Submitted:
03 June 2024
Posted:
03 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
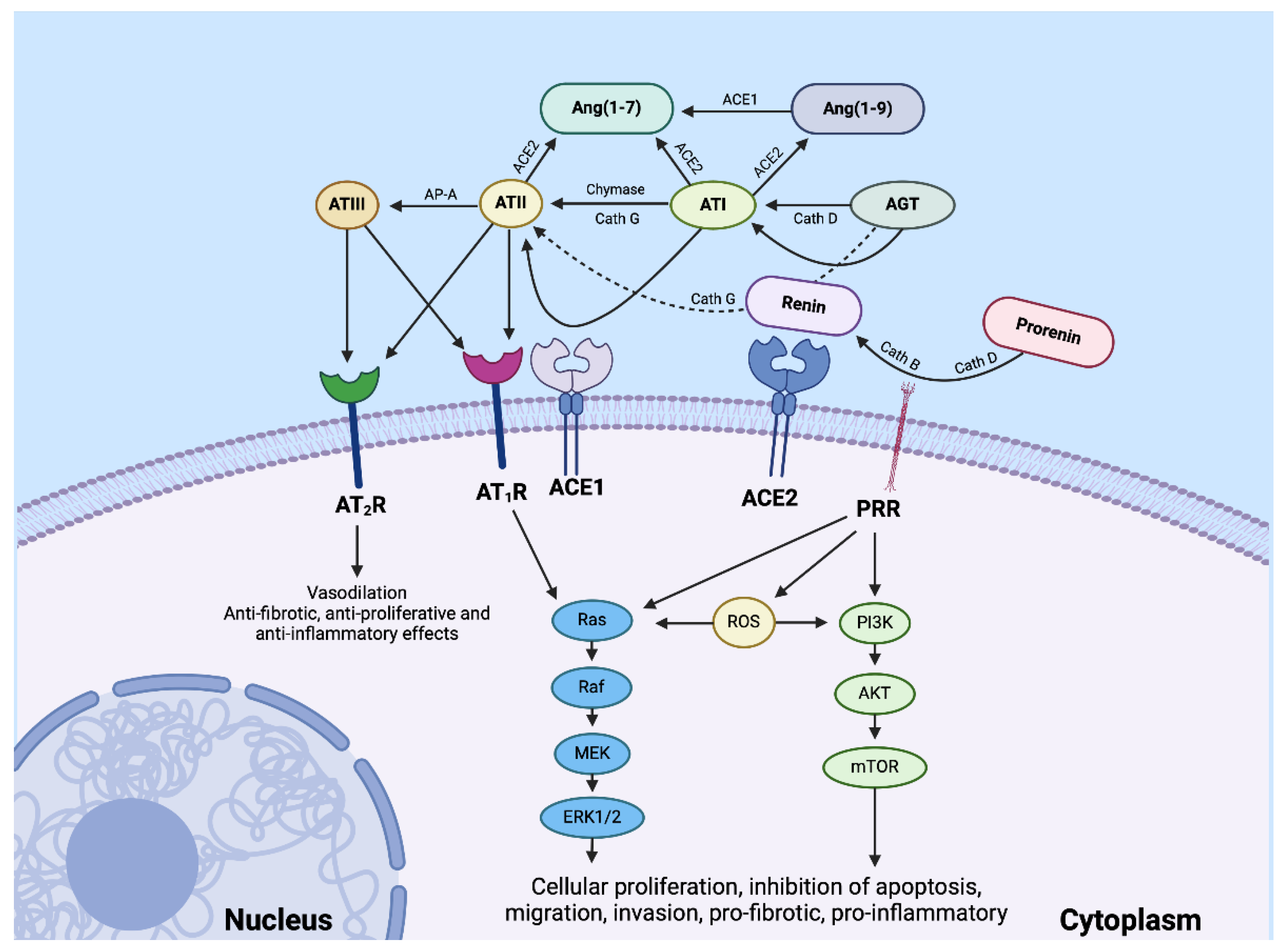
2. The Role of the Renin-Angiotensin System in Malignant Melanoma
2.1. The Role of the Renin-Angiotensin System in Malignant Melanoma Tumor Microenvironment
2.2. The Renin-Angiotensin System and Cancer Metastasis
3. Immunotherapy and Cancer Vaccines for Metastatic Malignant Melanoma
3.1. Immunotherapy
3.2. Cancer Vaccines
4. Concurrent Targeting of the Renin-Angiotensin System and Other Dysregulated Pathways
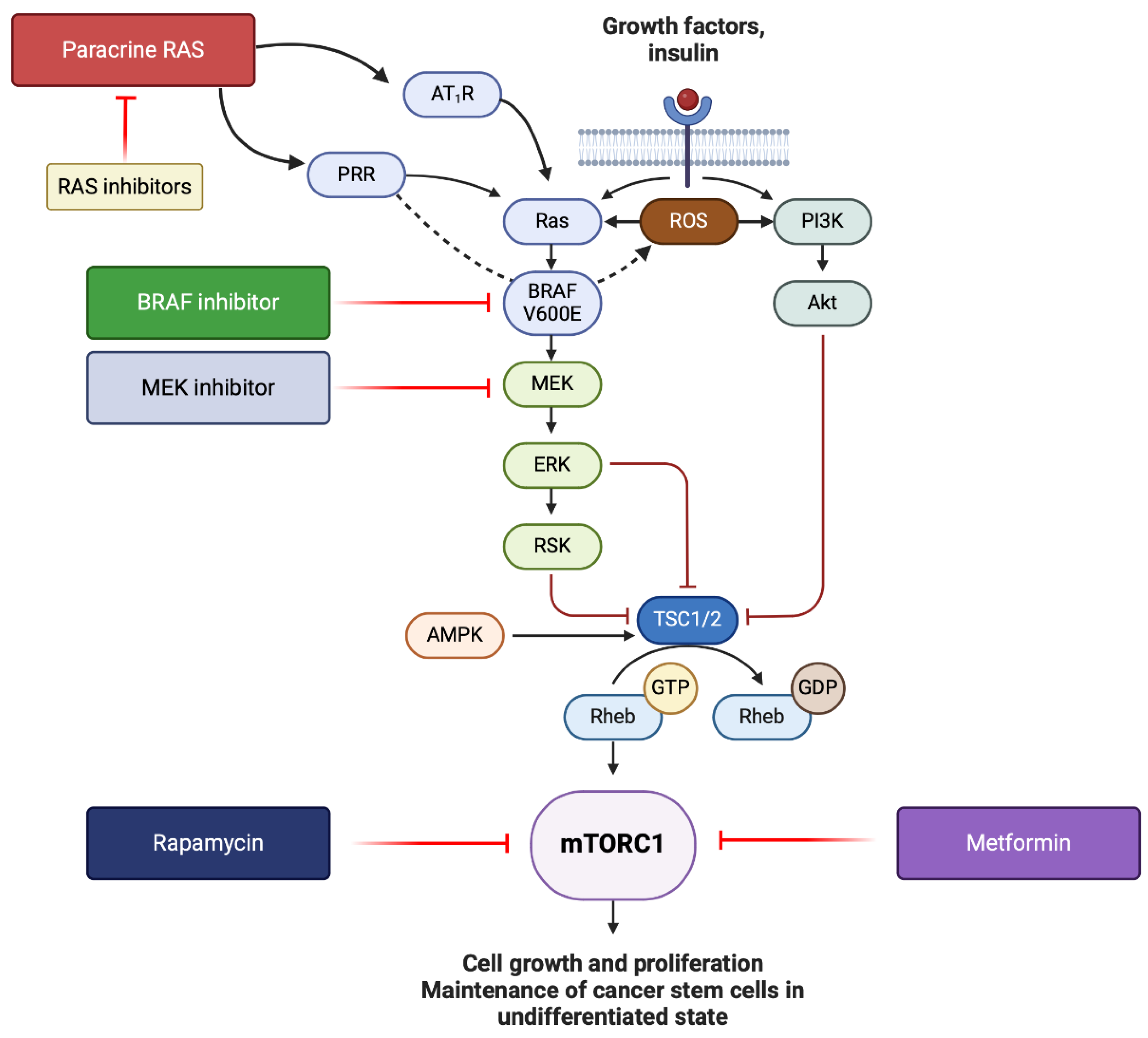
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 1: Diagnostics: Update 2022. Eur J Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J Invest Dermatol 2016, 136, 1161–1171. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Review: Ultraviolet Radiation and Skin Cancer. Int J Dermatol 2010, 49, 978–986. [Google Scholar] [CrossRef]
- https://www.tewhatuora.govt.nz/our-health-system/data-and-statistics/historical-cancer (Accessed on 25 June 2023) Historical Cancer Data.
- Serman, N.; Vranic, S.; Glibo, M.; Serman, L.; Bukvic Mokos, Z. Genetic Risk Factors in Melanoma Etiopathogenesis and the Role of Genetic Counseling: A Concise Review. Bosn J Basic Med Sci 2022, 22, 673–682. [Google Scholar] [CrossRef]
- Bauer, J.; Garbe, C. Acquired Melanocytic Nevi as Risk Factor for Melanoma Development. A Comprehensive Review of Epidemiological Data. Pigment Cell Res 2003, 16, 297–306. [Google Scholar] [CrossRef]
- Grob, J.J.; Gouvernet, J.; Aymar, D.; Mostaque, A.; Romano, M.H.; Collet, A.M.; Noe, M.C.; Diconstanzo, M.P.; Bonerandi, J.J. Count of Benign Melanocytic Nevi as a Major Indicator of Risk for Nonfamilial Nodular and Superficial Spreading Melanoma. Cancer 1990, 66, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Yoganandarajah, V.; Patel, J.; Schaijik, B. van; Bockett, N.; Brasch, H.D.; Paterson, E.; Sim, D.; Davis, P.F.; Roth, I.M.; Itinteang, T.; et al. Identification of Cancer Stem Cell Subpopulations in Head and Neck Metastatic Malignant Melanoma. Cells 2020, Vol. 9, Page 324 2020, 9, 324. [Google Scholar] [CrossRef]
- Sangster, A.B.; Chang-Mcdonald, B.; Patel, J.; Bockett, N.; Paterson, E.; Davis, P.F.; Tan, S.T. Expression of Cathepsins B and D by Cancer Stem Cells in Head and Neck Metastatic Malignant Melanoma. Melanoma Res 2021, 31, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Wickremesekera, A.C.; Brasch, H.D.; Lee, V.M.; Davis, P.F.; Woon, K.; Johnson, R.; Tan, S.T.; Itinteang, T. Expression of Cancer Stem Cell Markers in Metastatic Melanoma to the Brain. J Clin Neurosci 2019, 60, 112–116. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer Stem Cells Revisited. Nat Med 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Eyler, C.E.; Rich, J.N. Survival of the Fittest: Cancer Stem Cells in Therapeutic Resistance and Angiogenesis. J Clin Oncol 2008, 26, 2839–2845. [Google Scholar] [CrossRef]
- Gourab, G.; George, M.; Shakthika, S.; Hexin, C. Cancer Resistance to Immunotherapy: What Is the Role of Cancer Stem Cells? Cancer Drug Resist 2022, 5, 981–994. [Google Scholar] [CrossRef]
- Shackleton, M.; Quintana, E.; Fearon, E.R.; Morrison, S.J. Heterogeneity in Cancer: Cancer Stem Cells versus Clonal Evolution. Cell 2009, 138, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Kilmister, E.J.; Koh, S.P.; Weth, F.R.; Gray, C.; Tan, S.T. Cancer Metastasis and Treatment Resistance: Mechanistic Insights and Therapeutic Targeting of Cancer Stem Cells and the Tumor Microenvironment. Biomedicines 2022, 10, 2988. [Google Scholar] [CrossRef]
- Strashilov, S.; Yordanov, A. Aetiology and Pathogenesis of Cutaneous Melanoma: Current Concepts and Advances. Int J Mol Sci 2021, 22, 6395. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N Eng J Med 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N Eng J Med 2012, 367, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Ombra, M.N.; Colombino, M.; Casula, M.; Sini, M.C.; Manca, A.; Paliogiannis, P.; Ascierto, P.A.; Cossu, A. Multiple Molecular Pathways in Melanomagenesis: Characterization of Therapeutic Targets. Front Oncol 2015, 5, 151142. [Google Scholar] [CrossRef]
- Patel, H.; Yacoub, N.; Mishra, R.; White, A.; Yuan, L.; Alanazi, S.; Garrett, J.T. Current Advances in the Treatment of BRAF-Mutant Melanoma. Cancers (Basel) 2020, 12, 482. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, J.; Liu, S.; Shao, J.; Liu, Z.; Li, J.; Xiao, W. Role of the Tumor Microenvironment in Malignant Melanoma Organoids during the Development and Metastasis of Tumors. Front Cell Dev Biol 2023, 11, 1166916. [Google Scholar] [CrossRef]
- Huang, A.C.; Zappasodi, R. A Decade of Checkpoint Blockade Immunotherapy in Melanoma: Understanding the Molecular Basis for Immune Sensitivity and Resistance. Nat Immunol 2022, 23, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.H.; From, L.; Bernardino, E.; Mihm, M. The Histogenesis and Biologic Behavior of Primary Human Malignant Melanomas of the Skin. Cancer Res 1969, 29, 705–727. [Google Scholar] [PubMed]
- Thomas, N.E.; Busam, K.J.; From, L.; Kricker, A.; Armstrong, B.K.; Anton-Culver, H.; Gruber, S.B.; Gallagher, R.P.; Zanetti, R.; Rosso, S.; et al. Tumor-Infiltrating Lymphocyte Grade in Primary Melanomas Is Independently Associated with Melanoma-Specific Survival in the Population-Based Genes, Environment and Melanoma Study. J Clin Oncol 2013, 31, 4252–4259. [Google Scholar] [CrossRef] [PubMed]
- Maurer, D.M.; Butterfield, L.H.; Vujanovic, L. Melanoma Vaccines: Clinical Status and Immune Endpoints. Melanoma Res 2019, 29, 109–118. [Google Scholar] [CrossRef]
- Livingston, P.O.; Adluri, S.; Helling, F.; Yao, T.J.; Kensil, C.R.; Newman, M.J.; Marciani, D. Phase 1 Trial of Immunological Adjuvant QS-21 with a GM2 Ganglioside-Keyhole Limpet Haemocyanin Conjugate Vaccine in Patients with Malignant Melanoma. Vaccine 1994, 12, 1275–1280. [Google Scholar] [CrossRef]
- Ross, M.I.; Andtbacka, R.H.I.; Puzanov, I.; Milhem, M.M.; Collichio, F.A.; Delman, K.A.; Noyes, R.D.; Zager, J.S.; Cranmer, L.D.; Spitler, L.E.; et al. Patterns of Durable Response with Intralesional Talimogene Laherparepvec (T-VEC): Results from a Phase III Trial in Patients with Stage IIIb-IV Melanoma. J Clin Oncol 2014, 32, 9026–9026. [Google Scholar] [CrossRef]
- Stingl, G.; Brŏcker, E.B.; Mertelsmann, R.; Wolff, K.; Schreiber, S.; Kămpgen, E.; Schneeberger, A.; Dummer, W.; Brennscheid, U.; Veelken, H.; et al. Phase I Study to the Immunotherapy of Metastatic Malignant Melanoma by a Cancer Vaccine Consisting of Autologous Cancer Cells Transfected with the Human IL-2 Gene. Hum Gene Ther 1996, 7, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.E.; Sondak, V.K.; Bishop, D.K.; Nickoloff, B.J.; Mulligan, R.C.; Mulé, J.J. Adoptive Immunotherapy of Cancer with Activated Lymph Node Cells Primed in Vivo with Autologous Tumor Cells Transduced with the GM-CSF Gene. Hum Gene Ther 1996, 7, 773–792. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Eng J Med 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma (KEYNOTE-006): Post-Hoc 5-Year Results from an Open-Label, Multicentre, Randomised, Controlled, Phase 3 Study. Lancet Oncol 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Jacques Grob, J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Eng J Med 2015, 372, 2521–2553. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma (EORTC 1325-MG/KEYNOTE-054): Distant Metastasis-Free Survival Results from a Double-Blind, Randomised, Controlled, Phase 3 Trial. Lancet Oncol 2021, 22, 643–654. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer Follow-up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results from the EORTC 1325-MG/KEYNOTE-054 Trial. J Clin Oncol 2020, 38, 3925–3936. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G. V; et al. Pembrolizumab versus Placebo as Adjuvant Therapy in Completely Resected Stage IIB or IIC Melanoma (KEYNOTE-716): A Randomised, Double-Blind, Phase 3 Trial. Lancet 2022, 399, 1718–1729. [Google Scholar] [CrossRef]
- Kilmister, E.J.; Tan, S.T. The Role of the Renin–Angiotensin System in the Cancer Stem Cell Niche. J Histochem Cytochem 2021, 69, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Catarata, M.J.; Ribeiro, R.; Oliveira, M.J.; Robalo Cordeiro, C.; Medeiros, R. Renin-Angiotensin System in Lung Tumor and Microenvironment Interactions. Cancers (Basel) 2020, 12, 1457. [Google Scholar] [CrossRef]
- Kapoor-Narula, U.; Lenka, N. Cancer Stem Cells and Tumor Heterogeneity: Deciphering the Role in Tumor Progression and Metastasis. Cytokine 2022, 157, 155968. [Google Scholar] [CrossRef]
- Siljee, S.; Pilkington, T.; Brasch, H.D.; Bockett, N.; Patel, J.; Paterson, E.; Davis, P.F.; Tan, S.T. Cancer Stem Cells in Head and Neck Metastatic Malignant Melanoma Express Components of the Renin-Angiotensin System. Life 2020, 10, 268. [Google Scholar] [CrossRef]
- Bafaloukos, D.; Gazouli, I.; Koutserimpas, C.; Samonis, G. Evolution and Progress of MRNA Vaccines in the Treatment of Melanoma: Future Prospects. Vaccines (Basel) 2023, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yaguchi, T.; Ohmura, G.; Kobayashi, A.; Kawamura, N.; Iwata, T.; Kiniwa, Y.; Okuyama, R.; Kawakami, Y. Involvement of Local Renin-Angiotensin System in Immunosuppression of Tumor Microenvironment. Cancer Sci 2018, 109, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Almutlaq, M.; Alamro, A.A.; Alamri, H.S.; Alghamdi, A.A.; Barhoumi, T. The Effect of Local Renin Angiotensin System in the Common Types of Cancer. Front Endocrinol (Lausanne) 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Durik, M.; Sevá Pessôa, B.; Roks, A.J.M. The Renin–Angiotensin System, Bone Marrow and Progenitor Cells. Clin Sci 2012, 123, 205–223. [Google Scholar] [CrossRef]
- George, A.J.; Thomas, W.G.; Hannan, R.D. The Renin–Angiotensin System and Cancer: Old Dog, New Tricks. Nat Rev Cancer 2010, 10, 745–759. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol Rev 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Dikalova, A.; Clempus, R.; Lassègue, B.; Cheng, G.; McCoy, J.; Dikalov, S.; Martin, A.S.; Lyle, A.; Weber, D.S.; Weiss, D.; et al. Nox1 Overexpression Potentiates Angiotensin II-Induced Hypertension and Vascular Smooth Muscle Hypertrophy in Transgenic Mice. Circulation 2005, 112, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Tehranian, C.; Fankhauser, L.; Harter, P.N.; Ratcliffe, C.D.H.; Zeiner, P.S.; Messmer, J.M.; Hoffmann, D.C.; Frey, K.; Westphal, D.; Ronellenfitsch, M.W.; et al. The PI3K/Akt/MTOR Pathway as a Preventive Target in Melanoma Brain Metastasis. Neuro-Oncol 2022, 24, 213–225. [Google Scholar] [CrossRef]
- Renziehausen, A.; Wang, H.; Rao, B.; Weir, L.; Nigro, C. Lo; Lattanzio, L.; Merlano, M.; Vega-Rioja, A.; del Carmen Fernandez-Carranco, M.; Hajji, N.; et al. The Renin Angiotensin System (RAS) Mediates Bifunctional Growth Regulation in Melanoma and Is a Novel Target for Therapeutic Intervention. Oncogene 2019, 38, 2320–2336. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Meza, S.; Díaz, J.; Sandoval-Bórquez, A.; Valenzuela-Valderrama, M.; Díaz-Valdivia, N.; Rojas-Celis, V.; Contreras, P.; Huilcaman, R.; Ocaranza, M.P.; Chiong, M.; et al. AT2 Receptor Mediated Activation of the Tyrosine Phosphatase PTP1B Blocks Caveolin-1 Enhanced Migration, Invasion and Metastasis of Cancer Cells. Cancers (Basel) 2019, 11, 1299. [Google Scholar] [CrossRef]
- Ishikane, S.; Hosoda, H.; Nojiri, T.; Tokudome, T.; Mizutani, T.; Miura, K.; Akitake, Y.; Kimura, T.; Imamichi, Y.; Kawabe, S.; et al. Angiotensin II Promotes Pulmonary Metastasis of Melanoma through the Activation of Adhesion Molecules in Vascular Endothelial Cells. Biochem Pharmacol 2018, 154, 136–147. [Google Scholar] [CrossRef]
- Frampton, A.E.; Sivakumar, S. A New Combination Immunotherapy in Advanced Melanoma. N Eng J Med 2022, 386, 91–92. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Eng J Med 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.-G.; et al. Neoadjuvant–Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N Eng J Med 2023, 388, 813–823. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Eng J Med 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Nakamura, K.; Kiniwa, Y.; Okuyama, R. CCL5 Production by Fibroblasts through a Local Renin–Angiotensin System in Malignant Melanoma Affects Tumor Immune Responses. J Cancer Res Clin Oncol 2021, 147, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Versluis, J.M.; Menzies, A.M.; Sikorska, K.; Rozeman, E.A.; Saw, R.P.M.; van Houdt, W.J.; Eriksson, H.; Klop, W.M.C.; Ch’ng, S.; van Thienen, J.V.; et al. Survival Update of Neoadjuvant Ipilimumab plus Nivolumab in Macroscopic Stage III Melanoma in the OpACIN and OpACIN-Neo Trials. Ann Oncol 2023, 34, 420–430. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.X.; Chen, Y.X.; Wu, H.X.; Yin, L.; Zhao, Q.; Luo, H.Y.; Zeng, Z.L.; Qiu, M.Z.; Xu, R.H. Integrated Analysis of Single-Cell and Bulk RNA Sequencing Data Reveals a Pan-Cancer Stemness Signature Predicting Immunotherapy Response. Genome Med 2022, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Khattak, A.; Weber, J.S.; Meniawy, T.; Taylor, M.H.; Ansstas, G.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; Faries, M.B.; et al. Distant Metastasis-Free Survival Results from the Randomized, Phase 2 MRNA-4157-P201/KEYNOTE-942 Trial. J Clin Oncol 2023, 41, LBA9503–LBA9503. [Google Scholar] [CrossRef]
- Dillman, R.O.; Cornforth, A.N.; Nistor, G. Cancer Stem Cell Antigen-Based Vaccines: The Preferred Strategy for Active Specific Immunotherapy of Metastatic Melanoma? Expert Opin Biol Ther 2013, 13, 643–656. [Google Scholar] [CrossRef]
- Wang, J.; Nishiyama, A.; Matsuyama, M.; Wang, Z.; Yuan, Y. The (pro)Renin Receptor: A Novel Biomarker and Potential Therapeutic Target for Various Cancers. Cell Commun Signal 2020, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Noble, N.A.; Zhang, J.; Xu, C.; Border, W.A. Renin-Stimulated TGF-Beta1 Expression Is Regulated by a Mitogen-Activated Protein Kinase in Mesangial Cells. Kidney Int 2007, 72, 45–52. [Google Scholar] [CrossRef]
- Cardinale, J.P.; Sriramula, S.; Mariappan, N.; Agarwal, D.; Francis, J. Angiotensin II-Induced Hypertension Is Modulated by Nuclear Factor-ΚB in the Paraventricular Nucleus. Hypertension 2012, 59, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.L.; Cui, W. Proliferation, Survival and Metabolism: The Role of PI3K/AKT/MTOR Signalling in Pluripotency and Cell Fate Determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, W.; Zhang, G.; Kwong, L.; Lu, H.; Tan, J.; Sadek, N.; Xiao, M.; Zhang, J.; Labrie, M.; et al. Targeting MTOR Signaling Overcomes Acquired Resistance to Combined BRAF and MEK Inhibition in BRAF-Mutant Melanoma. Oncogene 2021 40:37 2021, 40, 5590–5599. [Google Scholar] [CrossRef]
- Tomic, T.; Botton, T.; Cerezo, M.; Robert, G.; Luciano, F.; Puissant, A.; Gounon, P.; Allegra, M.; Bertolotto, C.; Bereder, J.M.; et al. Metformin Inhibits Melanoma Development through Autophagy and Apoptosis Mechanisms. Cell Death Dis 2011, 2, e199. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Ichisaka, T.; Maeda, M.; Oshiro, N.; Hara, K.; Edenhofer, F.; Kiyama, H.; Yonezawa, K.; Yamanaka, S. MTOR Is Essential for Growth and Proliferation in Early Mouse Embryos and Embryonic Stem Cells. Mol Cell Biol 2004, 24, 6710–6718. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Okabe, I.; Bernard, D.J.; Wynshaw-Boris, A.; Nussbaum, R.L. Proliferative Defect and Embryonic Lethality in Mice Homozygous for a Deletion in the P110α Subunit of Phosphoinositide 3-Kinase. J Biol Biochem 1999, 274, 10963–10968. [Google Scholar] [CrossRef]
- Peng, X.-D.; Xu, P.-Z.; Chen, M.-L.; Hahn-Windgassen, A.; Skeen, J.; Jacobs, J.; Sundararajan, D.; Chen, W.S.; Crawford, S.E.; Coleman, K.G.; et al. Dwarfism, Impaired Skin Development, Skeletal Muscle Atrophy, Delayed Bone Development, and Impeded Adipogenesis in Mice Lacking Akt1 and Akt2. Genes Dev 2003, 17, 1352–1365. [Google Scholar] [CrossRef]
- Chaturvedi, M.M.; Sung, B.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. NF-ΚB Addiction and Its Role in Cancer: ‘One Size Does Not Fit All. ’ Oncogene 2011, 30, 1615–1630. [Google Scholar] [CrossRef]
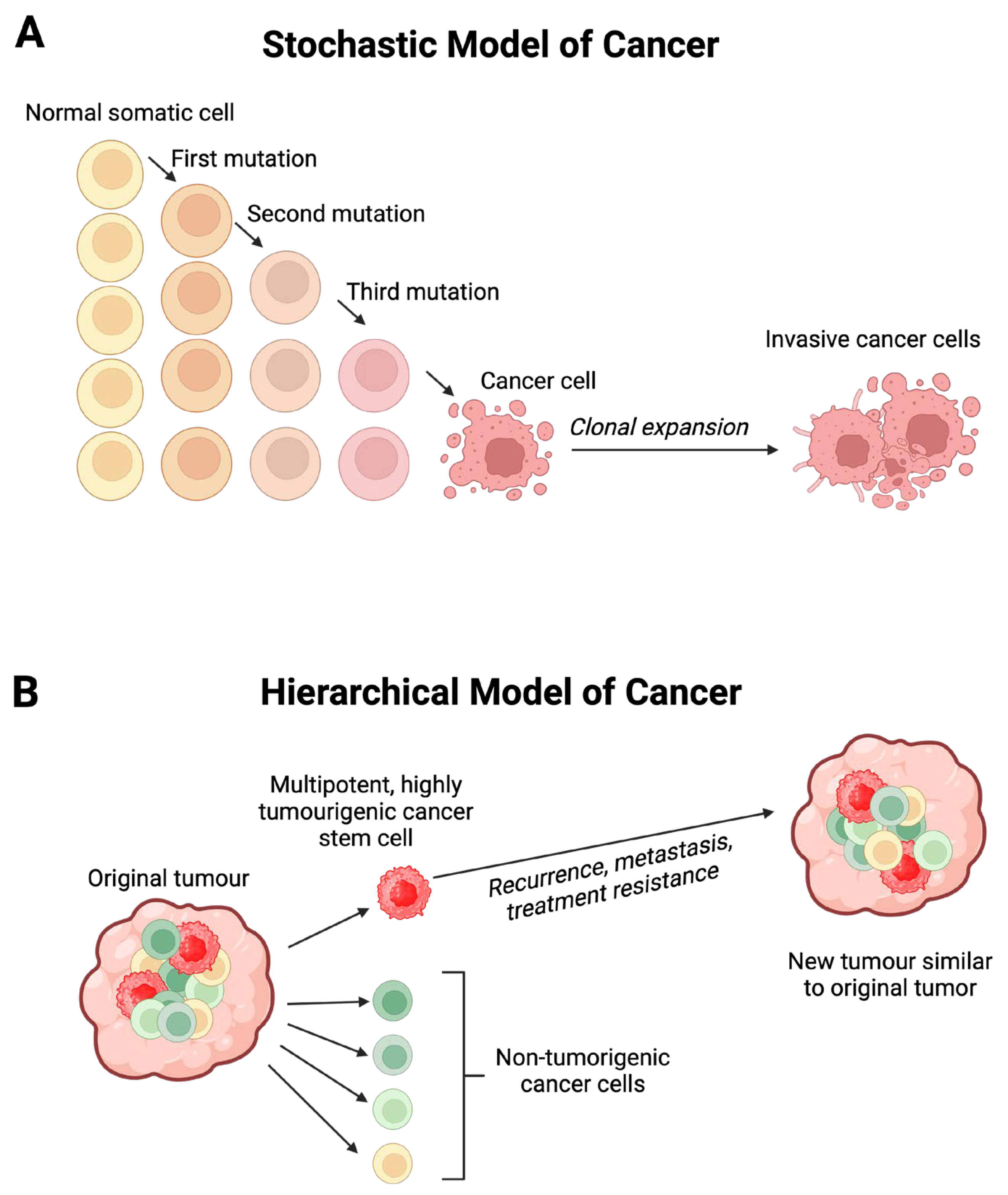
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




