1. Introduction
Gram-negative bacteremia is a significant public health threat, particularly caused by
Klebsiella spp.,
Escherichia coli, and
Pseudomonas aeruginosa. These pathogens, alongside
Staphylococcus aureus and
Streptococcus pneumoniae, are leading causes of global mortality associated with bacterial infections [
1]. The healthcare systems face considerable challenges due to the high mortality rates of these infections, which can reach high as 20-40% [
2,
3,
4]. In Colombia, there is increasing concern over Gram-negative bacteremia, particularly due to the rising issue of multidrug resistance [
5,
6]. A study in a tertiary care institution in Colombia reported a 29% mortality rate, with Gram-negative organisms being the most frequently isolated in 49.8% of cases, primarily affecting the vascular and urinary systems [
7]. To mitigate the impact of antimicrobial resistance, especially to carbapenems, antimicrobial stewardship programs promote carbapenem-sparing strategies through the use of active antibiotics such as piperacillin/tazobactam (PTZ) with good results [
8,
9]. However, this leads to an increase in the use of piperacillin/tazobactam and the need to introduce not only the innovative drug but other generics to facilitate access. In the literature, there is controversy about whether the generic PTZ molecules have a different activity to the innovative one, while some studies describe differences in in-vitro or animal models [
10,
11], in equivalence and clinical studies the data are inconclusive [
12,
13,
14]. It is crucial to conduct real-life evaluations of frequently used generic drugs with high clinical impact, such as PTZ. The present study aimed to describe the effectiveness of one generic piperacillin/tazobactam in treating Gram-negative bloodstream infection in adults through real-life study.
2. Materials and Methods
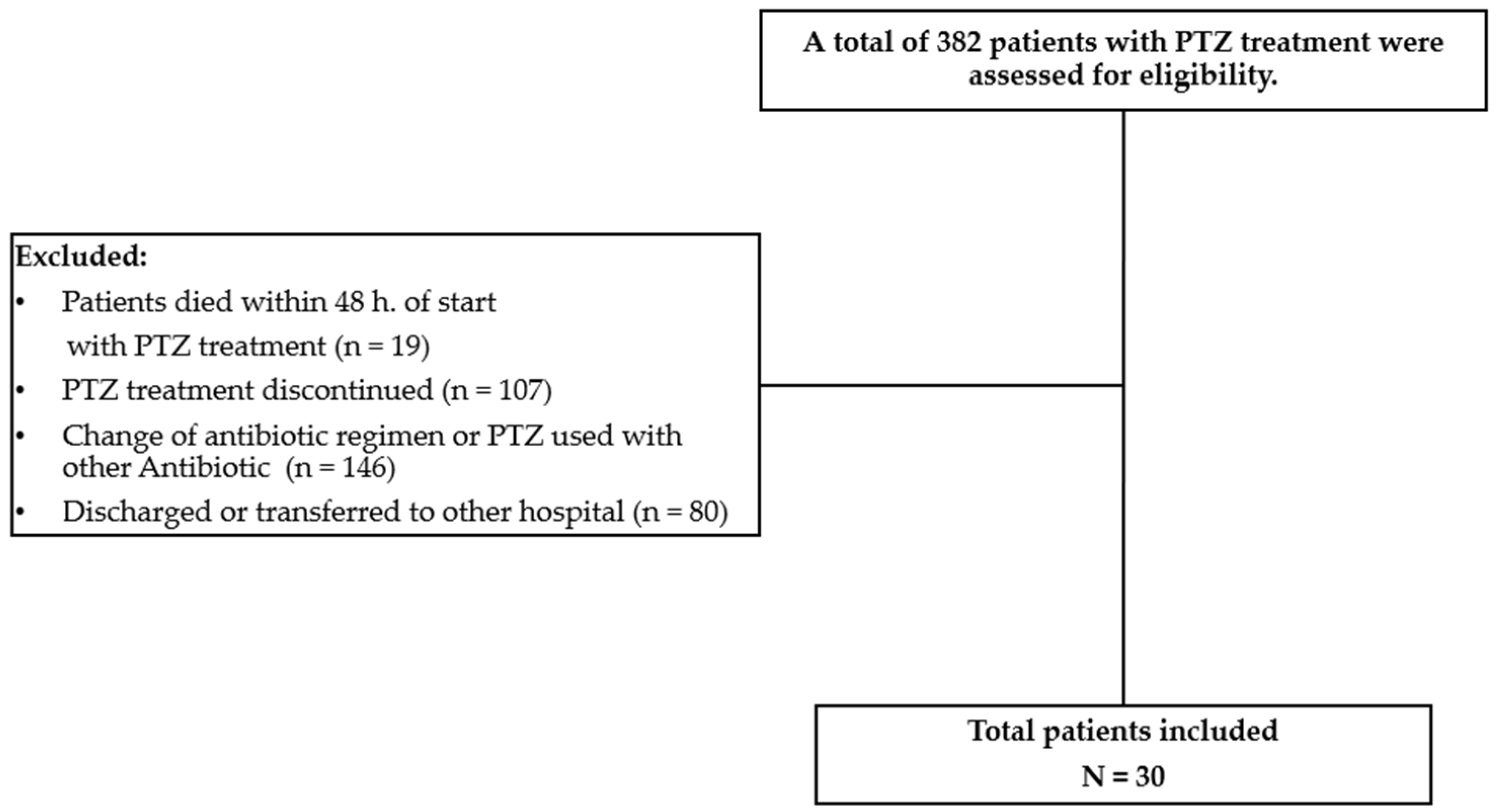
A descriptive phase IV study was conducted at a tertiary care hospital in Colombia from January to December 2019.
The study population included adult patients who were 18 years or older and had a diagnosis of bacteremia due to a Gram-negative bacillus that was confirmed by at least one positive blood culture, identified using the automated MIC Microscan Walkaway® method. For susceptibility, we evaluated the MIC reported for the system with a sensitive cutoff point of ≤ 16. The physician responsible for the patient’s care was the one who, at his discretion, selected the use of PZT for empirical management and, subsequently, based on the results of antimicrobial susceptibility, maintained or changed it.
The definitions of infection for each anatomical site were based on those proposed by Calandra and Cohen [
15], and the definition of sepsis was based on sepsis criteria 3 [
16]. To assess organ dysfunction, the APACHE II score was used. Patients were excluded if they had a high degree of immunosuppression (e.g., neutropenia or HIV-AIDS with a CD4/mm
3 cell count < 50 cells), had chronic infections requiring prolonged antibiotic administration, or were pregnant. Additionally, 1) patients who died before 48 hours from the start of PTZ, 2) receiving PTZ at the time of isolation, 3) using another antibiotic with activity against Gram-negative bacilli, or 4) with concomitant use of another antibiotic with activity against Gram- negative bacilli were also excluded, (
Figure 1) shows the flow diagram of patients included in this study.
Generic piperacillin/tazobactam (Penibectam®) was supplied as 4.5 g ampoules for intravenous administration at doses of 4.5 g every 6 hours. In patients with altered renal function (GFR less than 50 ml/min), the dose was adjusted according to the attending physician’s criteria.
Clinical and microbiological improvements were assessed whenever possible to determine the effectiveness of the study drug. Clinical response was deemed favorable when all signs and symptoms of sepsis or systemic inflammatory response (SIRS) had resolved completely or when there was partial improvement due to a decrease in the signs and symptoms of sepsis or SIRS without complete resolution of the infectious picture. The persistence of signs and symptoms of infection at the time of evaluation in the absence of another pathogen or causative agent was considered an unfavorable clinical response. A favorable microbiological response was established as the absence of isolation of the infection-causing organism in blood cultures collected seven days after the start of antibiotic treatment. In cases where the infection-causing organism persisted, the response was recorded as an unfavorable microbiological response. A complete favorable response was defined as the complete resolution of all signs and symptoms of sepsis and favorable clinical and microbiological responses. This evaluation was carried out during and at the end of treatment (admission, one week, two weeks, and 28 days). For patients who were not hospitalized at 28 days, the follow-up was conducted via a telephone call by one of the investigators.
Demographic and clinical characteristics were described, categorical variables presented as absolute and relative frequencies, and quantitative variables described with averages and standard deviation or median and interquartile range, depending on whether they had a normal distribution. Finally, an exploratory analysis was conducted using Cox regression for proportional risk models to explore and control for potential confounding variables related to cure and overall mortality. This study was approved by the Institutional Ethics Committee of Hospital Cardiovascular de Cundinamarca, meeting minutes No. 009, approved on 24 May 2018
3. Results
Thirty patients (median age, 62 years) were included in this observational clinical study. Most infections were acquired in the community, and the cardiovascular system was the most common site of infection. No significant prior use of antibiotics or previous surgery was observed in the evaluated patients.
The most common underlying diseases were hypertension, heart failure, and diabetes mellitus.
Table 1 displays the complete demographic data, including the patients’ baseline laboratory and clinical evaluation results, before the initiation of antibiotic therapy. The control laboratory values obtained upon admission to the cohort and on days 7 and 14 of hospitalization are also shown; they display median values within normal ranges for C-reactive protein (CRP) and do not indicate leukocytosis.
The main microorganisms isolated were
Serratia marcescens (33%) and
Escherichia coli (26%), followed by
Pseudomonas aeruginosa (13%) (
Table 2). Of the 29 isolated bacilli, 27 were susceptible, one (3.3%) was resistant, and one (3.3%) was considered intermediate susceptibility. However, given the clinical evolution, the attending clinical team continued PZT therapy.
The clinical response on day 7 was 66% (n = 20), and the microbiological response was 56.7% (n = 17), with the persistence of one pathogen (
S. marcescens) and isolation of a new pathogen (
S. hominis). In the second week of evaluation, clinical improvement was 70% (n = 21) and microbiological improvement was 60% (n = 18) without isolating new pathogens. At the end of follow-up, a clinical response was observed in 63.3% (n = 19) of patients, with a mortality rate of 23% (n = 7).
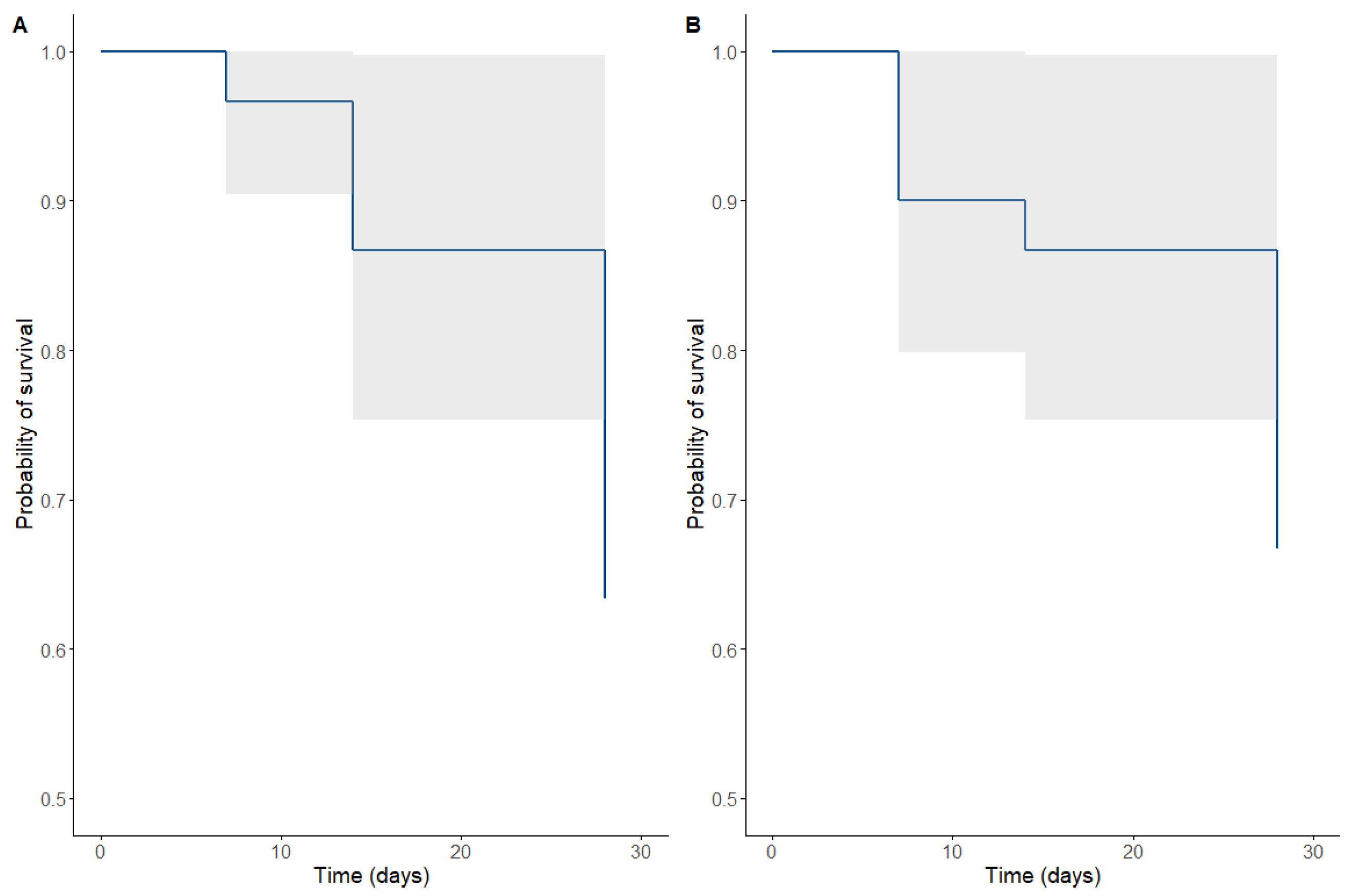
Figure 2 and
Figure 3 show patients’ clinical and microbiological responses receiving PTZ therapy. Of the patients, 73.3 % (n = 22) required management in the intensive care unit, with a median stay of 5 days (RIQ 2–15 days). The median duration of PTZ use was 10 days (RIQ 7-14).
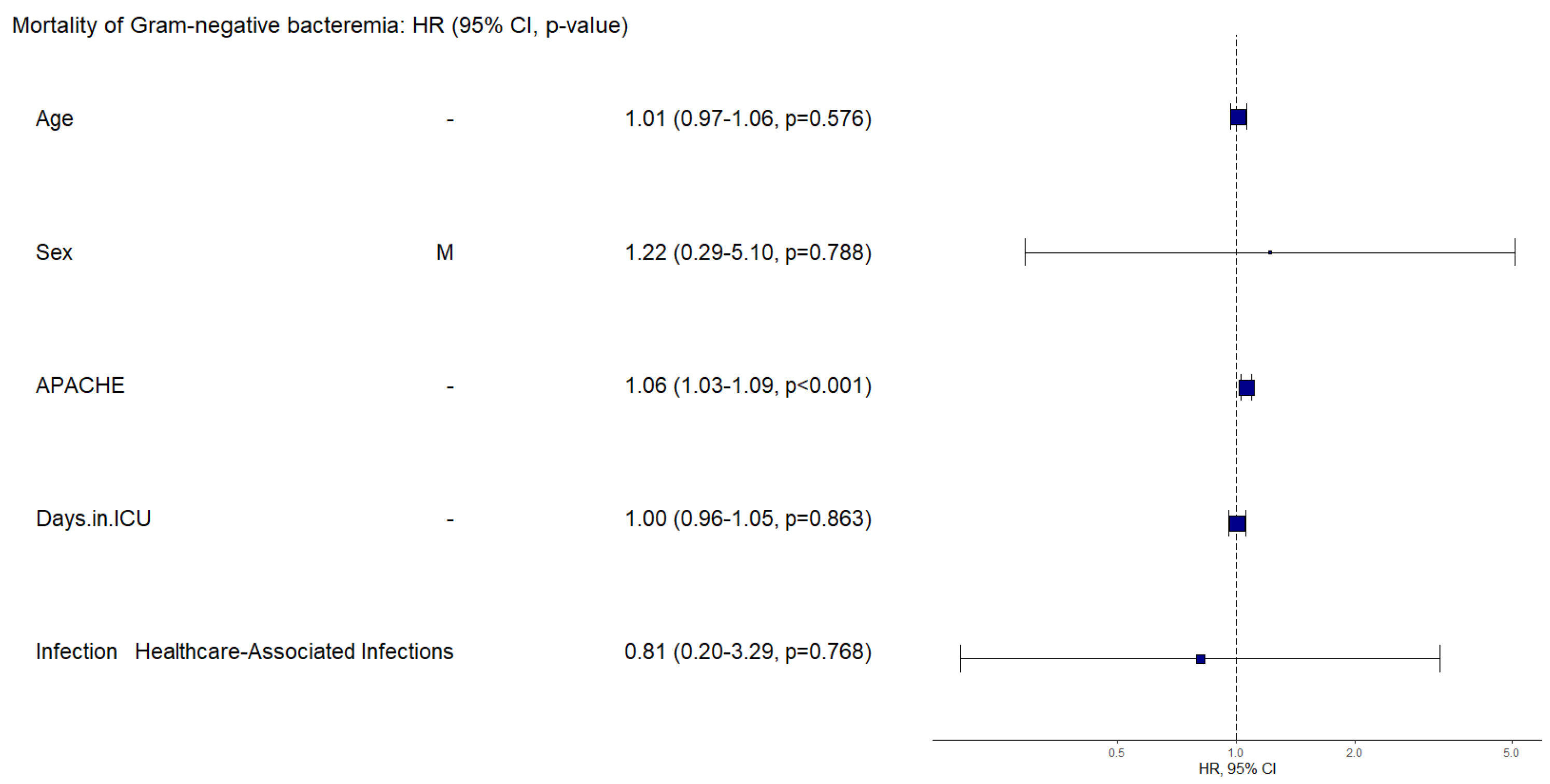
Mortality was assessed (
Figure 2) regarding HR related to clinical and paraclinical characteristics such as age, sex, APACHE II score, length of stay in the ICU, source of infection, presence of septic shock, and compromised organs. The APACHE II score was an important factor for patient mortality, with a HR of 1.06 (IC95% 1.03-1.13), p of 0.001. When adjusting for the site of infection as a confounding factor, we obtained an HR of 1.14 (IC95% 1.05-1.23), p=0.001, for the APACHE score, making it the most important factor in mortality.
4. Discussion
Given the emergence of carbapenem-resistant Gram-negative bacilli, WHO consider these pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance [
17] and prudent use of carbapenem-sparing antibiotics is becoming an important strategy in antimicrobial stewardship programs [
18]. PTZ is currently indicated for severe respiratory, intra-abdominal, urinary, and bacteremia infections [
19]. The increasing use of this antibiotic has led to the marketing of multiple molecules, with at least ten PZT products currently authorized for sale in Colombia [
20]. Although the utilization of generics drugs enables access to population, their introduction often leads to controversies concerning discrepancies that may arise compared to the innovative product. PZT is no exception, and several publications corroborate the equivalence or effectiveness of various generic products as well as some that have reported differences in potency or clinical effectiveness [
12,
13,
14].
The effectiveness of a generic formulation of PTZ at standard doses in managing bacteremia obtained in this study is consistent with the available literature. A clinical trial by Rafati et al. in patients with sepsis and critical illness showed a mortality rate of 25-30% [
21]. Another study by Abdul in 85 patients in the intensive care unit showed an 18.4%–42.6% rate, depending on the dosing scheme used in these patients [
22]. Several observational studies demonstrated similar clinical cure rates to ours with the dosing regimen used, with cure rates between 56.2% and 82% [
23,
24]. A study by Machado et al. evaluated the effectiveness of piperacillin/tazobactam in real-world patients who received therapy with various infections and found a mortality rate of 22.9%. The study included 37 patients with bacteremia, of whom 11 received a generic formulation of PTZ without further information on their outcomes [
25,
26].
There are barriers between developing and researching these molecules and their use in daily clinical practice. Some initiatives have been developed to take advantage of real-world evidence for the optimization and appropriate use of antibiotics and the development of clinical practice guidelines [
27,
28]. It is crucial to highlight that the clinical scenario under which the antibiotic is being evaluated in patients with confirmed bacteremia caused by Gram-negative bacteria is putting the drug to the test in terms of highly demanding pharmacokinetic and pharmacodynamic parameters. If the antibiotic does not act bactericidal quickly and appropriately, it can lead to serious complications and mortality.
Gram-negative Bacteremia is a serious complication that significantly increases mortality rates in infected patients [
19,
20,
21]. Our study’s mortality rate of 23% is within the expected range, highlighting the importance of quality generic product. The outcomes and response to therapy are dependent on the pharmacological behavior of the drug [
29] and are of greater importance in critically ill patients. Roberts et al. demonstrated that the behavior of piperacillin/tazobactam in patients with sepsis could be affected by an increased volume of distribution and protein binding, among others [
30], in which the real-world scenario and the outcomes achieved in this population serve as indicators of effectiveness. The results of this study demonstrate the therapeutic potential of this generic PTZ in managing severe infections. The observed outcomes align with the current understanding of the pharmacological behavior of PTZ in patients with sepsis. A mortality rate of 23% and evidence of clinical and microbiological improvement indicate that this generic PTZ (Penibactam
®) could be a suitable option for the target patient population.
The main limitations of this study are its descriptive nature, having been conducted at a single center, and the sample size, which allows for generating hypotheses, but not for drawing definitive conclusions regarding risk factors and mortality. Nonetheless, it is important to highlight the confirmed diagnosis, clear definitions used to evaluate the clinical response and microbiological outcomes, accompanied by strict patient follow-up with microbiological confirmation through culture, which contributes to the confirmation of the effectiveness of the study medication using rigorous standards and outcomes in a complex scenario. On the other hand, this study did not evaluate safety, given that we consider that both the PTZ molecule and this generic presentation have already been approved by the Colombian regulatory agency, INVIMA and that clinical concern is precisely focused on effectiveness.
5. Conclusions
The present study highlights the effectiveness of a generic piperacillin/tazobactam (Penibactam®) for treating Gram-negative bacteremia. This study included a critical patient population with significant underlying health conditions such as arterial hypertension, heart failure, and diabetes. Despite the high average APACHE II score indicating severe disease, this study revealed that the use of generic PTZ in a real-world clinical setting resulted in mortality and microbiological cure rates within the expected ranges over a 28 days of follow-up period that included both microbiological and clinical monitoring. These results are promising, especially considering the challenges in treating such infections and the need for cost-saving and accessible therapeutic options in clinical practice.
Supplementary Materials
No supplementary material is available for this study.
Author Contributions
Conceptualization, C.A. M.H. and L.C.; methodology, C.A. and L.C.; investigation, C.L, A.M, O.S.; data curation, L.C. A.M, J.K. and C.A.; writing—original draft preparation, J.K.; writing—review and editing, L.C., J.K., M.H and C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Alvarez & Gotuzzo CRO, which received an unrestricted grant of Farmalogica.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Hospital Cardiovascular de Cundinamarca, into session No 009, approved on 24 May 2018. All researchers who participated in this study complied with the national regulations (Resolution 8430 of 1993) that govern research activities and the recommendations of the institutional ethics committee.
Informed Consent Statement
The institutional ethics committee waived patient consent due to the observational nature of the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
To patients and healthcare professionals of Hospital Cardiovascular de Cundinamarca, especially microbiological laboratory and Intensive Care Unit.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ikuta, K.S.; Swetschinski, L.R.; Robles Aguilar, G.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Davis Weaver, N.; Wool, E.E.; Han, C.; Gershberg Hayoon, A.; et al. Global Mortality Associated with 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. The Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.L.; Anderson, M.T.; Mobley, H.L.T.; Bachman, M.A. Pathogenesis of Gram-Negative Bacteremia. Clin Microbiol Rev 2021, 34, e00234-20. [Google Scholar] [CrossRef] [PubMed]
- Quillici, M.C.B.; Resende, D.S.; Gonçalves, I.R.; Royer, S.; Sabino, S.S.; Almeida, V.F.D.; Ribas, R.M.; Gontijo Filho, P.P. Gram-Negative Bacilli Bacteremia: A 7 Year Retrospective Study in a Referral Brazilian Tertiary-Care Teaching Hospital. Journal of Medical Microbiology 2021, 70. [Google Scholar] [CrossRef]
- Deelen, J.W.T.; Rottier, W.C.; Van Werkhoven, C.H.; Woudt, S.H.S.; Buiting, A.G.M.; Dorigo-Zetsma, J.W.; Kluytmans, J.A.J.W.; Van Der Linden, P.D.; Thijsen, S.F.T.; Vlaminckx, B.J.M.; et al. The Burden of Bacteremic and Non-Bacteremic Gram-Negative Infections: A Prospective Multicenter Cohort Study in a Low-Resistance Country. Journal of Infection 2020, 81, 895–901. [Google Scholar] [CrossRef]
- Ovalle, M.V.; Saavedra, S.Y.; González, M.N.; Hidalgo, A.M.; Duarte, C.; Beltrán, M. Results of the national surveillance of antimicrobial resistance of Enterobacteriaceae and Gram negative bacilli in health care-associated infections in Colombia, 2012-2014. biomedica 2017, 37, 473. [Google Scholar] [CrossRef]
- Molina, F.J.; Díaz, C.A.; Barrera, L.; De La Rosa, G.; Dennis, R.; Dueñas, C.; Granados, M.; Londoño, D.; Ortiz, G.; Rodríguez, F.; et al. Microbiological Profile of Infections in the Intensive Care Units of Colombia (EPISEPSIS Colombia. Med Intensiva 2011Mar;35(2):75-83, 4.
- Sánchez-Pardo, S.; Ochoa-Díaz, A.F.; Rodríguez-Amaya, R.M.; Rojas-Garrido, E.M.; Rodríguez-Morales, A.J. Case fatality rate-related factors in patients with bacteremia hospitalized due medical conditions in an institution of third level in Colombia, 2014-2016. Rev Chilena Infectol 2020, 37, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Pallares, C.; Hernández-Gómez, C.; Appel, T.M.; Escandón, K.; Reyes, S.; Salcedo, S.; Matta, L.; Martínez, E.; Cobo, S.; Mora, L.; et al. Impact of Antimicrobial Stewardship Programs on Antibiotic Consumption and Antimicrobial Resistance in Four Colombian Healthcare Institutions. BMC Infect Dis 2022, 22, 420. [Google Scholar] [CrossRef] [PubMed]
- Gudiol, C.; Royo-Cebrecos, C.; Abdala, E.; Akova, M.; Álvarez, R.; Maestro-de La Calle, G.; Cano, A.; Cervera, C.; Clemente, W.T.; Martín-Dávila, P.; et al. Efficacy of β-Lactam/β-Lactamase Inhibitor Combinations for the Treatment of Bloodstream Infection Due to Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae in Hematological Patients with Neutropenia. Antimicrob Agents Chemother 2017, 61, e00164-17. [Google Scholar] [CrossRef]
- Moet, G.J.; Watters, A.A.; Sader, H.S.; Jones, R.N. Expanded Studies of Piperacillin/Tazobactam Formulations: Variations among Branded Product Lots and Assessment of 46 Generic Lots. Diagnostic Microbiology and Infectious Disease 2009, 65, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.A.; Agudelo, M.; Zuluaga, A.F.; Vesga, O. In Vivo Pharmacodynamics of Piperacillin/Tazobactam: Implications for Antimicrobial Efficacy and Resistance Suppression with Innovator and Generic Products. International Journal of Antimicrobial Agents 2017, 49, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, F.; Wulkersdorfer, B.; Oesterreicher, Z.; Bauer, M.; Al Jalali, V.; Nussbaumer-Pröll, A.; Wölfl-Duchek, M.; Jorda, A.; Lackner, E.; Reiter, B.; et al. Comparison of Pharmacokinetics and Stability of Generics of Cefepime, Linezolid and Piperacillin/Tazobactam with Their Respective Originator Drugs: An Intravenous Bioequivalence Study in Healthy Volunteers. Journal of Antimicrobial Chemotherapy 2022, 77, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Chen, C.-H.; Tu, C.-Y.; Chen, W.-C.; Kuo, L.-K.; Wang, Y.-T.; Fu, P.-K.; Ku, S.-C.; Fang, W.-F.; Chen, C.-M.; et al. Clinical Effectiveness of Branded versus Generic Piperacillin-Tazobactam for Treating Severe Community-Acquired Pneumonia. Journal of Infection and Public Health 2022, 15, 961–965. [Google Scholar] [CrossRef]
- Cotia, A.; Oliveira Junior, H.A.; Matuoka, J.Y.; Boszczowski, Í. Clinical Equivalence between Generic Versus Branded Antibiotics: Systematic Review and Meta-Analysis. Antibiotics 2023, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T.; Cohen, J. International Sepsis Forum Definition of Infection in the ICU Consensus Conference. International Sepsis Forum Consensus Conference on Definitions of Infection in the Intensive Care Unit. Crit Care Med 2005, 10, 10. [Google Scholar]
- Seymour, C. Assesment of Clinical Criteria for Sepsis. For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 11. [Google Scholar]
- World Health Organization WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance.; 2024; ISBN 978-92-4-009346-1.
- García-Betancur, J.C.; Appel, T.M.; Esparza, G.; Gales, A.C.; Levy-Hara, G.; Cornistein, W.; Vega, S.; Nuñez, D.; Cuellar, L.; Bavestrello, L.; et al. Update on the Epidemiology of Carbapenemases in Latin America and the Caribbean. Expert Review of Anti-infective Therapy 2021, 19, 197–213. [Google Scholar] [CrossRef] [PubMed]
- FDA ZOSYN (Piperacillin and Tazobactam) for Injection FULL PRESCRIBING INFORMATION 2017.
- INVIMA Sistema de Tramites En Linea - Consultas Publicas. Available online: https://www.invima.gov.co/atencion-al-ciudadano/consulta-avanzada-registros-sanitarios (accessed on 20 May 2024).
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus Short-Term Intravenous Infusion of Antipseudomonal β-Lactams for Patients with Sepsis: A Systematic Review and Meta-Analysis of Randomised Trials. The Lancet Infectious Diseases 2018, 18, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.-B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Abd Rahman, A.N.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A Prospective, Two-Centre, Open-Labelled Randomised Controlled Trial of Continuous versus Intermittent Beta-Lactam Infusion in Critically Ill Patients with Severe Sepsis. Intensive Care Med 2016, 42, 1535–1545. [Google Scholar] [CrossRef]
- Grant, E.M.; Kuti, J.L.; Nicolau, D.P.; Nightingale, C.; Quintiliani, R. Clinical Efficacy and Pharmacoeconomics of a Continuous-Infusion Piperacillin-Tazobactam Program in a Large Community Teaching Hospital. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2002, 22, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Jiménez, A.; Martín, M.M.; Iribarren, J.L.; Jiménez, J.J.; Mora, M.L. Clinical Cure of Ventilator-Associated Pneumonia Treated with Piperacillin/Tazobactam Administered by Continuous or Intermittent Infusion. International Journal of Antimicrobial Agents 2009, 33, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Machado-Alba, J.; Sanchez-Duque, J.; Gómez-González, J.; Moreno-Gutierrez, P.; Pantoja-Meneses, S.; Thahir-Silva, S.; Gaviria-Mendoza, A. Trends of Antibiotic Consumption in Intensive Care Units of Colombia, 2010-2016. Value in Health 2018, 21, S96–S97. [Google Scholar] [CrossRef]
- Machado-Alba, J.E.; Gaviria-Mendoza, A.; Machado-Duque, M.E. Results of the Effectiveness of Two Piperacillin-Tazobactam Molecules in the Real World. Int J Infect Dis 2018, 76, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Fabre, V.; Amoah, J.; Cosgrove, S.E.; Tamma, P.D. Antibiotic Therapy for Pseudomonas Aeruginosa Bloodstream Infections: How Long Is Long Enough? Clin Infect Dis 2019, 69, 2011–2014. [Google Scholar] [CrossRef] [PubMed]
- Steels, S.; Van Staa, T.P. The Role of Real-World Data in the Development of Treatment Guidelines: A Case Study on Guideline Developers’ Opinions about Using Observational Data on Antibiotic Prescribing in Primary Care. BMC Health Serv Res 2019, 19, 942. [Google Scholar] [CrossRef]
- Heffernan, A.J.; Mohd Sazlly Lim, S.; Lipman, J.; Roberts, J.A. A Personalised Approach to Antibiotic Pharmacokinetics and Pharmacodynamics in Critically Ill Patients. Anaesthesia Critical Care & Pain Medicine 2021, 40, 100970. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Roberts, M.S.; Robertson, T.A.; Dalley, A.J.; Lipman, J. Piperacillin Penetration into Tissue of Critically Ill Patients with Sepsis--Bolus versus Continuous Administration? Crit Care Med 2009, 37, 926–933. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).