1. Introduction
Due to the disposal standards and the need to protect the environmental quality of ecosystems, landfill leachate treatment plants adopt membrane separation processes (MSP), such as nanofiltration and reverse osmosis, to remove macro and micro-contaminants, complementing or replacing conventional treatment methods [
1]. However, the destination of landfill leachate membrane concentrate (LLMC) – the residual stream of MSP – is a critical aspect of the landfill leachate treatment loop and is challenging for landfill operators [
2]. The common practice in handling the LLMC is infiltration into landfilled waste. However, the recent literature indicates that membrane concentrate infiltration tends to impact the environmental and economic performance of the leachate treatment chain [
3,
4,
5].
Generally, membrane concentrates generated in landfills are high-saline streams (conductivity of 16,130–98,000 μS cm
-1; 890 – 15,400 mg/L sodium; 210 – 9,600 mg L
-1 potassium) and, depending on the leachate composition and its treatment layout, refractory organic pollutants such as lignin-like, lipids/proteins-like and unsaturated hydrocarbons are presented on it in high-level (719 – 4,500 mg L
-1 total organic carbon; 1,393 – 1,501 mg L
-1 humic substances) [
6]. Therefore, due to its complex nature and high pollution potential, cost-effective options for managing LLMC are urgent.
Thermal technologies such as submerged combustion evaporation and mechanical vapor recompression handle leachate concentrate streams [
7]. In these systems, an evaporation mother liquor is generated and should be managed appropriately [
2]. Likewise, evaporation pounds, used in warm regions to reduce the membrane concentration volume, produce secondary pollutants such as sludge directed to the landfill body [
8]. Inorganic salts of leachate evaporation residues are mainly K, Na, Mg, and Ca. Due to the complexity of these wastes, there is currently no feasible path for their treatment and disposal. The accumulation of evaporation residues leads to significant environmental contamination, equipment deterioration, and lack of economic benefit [
9].
The pyrolysis process has emerged as a management solution for valorizing different waste streams, such as sewage sludge, crop and agro-industrial residues, and industrial and municipal solid wastes [
10,
11,
12]. In this context, one of the emerging waste challenges is handling spent coffee ground (SCG) residues, which is a significant concern. SCG is a solid waste by-product from the coffee processing industry – the second-largest traded commodity after petroleum. The SCG waste is usually landfilled, open-burned with other coffee residues, or mixed with animal fodder. The carbon footprint and environmental burdens associated with the existing management practices challenge sustainability [
13,
14].
Pyrolysis is the thermochemical decomposition of carbon-based biomass under deficient concentration or absence of oxygen at a temperature higher than 400ºC and atmospheric pressure. Solid carbonaceous material named biochar, volatile organic substances that can be condensed to liquid phase (bio-oil), and mixed non-condensable gasses (CO, CO
2, CH
4, and H
2) (syngas) are produced in this process [
15]. The pyrolysis technology is categorized as fast, intermediate, and slow depending on heating rate, peak temperature, and residence time. Slow pyrolysis, also named carbonization, is the most employed method for char production because it offers the highest recovery of carbon-based material [
16,
17].
In recent scientific investigations, research has concentrated on utilizing char derived from commercial or specially prepared biochar sourced from organic feedstocks to purify landfill wastewater [
18,
19,
20,
21,
22]. Besides, the landfill leachate co-pyrolysis has been assessed in lab-scale experiments. Ben Hassen-Trabelsi et al. [
23] tested the co-pyrolysis of LLMC and sewage sludge for bio-oil and syngas recovery. The authors explored the possibility of recycling organics in biofuels, which could be exported or used as an energy source for the pyrolysis treatment. The results showed that adding LLMC into sewage sludge increased the bio-oil yield (from 25 wt% of sludge pyrolysis to 31 wt% for LLMC: sludge at 30:70 ratio by weight). In addition, the syngas had a sound hydrogen concentration and high light hydrocarbon content (methane and CnHm). The heating value increased from 8.48 MJ kg
-1 for sludge pyrolysis to 12.29 MJ kg
-1 for LLMC: sludge-30:70 co-pyrolysis [
23]. In a recent study, landfill leachate sludge was pyrolyzed to produce an adsorbent for chromium removal [
24]. Utilizing leachate waste streams in pyrolytic processes is promising, but literature on this topic is scarce. Therefore, our study contributes to this area of research by exploring the utilization of landfill leachate residues in the pyrolysis of SCG. It is acknowledged that inorganic compounds, such as alkali and alkaline earth metals (mainly Na, Mg, and Ca), can catalyze the pyrolytic reaction of biomasses, enhancing the char yield and morphological properties of the carbonaceous material produced [
25,
26,
27,
28,
29]. In that direction, we hypothesized that the concentrated leachate residue could be used as an additive to boost the yield and/or improve the char quality produced in the pyrolytic conversion. This research comparatively analyzes the slow pyrolysis of SCG using potassium hydroxide (KOH) and LLMC residue as additives. Morphological and thermal characterization were performed to discuss environmental benefits and potential applications. The experimental study showcased here represents the pioneering investigations into using LLMC as an additive in pyrolysis. This research extends and complements our previous findings [
30], shedding light on possible alternatives and ranging the spectrum for solid waste valorization.
2. Materials and Methods
2.1. Biomass and Additives
The LLMC was prepared following the procedure proposed by Grossule et al. [
31]. Hereafter, the sample was oven-dried at 105ºC for 24 h. The oven-dried residue presented water content and volatile solids/ total solids (VS/TS) ratio of 2 wt% and 51%, respectively. The LLMC solid was powdered and used in the pyrolysis tests. The proximate and ultimate analyses of LLMC residue are presented in
Table 1.
The SCG (100% Arabica blend) was provided by an Italian company. The sample was oven-dried overnight at 110ºC and stored in glass bottles. The potassium hydroxide (KOH) powder was obtained from Sigma-Aldrich. KOH is commonly used as an activating agent to develop porosity during thermal processes [
26]. This work employed KOH as the reference catalyzing agent for comparative analysis.
2.2. Pyrolysis Experimental Set-Up
Slow pyrolysis experiments were conducted using a lab-scale pyrolyzer at a heating rate of 45ºC min
-1. The bench-scale pyrolizer was positioned within an exhaust system operating at atmospheric pressure and ambient temperature (±20ºC). Pyrolysis conditions included an isothermal temperature of 600ºC, an inert gas flow of 100 cm
3 N
2 min
-1, and a residence time of 1 h. The peak temperature is a critical factor in the slow pyrolysis, significantly influencing the characteristics of the char product. Elevating the peak temperature appears to result in carbon materials with increased aromaticity, fixed carbon content, and porosity [
16]. At the same time, at temperatures higher than 800ºC, the quantity of carbon left on char is minimal, as observed in previous findings [
32]. Below 500ºC, biomass pyrolysis may produce biochar with low structural stability [
17]. The SCG and additives (i.e., KOH and LLMC residue) were pyrolyzed in a 1:1 ratio by weight. Experimental conditions were defined according to our preliminary results [
32]. Obtained chars were washed with deionized water on a paper filter and left to dry at ambient temperature (±20ºC). The activated-carbon yield was determined as a mass fraction of the initial biomass (Equation (1)). The activated-carbon materials were stored for morphological and thermal characterization.
Where m0 (in mass unit) is the initial mass of SCG and mi (in mass unit) is the mass of activated carbon produced in slow pyrolysis.
2.3. Characterization of Biomass and the Activated Materials
Moisture content (MC), volatile matter (VM), ash content, and fixed carbon (FC) were determined. The MC was measured by heating 1,000 mg of a sample at 105±5ºC for one hour in an oven; the VM was determined by heating the left residue at 950ºC for 6 min. Last, ash content was obtained by heating the samples in a furnace at 750ºC for 6 h. FC was calculated by difference following the ASTM method D1762 – 84/ 2021 [
33].
Scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM/EDS) of biomass and prepared activated-carbon materials was performed with a FEI-QUANTA200 instrument (Milan, Italy). The Brunauer-Emmett-Teller (BET) surface area and average pore size of SCG and prepared chars were calculated based on the N2 adsorption-desorption isotherms measured using a gas sorption analyzer (AutoChem II Modelo 2920, Micromeritics Instrument Ltd.).
Thermogravimetric (TG) analysis and decomposition profile were performed using TA Instruments equipment, model SDTQ600. The samples were weighed to around 5 mg. After that, they were heated from 20 to 1000ºC (in an alumina pan) at 20 °C min
-1 under an air gas flow rate of 100 mL min
-1.
Figure 1 illustrates a schematic diagram of the research steps.
3. Results and Discussion
3.1. Char Yield, Proximate Analysis, and Elemental Composition
Table 1 shows char yield and proximate content (wt%) of SCG, biochar, and activated chars using KOH and LLMC residue.
Table 2.
Yield and proximate analysis.
Table 2.
Yield and proximate analysis.
| |
SCG |
Biochar |
KOH-activated char |
LLMC-activated char |
| Mass yield (wt%) |
─ |
23.9 |
21.2 |
18.6 |
| MC (wt%) |
3.78 |
9.2 |
5.8 |
23.3 |
| VM (wt%) |
94.91 |
58.4 |
47.9 |
44.4 |
| Ashes (wt%) |
1.26 |
5.8 |
1.3 |
7.0 |
| FC (wt%) |
1.25 |
33.0 |
25.6 |
20.4 |
The biochar yields produced from SCG and activated chars were 23.9, 21.2, and 18.6%, respectively. A low mass yield of activated chars is obtained because of the high release of volatile matter catalyzed by oxidizing agents in slow pyrolysis (i.e., KOH and concentrated leachate residue). Alkali and alkaline earth metals shift the decomposition of biomass to lower temperatures while increasing the char and gas yields at the expense of bio-oil. As far as potassium additives in particular, they have been shown to promote the yields of low molecular compounds and gaseous species, which could justify the lower yield of KOH-activated char than SCG char in this study [
29]. For example, Wang et al. [
25] showed that potassium and sodium compounds promoted the reduction of char formation and made pyrolysis more exothermic.
Besides, the LLMC-activated char had a relatively lower mass yield than that obtained from KOH activation (18.6 vs. 21.2%). Alkali metals have been shown to induce a so-called synergistic effect that decreases the apparent activation energy of the pyrolysis reaction, promotes the yields of volatiles, and reduces the temperature of the maximum weight loss rate [
29]. Lower yield when concentrated leachate residue was employed could be due to the synergetic interactions between different LLMC metals (mainly Na and K) and the SCG [
34].
The proximate content of the SCG biomass is primarily dominated by volatile matter (94.91 wt%), moisture (3.78 wt%), ashes (1.26 wt%), and fixed carbon (1.25 wt%). The volatile matter was significantly reduced to 44 – 47 wt% for both activated chars, producing materials with a high fixed carbon content (20 – 33 wt%). High fixed carbon, closely related to stable carbon content, represents a beneficial feature that shows higher stability against environmental oxidation and thermal degradation [
21]. On the other hand, the high ash content of LLMC-activated char indicates that it may not be appropriated for cofiring or use as boiler fuel, as that may lead to fouling and corrosion in combustors [
35].
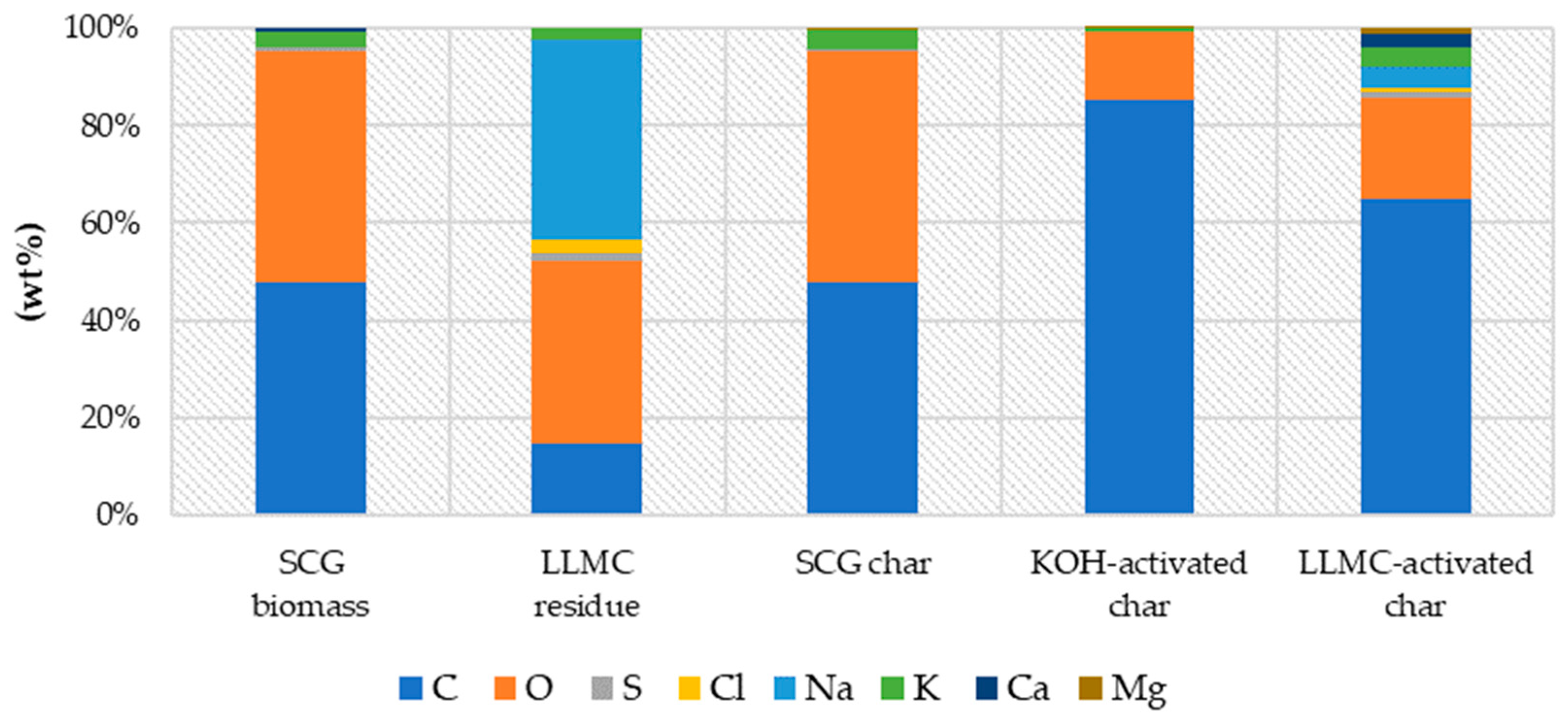
SEM/EDS was employed to observe the morphology and pore size of SCG, concentrated leachate residue, and prepared materials. EDS spectra were used to determine biomass and char composition (Table S1). The biochar obtained from SCG presented 46% wt% of carbon, and both activated chars were highly C-rich. The results showed similar carbon content in activated chars (>60 wt%) (
Figure 2). The final elemental composition of the LLMC-activated sample was the following (wt%): carbon (C) content of 63.97%, oxygen (O) content of 20.16%, sulfur (S) content of 1.43%, chloride (Cl) content of 0.47%, sodium content of 4.32%, potassium (K) content of 4.14%, calcium (Ca) content of 2.87%, and magnesium (Mg) content of 1%. Foreign elements were observed in the three prepared materials (i.e., Na, K, or S, <5% wt%).
3.2. SEM images and Porosity
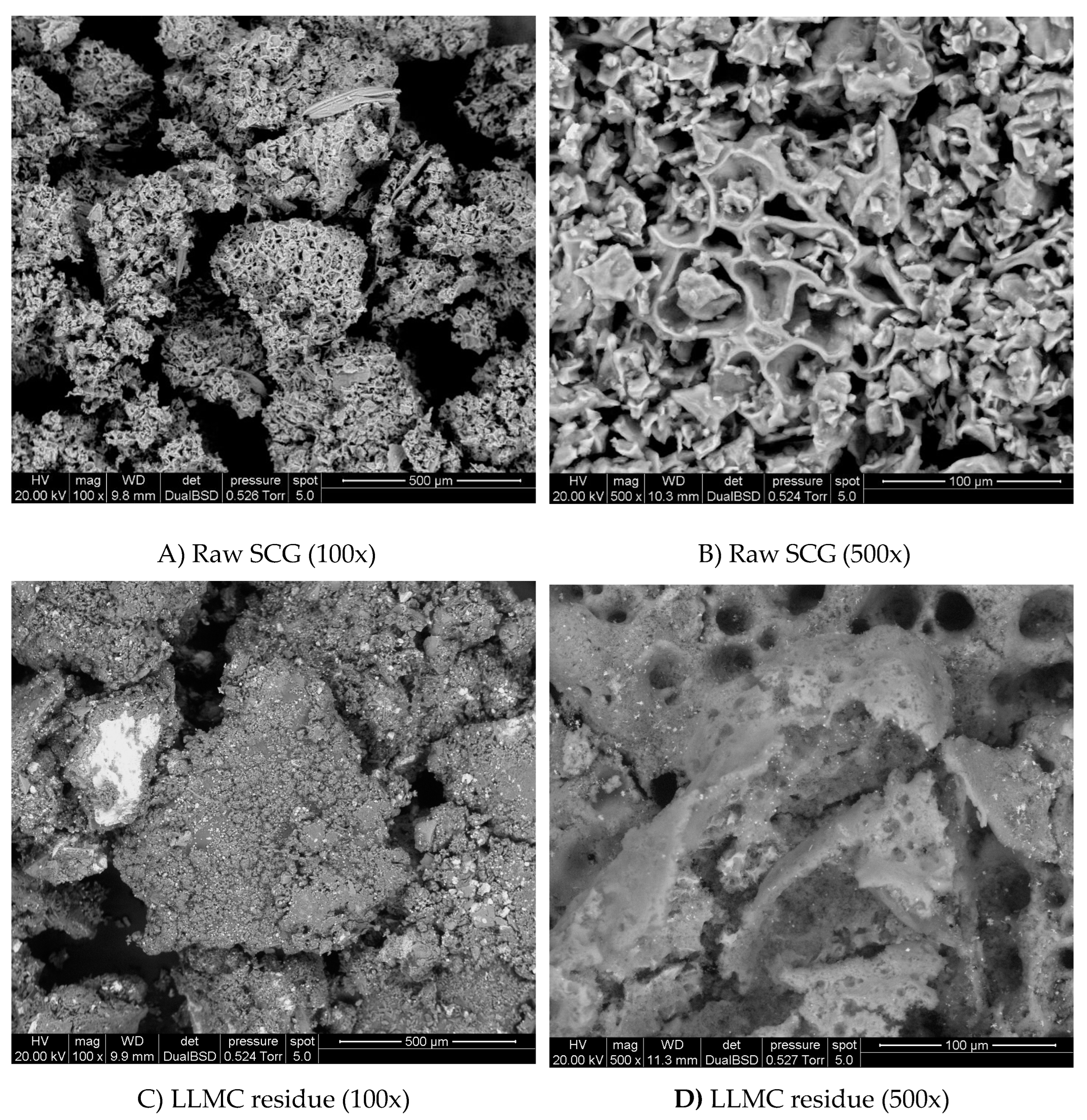
Scanning electron microscopy (SEM) was used to analyze the biomass and LLMC residue structure and surface topography (
Figure 3). The surface of the SCG is slightly porous and has a high surface roughness. The LLMC residue was brown and had an amorphous structure and irregular shape. From SEM images, many fractured particles with flaky substances are attached to them.
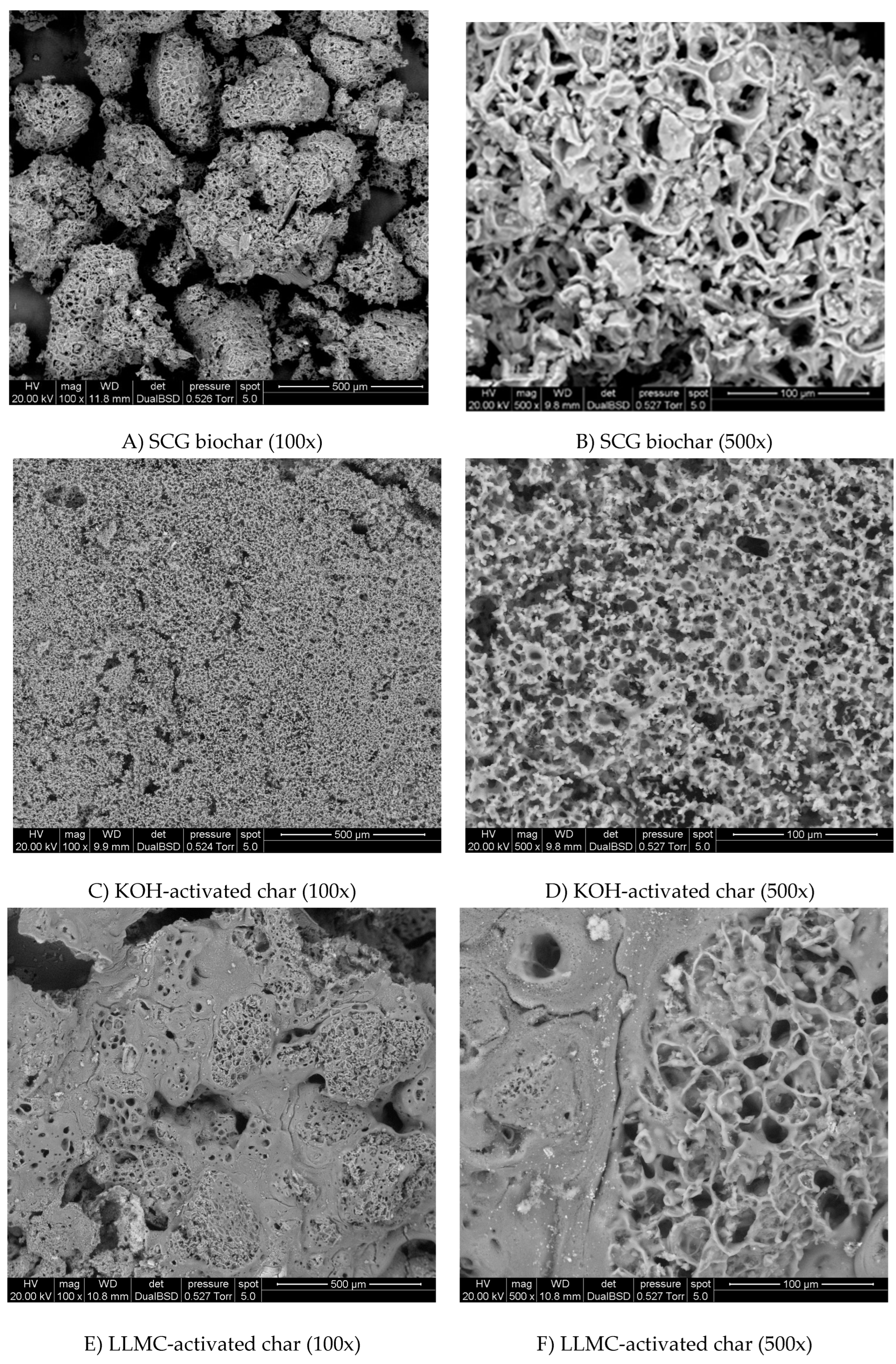
Following slow pyrolysis, biomass undergoes a notable transformation, manifesting a darker hue. SEM images of SCG, KOH-activated, and concentrated leachate-activated chars are illustrated in
Figure 4. The SCG char was denser than the precursor. From the SEM images, it owns a macro-porous structure. In contrast, the KOH-activated char has many micropores under its surface. In the LLMC-activated char, larger dispersed pores were observed, and discontinuous microstructures impregned with inorganic elements were portrayed. This portrayal suggests a unique structural arrangement compared to the other char samples.
Table 3 shows the BET surface area and average pore diameter of SCG and prepared chars at isothermal pyrolysis (600ºC) of 1 h.
The N
2 physisorption isotherms revealed that the SCG had a low BET surface area (4.5 m
2 g
-1). On the other hand, both activated chars exhibited high surface areas. In particular, the KOH-activated char displayed a higher specific surface area than the LLMC-activated char (1960 vs. 1130 m
2 g
-1). The BET surface areas for SCG and KOH-activated chars in this study were consistent with values reported in the literature (4.3 to 2230 m
2 g
-1) [
36,
37].
In terms of pore structure, KOH and LLMC-activated materials presented average pore diameters of 1.8 and 5.9 nm, respectively. According to the International Union of Pure and Applied Chemistry (IUPAC), KOH-activated char is classified as microporous. In contrast, LLMC-activated char is a mesoporous material [
38].
3.3. Thermal Analysis
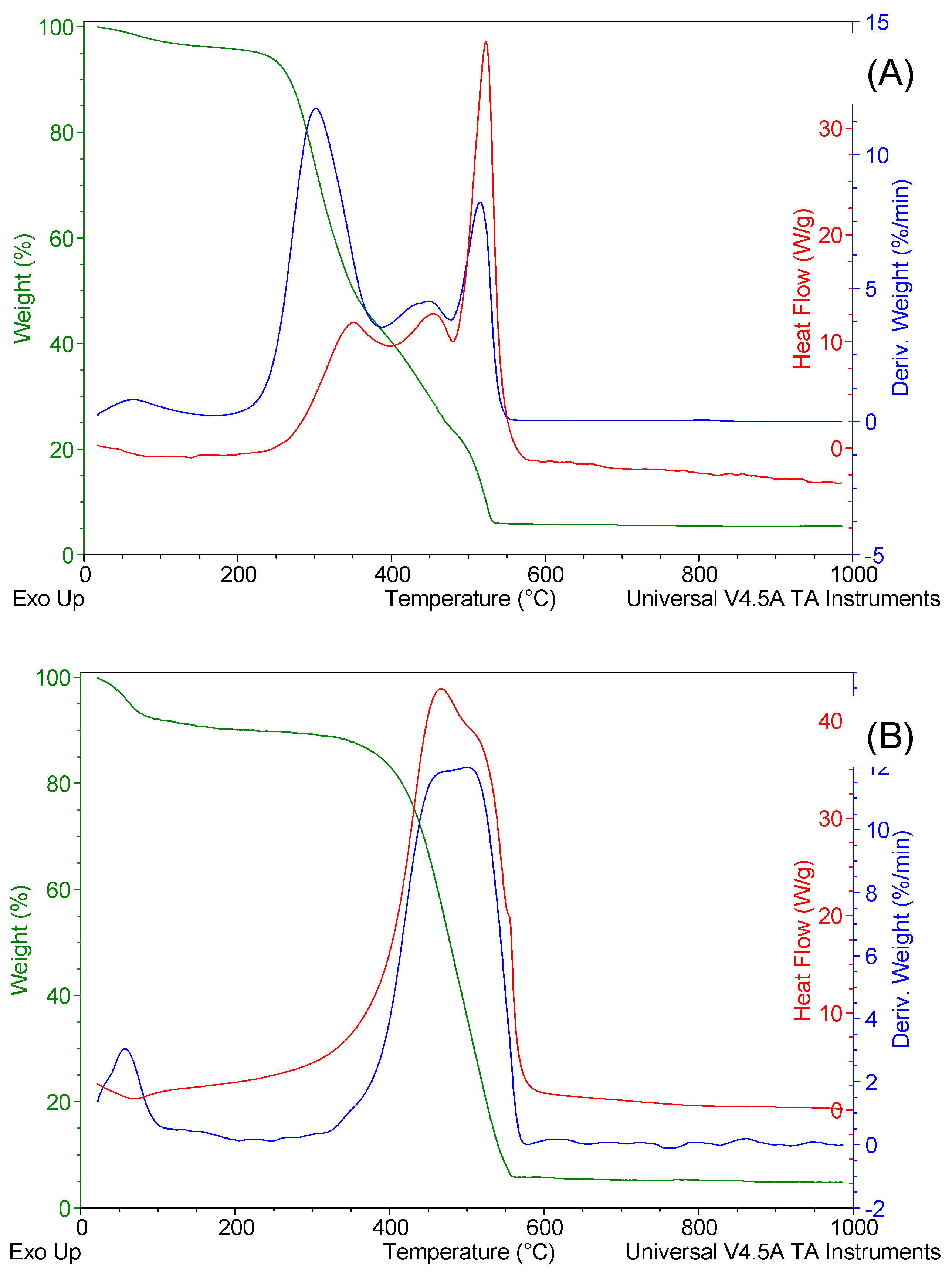
Figure 5 shows the TG, derivative thermogravimetry (DTG), and differential scanning calorimetry (DSC) curves for SCG and the produced biochar. Thermal analysis for SCG showed that free water loss occurred from 20 to 150 °C, followed by combustion of organics at around 580°C. The first DTG peak and its respective endothermic DSC at 150°C are linked to free water vaporization. DTG and exothermic DSC peaks from 150 to 580 °C are connected to the combustion of SCG organic compounds.
The most significant peaks in the DTG thermal decomposition of SCG include cellulose, lignin, and hemicellulose degradation [
28]. Wachter et al. [
39] also described three thermogravimetric peaks of the SCG in airflow. The first DTG peak was assigned to the degradation of hemicellulose, the second (lower) peak to cellulose, and the third peak to lignin decomposition. DTG peaks were observed at 337, 451°C, and 505°C [
39]. These values are very similar to those shown in Fig.5(A) (300°C and 425°C, 520ºC).
This study utilized the SCG biomass derived from Arabica coffee, and three distinct phases were observed during combustion. The first two peaks have almost the same heat flow, about 12.5 W g
-1, and the last peak has a higher heat flow of about 40 W g
-1. This finding corroborates the findings of Bejenari et al. [
40], who investigated the combustion of SCG from Arabica and Robusta coffee varieties. The combustion of Arabica coffee occurs in three stages. In addition, similar heat flows were recorded in their study [
40].
Similar behavior was observed for the produced char from that biomass. However, DTG and DSC peaks from 150 to 390 °C are not presented for SCG char, which the hemicellulose loss during the SCG pyrolysis can explain. Hemicellulose has a temperature decomposition of around 220ºC [
13]. Thus, the pyrolysis to which the SCG was submitted (600ºC) provided the total decomposition of hemicellulose and modified it thermally. In the char, it can be assumed that the first peak is linked to the thermo-oxidation of cellulose and lignin, and the last stage was probably the thermal decomposition of carbonaceous residues. Both the width of the curves and the width of the peaks are higher in the SCG char. It should be highlighted that the fixed-carbon content of char was more than 25-fold higher than that in the SCG (
Table 2). High-fixed carbon content materials often exhibit a heterogeneous structure with different types of bonds and functional groups. Thus, the decomposition process may break various chemical constituents over various temperatures, contributing to broader peaks in the DTG curve, as observed in this work [
41].
The amount of free water in SCG char was 9.18%. In the raw SCG, it is 3.87%, indicating that the SCG char can absorb more water on its external and internal surfaces due to its higher porosity. By DSC peak areas, the combustion enthalpy of SCG char is 18.11 MJ kg
-1. For SCG, it is 11.55 MJ kg
-1, showing that biochar will release more energy upon combustion. The enhanced SCG char stability and higher surface area than the raw biomass can explain this. Char formation is frequently promoted by intramolecular and intermolecular rearrangement processes, culminating in a material characterized by enhanced thermal stability [
42]. The high stability results in more complete combustion and higher energy release during combustion. Besides, the augmented surface area of char (463 vs. 4.5 m
2 g
-1) enhances combustion kinetics, enabling more effective utilization of its carbon content and releasing more energy [
43].
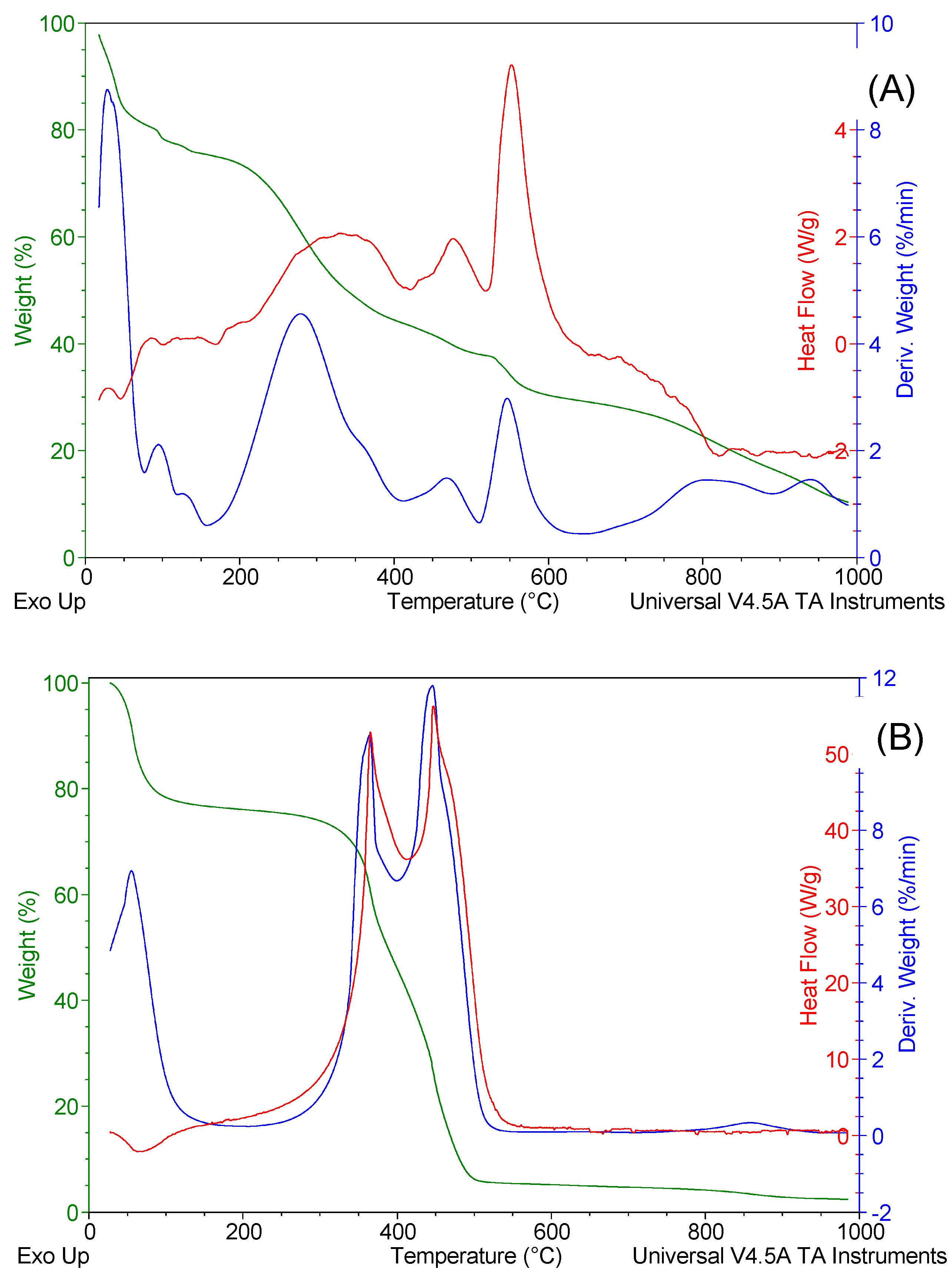
TG, DTG, and DSC curves of SCG + LLMC residue (1:1) and LLMC-activated char are illustrated in
Figure 6. For the LLMC-activated char, water loss occurred from 20 to 160 °C. Organics were combusted from 160 to 650°C. The curves showed that the hemicellulose degradation shifts toward lower temperatures (c.a. 280ºC), which indicates that the presence of the LLMC residue influences the thermal oxidation of the SCG. Inorganic salts, mainly in the LLMC residue, melt and vaporize above 650 °C. For example, magnesium chloride, potassium chloride, and sodium chloride have melting points of around 700, 770, and 800ºC, respectively [
44].
The LLMC-activated char exhibited loss of free water from room temperature up to 150 °C, identified by the DTG peak and endothermic DSC peak, followed by the combustion of pyrolyzed organic products between 150°C and 580°C, determined by the two DTG peaks and two exothermic DSC peaks. It was observed that the SCG char underwent combustion in a single DTG peak with two overlapping stages, peaking at around 500 °C (Fig. 5B). In contrast, the LLMC-activated char exhibited combustion in two stages, identified by two DTG peaks, peaking at temperatures of 360°C and 445°C (Fig. 6B). It has been underlined that alkali and alkaline metals, such as Na and K, alter the char reactivity and shift the thermal oxidation of carbon materials to lower temperatures [
25]. These elements on the carbon surface could act as the active sites for oxygen chemisorption, weakening C=C surface bonds and promoting the desorption of monoxide and carbon dioxide [
28]. We assumed that the content of alkali and alkaline metals in the LLMC residue contributed to the results obtained in this work. From thermal analysis, the water content and the combustion enthalpy in the LLMC-activated char were estimated at 23.25 wt% and 22.04 MJ kg
-1.
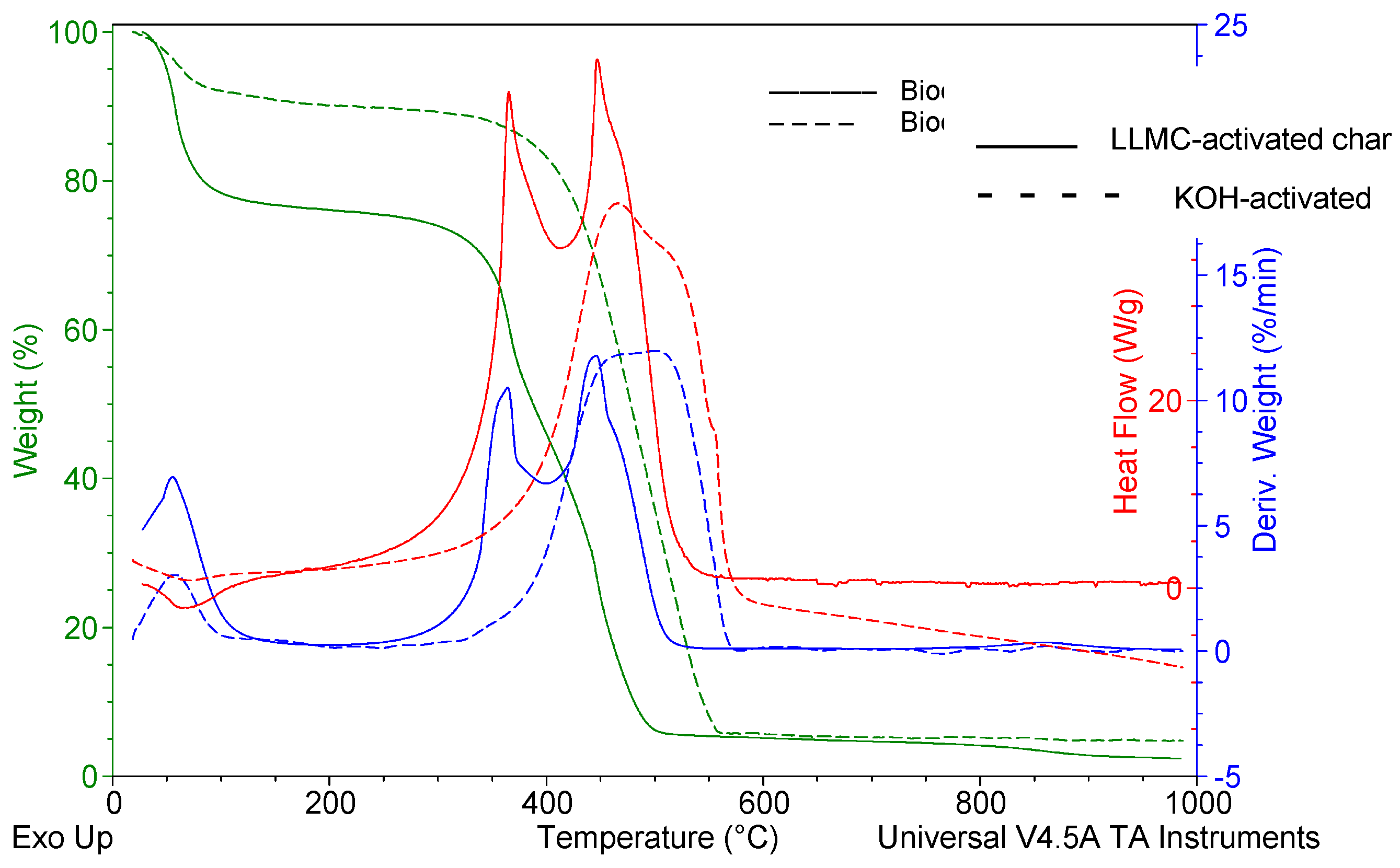
Comparing both activated chars, the DSC peaks of the LLMC-activated char were more substantial, meaning that the thermal oxidation of the LLMC-activated char is more exothermic than the KOH-activated char (
Figure 7). Besides, it was registered that the energy of the char activated using KOH was similar to the SCG char (18.4 MJ kg-1). These results are consistent with the literature [
40,
45]. All materials had net calorific values higher than the lowest limit of 16.50 MJ kg-1 stated in ISO 17225-1:2021, making it possible to direct them for usage as solid biofuels [
46].
4. Conclusions
This research focused on the slow pyrolysis of spent coffee ground biomass (SCG) using concentrated landfill leachate membrane concentrate (LLMC) as a pyrolytic additive. The conclusions from this work are: #1) The char yields in slow pyrolysis ranged from 18 to 24%. The char activated by LLMC demonstrates a mass yield lower than that achieved through KOH activation. Alkali metals have been shown to induce a so-called synergistic effect that promotes the yields of volatiles at the expense of char and oil recovery. Lower yield when LLMC residue was employed in pyrolysis could be due to the synergetic interactions between different LLMC metals (mainly Na and K) and the SCG; #2) Both activated chars showcased remarkable surface areas, with the KOH-activated char notably surpassing the specific surface area of the LLMC-activated char. This feature could render it invaluable for applications such as the adsorption of pollutants, catalyst support, and energy storage devices; and #3) the LLMC inorganic components catalyzed the carbonization of SCG, making thermal decomposition faster. By DSC peak areas, the combustion enthalpy of the activated char was estimated at 22 MJ kg-1. In addition, the elemental composition of the LLMC-activated sample identified the material as C-rich (64 wt%). Our results may aid in identifying potential industrial applications for SCG and LLMC residues, enabling the production of energy and high-value materials.
Author Contributions
Conceptualization, R.d.A., M.C.L., P.S. and R.B.; methodology, R.d.A., M.C.L., P.S. and R.B.; validation, R.d.A., M.C.L., P.S., R.B., M.M.V. and J.C.C.; formal analysis, R.d.A., M.C.L., P.S., R.B., M.M.V. and J.C.C.; investigation, R.d.A., F.L. and M.M.V.; writing—original draft preparation, R.d.A., and M.M.V.; writing—review and editing, R.d.A., and M.M.V..; supervision, M.C.L., P.S., R.B., B.R.Q., D.M.B, and J.C.C.; funding acquisition, M.C.L., P.S., R.B., R.d.A, and J.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPERJ – Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (Grant numbers E-26/200.065/2020; E-26/205.842/2022; E-26/205.843/2022) and IILA – Organização Italo Latino Americana (Grant number 40/1621).
Conflicts of Interest
The authors declare no conflict of interest.
References
- de Almeida, R.; de Souza Couto, J.M.; Gouvea, R.M.; de Almeida Oroski, F.; Bila, D.M.; Quintaes, B.R.; Campos, J.C. Nanofiltration Applied to the Landfill Leachate Treatment and Preliminary Cost Estimation. Waste Manag. Res. J. a Sustain. Circ. Econ. 2020, 38, 1119–1128. [CrossRef]
- Li, Q.; Cui, H.; Li, Y.; Song, X.; Liu, W.; Wang, Y.; Hou, H.; Zhang, H.; Li, Y.; Wang, F.; et al. Challenges and Engineering Application of Landfill Leachate Concentrate Treatment. Environ. Res. 2023, 231, 116028. [CrossRef]
- Gripa, E.; Daflon, S.D.A.; de Almeida, R.; da Fonseca, F.V.; Campos, J.C. Landfill Leachate Treatment by High-Pressure Membranes and Advanced Oxidation Techniques with a Focus on Ecotoxicity and by-Products Management: A Review. Process Saf. Environ. Prot. 2023, 173, 747–764. [CrossRef]
- de Almeida, R.; Porto, R.F.; Hinojosa, M.A.G.; Sanches, L.C.M.; Monteiro, B.B.; Conde, A.L.F.M.; Costa, A.M.; Quintaes, B.R.; Bila, D.M.; Campos, J.C. Monitoring of Experimental Landfill Cells with Membrane Concentrate Infiltration: A Systematic Assessment of Leachate Quality and Treatment Performance. Process Saf. Environ. Prot. 2022, 168, 1155–1165. [CrossRef]
- Chamem, O.; Fellner, J.; Zairi, M. Ammonia Inhibition of Waste Degradation in Landfills – A Possible Consequence of Leachate Recirculation in Arid Climates. Waste Manag. Res. J. a Sustain. Circ. Econ. 2020, 38, 1078–1086. [CrossRef]
- de Almeida, R.; Porto, R.F.; Quintaes, B.R.; Bila, D.M.; Lavagnolo, M.C.; Campos, J.C. A Review on Membrane Concentrate Management from Landfill Leachate Treatment Plants: The Relevance of Resource Recovery to Close the Leachate Treatment Loop. Waste Manag. Res. J. a Sustain. Circ. Econ. 2023, 41, 264–284. [CrossRef]
- Zhang, L.; Lavagnolo, M.C.; Bai, H.; Pivato, A.; Raga, R.; Yue, D. Environmental and Economic Assessment of Leachate Concentrate Treatment Technologies Using Analytic Hierarchy Process. Resour. Conserv. Recycl. 2019, 141, 474–480. [CrossRef]
- Keyikoglu, R.; Karatas, O.; Rezania, H.; Kobya, M.; Vatanpour, V.; Khataee, A. A Review on Treatment of Membrane Concentrates Generated from Landfill Leachate Treatment Processes. Sep. Purif. Technol. 2021, 259, 118182. [CrossRef]
- Wang, H.; Zhang, L.; Wang, J.; Li, M.; Dong, X.; Yue, D. Fabricating Functionalized Carbon Nitride Using Leachate Evaporation Residue and Its Adsorptive Application. Sep. Purif. Technol. 2024, 341, 126961. [CrossRef]
- Manyà, J.J.; Azuara, M.; Manso, J.A. Biochar Production through Slow Pyrolysis of Different Biomass Materials: Seeking the Best Operating Conditions. Biomass and Bioenergy 2018, 117, 115–123. [CrossRef]
- Manikandan, S.; Vickram, S.; Subbaiya, R.; Karmegam, N.; Woong Chang, S.; Ravindran, B.; Kumar Awasthi, M. Comprehensive Review on Recent Production Trends and Applications of Biochar for Greener Environment. Bioresour. Technol. 2023, 388, 129725. [CrossRef]
- Li, Y.; Gupta, R.; Zhang, Q.; You, S. Review of Biochar Production via Crop Residue Pyrolysis: Development and Perspectives. Bioresour. Technol. 2023, 369, 128423. [CrossRef]
- Johnson, K.; Liu, Y.; Lu, M. A Review of Recent Advances in Spent Coffee Grounds Upcycle Technologies and Practices. Front. Chem. Eng. 2022, 4, 1–15. [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [CrossRef]
- Conte, P.; Bertani, R.; Sgarbossa, P.; Bambina, P.; Schmidt, H.-P.; Raga, R.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P. Recent Developments in Understanding Biochar's Physical–Chemistry. Agronomy 2021, 11, 615. [CrossRef]
- Manyà, J.J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [CrossRef]
- Bertero, M.; Sedran, U. Coprocessing of Bio-Oil in Fluid Catalytic Cracking. In Recent Advances in Thermo-Chemical Conversion of Biomass; Elsevier, 2015; pp. 355–381 ISBN 9780444632906.
- Prado, L.L.; Melo, V.F.; Braga, M.C.; Motta, A.C. V.; Araújo, E.M. Pyrolyzed Sewage Sludge Used in the Decontamination of Landfill Leachate: Ammonium Adsorption. Int. J. Environ. Sci. Technol. 2023, 20, 9129–9142. [CrossRef]
- Celso Monteiro Zanona, V.R.; Rodrigues Barquilha, C.E.; Borba Braga, M.C. Removal of Recalcitrant Organic Matter of Landfill Leachate by Adsorption onto Biochar from Sewage Sludge: A Quali-Quantitative Analysis. J. Environ. Manage. 2023, 344, 118387. [CrossRef]
- Nav, T.Z.; Pümpel, T.; Bosch, D.; Bockreis, A. Insight into the Application of Biochars Produced from Wood Residues for Removing Different Fractions of Dissolved Organic Material (DOM) from Bio-Treated Wastewater. Environ. Technol. Innov. 2023, 32, 103271. [CrossRef]
- Yek, P.N.Y.; Li, C.; Peng, W.; Wong, C.S.; Liew, R.K.; Wan Mahari, W.A.; Sonne, C.; Lam, S.S. Production of Modified Biochar to Treat Landfill Leachate Using Integrated Microwave Pyrolytic CO2 Activation. Chem. Eng. J. 2021, 425, 131886. [CrossRef]
- Igwegbe, C.A.; Kozłowski, M.; Wąsowicz, J.; Pęczek, E.; Białowiec, A. Nitrogen Removal from Landfill Leachate Using Biochar Derived from Wheat Straw. Materials (Basel). 2024, 17, 928. [CrossRef]
- Ben Hassen-Trabelsi, A.; Kallel, A.; Ben Amor, E.; Cherbib, A.; Naoui, S.; Trabelsi, I. Up-Grading Biofuel Production by Co-Pyrolysis of Landfill Leachate Concentrate and Sewage Sludge Mixture. Waste and Biomass Valorization 2020, 11, 291–301. [CrossRef]
- Li, Y.; Chen, X.; Liu, L.; Liu, P.; Zhou, Z.; Huhetaoli; Wu, Y.; Lei, T. Characteristics and Adsorption of Cr(VI) of Biochar Pyrolyzed from Landfill Leachate Sludge. J. Anal. Appl. Pyrolysis 2022, 162, 105449. [CrossRef]
- Wang, J.; Zhang, M.; Chen, M.; Min, F.; Zhang, S.; Ren, Z.; Yan, Y. Catalytic Effects of Six Inorganic Compounds on Pyrolysis of Three Kinds of Biomass. Thermochim. Acta 2006, 444, 110–114. [CrossRef]
- Rosson, E.; Garbo, F.; Marangoni, G.; Bertani, R.; Lavagnolo, M.C.; Moretti, E.; Talon, A.; Mozzon, M.; Sgarbossa, P. Activated Carbon from Spent Coffee Grounds: A Good Competitor of Commercial Carbons for Water Decontamination. Appl. Sci. 2020, 10, 5598. [CrossRef]
- Wongmat, Y.; Wagner, D.R. Effect of Potassium Salts on Biochar Pyrolysis. Energies 2022, 15, 5779. [CrossRef]
- Safar, M.; Lin, B.-J.; Chen, W.-H.; Langauer, D.; Chang, J.-S.; Raclavska, H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Catalytic Effects of Potassium on Biomass Pyrolysis, Combustion and Torrefaction. Appl. Energy 2019, 235, 346–355. [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the Catalytic Effects of Alkali and Alkaline Earth Metals (AAEMs) Including Sodium, Potassium, Calcium and Magnesium on the Pyrolysis of Lignocellulosic Biomass and on the Co-Pyrolysis of Coal with Biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [CrossRef]
- de Almeida, R.; Lanero, F.; Lavagnolo, M.C.; Sgarbossa, P.; Bertani, R.; Vianna, M.M.; Campos, J.C. Thermal Characterization of Biochars Produced in Slow Co-Pyrolysis of Spent Coffee Ground and Concentrated Landfill Leachate Residue. In Proceedings of the ECP 2023; MDPI: Basel Switzerland, May 17 2023; p. 12.
- Grossule, V.; Fang, D.; Yue, D.; Lavagnolo, M.C.; Raga, R. Preparation of Artificial MSW Leachate for Treatment Studies: Testing on Black Soldier Fly Larvae Process. Waste Manag. Res. J. a Sustain. Circ. Econ. 2022, 0734242X2110667. [CrossRef]
- Almeida, R. De Landfill Leachate Treatment by Membrane-Based Technologies: Cost-Benefit Analysis, Membrane Concentrate Management, and Perspectives, Universidade Federal do Rio de Janeiro, 2022.
- ASTM Standard Test Method for Chemical Analysis of Wood Charcoal 2021, 2.
- Rijo, B.; Soares Dias, A.P.; de Jesus, N.; Pereira, M.F. Home Trash Biomass Valorization by Catalytic Pyrolysis. Environments 2023, 10, 186. [CrossRef]
- Huang, C.-W.; Li, Y.-H.; Xiao, K.-L.; Lasek, J. Cofiring Characteristics of Coal Blended with Torrefied Miscanthus Biochar Optimized with Three Taguchi Indexes. Energy 2019, 172, 566–579. [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [CrossRef]
- Alcaraz, L.; Escudero, M.E.; Alguacil, F.J.; Llorente, I.; Urbieta, A.; Fernández, P.; López, F.A. Dysprosium Removal from Water Using Active Carbons Obtained from Spent Coffee Ground. Nanomaterials 2019, 9, 1372. [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterization of Porous Solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [CrossRef]
- Wachter, I.; Rantuch, P.; Drienovský, M.; Martinka, J.; Ház, A.; Štefko, T. Determining the Activation Energy of Spent Coffee Grounds By the Thermogravimetric Analysis. J. Chem. Technol. Metall. 2022, 57, 1006–1018.
- Bejenari, V.; Marcu, A.; Ipate, A.-M.; Rusu, D.; Tudorachi, N.; Anghel, I.; Şofran, I.-E.; Lisa, G. Physicochemical Characterization and Energy Recovery of Spent Coffee Grounds. J. Mater. Res. Technol. 2021, 15, 4437–4451. [CrossRef]
- Protásio, T. de P.; Melo, I.C.N.A. de; Guimarães Junior, M.; Mendes, R.F.; Trugilho, P.F. Thermal Decomposition of Torrefied and Carbonized Briquettes of Residues from Coffee Grain Processing. Ciência e Agrotecnologia 2013, 37, 221–228. [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar Production Techniques Utilizing Biomass Waste-Derived Materials and Environmental Applications – A Review. J. Hazard. Mater. Adv. 2022, 7, 100134. [CrossRef]
- Leng, L.; Huang, H. An Overview of the Effect of Pyrolysis Process Parameters on Biochar Stability. Bioresour. Technol. 2018, 270, 627–642. [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; Haynes, W.M., Ed.; CRC Press, 2014; ISBN 9780429170195.
- Ben Abdallah, A.; Ben Hassen Trabelsi, A.; Navarro, M.V.; Veses, A.; García, T.; Mihoubi, D. Pyrolysis of Tea and Coffee Wastes: Effect of Physicochemical Properties on Kinetic and Thermodynamic Characteristics. J. Therm. Anal. Calorim. 2023, 148, 2501–2515. [CrossRef]
- ISO 17225-1 International Standard for Solid Biofuels — Fuel Specifications and Classes. 2021, 2021.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).