1. Introduction
Since the discovery of Bakelite in 1907, there has been increasing interest in macromolecular science to exploit the beneficial characteristics of plastics, including their low density, lightweight, high tensile strength, low heat and electrical conductivity, and resistance to degradation. This interest has been fueled by technological progress. As time has passed, plastics have taken the place of traditional materials like wood, metals, and paper in important uses. Consequently, plastics are used in several fields such as biomedicine, healthcare, automotive and aerospace industries, and environmental sciences (Khan et al., 2022).
The output of plastics worldwide has consistently grown, rising from 5 million metric tons (Mt) in the 1950s to over 300 Mt in 2015 (Hamidian et al., 2021). They usually exist as a mix of polymers, pigments, superplasticizers, and flame retardants (Z. Xu et al., 2021).
Microplastics (MP) are small particles from several origins usually less than 5000μm (Sol et al., 2020; Wolff et al., 2019). MPs are categorized based on size, form, polymer characteristics, and source. According to the source, microplastics can be further classified as primary or secondary. Secondary MPs are created by the breakdown of bigger plastics through some processes (physical, chemical, or biological) (Ziajahromi et al., 2017). Secondary sources of MPs include home and industrial items, vehicle tires, color flakiness, and fragmentation during plastic production (Hamidian et al., 2021). Primary MPs are found in cleaning and cosmetic items, paints, etc (Sol et al., 2020; Wei et al., 2020).
Polymer MP types include polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinylchloride (PVC), polyacrylamide (PAM), and polyethylene terephthalate. MPs may be carriers of harmful compounds including Persistent organic pollutants and heavy metals (Novotna et al., 2019; Pivokonský et al., 2020; Wu et al., 2021). MP has been extensively investigated for its ability to adsorb and transport both inorganic and organic contaminants into various species. Although slower than dissolved organic materials, the rate of desorption depends on the content (Bayo et al., 2020). Due to their small size and large specific surface area, they have the ability to adsorb contaminants such as polycyclic aromatic hydrocarbons, heavy metals, polybrominated diphenyl ethers, and pharmaceutical and personal care products from the environment (Enfrin et al., 2019; Wang et al., 2020; Wei et al., 2020). Consequently, microplastics consistently induce chronic toxicity by accumulating in organisms (Khan et al., 2022; Sun et al., 2019).
MP identification mechanisms include visual observations, Fourier Transform Infrared (FT-IR) or Raman Spectroscopy, although these methods have different degrees of efficiency (Ziajahromi et al., 2017). Several MP treatment technologies have been devised, and membrane separations, microbial and treatment, and micromotor approaches have been used recently.
MPs pose a significant risk to the environment due to their durability and resilience (Iyare et al., 2020; Padervand et al., 2020; Wolff et al., 2019). MPs create concerns since they can be mistaken for food and eaten by marine species. MP use can harm both vertebrates and invertebrates' growth, reproduction and overall health. Damage to the digestive tract can result in intestinal blockage, diminished enzyme production, and negative hormone levels (Khan et al., 2022; Novotna et al., 2019; Sun et al., 2019; Wolff et al., 2019).
Wastewater treatment plants (WWTPs) are the principal receivers of microplastics from land through pipe networks before they enter natural aquatic systems. These plants transform primary microplastics into secondary microplastics (Hamidian et al., 2021; Jiang et al., 2022; Liu et al., 2021). WWTPs serve as both barriers and entry points for microplastics (MPs) into soil and water through sludge disposal and outflow (Jiang et al., 2022). Microplastics from sewage treatment may remain in sludge, which can then be put to land for agricultural purposes (Iyare et al., 2020). WWTPs have a significant role in the transport and diffusion of MPs, prompting extensive research into their particle abundance. High concentrations of MP particles in water can clog and destroy WTP filter systems due to particle caking or wear. WWTPs use shear forces to break down MPs, lowering the number of dangerous MPs released into the water (Enfrin et al., 2019).
Some success has been recorded in MP degradation by treatment with Hydrochloric acid (HCl), Hydrogen trioxonitrate (V) acid (HNO3), Sodium hydroxide (NaOH), Potassium hydroxide (KOH), and enzymes (Raju et al., 2020). They could also be degraded into smaller particles through both abiotic (UV radiation) and biotic (biodegradation) mechanisms (Wolff et al., 2019). Microbial degradation of MPs involves breakdown into oligomers, dimers, and monomers under conditions such as temperature, salinity, pH, and moisture content (Krishnan et al., 2023).
The various methods for eliminating microplastics from wastewater include screening and filtration, rapid sand filtration, dissolved air flotation, membrane processes, membrane bioreactor, electrocoagulation, and activated sludge process (Krishnan et al., 2023).
Coagulation and granular media filtration are the primary methods used in traditional treatment to remove MPs. Coagulants are chosen to counteract the electric charges on organic matter and pathogens. However, since the surface properties of MPs differ and the treatment process is not specifically tailored for their elimination, the coagulants used for removing organics may not be the most effective for microplastics. Filtration eliminates residual particles and flocs that persist after clarity. A typical change made to traditional treatment techniques involves substituting sand/anthracite filter media with Granular Activated Carbon (GAC) to enhance the removal of organic substances. GAC procedures can be enhanced to facilitate the establishment of biofilms, which can effectively remove organic substances once the initial capacity for adsorption has been depleted. Ozone can enhance the effectiveness of biofiltration by decomposing natural organic matter (NOM) into substances that are more easily degraded by biological processes (Cherniak et al., 2022). Although WWTPs have excellent MP removal efficiency partly due to MP partitioning from liquid to sludge, total removal from wastewater remains unobtainable (Jiang et al., 2022; Raju et al., 2020; Wu et al., 2021).
This review examines both traditional and innovative treatment techniques employed to eliminate microplastics (MPs) from water and evaluates their efficacy in treatment of microplastics. This review further focuses on one of the MP treatment systems that has demonstrated effectiveness in containing microplastics.
2. Sources and Detection of Microplastics
2.1. Sources
Variations in form, size, weight, and density of MPs make them easily transported and dispersed by storm sewers, wind, and other natural currents (Padervand et al., 2020). Wastewater treatment facilities are another important source of microplastic emission. Microplastics can get through wastewater treatment units and accumulate in the aquatic environment, while larger plastic particles are effectively eliminated (Padervand et al., 2020) (Khan et al., 2022). WWTPs can remove up to 99% of microplastics from wastewater but they are still found in sewage sludge or biosolids, which have become a primary source of fertilizers as reported in America, Europe, and Australia (Khan et al., 2022). In a recent experiment, MPs were extracted from sludge using a three-step process of purification, density separation, and filtration (Alavian Petroody et al., 2021).
The most commonly identified MPs in wastewater were PET, PE, PP, and PS. Humans mostly use and consume these materials, which contribute to the issue. Polyester (PE/PES) and polyamide (PA) make up a significant component of MPs detected in wastewater (Zhang et al., 2020). PP is commonly used for food packaging, containers, and bags. PE is used for plastic bottles and toys. PVC is used in buildings, PS in electronics, and PES in clothing (Khan et al., 2022) (Sun et al., 2019).
Common forms of MPs include fibers, pellets, spheres, plastic beads, foams, pieces, and films (Khan et al., 2022) (Novotna et al., 2019) (Iyare et al., 2020). Alternatively, the most common microplastics identified in wastewater are fragments and fibers, followed by microbeads and film. Foam is rarely present (Zhang et al., 2020). Microplastic particles (MPPs) differ in origin and abundance from microplastic fibers (MPFs), which are ribbon-shaped with shredded ends (Magni et al., 2019). The clothing industry is principally responsible for the emission of microfibers, which are generated during various production stages. Other sources include oil and gas usage and industrial abrasives (Khan et al., 2022). In a research by Wang et al., (2020), fibers accounted for 53.9-73.9% of microplastics in raw water, outnumbering the spheres and fragments which contributed 8.6-20.6% and 17.6-25.5%, respectively.
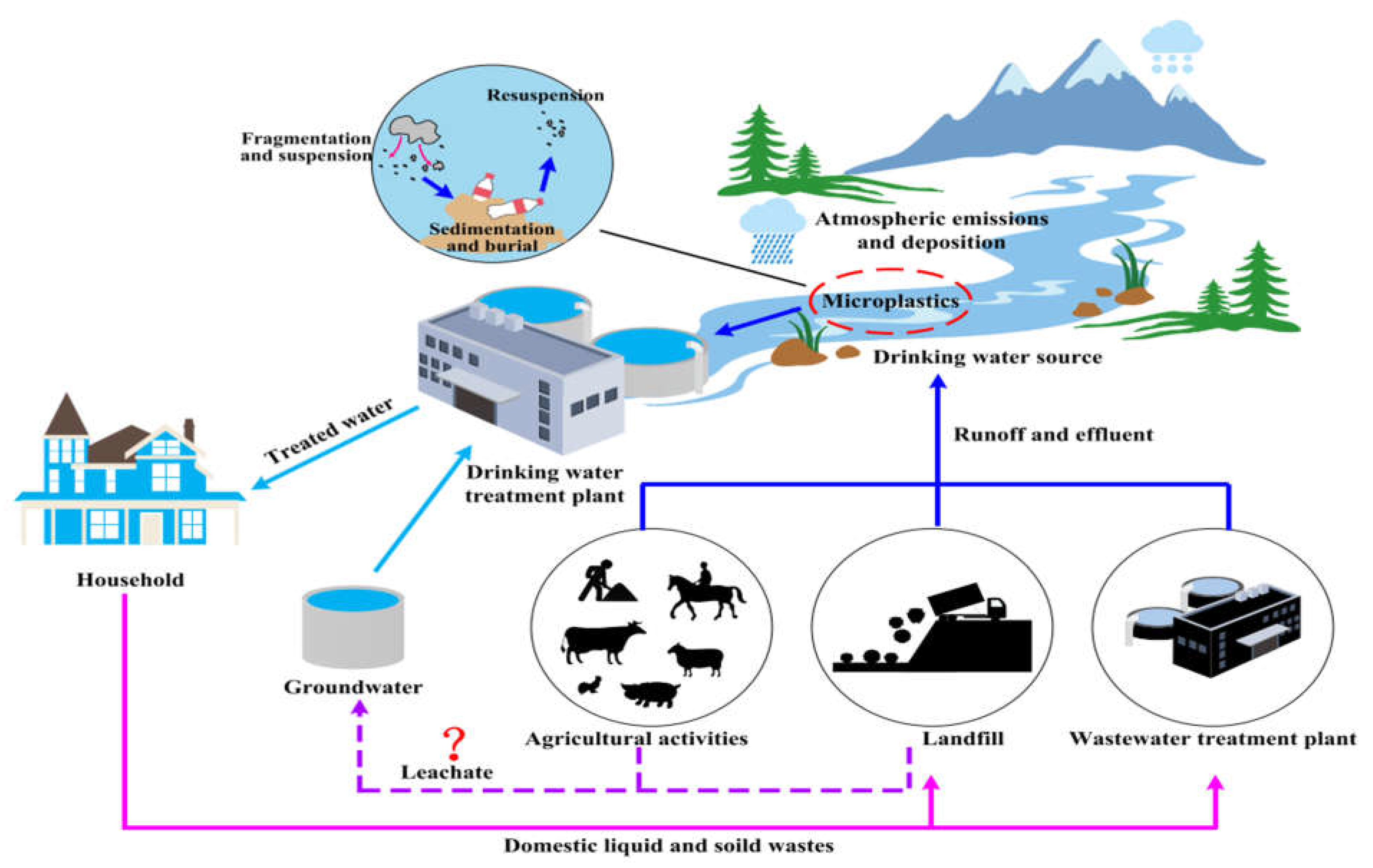
Figure 1.
shows the sources and movement of microplastics in raw and treated drinking water. Human-induced causes such as agricultural operations and wastewater discharge are more prevalent. The question of whether leachate can transfer microplastics into groundwater requires further discussion (adapted from Shen et al., 2020).
Figure 1.
shows the sources and movement of microplastics in raw and treated drinking water. Human-induced causes such as agricultural operations and wastewater discharge are more prevalent. The question of whether leachate can transfer microplastics into groundwater requires further discussion (adapted from Shen et al., 2020).
2.2. Sampling and Detection
MPs have been detected using several approaches, such as sieving, chemical digestion, density separation, and filtration (Khan et al., 2022) (Novotna et al., 2019).
Microplastics are often collected using sieving or filtering with different mesh/pore diameters to identify their size categories (Gatidou et al., 2019). Ziajahromi et al., (2017) used four replaceable stainless-steel mesh screens (simple Dutch weave) as sampling apparatus with diameters of 500, 190, 100, and 25 μm. To prevent overestimation of MP numbers, particles on steel mesh screens with a mesh size of 37 mm were dyed with 0.2 mg/mL of Bengal Rose solution. To identify MPs, needles were used to distinguish fibers (longer than diameter and about the same thickness) and particles (spherical, shapeless, and sometimes porous). The color and number of each were recorded. Micro Raman spectroscopy was used to evaluate the structure of suspected MPs (Alavian Petroody et al., 2021). A review on research on microplastics in wastewater by Gatidou et al., (2019) has been focused on North Europe, the United States, and Australia. Microplastic levels in raw and treated wastewater were between 1-3160 and 0.0007-125 particles per liter, respectively.
Hydrogen peroxide is commonly used to digest organic matter from wastewater. To make the digestion fast, Fenton’s regent is usually added. Acid and alkaline treatments have been found to give good digestion results when applied carefully. Enzymatic decomposition is a developing method to remove organic substances from MPs. Inorganic matter digestion is usually done by density separation involving salts such as NaCl, ZnCl2, NaI, and NaBr (Hamidian et al., 2021). To eliminate organic material from the sample matrix, Conley et al., (2019) added hydrogen peroxide (H2O2(aq)) to the samples, at 65 degrees Celsius. HCl was added to dried samples and reacted over 30 minutes at 65 °C to dissolve inorganics and non-plastic components. According to Magni et al., (2019), MPs were separated from particulate matter using a sodium chloride (NaCl) hypersaline solution based on a density gradient. To purify the sample, a 30% solution of hydrogen peroxide (H2O2) was added until it reached 3% of the volume. The samples were heated to 70°C and agitated for 5 hours. This process was repeated until a clear sample was achieved. The samples were held at 70°C to fully evaporate the H2O2 and water (Alavian Petroody et al., 2021).
In recent years, MP detection has advanced using spectroscopy and thermo-analytical approaches. Spectroscopy can identify particle chemical composition, however certain compounds, such as dyes, can mask the material's spectra and make identification difficult. The presence of a dye can identify a microparticle as anthropogenic, but it may not always be plastic. Raman spectroscopy and Fourier-Transform Infrared (FTIR) are commonly employed to identify MPs, with Raman spectroscopy capable of detecting up to 1 μm (Khan et al., 2022) (Iyare et al., 2020). Examples of mass-based methods include thermal degradation with chromatography/mass spectrometry, pyrolysis, thermal desorption-gas chromatography-TED-GC/MS mass spectrometry, thermal desorption-proton transfer reaction-TD-PTR/MS mass spectrometry, matrix-assisted laser desorption/MALDI-ToF/MS time-of-flight mass spectrometry, and vibrational spectroscopy. They are better used with other methods as they are destructive (Khan et al., 2022). Microplastics were counted using a stereomicroscope equipped with UV and white light, as well as a digital camera (Conley et al., 2019). In a recent experiment to identify MPs, a stereomicroscope was used to inspect wastewater and sludge filters after organic matter digestion. To discriminate between plastic and non-plastic components, all suspected plastic particles and fibers were manually collected and examined on a clean filter. Smaller particles, particularly those between 30 μm and 10 μm, may be underestimated (sometimes up to 70% error) despite experienced researchers' stringent visual technique. Visual examination of particles before chemical characterization is important for isolating them from filters that cannot be entirely removed of organic materials and/or mineral components without using destructive or costly procedures (Magni et al., 2019). Talvitie et al., (2017) examined all samples under a stereo microscope. Textile fibers and plastic particles were counted and classified into fragments, flakes, films, and spheres, with colors reported. Particles having cellular structures and soft, readily degraded (organic) components were eliminated from further analysis. The chemical composition of pre-selected particles was examined using imaging FTIR spectroscopy (X. Xu et al., 2019).
2.3. Physical and Chemical Properties of Microplastics in Water (Enfrin et al., 2019)
Large plastic items are fragmented through a variety of mechanisms, either individually or collectively, including photo-oxidation by UV light, hydrolysis, mechanical fracture due to sand abrasion or water turbulence, and bio-assimilation by microorganisms. UV light in water and outdoor circumstances can cause oxidation of plastic pellets like PE, PP, PS, PET, and PLA. Hydrolysis is one of the primary degradation processes for polymers containing heteroatoms, including poly(urethane) (PU) and PET (Enfrin et al., 2019).
The fragmentation mechanism is determined by ambient conditions, polymer material, and additives in the plastic, all of which can impact the material's physicochemical qualities. Antioxidants and UV stabilizers, such as Bisphenol A and Nonylphenol, are used to prevent plastic polymers from fragmenting due to UV radiation oxidation (Enfrin et al., 2019).
A study found that plastic litter produced MPs within 8 weeks of contact to salt marshes, despite the fact that breakdown of plastics in the environment often takes decades. Bacillus sp. and Rhodococcus sp. strains degraded PP particles by 6.3% over 40 days, while I. sakaiensis degraded nearly an entire PET film of 60 mg in 6 weeks at 30°C (Enfrin et al., 2019).
PE and PP are hydrophobic materials, which like to blend in water. Nonetheless, the nanoscale and micrometer sizes of NPs/MPs will have a substantial impact on the stability of these agglomerates in water (Enfrin et al., 2019). The Derjaguin, Landau, Verwey Overbeek (DLVO) theory describes colloid stability in water and applies to NPs and MPs under 1 mm in size. The interparticle distance h determines the balance of electrostatic repulsion energy Vedl and Van der Waals attraction energy, leading to particle stability, agglomeration, or aggregation in suspension as shown in (1). This equation can be used to investigate interactions of MPs/NPs in water. MPs potential energy is decreased and interparticle reduced until agglomeration whereby they are changeably dense (secondary minimum). Kinetic energy is caused by motion and particles are unchangeably dense (primary minimum).
This theory can be influenced by several factors, including particle surface charge and size, as well as the ionic strength of the liquid phase. A charged surface stabilizes the system by increasing electrostatic repulsion, while high particle concentrations diminish interparticle distance, leading to agglomeration. NPs with a high surface charge density agglomerate in a loose pattern when the surface charge exceeds 30 mV (Figure 1b). The particles' electrical double layer (EDL) affect the liquid phase's ionic strength. The hydrophobic characteristic of plastic materials in water leads to increased adsorption of hydroxide ions, resulting in a negatively charged particle surface. Suspended MPs will probably exist either as single particles or loose particles. In conclusion, shear force is able to reverse homogenous agglomeration of MPs in water.
There is also a possibility of heterogenous combination with bacteria or organic matters. When PS NPs and MPs were exposed to bacterial glycoprotein exopolymeric substances (EPS), they formed agglomerates with the microbial population due to polymer chain entanglement. Low EPS concentrations helped disperse NPs and MPs by removing their hydrophobic characteristics, but did not create enough entanglements to cause agglomeration. Aquatic microorganisms create EPS, which is a major component of water's dissolved organic matter. Additionally, plastic additives and chemical adsorption in water alter particle surface chemistry, impacting agglomeration and dispersion behavior. New functional groups such as chloro-halides, aliphatic and aromatic hydrocarbons have been formed due to the lipophilic nature of some MPs.
2.4. Composition
Microplastics contain a wide range of chemical additives, including bisphenol A, phthalates, and polybrominated diphenyl ethers, which are used to increase plasticity in raw plastic production. These additives are endocrine disruptors, which means they may have harmful effects when released (Padervand et al., 2020).
Plasticizer concentrations in plastic debris on remote and urban beaches range from 35 to 700 ng/g for bisphenol A, 0.1 to 400 ng/g for polybrominated diphenyl ethers, and up to 3940 ng/g for phthalates. The leaching of bisphenol A and nonylphenol from silicone and polycarbonate microplastics was reported (Padervand et al., 2020).
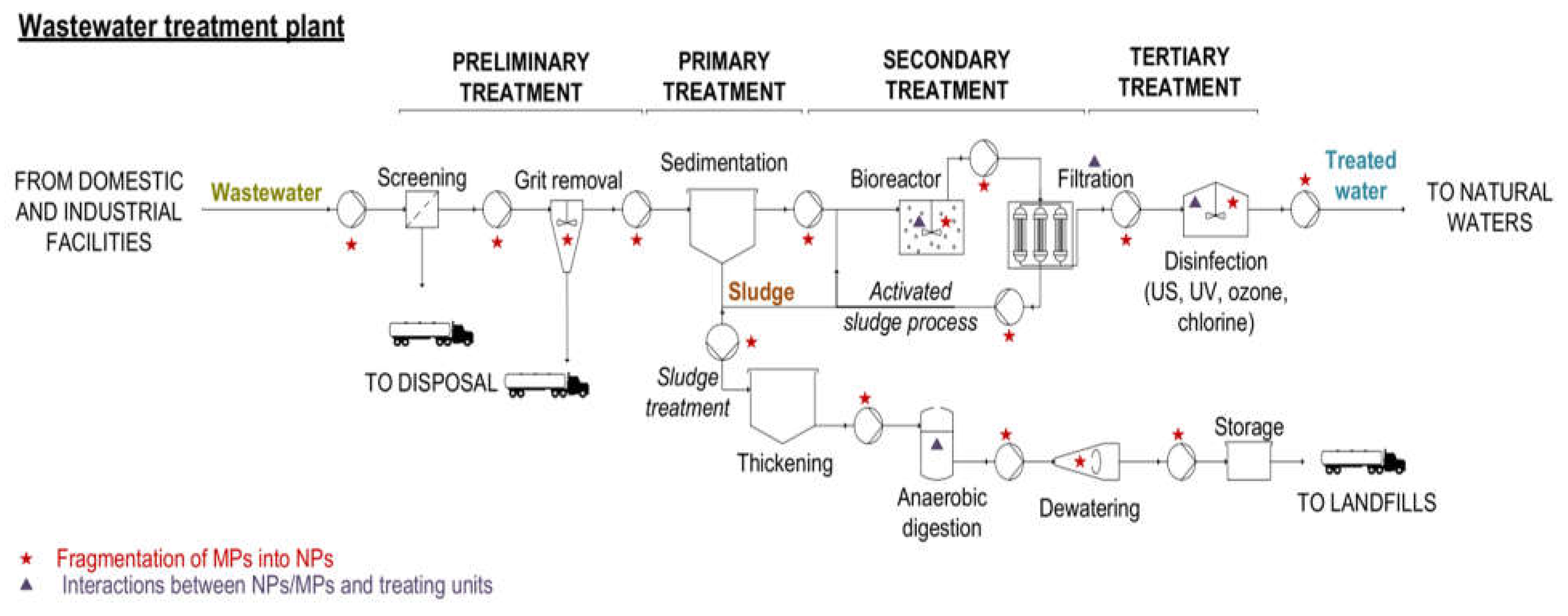
Figure 1.
Fate of nano/microplastics through waste water treatment plant and potential risks in term of microplastics fragmentation and cleaning performance reduction (adapted from Enfrin et al., 2019).
Figure 1.
Fate of nano/microplastics through waste water treatment plant and potential risks in term of microplastics fragmentation and cleaning performance reduction (adapted from Enfrin et al., 2019).
3. Treatment Technologies
The wastewater treatment plants (WWTPs) utilized various treatment technologies, including preliminary treatment processes such as primary treatment processes such as primary settling treatment, grit and grease treatment. Secondary treatment processes such as A2O, biofilters, and other bioreactors were also employed. Additionally, tertiary treatment processes such as UV, O3, chlorination, biologically active filters (BAFs), disc filters (DFs), and rapid sand filters (RSFs) were utilized (Liu et al., 2021).
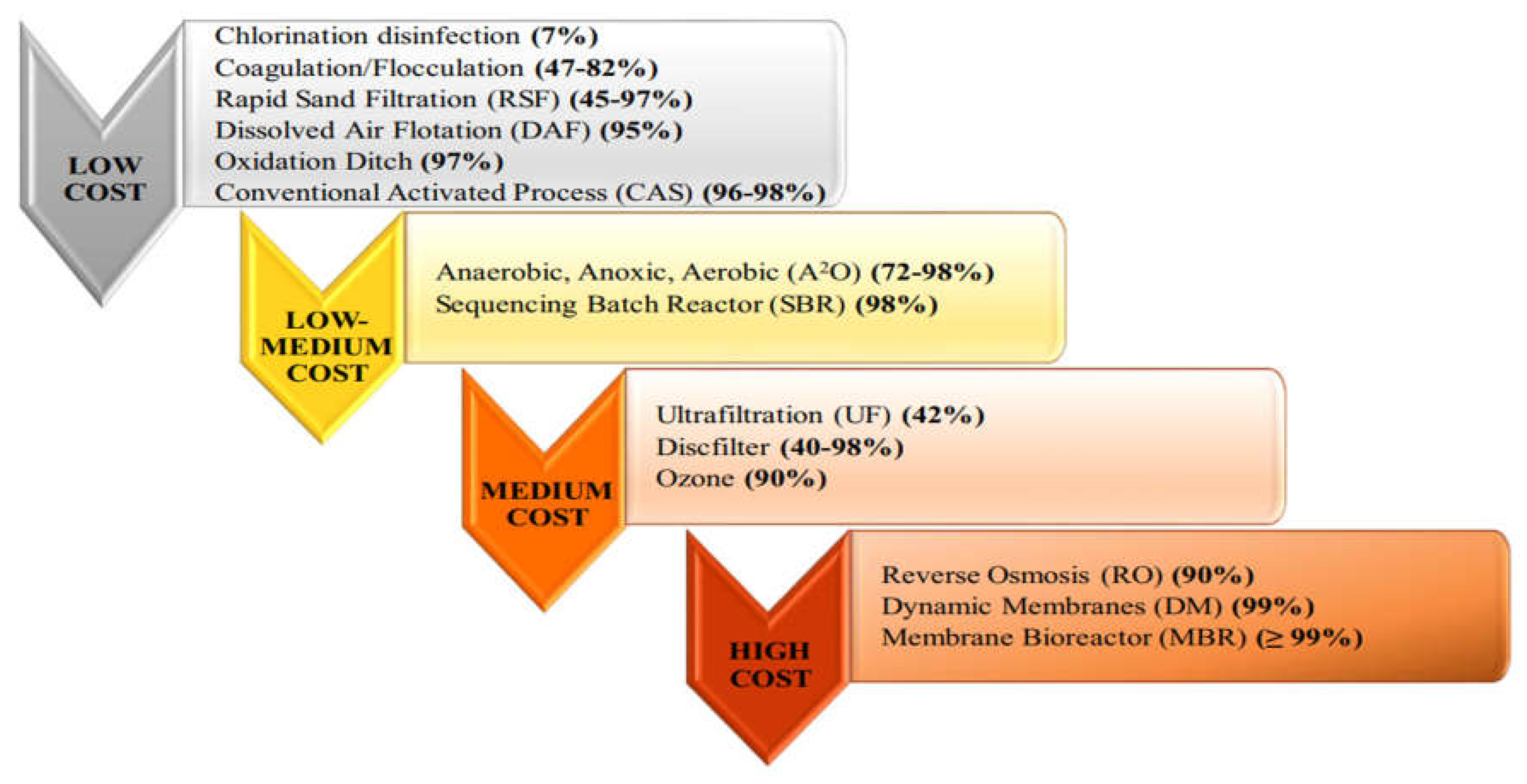
Figure 2.
Removal efficiency of microplastics during different wastewater treatment processes inWWTPs and overview of the costs of the technologies employed (adapted from 3Liu et al., 2021; Magni et al., 2019; Talvitie et al., 2017; Ziajahromi et al., 2017).
Figure 2.
Removal efficiency of microplastics during different wastewater treatment processes inWWTPs and overview of the costs of the technologies employed (adapted from 3Liu et al., 2021; Magni et al., 2019; Talvitie et al., 2017; Ziajahromi et al., 2017).
Figure 3.
Classification of different stages and general outline of a wastewater treatment plant.
Figure 3.
Classification of different stages and general outline of a wastewater treatment plant.
3.1. Preliminary and Primary Treatment
Preliminary treatment is the process of eliminating substances that have the potential to harm or hinder the effectiveness of subsequent procedures. These substances include rags, sticks, floatables, grit, and grease (Z. Xu et al., 2021) (Zhang et al., 2020). The pre-treatment had the most significant influence on the distribution of microplastics sizes, as it was able to efficiently eliminate bigger microplastics. MPs with a higher density than wastewater, such as PBT, PET, and PVC, can be easily removed using physical sedimentation (Z. Xu et al., 2021). In their study, Dris et al. (2015) saw a significant reduction in the proportion of large particles (1000 μm-5000 μm) from 45% to 7% following the first treatment. Khan et al., (2022) reported that it is possible to remove between 40.7% and 97.9% of microplastics from wastewater after the pretreatment stage. It was also demonstrated that pre-treatment methods are more successful in eliminating fibers compared to fragments from wastewater. The relative abundance of fibers decreases following pre-treatment (Sun et al., 2019). The initial phase of treatment involves the physical extraction of pollutants, whereas the primary treatment focuses on the physical and chemical elimination of chemicals that float or settle. In contrast, the secondary treatment stage mostly relies on biological means to eliminate contaminants (Hamidian et al., 2021; Iyare et al., 2020).
Preliminary treatment methods like as sedimentation, screening, and flotation have been shown to eliminate between 35% to 59% of microplastics. Additionally, primary treatment techniques like adsorption, coagulation, and ozonation have the potential to remove around 50% to 98% of microplastics. Studies have shown that around 65% of microplastics are removed during the standard initial treatment phase. The microplastic removal efficiency of 92% and 74% were recorded during the first stage of wastewater treatment plant, respectively. Granule and fragment-shaped particles were found to have a removal effectiveness of 91%, whereas fibers and pellets had 79% and 83%, respectively. Because of their smooth texture, fibers and pellets were more difficult to grab by solid flocs than granules and pieces, which had twisted and angular shapes (Krishnan et al., 2023).
3.2. Secondary Treatment
Secondary treatment involves biological activities that remove residual suspended and dissolved particles in wastewater after primary treatment. Secondary treatment eliminates microplastics through trapping in solid flocs, sedimentation in clarifiers, and consumption by microorganisms like protozoa and metazoans (Hamidian et al., 2021). Smaller microplastics (106-300 μm) are more effectively removed than larger microplastics (>300 μm) due to their retention in the grit and grease removal stage and easy adsorption to sticky medium like biofilm or floc (Iyare et al., 2020). In the secondary treatment stage, MPs are expected to be impacted by physical treatment, regardless of the biological method used, sometimes removal percentages are reduced up to 14% (Krishnan et al., 2023; Talvitie et al., 2017).
3.3. Tertiary Treatment
Tertiary treatment procedures remove inorganic and organic pollutants above secondary treatment standards, often for discharge consent or reuse requirements. The tertiary treatment may give significant further polishing for microplastics removal (Krishnan et al., 2023). The selected tertiary WWTPs utilized several filtering techniques such as depth, surface, biological, membrane, and dissolved air flotation. Other systems for treating primary effluent include disc filters (DF), rapid sand filters (RSF), and membrane bioreactors (MBRs). It was suggested that microplastic removal should consider both mass and particle number. Ultrafiltration and reverse osmosis (RO) effectively remove particles larger than 190 µm (Iyare et al., 2020).
In a report from Jiang et al., (2022), tertiary treatment techniques include coagulation/flocculation, membrane-related technology, and disinfection. The three WWTPs under consideration use a high-efficiency clarifier, a clarifier, a flocculation tank, and a radial flow sedimentation tank to remove MPs at rates of 23.7%, 56.4%, and 11.6%, respectively. MPs could be trapped by the flocculant through hydrogen bonding, electrostatic force, and van der Waals force. Membrane-related technologies include cloth-media filtration, deep-bed filter, high-efficiency filter, and fiber filter. Following tertiary treatment by Sun et al., (2019), microplastics in wastewater reduced to 0.2%-2% of the influent level. Microplastics removal efficiency varies with treatment procedure, with membrane-related technologies outperforming others. Talvitie et al., (2017) investigated the efficiency of tertiary treatment procedures, including disc filter (DF), rapid sand filtration (RSF), and dissolved air flotation (DAF) for secondary effluent and membrane bioreactor (MBR) for primary effluent. The study indicated that MBR had the best removal efficiency (99.9%), followed by RSF and DAF at 97% and 95%. The elimination efficacy of DF ranged from 40% to 98.5%.
Grit Chamber
Desanding is a preliminary treatment used in wastewater treatment plants to remove suspended particles, oils, and grease. However, depending on its design and operation, it can also effectively remove or fragment microplastics (Krishnan et al., 2023). Aerated grit chambers may remove 21.4% to 30% of MP, while rotating grit chambers can increase concentration in water up to 50%. Mechanical agitation in the rotary desander presumably causes microplastic fragmentation (Khan et al., 2022).
Primary sedimentation
After preparatory treatment, primary sedimentation is one of the most effective methods for removing MP, with up to 98% effectiveness (Zhang et al., 2020). Liu reported a removal efficiency of 40.7% following a primary settler in a wastewater treatment facility, with primarily MP in the form of fiber in the influent (Khan et al., 2022).
Coagulation-Flocculation-Sedimentation
The technique involves injecting a coagulant to neutralize the charge of colloidal particles and disrupt their stability. The first phase involves destabilizing colloids by neutralizing their charges, which leads to the production of bigger particles (Khan et al., 2022). Following that, in flocculation, the clots are linked together to enhance their volume and enable sedimentation. This procedure, often used in water and wastewater treatment, effectively removes microplastics (MPs) using aluminum and iron-based compounds (Z. Xu et al., 2021).
Biological Treatment
Secondary treatment in wastewater plants can help remove MPs by degrading organic matter and forming aggregates in a sedimentation tank (Krishnan et al., 2023). Common biological treatment procedures include activated sludge process (ASP), anaerobic-anoxic-oxic (A2O), oxidation ditch (OD), and biofilter. (Khan et al., 2022).
Following secondary sedimentation, the typical activated sludge procedure (ASP) removes 75%-91.9% of microplastics (Khan et al., 2022). These values are higher than other biological treatments reported. MP removal in this technique relies on adsorption and aggregation in activated sludge flocs, as microbes do not often break down MPs (Zhang et al., 2020).
Membrane Bioreactor
This process combines membrane filtration (pore size: 0.1-0.4 μm) for solid-liquid separation with a traditional activated sludge process. It is a contemporary treatment that has lately gained popularity in wastewater treatment plants (Krishnan et al., 2023) due to its low cost and high efficacy in the removal of microplastics. It has been reported that removal percentages can reach 99.5% (Talvitie et al., 2017). The biofilm support side of the system retained the majority of microplastics, showing that adsorption played a significant role in their removal (Khan et al., 2022) (Krishnan et al., 2023).
Rapid Sand Filtration
This technology is widely employed in the drinking and wastewater treatment sector due to its low operating and maintenance costs and ability to remove impurities quickly and efficiently (Krishnan et al., 2023). In wastewater treatment plants, quick sand filtration is typically used in the tertiary stage, with a reported removal rate of 98.9% following flocculation (Khan et al., 2022).
Reverse Osmosis
Reverse osmosis is a membrane technology that is widely used in water purification to separate dissolved solids such as ions from a given solution (Krishnan et al., 2023). The operating principle involves applying high pressure (10-100 bar) to a concentrated solution, forcing water through a semi-permeable membrane while retaining the remaining chemicals. The membrane separates water components (concentrate) and produces a diluted solution with low dissolved solids (permeate) on the other side (Khan et al., 2022). A preliminary stage, such as pretreatment with coagulants, disinfectants, and oxidizing agents, is frequently required to maintain consistent flux rates, limit the effects of fouling, and increase the shelf life of the reverse osmosis unit (Krishnan et al., 2023)
Electrocoagulation
Electrocoagulation offers an alternative to traditional water treatment technologies. This method generates metal ions, often iron or aluminum, by electro-dissolving a metal electrode (Krishnan et al., 2023). The metal ions formed (typically Al3+ or Fe2+) then react with the hydroxide ions to form the coagulant molecules Al(OH)3 or Fe(OH)2. The coagulants created will neutralize the dissolved colloidal particles, forming floccules in which certain dissolved pollutants can be absorbed. While electrocoagulation appears to be a successful method for removing microplastics, further research in real-world settings is needed (Khan et al., 2022).
Chemical Oxidation
In the chemical oxidation process, agents that may obtain electrons are used, i.e. they are formed while oxidizing other chemicals that are typically pollutants in the water treatment region. Chlorine derivatives, peroxides, certain acids, and ozone are only a few examples of oxidizing agents. Ozone (O3) is a gas that can be used to clean both drinking water and wastewater since it is a strong oxidizing agent that reacts with oxidizable molecules, both organic and inorganic. Ozone decomposition in water produces the OH− radical, which oxidizes and modifies the surface of microplastics. This action is enhanced when ozone and hydrogen peroxide are employed together (Khan et al., 2022).
UV-Oxidation
This comprises of a series of intricate processes in which the combined effect of UV radiation and oxygen is prominent. Photo-oxidation causes the polymer chains to degrade, resulting in a change in their topographical and chemical properties, making the material more brittle and, at a later stage, leading to mechanical failure such as crack development and fragmentation (Khan et al., 2022).
Photocatalytic degradation involves bombarding a semiconductor, such as TiO2, with photons. This generates positively charged holes, which combine with water to produce OH• (Krishnan et al., 2023; Z. Xu et al., 2021).
Electrochemical Process
Microplastics are degraded into non-toxic compounds through in-situ formation of oxidizing radicals, such as hydroxyls (OH−), using direct and indirect electrochemical processes (Khan et al., 2022).
Plasma
This technology is widely regarded as highly effective for treating water. When plasma is produced, the temperature of the water rises. However, due to limited space, the plasma cannot expand, causing the system's pressure to rise. Finally, due to the water's low coefficient of expansion, shock waves are generated. Plasma factors like as voltage, distance between electrodes, gas, pH, solution conductivity, and reagents (e.g., semiconductors or Fenton reagents) might impact contaminant removal effectiveness (Khan et al., 2022).
Fenton and its variants
Fenton's reagent is a sophisticated oxidation process that generates oxidizing agents (•OH radicals) in an acidic medium by combining iron salts and hydrogen peroxide. The electro-Fenton method consists of four steps, the most promising of which uses Fenton's reagent to create hydroxyl radicals in an electrolytic cell. Ferrous ions are then replenished by reducing ferric ions at the cathode (Khan et al., 2022).
Figure 4.
Schematic diagram of a Membrane bioreactor.
Figure 4.
Schematic diagram of a Membrane bioreactor.