Submitted:
31 May 2024
Posted:
04 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mechanisms of Ghrelin/GHSR System in Depressive Disorder
2.1. Links between Monoamine Neurotransmitter Receptors and Ghrelin
2.2. The Ghrelin/GHSR System Mediates the Inflammatory Response to Depression
2.3. The Ghrelin/GHSR System Promotes Neurogenesis in Depression
2.4. Regulation of Astrocyte Physiology by the Ghrelin/GHSR System
2.5. The Role of the Ghrelin/GHSR System in Endocrine Disruption in Depression
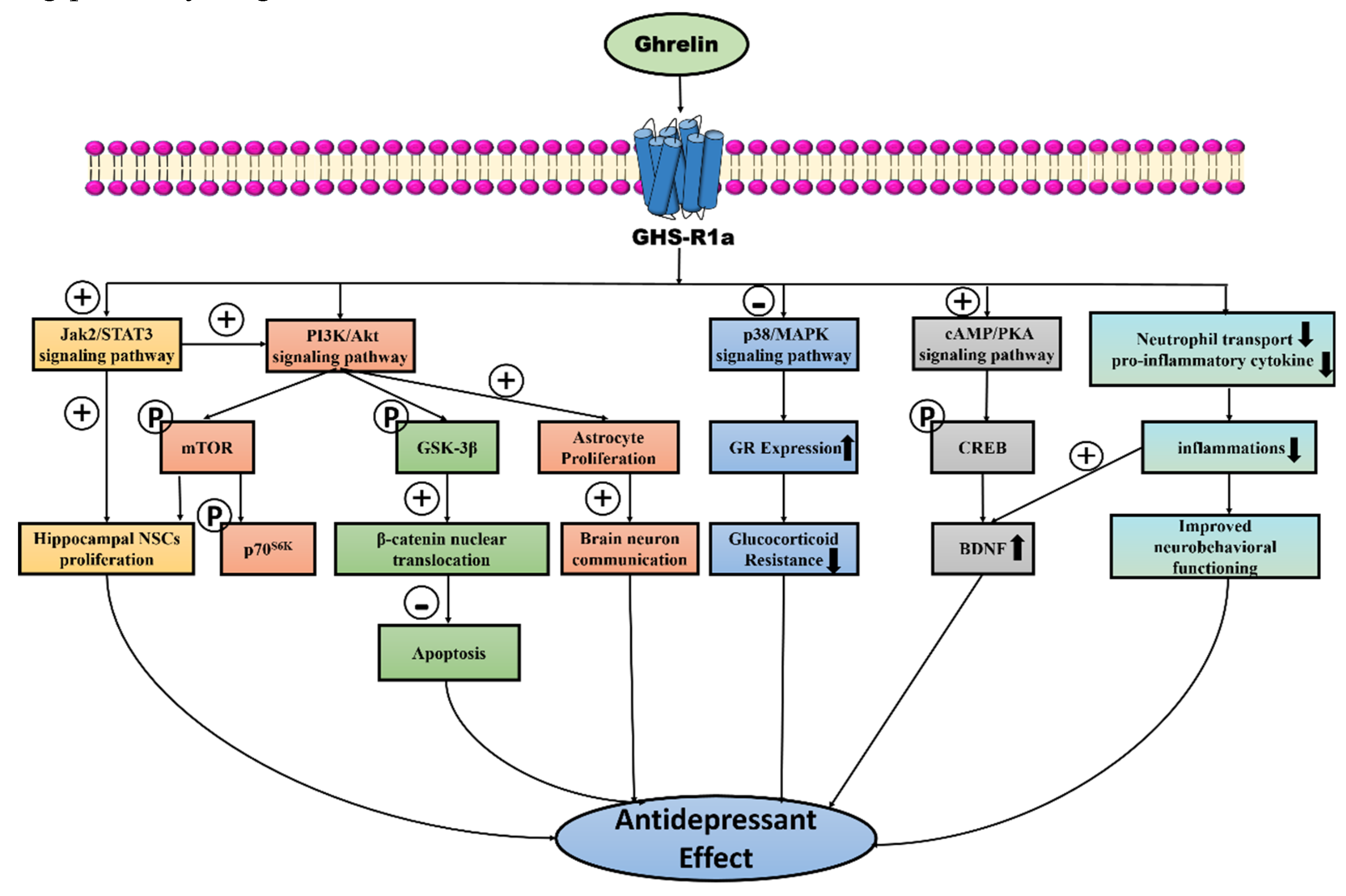
3. Signalling Pathways Induced by Ghrelin/GHSR1a System in Depression
3.1. cAMP-CREB-BDNF Signaling Pathway
3.2. p38-MAPK Signaling Pathway
3.3. PI3K/Akt Signaling Pathway
3.4. Jak2-STAT3 Signaling Pathway
4. Ghrelin/GHSR as a Therapeutic Target for Depressive Disorder
5. Conclusions and Future Direction
Author Contributions
Disclosure Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- A.D. Howard, S.D. Feighner, D.F. Cully, J.P. Arena, P.A. Liberator, C.I. Rosenblum, M. Hamelin, D.L. Hreniuk, O.C. Palyha, J. Anderson, P.S. Paress, C. Diaz, M. Chou, K.K. Liu, K.K. McKee, S.S. Pong, L.Y. Chaung, A. Elbrecht, M. Dashkevicz, R. Heavens, M. Rigby, D.J. Sirinathsinghji, D.C. Dean, D.G. Melillo, A.A. Patchett, R. Nargund, P.R. Griffin, J.A. DeMartino, S.K. Gupta, J.M. Schaeffer, R.G. Smith, L.H. Van der Ploeg, A receptor in pituitary and hypothalamus that functions in growth hormone release, Science, 273 (1996) 974-977. [CrossRef]
- Kojima, M.; Kangawa, K. Ghrelin: Structure and Function. Physiological Reviews 2005, 85, 495-522. [CrossRef]
- Albarrán-Zeckler, R.G.; Smith, R.G. The Ghrelin Receptors (GHS-R1a and GHS-R1b). In The Ghrelin System, 2013; pp. 5-15. [CrossRef]
- Sun, Y.; Asnicar, M.; Smith, R.G. Central and Peripheral Roles of Ghrelin on Glucose Homeostasis. Neuroendocrinology 2007, 86, 215-228. [CrossRef]
- Gortan Cappellari, G.; Barazzoni, R. Ghrelin forms in the modulation of energy balance and metabolism. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity 2018, 24, 997-1013. [CrossRef]
- Vinci, M.C.; Fujimura, K.; Wakino, S.; Minakuchi, H.; Hasegawa, K.; Hosoya, K.; Komatsu, M.; Kaneko, Y.; Shinozuka, K.; Washida, N.; et al. Ghrelin Protects against Renal Damages Induced by Angiotensin-II via an Antioxidative Stress Mechanism in Mice. PLoS ONE 2014, 9. [CrossRef]
- Laviano, A.; Molfino, A.; Rianda, S.; Rossi Fanelli, F. The growth hormone secretagogue receptor (Ghs-R). Curr Pharm Des 2012, 18, 4749-4754. [CrossRef]
- Leung, P.-K.; Chow, K.B.S.; Lau, P.-N.; Chu, K.-M.; Chan, C.-B.; Cheng, C.H.K.; Wise, H. The truncated ghrelin receptor polypeptide (GHS-R1b) acts as a dominant-negative mutant of the ghrelin receptor. Cellular Signalling 2007, 19, 1011-1022. [CrossRef]
- Chan, C.-B.; Leung, P.-K.; Wise, H.; Cheng, C.H.K. Signal transduction mechanism of the seabream growth hormone secretagogue receptor. FEBS Letters 2004, 577, 147-153. [CrossRef]
- Ting-Ting, T.; Ming-Xia, B.; Mei-Ning, D.; Xiao-Yi, Z.; Ling, C.; Xue, X.; Qian, J.; Xi, C.; Chun-Ling, Y.; Xi-Xun, D.; et al. Quinpirole ameliorates nigral dopaminergic neuron damage in Parkinson's disease mouse model through activating GHS-R1a/D(2)R heterodimers. Acta Pharmacol Sin 2023, 44. [CrossRef]
- Xiaoli, L.; Xia, L.; Xinyou, Z.; Huaiyu, Y.; Lijun, S.; Minmin, H.; Xiaoqi, C.; Mingxuan, Z.; Katrina, W.-G.; Tiantian, J.; et al. Olanzapine attenuates 5-HT2cR and GHSR1a interaction to increase orexigenic hypothalamic NPY: Implications for neuronal molecular mechanism of metabolic side effects of antipsychotics. Behav Brain Res 2024, 463. [CrossRef]
- Xue, Q.; Bai, B.; Ji, B.; Chen, X.; Wang, C.; Wang, P.; Yang, C.; Zhang, R.; Jiang, Y.; Pan, Y.; et al. Ghrelin Through GHSR1a and OX1R Heterodimers Reveals a Gαs-cAMP-cAMP Response Element Binding Protein Signaling Pathway in Vitro. Front Mol Neurosci 2018, 11, 245. [CrossRef]
- Baxter, L.C. Appetite Changes in Depression. American Journal of Psychiatry 2016, 173, 317-318. [CrossRef]
- Crouse, J.J.; Carpenter, J.S.; Song, Y.J.C.; Hockey, S.J.; Naismith, S.L.; Grunstein, R.R.; Scott, E.M.; Merikangas, K.R.; Scott, J.; Hickie, I.B. Circadian rhythm sleep–wake disturbances and depression in young people: implications for prevention and early intervention. The Lancet Psychiatry 2021, 8, 813-823. [CrossRef]
- Rakel, R.E. Depression. Prim Care 1999, 26, 211-224. [CrossRef]
- Duman, R.S.; Voleti, B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends in Neurosciences 2012, 35, 47-56. [CrossRef]
- Hauenstein, E.J. Depression in adolescence. J Obstet Gynecol Neonatal Nurs 2003, 32, 239-248. [CrossRef]
- Alexopoulos, G.S. Depression in the elderly. The Lancet 2005, 365, 1961-1970. [CrossRef]
- Park, C.; Rosenblat, J.D.; Brietzke, E.; Pan, Z.; Lee, Y.; Cao, B.; Zuckerman, H.; Kalantarova, A.; McIntyre, R.S. Stress, epigenetics and depression: A systematic review. Neuroscience & Biobehavioral Reviews 2019, 102, 139-152. [CrossRef]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199-229. [CrossRef]
- MacQueen, G.; Frodl, T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Molecular Psychiatry 2010, 16, 252-264. [CrossRef]
- Salvat-Pujol, N.; Labad, J.; Urretavizcaya, M.; de Arriba-Arnau, A.; Segalàs, C.; Real, E.; Ferrer, A.; Crespo, J.M.; Jiménez-Murcia, S.; Soriano-Mas, C.; et al. Hypothalamic-pituitary-adrenal axis activity and cognition in major depression: The role of remission status. Psychoneuroendocrinology 2017, 76, 38-48. [CrossRef]
- Nierenberg, A.A. Current perspectives on the diagnosis and treatment of major depressive disorder. Am J Manag Care 2001, 7, S353-366.
- Pruckner, N.; Holthoff-Detto, V. Antidepressant pharmacotherapy in old-age depression—a review and clinical approach. European Journal of Clinical Pharmacology 2017, 73, 661-667. [CrossRef]
- Gonda, X.; Dome, P.; Neill, J.C.; Tarazi, F.I. Novel antidepressant drugs: Beyond monoamine targets. CNS Spectrums 2021, 28, 6-15. [CrossRef]
- Hashimoto, K.; Naudet, F.; Maria, A.S.; Falissard, B. Antidepressant Response in Major Depressive Disorder: A Meta-Regression Comparison of Randomized Controlled Trials and Observational Studies. PLoS ONE 2011, 6. [CrossRef]
- Li, Y.H.; Qing-Xiu, L.; Wang, J.S.; Xiang, H.; Zhang, R.F.; Huang, C.Q. Ghrelin improves cognition via activation of the cAMP- CREB signalling pathway in depressed male C57BL/6J mice. Int J Neurosci 2023, 133, 1233-1241. [CrossRef]
- Bianconi, S.; Poretti, M.B.; Rodríguez, P.; Maestri, G.; Rodríguez, P.E.; de Barioglio, S.R.; Schiöth, H.B.; Carlini, V.P. Ghrelin restores memory impairment following olfactory bulbectomy in mice by activating hippocampal NMDA1 and MAPK1 gene expression. Behav Brain Res 2021, 410, 113341. [CrossRef]
- Guo, L.; Niu, M.; Yang, J.; Li, L.; Liu, S.; Sun, Y.; Zhou, Z.; Zhou, Y. GHS-R1a Deficiency Alleviates Depression-Related Behaviors After Chronic Social Defeat Stress. Front Neurosci 2019, 13, 364. [CrossRef]
- Hirschfeld, R.M. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry 2000, 61 Suppl 6, 4-6.
- Jiang, H.; Betancourt, L.; Smith, R.G. Ghrelin Amplifies Dopamine Signaling by Cross Talk Involving Formation of Growth Hormone Secretagogue Receptor/Dopamine Receptor Subtype 1 Heterodimers. Molecular Endocrinology 2006, 20, 1772-1785. [CrossRef]
- Hansson, C.; Alvarez-Crespo, M.; Taube, M.; Skibicka, K.P.; Schmidt, L.; Karlsson-Lindahl, L.; Egecioglu, E.; Nissbrandt, H.; Dickson, S.L. Influence of ghrelin on the central serotonergic signaling system in mice. Neuropharmacology 2014, 79, 498-505. [CrossRef]
- Sakata, I.; Gong, Z.; Ikenoya, C.; Takemi, S.; Sakai, T. The study of ghrelin secretion and acyl-modification using mice and ghrelinoma cell lines. Endocr J 2017, 64, S27-s29. [CrossRef]
- Zhao, T.J.; Sakata, I.; Li, R.L.; Liang, G.; Richardson, J.A.; Brown, M.S.; Goldstein, J.L.; Zigman, J.M. Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci U S A 2010, 107, 15868-15873. [CrossRef]
- Granado, M.; Priego, T.; Martín, A.I.; Villanúa, M.Á.; López-Calderón, A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. American Journal of Physiology-Endocrinology and Metabolism 2005, 288, E486-E492. [CrossRef]
- Schiepers, O.J.G.; Wichers, M.C.; Maes, M. Cytokines and major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2005, 29, 201-217. [CrossRef]
- Hodes, G.E.; Ménard, C.; Russo, S.J. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiology of Stress 2016, 4, 15-22. [CrossRef]
- Karmiris, K.; Koutroubakis, I.E.; Xidakis, C.; Polychronaki, M.; Voudouri, T.; Kouroumalis, E.A. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis 2006, 12, 100-105. [CrossRef]
- Kerem, M.; Bedirli, A.; Pasaoglu, H.; Unsal, C.; Yilmaz, T.U.; Ofluoglu, E.; Sahin, T.T. Role of Ghrelin and Leptin in Predicting the Severity of Acute Pancreatitis. Digestive Diseases and Sciences 2007, 52, 950-955. [CrossRef]
- Zhao, D.; Zhan, Y.; Zeng, H.; Moyer, M.P.; Mantzoros, C.S.; Pothoulakis, C. Ghrelin stimulates interleukin-8 gene expression through protein kinase C-mediated NF-κB pathway in human colonic epithelial cells. Journal of Cellular Biochemistry 2005, 97, 1317-1327. [CrossRef]
- Otero, M. Chronic inflammation modulates ghrelin levels in humans and rats. Rheumatology 2003, 43, 306-310. [CrossRef]
- Cheyuo, C.; Wu, R.; Zhou, M.; Jacob, A.; Coppa, G.; Wang, P. Ghrelin Suppresses Inflammation and Neuronal Nitric Oxide Synthase in Focal Cerebral Ischemia Via the Vagus Nerve. Shock 2011, 35, 258-265. [CrossRef]
- Waseem, T.; Duxbury, M.; Ito, H.; Ashley, S.W.; Robinson, M.K. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 2008, 143, 334-342. [CrossRef]
- Dixit, V.D.; Schaffer, E.M.; Pyle, R.S.; Collins, G.D.; Sakthivel, S.K.; Palaniappan, R.; Lillard, J.W.; Taub, D.D. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. Journal of Clinical Investigation 2004, 114, 57-66. [CrossRef]
- Lin, L.; Lee, J.H.; Buras, E.D.; Yu, K.; Wang, R.; Smith, C.W.; Wu, H.; Sheikh-Hamad, D.; Sun, Y. Ghrelin receptor regulates adipose tissue inflammation in aging. Aging (Albany NY) 2016, 8, 178-191. [CrossRef]
- Guo, L.; Niu, M.; Yang, J.; Li, L.; Liu, S.; Sun, Y.; Zhou, Z.; Zhou, Y. GHS-R1a Deficiency Alleviates Depression-Related Behaviors After Chronic Social Defeat Stress. Frontiers in Neuroscience 2019, 13. [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894-902. [CrossRef]
- Schmidt, H.D.; Shelton, R.C.; Duman, R.S. Functional Biomarkers of Depression: Diagnosis, Treatment, and Pathophysiology. Neuropsychopharmacology 2011, 36, 2375-2394. [CrossRef]
- Patas, K.; Penninx, B.W.J.H.; Bus, B.A.A.; Vogelzangs, N.; Molendijk, M.L.; Elzinga, B.M.; Bosker, F.J.; Oude Voshaar, R.C. Association between serum brain-derived neurotrophic factor and plasma interleukin-6 in major depressive disorder with melancholic features. Brain, Behavior, and Immunity 2014, 36, 71-79. [CrossRef]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000, 47, 351-354. [CrossRef]
- Zarate, C.A., Jr.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006, 63, 856-864. [CrossRef]
- Li, N.; Lee, B.; Liu, R.-J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.-Y.; Aghajanian, G.; Duman, R.S. mTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science 2010, 329, 959-964. [CrossRef]
- Park, S.; Kim, H.; Lee, D.; Ju, S.; Seo, S.; Lee, D.H.; Kim, E.; Chung, H. Ghrelin Inhibits Apoptosis in Hypothalamic Neuronal Cells during Oxygen-Glucose Deprivation. Endocrinology 2007, 148, 148-159. [CrossRef]
- Lee, J.Y.; Chung, H.; Yoo, Y.S.; Oh, Y.J.; Oh, T.H.; Park, S.; Yune, T.Y. Inhibition of Apoptotic Cell Death by Ghrelin Improves Functional Recovery after Spinal Cord Injury. Endocrinology 2010, 151, 3815-3826. [CrossRef]
- Li, E.; Chung, H.; Kim, Y.; Kim, D.H.; Ryu, J.H.; Sato, T.; Kojima, M.; Park, S. Ghrelin directly stimulates adult hippocampal neurogenesis: implications for learning and memory. Endocrine Journal 2013, 60, 781-789. [CrossRef]
- Walker, A.K.; Rivera, P.D.; Wang, Q.; Chuang, J.C.; Tran, S.; Osborne-Lawrence, S.; Estill, S.J.; Starwalt, R.; Huntington, P.; Morlock, L.; et al. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Molecular Psychiatry 2014, 20, 500-508. [CrossRef]
- Chuang, J.-C.; Perello, M.; Sakata, I.; Osborne-Lawrence, S.; Savitt, J.M.; Lutter, M.; Zigman, J.M. Ghrelin mediates stress-induced food-reward behavior in mice. Journal of Clinical Investigation 2011, 121, 2684-2692. [CrossRef]
- Cuellar, J.N.; Isokawa, M. Ghrelin-induced activation of cAMP signal transduction and its negative regulation by endocannabinoids in the hippocampus. Neuropharmacology 2011, 60, 842-851. [CrossRef]
- Diano, S.; Farr, S.A.; Benoit, S.C.; McNay, E.C.; da Silva, I.; Horvath, B.; Gaskin, F.S.; Nonaka, N.; Jaeger, L.B.; Banks, W.A.; et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nature Neuroscience 2006, 9, 381-388. [CrossRef]
- Bayliss, J.A.; Andrews, Z.B. Ghrelin is neuroprotective in Parkinson’s disease: molecular mechanisms of metabolic neuroprotection. Therapeutic Advances in Endocrinology and Metabolism 2013, 4, 25-36. [CrossRef]
- Lee, J.; Lim, E.; Kim, Y.; Li, E.; Park, S. Ghrelin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Journal of Endocrinology 2010, 205, 263-270. [CrossRef]
- Fornaro, M.; Escelsior, A.; Rocchi, G.; Conio, B.; Magioncalda, P.; Marozzi, V.; Presta, A.; Sterlini, B.; Contini, P.; Amore, M.; et al. BDNF plasma levels variations in major depressed patients receiving duloxetine. Neurological Sciences 2014, 36, 729-734. [CrossRef]
- Banasr, M.; Duman, R.S. Glial Loss in the Prefrontal Cortex Is Sufficient to Induce Depressive-like Behaviors. Biological Psychiatry 2008, 64, 863-870. [CrossRef]
- Cotter, D.; Mackay, D.; Landau, S.; Kerwin, R.; Everall, I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 2001, 58, 545-553. [CrossRef]
- Czéh, B.; Simon, M.; Schmelting, B.; Hiemke, C.; Fuchs, E. Astroglial Plasticity in the Hippocampus is Affected by Chronic Psychosocial Stress and Concomitant Fluoxetine Treatment. Neuropsychopharmacology 2005, 31, 1616-1626. [CrossRef]
- Choudary, P.V.; Molnar, M.; Evans, S.J.; Tomita, H.; Li, J.Z.; Vawter, M.P.; Myers, R.M.; Bunney, W.E., Jr.; Akil, H.; Watson, S.J.; et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A 2005, 102, 15653-15658. [CrossRef]
- Banasr, M.; Chowdhury, G.M.I.; Terwilliger, R.; Newton, S.S.; Duman, R.S.; Behar, K.L.; Sanacora, G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Molecular Psychiatry 2008, 15, 501-511. [CrossRef]
- Volterra, A.; Meldolesi, J. Astrocytes, from brain glue to communication elements: the revolution continues. Nature Reviews Neuroscience 2005, 6, 626-640. [CrossRef]
- Murphy, S.; Pearce, B. Functional receptors for neurotransmitters on astroglial cells. Neuroscience 1987, 22, 381-394. [CrossRef]
- Porter, J.T.; McCarthy, K.D. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol 1997, 51, 439-455. [CrossRef]
- Rose, C.R.; Blum, R.; Pichler, B.; Lepier, A.; Kafitz, K.W.; Konnerth, A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature 2003, 426, 74-78. [CrossRef]
- Zhang, J.M.; Wang, H.K.; Ye, C.Q.; Ge, W.; Chen, Y.; Jiang, Z.L.; Wu, C.P.; Poo, M.M.; Duan, S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 2003, 40, 971-982. [CrossRef]
- Matyash, V.; Filippov, V.; Mohrhagen, K.; Kettenmann, H. Nitric Oxide Signals Parallel Fiber Activity to Bergmann Glial Cells in the Mouse Cerebellar Slice. Molecular and Cellular Neuroscience 2001, 18, 664-670. [CrossRef]
- Araque, A.; Martín, E.D.; Perea, G.; Arellano, J.I.; Buño, W. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci 2002, 22, 2443-2450. [CrossRef]
- Bezzi, P.; Volterra, A. A neuron-glia signalling network in the active brain. Curr Opin Neurobiol 2001, 11, 387-394. [CrossRef]
- Halassa, M.M.; Haydon, P.G. Integrated Brain Circuits: Astrocytic Networks Modulate Neuronal Activity and Behavior. Annual Review of Physiology 2010, 72, 335-355. [CrossRef]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 1999, 22, 208-215. [CrossRef]
- Fuente-Martín, E.; García-Cáceres, C.; Argente-Arizón, P.; Díaz, F.; Granado, M.; Freire-Regatillo, A.; Castro-González, D.; Ceballos, M.L.; Frago, L.M.; Dickson, S.L.; et al. Ghrelin Regulates Glucose and Glutamate Transporters in Hypothalamic Astrocytes. Scientific Reports 2016, 6. [CrossRef]
- Baquedano, E.; Chowen, J.A.; Argente, J.; Frago, L.M. Differential effects of GH and GH-releasing peptide-6 on astrocytes. Journal of Endocrinology 2013, 218, 263-274. [CrossRef]
- Dixit, V.D.; Weeraratna, A.T.; Yang, H.; Bertak, D.; Cooper-Jenkins, A.; Riggins, G.J.; Eberhart, C.G.; Taub, D.D. Ghrelin and the Growth Hormone Secretagogue Receptor Constitute a Novel Autocrine Pathway in Astrocytoma Motility. Journal of Biological Chemistry 2006, 281, 16681-16690. [CrossRef]
- Fischer, R.; Maier, O. Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxidative Medicine and Cellular Longevity 2015, 2015, 1-18. [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences 2008, 31, 464-468. [CrossRef]
- Vandoolaeghe, E.; Maes, M.; Vandevyvere, J.; Neels, H. Hypothalamic-pituitary-thyroid-axis function in treatment resistant depression. J Affect Disord 1997, 43, 143-150. [CrossRef]
- Bartalena, L.; Placidi, G.F.; Martino, E.; Falcone, M.; Pellegrini, L.; Dell'Osso, L.; Pacchiarotti, A.; Pinchera, A. Nocturnal serum thyrotropin (TSH) surge and the TSH response to TSH-releasing hormone: dissociated behavior in untreated depressives. J Clin Endocrinol Metab 1990, 71, 650-655. [CrossRef]
- Fischer, S.; Ehlert, U.; Amiel Castro, R. Hormones of the hypothalamic-pituitary-gonadal (HPG) axis in male depressive disorders – A systematic review and meta-analysis. Frontiers in Neuroendocrinology 2019, 55. [CrossRef]
- Han, Y.; Gu, S.; Li, Y.; Qian, X.; Wang, F.; Huang, J.H. Neuroendocrine pathogenesis of perimenopausal depression. Frontiers in Psychiatry 2023, 14. [CrossRef]
- Kluge, M.; Schmidt, D.; Uhr, M.; Steiger, A. Ghrelin suppresses nocturnal secretion of luteinizing hormone (LH) and thyroid stimulating hormone (TSH) in patients with major depression. Journal of Psychiatric Research 2013, 47, 1236-1239. [CrossRef]
- Kluge, M.; Riedl, S.; Uhr, M.; Schmidt, D.; Zhang, X.; Yassouridis, A.; Steiger, A. Ghrelin affects the hypothalamus–pituitary–thyroid axis in humans by increasing free thyroxine and decreasing TSH in plasma. European Journal of Endocrinology 2010, 162, 1059-1065. [CrossRef]
- Gupta, D.; Chuang, J.-C.; Mani, B.K.; Shankar, K.; Rodriguez, J.A.; Osborne-Lawrence, S.; Metzger, N.P.; Zigman, J.M. β1-adrenergic receptors mediate plasma acyl-ghrelin elevation and depressive-like behavior induced by chronic psychosocial stress. Neuropsychopharmacology 2019, 44, 1319-1327. [CrossRef]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nature Reviews Neurology 2009, 5, 311-322. [CrossRef]
- Keifer, J. Regulation of AMPAR trafficking in synaptic plasticity by BDNF and the impact of neurodegenerative disease. J Neurosci Res 2022, 100, 979-991. [CrossRef]
- Zhang, K.; Wang, F.; Zhai, M.; He, M.; Hu, Y.; Feng, L.; Li, Y.; Yang, J.; Wu, C. Hyperactive neuronal autophagy depletes BDNF and impairs adult hippocampal neurogenesis in a corticosterone-induced mouse model of depression. Theranostics 2023, 13, 1059-1075. [CrossRef]
- Liu, S.; Tao, G.; Zhou, C.; Wang, Q.; Wang, W.; Fei, X. Ketamine inhibits neuronal differentiation by regulating brain-derived neurotrophic factor (BDNF) signaling. Toxicol In Vitro 2021, 72, 105091. [CrossRef]
- Perea Vega, M.L.; Sanchez, M.S.; Fernández, G.; Paglini, M.G.; Martin, M.; de Barioglio, S.R. Ghrelin treatment leads to dendritic spine remodeling in hippocampal neurons and increases the expression of specific BDNF-mRNA species. Neurobiology of Learning and Memory 2021, 179. [CrossRef]
- Baj, G.; Leone, E.; Chao, M.V.; Tongiorgi, E. Spatial segregation of BDNF transcripts enables BDNF to differentially shape distinct dendritic compartments. Proc Natl Acad Sci U S A 2011, 108, 16813-16818. [CrossRef]
- Aid, T.; Kazantseva, A.; Piirsoo, M.; Palm, K.; Timmusk, T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res 2007, 85, 525-535. [CrossRef]
- Duman, R.S.; Heninger, G.R.; Nestler, E.J. A molecular and cellular theory of depression. Arch Gen Psychiatry 1997, 54, 597-606. [CrossRef]
- Banerjee, R.; Ghosh, A.K.; Ghosh, B.; Bhattacharyya, S.; Mondal, A.C. Decreased mRNA and Protein Expression of BDNF, NGF, and their Receptors in the Hippocampus from Suicide: An Analysis in Human Postmortem Brain. Clin Med Insights Pathol 2013, 6, 1-11. [CrossRef]
- Paska, A.V.; Zupanc, T.; Pregelj, P. The role of brain-derived neurotrophic factor in the pathophysiology of suicidal behavior. Psychiatr Danub 2013, 25 Suppl 2, S341-344.
- Dowlatshahi, D.; MacQueen, G.M.; Wang, J.F.; Young, L.T. Increased temporal cortex CREB concentrations and antidepressant treatment in major depression. Lancet 1998, 352, 1754-1755. [CrossRef]
- Nakagawa, S.; Kim, J.E.; Lee, R.; Malberg, J.E.; Chen, J.; Steffen, C.; Zhang, Y.J.; Nestler, E.J.; Duman, R.S. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci 2002, 22, 3673-3682. [CrossRef]
- Lee, B.H.; Kim, Y.K. BDNF mRNA expression of peripheral blood mononuclear cells was decreased in depressive patients who had or had not recently attempted suicide. J Affect Disord 2010, 125, 369-373. [CrossRef]
- Fan, J.; Li, B.J.; Wang, X.F.; Zhong, L.L.; Cui, R.J. Ghrelin produces antidepressant-like effect in the estrogen deficient mice. Oncotarget 2017, 8, 58964-58973. [CrossRef]
- Lizama, C.; Lagos, C.F.; Lagos-Cabré, R.; Cantuarias, L.; Rivera, F.; Huenchuñir, P.; Pérez-Acle, T.; Carrión, F.; Moreno, R.D. Calpain inhibitors prevent p38 MAPK activation and germ cell apoptosis after heat stress in pubertal rat testes. J Cell Physiol 2009, 221, 296-305. [CrossRef]
- Peng, Z.; Wang, H.; Zhang, R.; Chen, Y.; Xue, F.; Nie, H.; Chen, Y.; Wu, D.; Wang, Y.; Wang, H.; et al. Gastrodin ameliorates anxiety-like behaviors and inhibits IL-1beta level and p38 MAPK phosphorylation of hippocampus in the rat model of posttraumatic stress disorder. Physiol Res 2013, 62, 537-545. [CrossRef]
- Zoga, M.; Oulis, P.; Chatzipanagiotou, S.; Masdrakis, V.G.; Pliatsika, P.; Boufidou, F.; Foteli, S.; Soldatos, C.R.; Nikolaou, C.; Papageorgiou, C. Indoleamine 2,3-dioxygenase and immune changes under antidepressive treatment in major depression in females. In Vivo 2014, 28, 633-638.
- Zhou, W.; Dantzer, R.; Budac, D.P.; Walker, A.K.; Mao-Ying, Q.L.; Lee, A.W.; Heijnen, C.J.; Kavelaars, A. Peripheral indoleamine 2,3-dioxygenase 1 is required for comorbid depression-like behavior but does not contribute to neuropathic pain in mice. Brain Behav Immun 2015, 46, 147-153. [CrossRef]
- Cheng, Y.; Qiao, Z.; Dang, C.; Zhou, B.; Li, S.; Zhang, W.; Jiang, J.; Song, Y.; Zhang, J.; Diao, D. p38 predicts depression and poor outcome in esophageal cancer. Oncol Lett 2017, 14, 7241-7249. [CrossRef]
- Irusen, E.; Matthews, J.G.; Takahashi, A.; Barnes, P.J.; Chung, K.F.; Adcock, I.M. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol 2002, 109, 649-657. [CrossRef]
- Holsboer, F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000, 23, 477-501. [CrossRef]
- Liu, C.; Huang, J.; Li, H.; Yang, Z.; Zeng, Y.; Liu, J.; Hao, Y.; Li, R. Ghrelin accelerates wound healing through GHS-R1a-mediated MAPK-NF-κB/GR signaling pathways in combined radiation and burn injury in rats. Sci Rep 2016, 6, 27499. [CrossRef]
- Han, Q.Q.; Huang, H.J.; Wang, Y.L.; Yang, L.; Pilot, A.; Zhu, X.C.; Yu, R.; Wang, J.; Chen, X.R.; Liu, Q.; et al. Ghrelin exhibited antidepressant and anxiolytic effect via the p38-MAPK signaling pathway in hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 2019, 93, 11-20. [CrossRef]
- Bruchas, M.R.; Schindler, A.G.; Shankar, H.; Messinger, D.I.; Miyatake, M.; Land, B.B.; Lemos, J.C.; Hagan, C.E.; Neumaier, J.F.; Quintana, A.; et al. Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 2011, 71, 498-511. [CrossRef]
- Schoenfeld, T.J.; Cameron, H.A. Adult neurogenesis and mental illness. Neuropsychopharmacology 2015, 40, 113-128. [CrossRef]
- Peltier, J.; O'Neill, A.; Schaffer, D.V. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol 2007, 67, 1348-1361. [CrossRef]
- Doble, B.W.; Woodgett, J.R. GSK-3: tricks of the trade for a multi-tasking kinase. Journal of Cell Science 2003, 116, 1175-1186. [CrossRef]
- Hart, M.J.; de los Santos, R.; Albert, I.N.; Rubinfeld, B.; Polakis, P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol 1998, 8, 573-581. [CrossRef]
- Ryu, J.K.; Choi, H.B.; Hatori, K.; Heisel, R.L.; Pelech, S.L.; McLarnon, J.G.; Kim, S.U. Adenosine triphosphate induces proliferation of human neural stem cells: Role of calcium and p70 ribosomal protein S6 kinase. J Neurosci Res 2003, 72, 352-362. [CrossRef]
- Chung, H.; Li, E.; Kim, Y.; Kim, S.; Park, S. Multiple signaling pathways mediate ghrelin-induced proliferation of hippocampal neural stem cells. Journal of Endocrinology 2013, 218, 49-59. [CrossRef]
- Chung, H.; Seo, S.; Moon, M.; Park, S. Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3β and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen–glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. Journal of Endocrinology 2008, 198, 511-521. [CrossRef]
- Ferreira-Marques, M.; Carvalho, A.; Cavadas, C.; Aveleira, C.A. PI3K/AKT/MTOR and ERK1/2-MAPK signaling pathways are involved in autophagy stimulation induced by caloric restriction or caloric restriction mimetics in cortical neurons. Aging (Albany NY) 2021, 13, 7872-7882. [CrossRef]
- Hou, Y.; Wang, K.; Wan, W.; Cheng, Y.; Pu, X.; Ye, X. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis 2018, 5, 245-255. [CrossRef]
- Zhu, H.; Jian, Z.; Zhong, Y.; Ye, Y.; Zhang, Y.; Hu, X.; Pu, B.; Gu, L.; Xiong, X. Janus Kinase Inhibition Ameliorates Ischemic Stroke Injury and Neuroinflammation Through Reducing NLRP3 Inflammasome Activation via JAK2/STAT3 Pathway Inhibition. Front Immunol 2021, 12, 714943. [CrossRef]
- Zhang, W.; Xu, M.; Chen, F.; Su, Y.; Yu, M.; Xing, L.; Chang, Y.; Yan, T. Targeting the JAK2-STAT3 pathway to inhibit cGAS-STING activation improves neuronal senescence after ischemic stroke. Exp Neurol 2023, 368, 114474. [CrossRef]
- Ring, R.H.; Malberg, J.E.; Potestio, L.; Ping, J.; Boikess, S.; Luo, B.; Schechter, L.E.; Rizzo, S.; Rahman, Z.; Rosenzweig-Lipson, S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006, 185, 218-225. [CrossRef]
- Weissman, M.M.; Klerman, G.L. Sex differences and the epidemiology of depression. Arch Gen Psychiatry 1977, 34, 98-111. [CrossRef]
- Bleickardt, C.J.; Mullins, D.E.; Macsweeney, C.P.; Werner, B.J.; Pond, A.J.; Guzzi, M.F.; Martin, F.D.; Varty, G.B.; Hodgson, R.A. Characterization of the V1a antagonist, JNJ-17308616, in rodent models of anxiety-like behavior. Psychopharmacology (Berl) 2009, 202, 711-718. [CrossRef]
- Serradeil-Le Gal, C.; Wagnon, J., 3rd; Tonnerre, B.; Roux, R.; Garcia, G.; Griebel, G.; Aulombard, A. An overview of SSR149415, a selective nonpeptide vasopressin V(1b) receptor antagonist for the treatment of stress-related disorders. CNS Drug Rev 2005, 11, 53-68. [CrossRef]
- Lutter, M.; Sakata, I.; Osborne-Lawrence, S.; Rovinsky, S.A.; Anderson, J.G.; Jung, S.; Birnbaum, S.; Yanagisawa, M.; Elmquist, J.K.; Nestler, E.J.; et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 2008, 11, 752-753. [CrossRef]
- Zhang, Y.; Zhu, M.Z.; Qin, X.H.; Zeng, Y.N.; Zhu, X.H. The Ghrelin/Growth Hormone Secretagogue Receptor System Is Involved in the Rapid and Sustained Antidepressant-Like Effect of Paeoniflorin. Front Neurosci 2021, 15, 631424. [CrossRef]
- Wu, R.; Xiao, D.; Shan, X.; Dong, Y.; Tao, W.W. Rapid and Prolonged Antidepressant-like Effect of Crocin Is Associated with GHSR-Mediated Hippocampal Plasticity-related Proteins in Mice Exposed to Prenatal Stress. ACS chemical neuroscience 2020, 11, 1159-1170. [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Abdeen, A.; Ibrahim, S.F.; Mani, V.; Iqbal, M.S.; Bhatia, S.; et al. Exploring the role of neuropeptides in depression and anxiety. Prog Neuropsychopharmacol Biol Psychiatry 2022, 114, 110478. [CrossRef]
- Jackson, T.M.; Ostrowski, T.D.; Middlemas, D.S. Intracerebroventricular Ghrelin Administration Increases Depressive-Like Behavior in Male Juvenile Rats. Front Behav Neurosci 2019, 13, 77. [CrossRef]
- Hansson, C.; Haage, D.; Taube, M.; Egecioglu, E.; Salomé, N.; Dickson, S.L. Central administration of ghrelin alters emotional responses in rats: behavioural, electrophysiological and molecular evidence. Neuroscience 2011, 180, 201-211. [CrossRef]
- Jensen, M.; Ratner, C.; Rudenko, O.; Christiansen, S.H.; Skov, L.J.; Hundahl, C.; Woldbye, D.P.; Holst, B. Anxiolytic-Like Effects of Increased Ghrelin Receptor Signaling in the Amygdala. The international journal of neuropsychopharmacology 2016, 19. [CrossRef]
- Mahbod, P.; Smith, E.P.; Fitzgerald, M.E.; Morano, R.L.; Packard, B.A.; Ghosal, S.; Scheimann, J.R.; Perez-Tilve, D.; Herman, J.P.; Tong, J. Desacyl Ghrelin Decreases Anxiety-like Behavior in Male Mice. Endocrinology 2018, 159, 388-399. [CrossRef]
- Pawar, G.R.; Agrawal, Y.O.; Nakhate, K.T.; Patil, C.R.; Sharma, C.; Ojha, S.; Mahajan, U.B.; Goyal, S.N. Ghrelin alleviates depression-like behaviour in rats subjected to high-fat diet and diurnal rhythm disturbance. Am J Transl Res 2022, 14, 7098-7108.
- Sun, N.; Mei, Y.; Hu, Z.; Xing, W.; Lv, K.; Hu, N.; Zhang, T.; Wang, D. Ghrelin attenuates depressive-like behavior, heart failure, and neuroinflammation in postmyocardial infarction rat model. Eur J Pharmacol 2021, 901, 174096. [CrossRef]
- Huang, H.J.; Chen, X.R.; Han, Q.Q.; Wang, J.; Pilot, A.; Yu, R.; Liu, Q.; Li, B.; Wu, G.C.; Wang, Y.Q.; et al. The protective effects of Ghrelin/GHSR on hippocampal neurogenesis in CUMS mice. Neuropharmacology 2019, 155, 31-43. [CrossRef]
| Animal and Stress paradigm | Behavioral test | Intervention | Signal molecules | Effects | Reference |
| Mice CSDS | FST, TST | GHSR1a knock-out | BDNF↓, IL-6↑ | Pro-depression effect | [29] |
| Rat | FST, TST | i.c.v injection of ghrelin | HPA↑ | Immobility time↑ in TST | [133,134] |
| Male C57BL/J6 mice, RS | TST OFT FST |
rAAV-Mediated Overexpression of GHSR1a | c-Fos↑ | Antidepressant-like effect | [135] |
| Male mice, Restraint stress | EPM | Ghrelin KO | pERK↓ | Decreases Anxiety-like Behavior |
[136] |
| C57BL/J6 mice, Prenatal stress | OFT,TST FST,SPT |
i.p. injection of crocin | PI3K/Akt↑ mTOR↑ |
Antidepressant-like effect | [131] |
| Male SD rats, HFD and DDR | FST OFT EPM |
Intra-VTA administration of ghrelin | TNF-α↓ IL-1β↓ IL-6↓ |
Alleviates depression-like behaviour | [137] |
| Male SD rats, CAO | SPT,OFT EPM |
subcutaneously injection of ghrelin | Iba-1↓ GFAP↓ |
Attenuates depressive-like behavior | [138] |
| Male C57BL/J6 mice, CSDS | SIT, FST,OFT EPM |
Intrahippocampal ghrelin infusions, AAV-siRNA of GHSR1a |
p38-MAPK↓ | Antidepressant effect | [112] |
| Male C57BL/J6 mice | TST FST |
Lateral ventricle injection of ghrelin | CREB↑ BDNF↑ |
Improves cognition Antidepressant-like effects |
[27] [103] |
| Adult female mice,OB | OFT TST |
Ghrelin into the hippocampus | MAPK↑ CaMKIIa↑ |
Antidepressant-like effects | [28] |
| Male C57BL/J6 mice,CUMS | OFT EPM FST |
Intraperitoneally (i.p.) injection of ghrelin, GHSR knockdown | PI3K/Akt ↑ CDK2↑ CyclinD1↑ |
Spine density↑ Proliferation of hippocampal NSCs Neurogenesis↑ |
[139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





