retrospective study. Lancet Oncol. 2024, 25, 175–183. [Google Scholar] [CrossRef]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholoma, M.; Ezziddin, S. (225)Ac-PSMA-617/(177)Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 721–728. [Google Scholar] [CrossRef]
- Banda, A.; Prive, B.M.; Allach, Y.; Uijen, M.J.K.; Peters, S.M.B.; Loeff, C.C.; Gotthardt, M.; Muselaers, C. .J.; Witjes, J.A.; van Oooprt, I.M.; et al. PSMA-RLT in Patients with Metastatic Hormone-Sensitive Prostate Cancer: A Retrospective Study. Cancers 2023, 15, 297. [Google Scholar] [CrossRef]
- Langbein, T.; Kulkarni, H.R.; Schuchardt, C.; Mueller, D.; Volk, G.F.; Baum, R.P. Salivary Gland Toxicity of PSMA-Targeted Radioligand Therapy with (177)Lu-PSMA and Combined (225)Ac- and (177)Lu-Labeled PSMA Ligands (TANDEM-PRLT) in Advanced Prostate Cancer: A Single-Center Systematic Investigation. Diagnostics 2022, 12, 1986. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Hofman, M.S.; Emmett, L.; Calais, J.; Osborne, J.R.; Iravani, A.; Koo, P.; Lindenberg, L.; et al. Joint EANM/SNMMI procedure guideline for the use of (177)Lu-labeled PSMA-targeted radioligand-therapy ((177)Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2830–2845. [Google Scholar] [CrossRef]
- Tobias, A. Meta-analysis of p values. Stata Technical Bulletin 1999, 49, 15–17. [Google Scholar]
- Suman, S.; Parghane, R.V.; Joshi, A.; Prabhash, K.; Talole, S.; Basu, S. Combined 177Lu-PSMA-617 PRLT and abiraterone acetate versus 177Lu-PSMA-617 PRLT monotherapy in metastatic castration-resistant prostate cancer. An observational study comparing the response and durability. Prostate 2021, 81, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Zambelli, J.; Lara, M.K.; Wolf, T.H.; McDonald, A.; Lee, E.; Abou-Elkacem, L.; Gordon, E.J.; Baum, R.P. Case Report: long-term complete response to PSMA-targeted radioligand therapy and abiraterone in a metastatic prostate cancer patient. Front. Oncol. 2023, 13, 1192792. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Subramaniam, S.; Crumbaker, M.; Nguyen, A.; Joshua, A.M.; Weickhardt, A.; Lee, S.T.; Ng, S.; Francis, R.J.; Goh, J.C.; et al. [(177)Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 563–571. [Google Scholar] [CrossRef]
- Satapathy, S.; Mittal, B.R.; Sood, A.; Das, C.K.; Mavuduru, R.S.; Goyal, S.; Shukla, J.; Singh, S.K. (177)Lu-PSMA-617 versus docetaxel in chemotherapy-naive metastatic castration-resistant prostate cancer: a randomized, controlled, phase 2 non-inferiority trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.C.; Castellano Gauna, D.E.; Herman, K.; de Bono, J.S.; Shore, N.D.; Chi, K.N.N.; Crosby, N.; Piulats Rodriguez, J.M.; Flechon, A.; et al. LBA13 phase 3 trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore. Ann. Oncol. 2023, 34, S1324–S1325. [Google Scholar] [CrossRef]
- Sartor, O.; Fizazi, K.; Herrmann, K.; Morris, M.J. Design Considerations in the PSMAfore Trial. J. Nucl. Med. 2024, 65, 226–227. [Google Scholar] [CrossRef]
- Hansen, A.R.; Probst, S.; Tulane, R.F.; Osman, M.M.; Delpassand, E.S.; Viglianti, B.L.; Michalski, J.; Beauregard, J.-M.-; Oz, O.K.; Courtney, K.; et al. 1400P Efficacy and Safety of 177Lu PNT2002 Prostate-Specific Membrane Antigen (PSMA) Therapy in Metatatic Castration-Resistant Prostate Cancer (mCRPC) Initial Results from SPLASH. Ann. Oncol. 2022, 33, S1185. [Google Scholar] [CrossRef]
- Satapathy, S.; Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Bal, C. [(177)Lu]Lu-PSMA-617 as first-line systemic therapy in patients with metastatic castration-resistant prostate cancer: a real-world study. Eur. J. Nucl. Med. Mol. Imaging 2024. [Google Scholar] [CrossRef]
- Oprea-Lager, D.E.; MacLennan, S.; Bjartell, A.; Briganti, A.; Burger, I.A.; de Jong, I.; De Santis, M.; Eberlein, U.; Emmett, L.; Fizazi, K.; et al. European Association of Nuclear Medicine Focus 5: Consensus on Molecular Imaging and Theranostics in Prostate Cancer. Eur. Urol. 2024, 85, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Mattana, F.; Muraglia, L.; Barone, A.; Colandrea, M.; Saker Diffalah, Y.; Provera, S.; Cascio, A.S.; Omodeo Sale, E.; Ceci, F. Prostate-Specific Membrane Antigen-Targeted Therapy in Prostate Cancer: History, Combination Therapies, Trials, and Future Perspective. Cancers 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.W.; Singh, A.; Kulkarni, H.R.; Niepsch, K.; Billah, B.; Baum, R.P. Clinical outcomes of (177)Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J. Nucl, Med. 2019, 60, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Seifert, R.; Kessel, K.; Bogemann, M.; Kulkarni, H.R.; Zhang, J.; Gerke, C.; Fimmers, R.; et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [(177)Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial). Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 113–122. [Google Scholar] [CrossRef]
- Satapathy, S.; Sahoo, R.K.; Bal, C. [(177)Lu]Lu-PSMA-radioligand therapy efficacy outcomes in taxane-naive versus taxane-treated patients with metastatic castration-resistant prostate cancer: a systematic review and metaanalysis. J, Nucl, Med, 2023, 64, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Mader, N.; Schoeler, C.; Pezeshkpour, N.; Klimek, K.; Groener, D.; Happel, C.; Tselis, N.; Mandel, P.; Grunwald, F.; Sabet, A. Intermittent radioligand therapy with (177)Lu-PSMA-617 for oligometastatic castration-resistant prostate cancer. Cancers 2023, 15, 4605. [Google Scholar] [CrossRef] [PubMed]
- Edler von Eyben, F.; Singh, A.; Zhang, J.; Nipsch, K.; Meyrick, D.; Lenzo, N.; Kairemo, K.; Joensuu, T.; Virgolini, I.; Soydal, C.; et al. (177)Lu-PSMA radioligand therapy of predominant lymph node metastatic prostate cancer. Oncotarget 2019, 10, 2451–2461. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Kulkarni, H.R.; Baum, R.P. Metastatic extent predicts survival as patients with metastatic castration-resistant prostate cancer are treated with (177)Lu-PSMA radioligand therapy. Theranostics 2020, 10, 4900–4902. [Google Scholar] [CrossRef]
- Yaxley, W.J.; McBean, R.; Wong, D.; Grimes, D.; Vasey, P.; Frydenberg, M.; Yaxley, J.W. Should Lutetium-prostate specific membrane antigen radioligand therapy for metastatic prostate cancer be used earlier in men with lymph node only metastatic prostate cancer? Investig. Clin. Urol. 2021, 62, 650–657. [Google Scholar] [CrossRef]
- Kostos, L.; Buteau, J.P.; Hofman, M.S.; Azad, A.A. Determinants of outcome following PSMA-based radioligand therapy and mechanisms of resistance in patients with metastatic castration-resistant prostate cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231179309. [Google Scholar] [CrossRef]
- Stuparu, A.D.; Capri, J.R.; Meyer, C.A.L.; Le, T.M.; Evans-Axelsson, S.L.; Current, K.; Lennox, M.; Mona, C.E.; Fendler, W.P.; Calais, J.; et al. Mechanisms of Resistance to Prostate-Specific Membrane Antigen-Targeted Radioligand Therapy in a Mouse Model of Prostate Cancer. J. Nucl. Med. 2021, 62, 989–995. [Google Scholar] [CrossRef]
- De Giorgi, U.; Sansovini, M.; Severi, S.; Nicolini, S.; Monti, M.; Gurioli, G.; Foca, F.; Casadei, C.; Conteduca, V.; Celli, M.; et al. Circulating androgen receptor gene amplification and resistance to (177)Lu-PSMA-617 in metastatic castration-resistant prostate cancer: results of a Phase 2 trial. Br. J, Cancer 2021, 125, 1226–1232. [Google Scholar] [CrossRef]
- Chaiswing, L.; Weiss, H.L.; Jayswal, R.D.; Clair, D.K.S.; Kyprianou, N. Profiles of Radioresistance Mechanisms in Prostate Cancer. Crit. Rev. Oncog. 2018, 23, 39–67. [Google Scholar] [CrossRef]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Roviello, G.; Catalano, M.; Santi, R.; Santoni, M.; Galli, I.C.; Amorosi, A.; Polom, W.; De Giorgi, U.; Nesi, G. Neoadjuvant Treatment in Muscle-Invasive Bladder Cancer: From the Beginning to the Latest Developments. Front. Oncol. 2022, 12, 912699. [Google Scholar] [CrossRef]
- Horesh, N.; Freund, M.R.; Garoufalia, Z.; Gefen, R.; Nagarajan, A.; Suarez, E.; Emile, S.H.; Wexner, S.D. Total Neoadjuvant Therapy Is a Predictor for Complete Pathological Response in Patients Undergoing Surgery for Rectal Cancer. J. Gastrointest. Surg. 2022, 26, 2579–2584. [Google Scholar] [CrossRef]
- Schulman, C.C.; Debruyne, F.M.; Forster, G.; Selvaggi, F.P.; Zlotta, A.R.; Witjes, W.P. 4-Year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur. Urol. 2000, 38, 706–713. [Google Scholar] [CrossRef]
- Carles, J.; Gallardo, E.; Domenech, M.; Font, A.; Bellmunt, J.; Figols, M.; Mellado, B.; Saez, M.I.; Suarez, C.; Mendez, M.J.; et al. Phase 2 Randomized Study of Radiation Therapy and 3-Year Androgen Deprivation With or Without Concurrent Weekly Docetaxel in High-Risk Localized Prostate Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 344–352. [Google Scholar] [CrossRef]
- Perera, M.B.; Beech, B.B.; De Jesus Escano, M.; Gmelich, C.; Yip, Y.; Boorjan, S.A.; Eastham, J. Neoadjuvant Systemic Therapy Prior to Radical Prostatectomy for Clinically Localized High-Risk Prostate Cancer. Front. Oncol. 2022. [Google Scholar] [CrossRef]
- Eapen, R.S.; Buteau, J.P.; Jackson, P.; Mitchell, C.; Oon, S.F.; Alghazo, O.; McIntosh, L.; Dhiantravan, N.; Scalzo, M.J.; O'Brien, J.; et al. Administering [(177)Lu]Lu-PSMA-617 Prior to Radical Prostatectomy in Men with High-risk Localised Prostate Cancer (LuTectomy): A Single-centre, Single-arm, Phase 1/2 Study. Eur. Urol. 2024, 85, 217–226. [Google Scholar] [CrossRef]
- Golan, S.; Frumer, M.; Zohar, Y.; Rosenbaum, E.; Yakimov, M.; Kedar, D.; Margel, D.; Baniel, J.; Steinmetz, A.P.; Groshar, D.; et al. Neoadjuvant (177)Lu-PSMA-I&T Radionuclide Treatment in Patients with High-risk Prostate Cancer Before Radical Prostatectomy: A Single-arm Phase 1 Trial. Eur. Urol. Oncol. 2023, 6, 151–159. [Google Scholar]
- Muller, C.; van der Meulen, N.P.; Schibli, R. Opportunities and potential challenges of using terbium-161 for targeted radionuclide therapy in clinics. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3181–3184. [Google Scholar] [CrossRef]
- Baum, R.P.; Singh, A.; Kulkarni, H.R.; Bernhardt, P.; Ryden, T.; Schuchardt, C.; Gracheva, N.; Grundler, P.V.; Koster, U.; Muller, D.; et al. First-in-Humans Application of (161)Tb: A Feasibility Study Using (161)Tb-DOTATOC. J. Nucl. Med, 2021, 62, 1391–1397. [Google Scholar] [CrossRef]
- Schaefer-Schuler, A.; Burgard, C.; Blickle, A.; Maus, S.; Petrescu, C.; Petto, S.; Bartholoma, M.; Stemler, T.; Ezziddin, S.; Rosar, F. [(161)Tb]Tb-PSMA-617 radioligand therapy in patients with mCRPC: preliminary dosimetry results and intra-individual head-to-head comparison to [(177)Lu]Lu-PSMA-617. Theranostics 2024, 14, 1829–1840. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Scott, A.M. (161)Tb-PSMA Unleashed: a Promising New Player in the Theranostics of Prostate Cancer. Nucl. Med. Mol, Imaging 2023, 57, 168–171. [Google Scholar] [CrossRef]
- Budeau, J.P.; Kostos, A.; Aliiour, R.; Jackson, P.; McIntouch, L.; Emmerson, P.; Husted, M.B.; et al. VIOLET: A phase I/II trial evaluation of radioligand treatment in men with metastatic castration-resistant prostatecancer with [161Tb]Tb-PSMA I&T. J. Clin. Oncol. 2023, 41 (suppl), TPS281. [Google Scholar]
- Sandhu, S.L.; Josha, A.; Emmett, L.; Crumbaker, M.A.; Bressel, M.; Huynh, R.; Banks, P.; Wallace, R.; Hemd, D.; Interjeeh, A.; et al. LuPARP: Phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPPC). J. Clin. Oncol. 2023; 41 (Suppl). [Google Scholar]

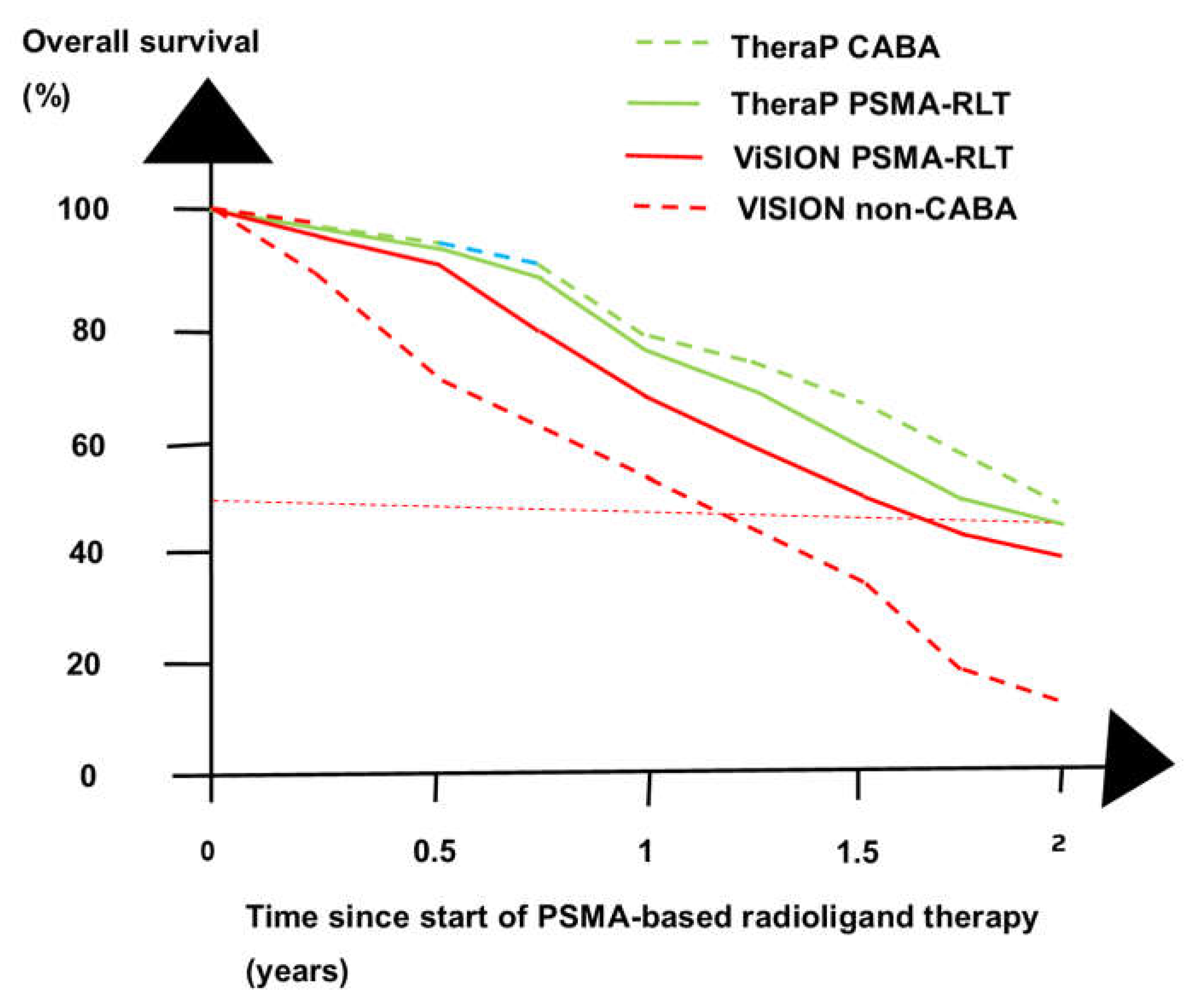
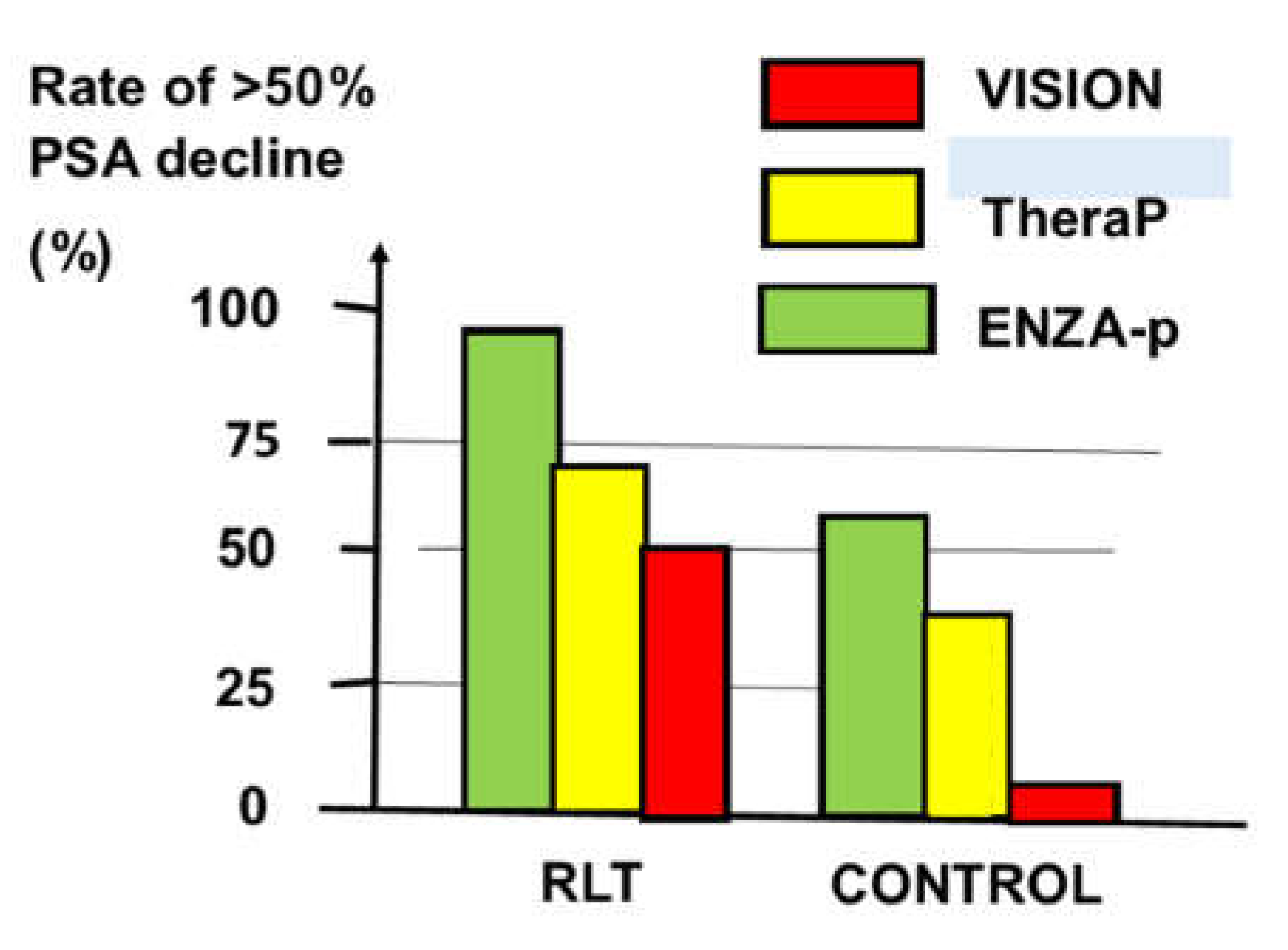
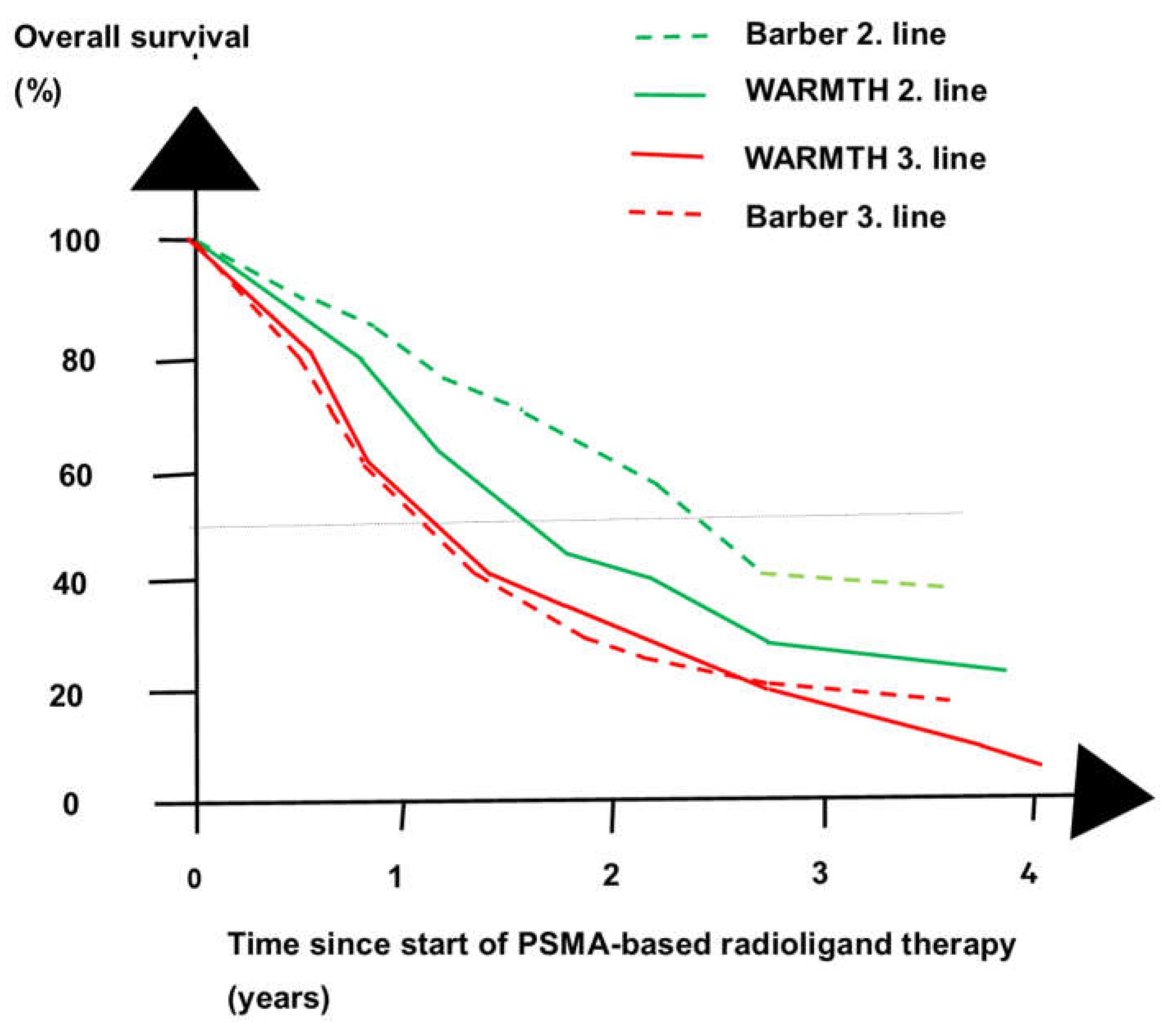
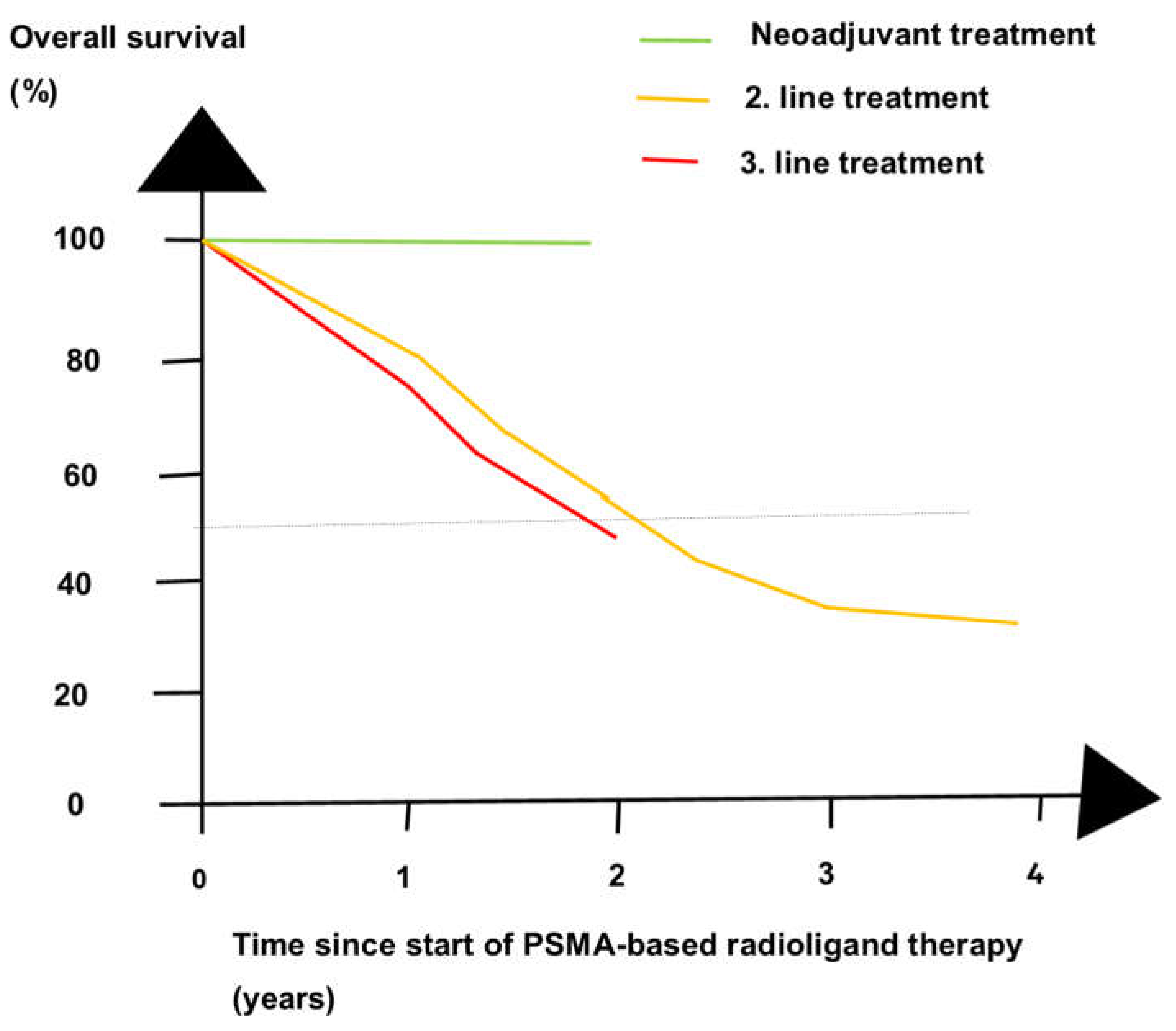
| Phase of Treatment |
Author/NCT Number | Reference | Name of Trial |
Type of RLT | No of Patients |
|---|---|---|---|---|---|
| First-line treatment | Emmett 2024 | [27] | ENZA-p | PSMA-617 | 162 |
| Second-line treatment | Suman 2021 | [25] | NR | PSMA-617 | 58 |
| NCT04689838 | [29] | PSMAfore | PSMA-617 | 467 | |
| Hansen 2022 | [31] | SPLASH | PSMA I&T | 412 | |
| Satapathy 2023 | [28] | NR | 40 | ||
| Overall total | 1139 |
| Trial | Reference | Radiological Progression Free Survival (Months) |
Hazard Ratio | Single p Value | Combined p Value |
|---|---|---|---|---|---|
| PSMAfore | [29] | 12 vs. 5.6 | 0.41 | <0.0001 | |
| Suman | [25] | >18 vs. 7 | NR | <0.0005 | |
| SPLASH | [31] | 9.5 vs. 6 | 0.71 | 0.0088 | |
| Combined | 6 x 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





