1. Introduction
Melatonin has been classified as an animal hormone since its discovery as a small-molecule growth regulator in the bovine pineal gland in 1958 [
1]. It did not receive attention from the plant scientific community until its discovery in
Pharbitis nil as well as the discovery of its related growth regulators [
2]. Melatonin is widely present in higher plants and can regulate circadian and photoperiod responses by scavenging reactive oxygen species (ROS) to protect leaves from peroxide effect [
3]. In 2018, Arabidopsis cell membrane melatonin receptors were discovered, and the status of melatonin as a novel phytohormone gradually started to be recognized by the academia [
4].
In animals, melatonin regulates the circadian cycle, mood, sleep, body temperature, and immune function [
3,
5,
6]. Recent research has discovered that melatonin, a hormone that was initially known for its regulatory functions in mammalian circadian rhythms, has a broad range of functions in plants as well. Notably, melatonin is linked to fruit development and the regulation of plant senescence progression. It is also involved in various physiological processes, such as leaf growth, reproduction, and stress response. In this review, we present the current knowledge on melatonin biosynthesis, action and synthetic mechanisms, its role in plants, and new research developments in melatonin signal transduction pathways, and we prospect its future applicability in agricultural production.
2. Melatonin Biosynthesis
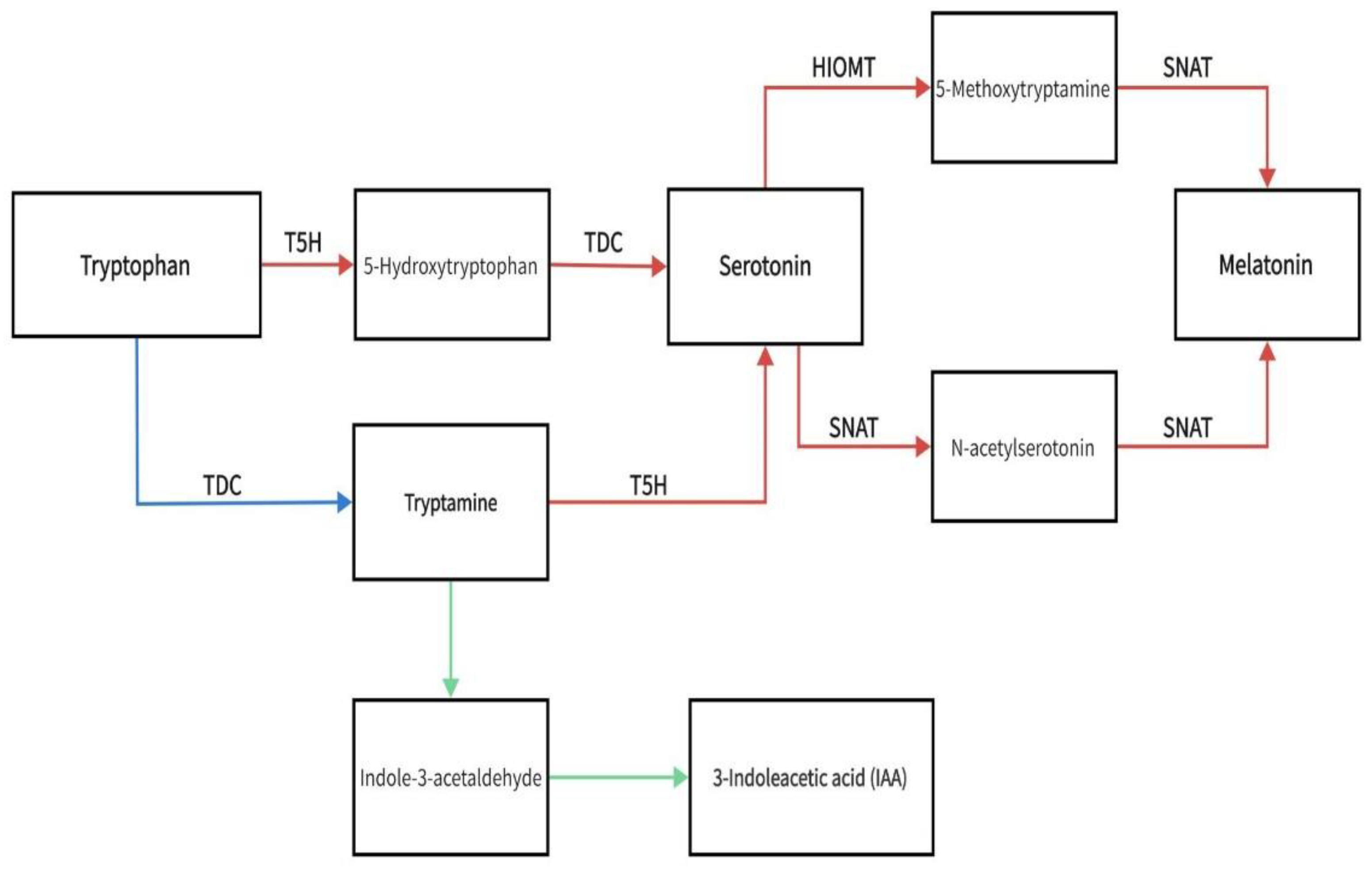
The melatonin precursor molecule tryptophan is converted to 5-hydroxytryptophan in the vertebrate pineal gland via catalysis by tryptophan-5-hydroxylase, which is the primary mechanism for melatonin generation in the pineal gland [
7]. This tryptophan route was later identified in plants, in which 5-hydroxytryptophan is transformed into serotonin, which is then turned into N-acetylserotonin via catalysis by serotonin N-acetyltransferase (SNAT). Eventually, oxindole-o-methyltransferase/acetylserotonin methyltransferase (HIOMT/ASMT) catalyzes the transformation of N-acetylserotonin to melatonin (N-acetyl-5-methoxytryptamine) [
8].
Figure 1 shows melatonin and 3-indoleacetic acid (IAA) biosynthetic pathway. The red arrows represent the melatonin synthesis pathway, the green arrows are the IAA synthesis pathway, and the blue arrow identifies the synthesis pathway shared by both molecules. Both Melatonin and IAA are synthesized from tryptophan. Tryptophan is converted into 5-hydroxytryptophan and tryptamine before the pathway branches into IAA biosynthesis. Tryptamine and 5-hydroxytryptophan are then synthesized serotonin and melatonin. Biosynthetic enzymes: TDC, tryptophan decarboxylase; T5H, tryptophan 5-hydroxylase; SNAT, serotonin N-acetyltransferase; HIOMT, hydroxyindole-O-methyltransferase.
Melatonin is biosynthesized in most of plant tissues [
8,
9,
10]. The biosynthesis of melatonin in plants has been the subject of several recent studies. Byeon et al. (2014) demonstrated the presence of melatonin in rice plants and showed that rice contains genes that encode all the enzymes responsible for the production of melatonin [
8]. While the presence of melatonin in plants has been established, the specific details of its biosynthesis remain unclear. The biosynthesis of melatonin in plants may follow a similar pathway to its synthesis in animals, involving tryptophan as the precursor molecule [
11]. However, the specifics of the melatonin synthetic route require further study.
3. Effects of Melatonin on Plant Growth
Several studies have shown that melatonin can regulate plant physiological functions; it generally promotes root, shoot, and explant growth [
12,
13]. Hernández-Ruiz et al. provided compelling evidence for the direct involvement of melatonin in the promotion of plant organ growth. Specifically, melatonin was found to increase the length of the roots and coleoptiles of monocots and exhibit high relative auxinic concentration (10%–55%) [
13].
Melatonin concentrations were found to be relatively low (nearly 15 ng/g FW) in the first and third stages of sweet cherry fruit development, but substantially higher (reaching a peak value of 35.6 ng/g FW) in the second stage [
14]. In this stage, the cells elongate significantly, cell volume expands, embryo develops, and seeds germinate more rapidly. In addition, RT-PCR experiments revealed that the PaTDC gene, which encodes the rate-limiting enzyme in melatonin synthesis, has a higher expression level of this gene during the second phase than during the first and last stages. Therefore, melatonin is likely involved in and contributes to fruit development processes. However, these phenomena and phenotypes require further study to reveal the mechanisms by which melatonin affects crop development.
Another study revealed the mechanism of melatonin signaling during maize growth from the perspective of glucose metabolism, demonstrating that 10 μM melatonin promoted increases in leaf length and root length to root weight ratio, whereas 100 μM of melatonin resulted in growth inhibition [
15]. At a low concentration (10 μM), melatonin stimulates the export of triose phosphate (a raw material for sucrose synthesis) from the chloroplast to the cytoplasm by upregulating the activity of the Calvin cycle enzymes. Experimental results indicated that the enzymatic activity related to sucrose synthesis in the source was upregulated, resulting in the accumulation of a large amount of sucrose which could be either intracellularly stored or transported to other sites for glycolysis to release energy or for storage. In contrast, sucrose metabolism is not affected by high melatonin levels. However, there was a significant downregulation of enzyme indicators related to photosynthesis and starch metabolism in the source, which decreased the amount of triose entering the cytosol along with a decrease in sucrose content. Additionally, hexokinase was shown to be downregulated in response to high concentrations of melatonin, hindering plant utilization of sucrose in a glycolytic manner. In summary, at the physiological level of growth, very high concentrations of melatonin had notably different effects from those of low concentrations of melatonin, mainly through the inhibition of the Calvin cycle, starch anabolism, glycolysis-related enzyme activities, and expression levels.
4. Effects of Melatonin on Plant Reproduction
The general method of ex vivo preserving tree species is cryopreservation. However, plant somatic cells can suffer from water stress, desiccation, and cryoinjury during cryopreservation, resulting in a variety of growth problems during the process of redifferentiation after the thawing of cryopreserved materials. It was discovered that treating immature shoots with 0.1–0.5 µM of melatonin in preculture and a redifferentiation medium for 24 hours resulted in a considerable increase in their redifferentiation ability compared to that of untreated nodes. Similarly, the redifferentiation ability of salidroside-pre-frozen calli increased following a 0.1 µM melatonin treatment [
16].
Polyethylene glycol (PEG) can be used to simulate drought stress by controlling the water potential (ψw) [
17]. When cucumber seeds were germinated in an 18% PEG + 100 µM melatonin solution, the seed germination rate was approximately 7% higher than that in the 18% PEG + water group, demonstrating that melatonin might alleviate water stress and promote seed germination [
18]. In addition, melatonin (1 µM) improved cucumber reproduction and increased germination under salt stress via regulating abscisic acid (ABA) and gibberellin (GA) synthesis [
19]. When applied exogenously, melatonin was shown to boost the tolerance of the grape root system to water stress, thereby encouraging growth. Melatonin was also found to maintain the normal structure of chloroplast internal lamellar systems and prevent ultrastructural damage caused by drought stress [
20]. Melatonin treatment frequently increases seed germination 2–3 times compared to that of untreated seeds [
21]. Although melatonin has been repeatedly shown to promote seed germination, the mechanism underlying this effect is still unclear.
5. Effects of Melatonin on Plant Stress Tolerance
5.1. Plant Disease Resistance
Plant diseases lead to the greatest productivity and economic losses in agriculture worldwide, and numerous organizations have developed various techniques to manage plant diseases. Exogenous administration of melatonin (0.05–0.5 mM) modifies the activity of antioxidant and plant defense-related enzymes and enhances resistance to apple blotch, one of the serious plant diseases [
22]. Furthermore, Ishihara et al. (2008) discovered that activating the tryptophan synthesis pathway led to an increase in serotonin levels within rice leaves. This increase in serotonin ultimately inhibited the development of fungal hyphae within rice leaf tissues, resulting in a highly efficient defense mechanism [
23]. Although the authors did not mention melatonin, this possibility cannot be ruled out, because serotonin is a biosynthetic precursor of melatonin [
7,
9,
13,
24]. In another study, researchers applied 10 µM melatonin to reduce the expression of genes associated with disease in Arabidopsis, such as PR1, PDF1.2, and ICS1 [
25]. This provides evidence that melatonin may act as a signaling molecule that helps plants resist infections.
5.2. Phytoharmful Chemical Stress Pathways
One of the biochemical indicators of senescence is chlorophyll degradation and loss, and the positive effects of melatonin on this process have been reported [
26]. According to one study, melatonin delays drought- and dark-induced leaf senescence in apples by preserving photosystem II performance and suppressing chlorophyll content loss under stress conditions. Melatonin was found to lower chlorophyll enzyme gene expression in Arabidopsis leaves treated with paraquat (PQ), indicating that chlorophyllase is involved in the light-regulated process of chlorophyll breakdown [
27]. In addition, a transcriptome analysis revealed the upregulation of many genes involved in pathways about stress-related phytohormones (e.g., jasmonic and abscisic acids), which may also indicate that melatonin elicits multiple stress responses in plants [
28].
Szafrańska et al. (2017) also found that the levels of chlorophyll A and B, as well as and carotenes (CARs) were higher after than before a melatonin treatment, which may confirm that melatonin positively affects the biosynthesis of chlorophyll and CARs [
27]. After a PQ treatment for 24 h, the pheophytin contents, which are related to photosynthesis, in the leaf discs significantly increased in th e control group (to over 0.4 mg/g FW), whereas only a slight increase was observed in the melatonin-treated group. These findings show the beneficial effect of melatonin in slowing down chlorophyll decomposition via the formation of pheophytins under PQ-induced oxidative stress. Taken together, these studies showed that melatonin protects the chlorophyll content of pea leaves during PQ-induced oxidative stress by delaying chlorophyll degradation and accelerating its de novo synthesis.
5.3. High Nitrate Stress Pathways
Nitrogen is a key element in the synthesis of nucleic acid molecules, proteins, chlorophyll, multiple hormones, and secondary metabolites [
29]. Consequently, nitrogen assimilation efficiency and availability significantly influence plant growth, development, and metabolism [
30]. Most plants obtain inorganic nitrogen from nitrate and ammonia nitrogen [
31]. Excess nitrate-nitrogen increases ion toxicity and osmotic, secondary, and ionic stress, reduces photosynthesis and enzyme activity, and decreases crop output quantity and quality. When nitrogen intake exceeds the plant digestion capability, large levels of ammonium and nitrate nitrogen accumulate in the plant, causing plant salt toxicity and even secondary soil salinization, all of which harm plant growth [
32].
In a recent study, researchers divided alfalfa seedlings into three groups of nine seedlings: the control group (CK), high nitrate group (HN), and high nitrate + melatonin group (HN + MT), and they measured several phenotypic parameters of seedlings in these three groups [
33]. Their findings indicated that melatonin improved alfalfa growth and development under high nitrate stress. Melatonin participates in free radical scavenging activities to protect the cells from the harmful effects of severe nitrate stress. Furthermore, under nitrate stress, melatonin directly increased the activities of glutamine synthetase (GS), glutamate synthase (GOGAT), and other enzymes. Proline is a multifunctional chemical that helps plants improve and enhance their drought resistance. It accumulates during stress and is destroyed to supply energy to support plant growth after the stress is relieved [
34]. When melatonin relieved high-nitrate stress, the proline levels increased again.
Adenosine triphosphate (ATP) is a crucial signaling molecule in cell communication and directly supplies energy for cell metabolism, gene expression, and other energy-consuming pathways in all living organisms [
35,
36,
37]. Under high nitrate stress, alfalfa consumed more adenosine triphosphate and generated adenosine diphosphate as well as adenosine monophosphate than those consumed and generated under control. Melatonin increased the adenosine triphosphate re-synthesis pathway, protecting alfalfa from high nitrate concentrations, which provide insight into the mechanisms that plants use to respond to stress and to maintain their metabolic activities under adverse environmental conditions.
Overall, melatonin plays a crucial role in mitigating the negative impact of high nitrate stress on plant physiology. By enhancing the activity and expression of nitrogen-metabolizing enzymes, melatonin effectively curbs the production of harmful nitrate-nitrogen and ammonia-nitrogen in alfalfa. Moreover, the hormone helps to maintain the energy homeostasis of the plant and safeguards it from nitrate-induced damage.
6. Coordination of Carbon and Nitrogen Metabolism
Nitrogen is a significant element found in living organisms; it cannot be used directly but permeates cells in the form of ammonium ions through various nitrogenous molecules (mostly proteins and nucleic acids). It is absorbed by plants as nitrate or ammonium ions via the root system [
38,
39]. Nitrates, being the principal nitrogen source for plant species, are first converted into nitrite by nitrate reductase (NR) in the cytosol, before being transformed into ammonium by nitrite reductase (NiR). The ammonium ion rapidly combines with ketoglutarate to generate glutamine via the combined activity of glutamine synthetase (GS), glutamate oxoglutarate transaminase (GOGAT), and glutamate dehydrogenase (GDH) [
40]. Intermediate metabolites in these pathways are required for plant growth because they serve as precursors in the synthesis of amino acids and almost all nitrogenous molecules. Photosynthesis and mitochondria provide ATP, reducing agents, and carbon skeletons for nitrogen absorption and amino acid biosynthesis [
38,
41]. Consequently, it is vital for plant development to maintain the balance between nitrogen and carbon metabolism.
In one study on maize seedling growth, the researcher sprayed an equal number of maize seedlings with 10, 100, and 1000 μM of melatonin and showed that melatonin administration to seedlings considerably increased root length, plant height, leaf area, soluble carbohydrate and protein content, and total chlorophyll content [
42]. Melatonin was shown to increase the rates of biosynthetic processes and stimulate plant growth.
Molecular-level studies have demonstrated that melatonin addition enhanced the activities of nitrate reductase, glutamate synthetase, nitrite reductase, and glutamine synthetase, as well as gene expression. The limiting enzyme in nitrogen assimilation is nitrate reductase, which catalyzes the reduction of nitrate to nitrite. Increased activity of this enzyme helps prevent excess nitrate buildup by coordinating the carbon and nitrogen metabolism, whereas nitrite reductase further reduces nitrite to ammonium, and low levels of ammonium favor the carbon and nitrogen metabolism [
14,
43,
44]. Studies have revealed that melatonin increased plant nitrate levels and that the conversion of nitrate to nitrite was significantly accelerated because of the increased nitrate reductase activity, which was also upregulated, catalyzing the conversion of nitrite to ammonium, which decreased the nitrite concentration. Melatonin was eventually found to improve plant usage of nitrate nitrogen by enhancing the activity of enzymes involved in nitrogen assimilation.
In summary, the link between carbon and nitrogen metabolism is strong and melatonin has made significant contributions to the coordinated management of carbon and nitrogen metabolism.
7. Melatonin interacts with other phytohormones
Melatonin acts synergistically or antagonistically with multiple phytohormones to regulate plant growth and development. For instance, when the plant body receives a certain intensity of melatonin signal, it can activate the auxin synthesis pathway, exhibiting a synergistic effect with auxin. Under stress conditions such as drought and unsuitable temperature, however, melatonin inhibits the expression of auxin synthesis-related enzymes [
45,
46]. This results in a reduction in metabolic levels, inhibition of plant growth, and improved plant resistance.
Melatonin has been shown to be particularly effective in mitigating the effects of drought stress. Abscisic acid (ABA) is one of the most important hormones that is affected by drought stress, and exogenous melatonin is capable of inhibiting abscisic acid synthesis and reducing ABA levels during drought. The NCED3 gene, which encodes an epoxycarotenoid oxygenase, is a key enzyme in the ABA synthesis pathway. Studies have shown that NCED3 expression is upregulated under drought conditions in various plants, leading to the synthesis of large amounts of ABA and exacerbation of plant senescence [
47]. Melatonin works to downregulate NCED3 expression, activate genes of ABA catabolic enzymes such as CYP707, reduce ABA levels in plants, and delay plant senescence under adverse stresses [
48,
49].
Together, melatonin interacts with other phytohormones (e.g. IAA, GA) to regulate plant growth and development, and it is particularly effective in mitigating the effects of drought stress by suppressing ABA synthesis and delaying plant senescence. As such, melatonin may hold significant potential for improving crop yields and enhancing plant resistance to environmental stresses.
8. Signaling Pathways Upstream of Melatonin in Plants
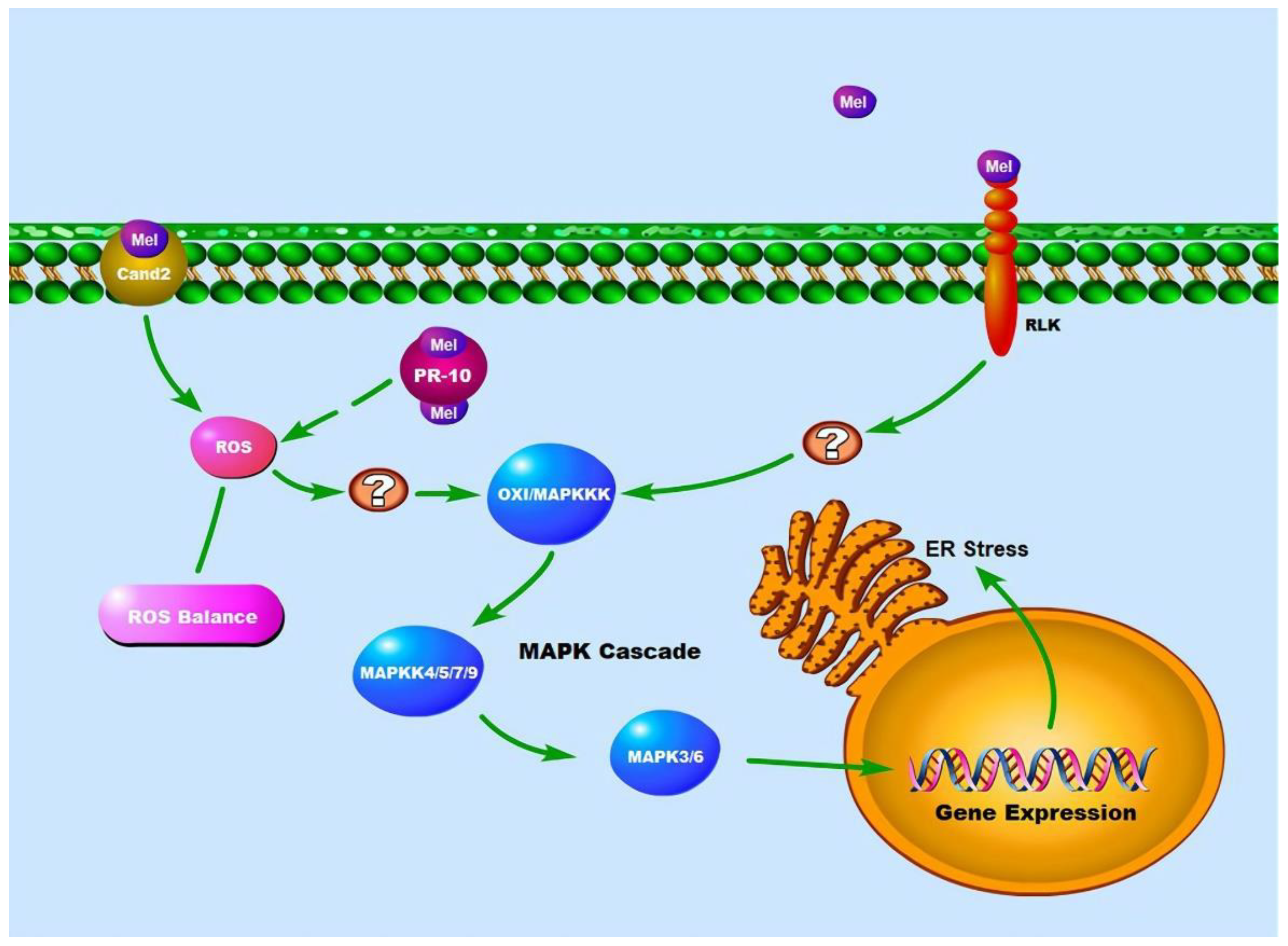
Cand2 (also known as GPCR) plays an essential role as a melatonin receptor in Arabidopsis. Recently, studies have demonstrated that melatonin interacts with Cand2 receptors situated on the plasma membrane to cause the stomatal closure in Arabidopsis. This closure occurs via two essential signaling pathways, the ROS and Ca
2+ signaling pathways. However, when Cand2 knockout mutants are studied, it is observed that this process does not take place [
4]. ROS signaling regulates several cellular pathways; for example, it can boost plant resilience to disease and promote plant development by modulating polyamine metabolic pathways [
50]. This research might provide new insights into how melatonin improves immunological function and promotes plant development.
The Cand2 receptors also stimulate the mitogen-activated protein kinase (MAPK) signaling cascade, either directly or indirectly. Melatonin-mediated MAPK cascade pathways have been investigated under two stress conditions: pathogen infection and endoplasmic reticulum stress [
51,
52]. When Arabidopsis was challenged by pathogens, melatonin production transiently enhanced, which activates MAPKKKs, including OXI1, resulting in the activation of different genes via the MAPK4/5/7/9 and MAPK3/6 signaling cascades (
Figure 2). MAPK3/6 double mutants exhibit no melatonin-mediated pathogen resistance. However, SNAT1 knockout mutants show considerably decreased MAPK3/6 activation, leading to enhanced pathogen susceptibility [
51,
52]. Melatonin was also linked to the activation of the endoplasmic reticulum (ER) resident chaperones and ER stress [
53]. Melatonin-mediated stress resistance was eliminated in MAPK3/6 knockout mutants, whereas ER stress-induced ER chaperone gene expression decreased in SNAT1 knockout mutants, demonstrating that the MAPK cascade is involved in melatonin-mediated ER stress tolerance.
The widespread Receptor Like Kinases (RLKs) on the plasma membrane may act as another melatonin receptor in Arabidopsis [
51]. RLKs are a class of transmembrane receptor proteins whose structure includes a transmembrane region, a cytoplasmic kinase domain, and an extracellular signaling molecule binding domain [
54]. Studies have shown that the major RLKs in Arabidopsis is FLS2 receptor. There is evidence that the FLS2 receptor stimulates the expression of several defense-related genes, including glutathione S-transferase 1 (GST1), PR1, and PR5, through activating the MAPK cascade and WRKY transcription factors via FLG22 binding [
55]. However, whether the signaling transduction after melatonin binding to FLS2 also occurs via the FLS2 pathway and the detailed process of gene expression activation is still an aspect of investigation.
Pathogenesis-related proteins (PR-10) are a group of proteins that are biosynthesized in plants upon exposure to various stresses, including pathogen attack, abiotic stress, and hormonal signals. PR-10 proteins are known to possess antimicrobial activity and have been extensively studied for their role in plant defense mechanisms [
56]. However, recent studies have suggested that PR-10 proteins may also play a key role in plant physiology through their interaction with melatonin [
57,
58]. The binding of melatonin to PR-10 proteins has been shown to have a correlation with reactive oxygen species (ROS) levels in plant cells. The molecular mechanisms involved in the modification period after PR-10 binds melatonin to ROS levels are still being explored. It has been suggested that this interaction may modulate the activity of enzymes involved in ROS metabolism, leading to changes in ROS levels in plant cells, and thus activation of the MAPK pathway [
59]. Further research is needed to fully elucidate the molecular mechanisms involved in this process and to determine the physiological significance of this interaction in plant growth and development.
To date, research on plant melatonin receptors remains relatively limited, particularly with regard to signal transduction pathways downstream of melatonin. The controversies and lack of exploration regarding these pathways have become an interesting subject in the fields of plant cytology and physiological biochemistry. It is expected that future studies will focus on elucidating more details about the molecular mechanisms and signaling pathways involved in melatonin's effects on plant growth and development.
9. Discussion
In this review, the focus is on the diverse roles of melatonin, which is a pleiotropic, amphipathic molecule and a chemically indoleamine in nature. The paper highlights its significance in regulating glucose metabolism, nitrogen metabolism, carbon and nitrogen metabolism coordination, and external stress resistance in plants. Additionally, the effects of exogenous melatonin application on downstream metabolic pathways in plant cells are discussed. The latest advances in understanding the signal transduction pathway upstream of melatonin are also reported. It is worth noting that melatonin plays an important role in various physiological processes of plant growth and development, as well as in stress response.
Since the discovery of plant melatonin receptors and the proposal of signal transduction pathways, the physiological and biochemical effects of melatonin in plants have been extensively researched. Like other phytohormones, melatonin has multifaceted functions in plant physiology. It plays a crucial role in regulating plant growth and development by promoting cell division and elongation, stimulating the production of plant hormones such as auxins, and inhibiting abscisic acid synthesis. These hormones are involved in various plant processes, including seed germination, leaf and stem growth, and stress tolerance. Additionally, melatonin is essential for plant biochemistry and physiology, particularly in the context of field crops. Research has shown that melatonin may contribute to crop productivity and nutritional value and help address global food security issues.
Although current studies have preliminarily revealed the synthesis, signal transduction pathways, and major physiological functions of melatonin in plants, a clear picture of melatonin signal transduction, the tissue specificity of melatonin synthesis, response, and how melatonin regulates gene expression remain to be investigated. In 1977, the birth of the next-generation sequencing technology (Sanger sequencing) marked the possibility of gene sequencing, which progressed from the end feet method, microarray chips, to the development of higher throughput next-generation sequencing (NGS) technologies such as next-generation sequencing and three generation sequencing. The advantage of three-generation sequencing technology to directly sequence RNA not only facilitates transcriptome research but also facilitates the development of novel research methods such as single-cell sequencing (scRNA-seq) and spatial transcriptome. To date, Hi-C sequencing technology, developed based on ''circularization enhancement'', and Spatial Enhanced Resolution Omics sequencing (Stereo-seq) technology enables ultra-high precision dissection of gene and cell-changing processes during life development in both temporal and spatial dimensions [
60,
61]. Currently, the deconvolution algorithms that have rarely been reported in plant research implement to estimate the cellular composition of a tissue by using bulk RNA-seq data, which will alleviate the cost and improve the efficiency of single-cell analysis [
62]. The methods above will be important tools for studying alterations in the genomic structure and function of plant cells under the actions of melatonin, as well as the functions of melatonin synthesis and response to changes in gene expression and the molecular functions of the expressed products, so as to construct a precise spatiotemporal picture of the processes involved in the synthesis and regulation of melatonin in plants.
In this comprehensive review, we delve into the latest research on the contribution of melatonin to plant stress resistance and disease prevention. The research has shown that melatonin, at specific concentrations, can improve the activities of enzymes involved in the carbon-nitrogen metabolism pathway. Additionally, it can inhibit disease-related gene expression, and promote chlorophyll stability, thereby improving plants' resistance to adverse stresses. We must note, however, that there is still a notable dearth of studies on the role of melatonin in controlling plant microbial and insect-related diseases. Future research will undoubtedly focus on identifying the precise function of melatonin in assisting disease and pest control. Through such investigations, we anticipate the development of novel plant growth-regulating products incorporating melatonin, which can be commercialized to control crop growth and enhance yields. Thus, melatonin applications can play an instrumental part in promoting sustainable agriculture practices, facilitating the growth and maturation of crops, as well as sustaining the environment.
Author Contributions
Conceptualization, Dawei Shi; methodology, Lejia Zhao and Ruijia Zhang.; investigation, Lejia Zhao and Ruijia Zhang; resources, Dawei Shi; writing—original draft preparation, Dawei Shi, Qiaofeng Song, Lejia Zhao and Ruijia Zhang ; writing—review and editing, Dawei Shi and Qiaofeng Song; supervision, Qiaofeng Song. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 31300572).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lerner, A. B.; Case, J. D.; Takahashi, Y.; Lee, T. H.; Mori, W. Isolation of melatonin, a pineal factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Van Tassel, D. L.; Roberts, N. J.; O’Neill, S. D. Melatonin from higher plants: isolation and identification of N-acetyl-5-methoxytryptamine. Plant Physiol. 1995, 108, 101. [Google Scholar]
- Reiter, R. J.; Tan, D. X.; Zhou, Z.; Cruz, M. H. C.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: assisting plants to survive and thrive. Molecules. 2015, 20, 7396–7437. [Google Scholar] [CrossRef]
- Wei, J.; Li, D. X.; Zhang, J. R.; Shan, C.; Rengel, Z.; Song, Z. B.; et al. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Maronde, E.; Stehle, J. The mammalian pineal gland: known facts, unknown facets. Trends Endocr. Metabol. 2007, 18, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Madrid, J. A.; Tan, D. X.; Reiter, R. J. Melatonin, the circadian multioscillator system and health: the need for detailed analysis of peripheral melatonin signal. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Falcón, J.; Besseau, L.; Fuentès, M.; Sauzet, S.; Magnanou, E.; Boeuf, G. Structural and functional evolution of the pineal melatonin system in vertebrates. Ann. N.Y. Acad. Sci. 2009, 163, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Lee, H. Y.; Lee, K.; Park, S.; Back, K. Cellular localization and kinetics of the rice melatonin biosynthetic enzymes SNAT and ASMT. J. Pineal Res. 2014, 56, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, K.; Kim, Y. S.; Back, K. Tryptamine 5-hydroxylase-deficient Sekiguchi rice induces synthesis of 5-hydroxytryptophan and N-acetyltryptamine but decreases melatonin biosynthesis during senescence process of detached leaves. J. Pineal Res. 2012, 52, 211–216. [Google Scholar] [CrossRef]
- Arnao, M. B.; Hernandez-Ruiz, J. Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Tan, D. X.; Reiter, R. J.; Back, K. Predominance of 2-hydroxymelatonin over melatonin in plants. J. Pineal Res. 2015, 59, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.; Campbell, S. S. B.; Saxena, P. K. The role of serotonin and melatonin in plant morphogenesis. Regulation of auxin-induced root organogenesis in in vitro-cultured explants of Hypericum perforatum L. In vitro Cell Dev. Biol. Plant. 2001, 37, 786-793. [CrossRef]
- Hernández-Ruiz, J.; Cano, A.; Arnao, M. B. Melatonin acts as a growth-stimulating compound in some monocot species. J. Pineal Res. 2005, 39, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Sun, Y.; Wang, D.Y.; Zheng, J. Effects of exogenous melatonin on nitrogen metabolism in cucumber seedlings under high temperature stress. Plant Physiol. J. 2012, 48, 557–564. [Google Scholar]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; et al. Unveiling the mechanism of melatonin impacts on maize seedling growth: sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, L. W.; Wang, W. M.; Saxena, P. K.; Liu, C. Z. Melatonin improves the survival of cryopreserved callus of Rhodiola crenulata. J. Pineal Res. 2011, 50, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno, A.; Oosterhuis, D.; Stewart, J. Physiological responses of cotton leaves and roots to water deficit induced by polyethylene glycol. Environ Exp Bot. 1998, 40, 29–41. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H. J.; Weeda, S.; Yang, C.; Yang, Z. C.; et al. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. J.; Zhang, N.; Yang, R. C.; Wang, L.; Sun, Q. Q.; Li, D. B.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus). J. Pineal Res. 2014, 57, 269279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F. M.; Teng, F. X.; Zhi, Z. W.; Yu, L. F.; Zhu, M. X.; Zhen, W. Z. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Herrera, T.; Benítez, V.; Arribas, S. M. ; Lopez de Pablo; Angel, L.; et al. Estimation of scavenging capacity of melatonin and otherantioxidants: contribution and evaluation in germinated seeds. Food Chem. 2015, 170, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, P.; Li, M.; Ke, X.; Li, C.; Liang, D.; et al. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J. Pineal Res. 2013, 54, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Hashimoto, Y.; Tanaka, C.; Dubouzet, J. G.; Nakao, T.; Matsuda, F.; et al. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008, 54, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R. J. Melatonin synthesis: multiplicity of regulation. Adv. Exp. Med. Biol. 1991, 294, 149–58. [Google Scholar] [CrossRef]
- Lee, H. Y.; Byeon, Y.; Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 2014, 57, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Li, C. H.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Katarzyna, S.; Russel, J. R.; Malgorzata, M. P. Melatonin Improves the Photosynthetic Apparatus in Pea Leaves Stressed by Paraquat via Chlorophyll Breakdown Regulation and Its Accelerated de novo Synthesis. Front. in Plant Science. 2017, 8, 878. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G. A.; et al. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE. 2014, 9, e93462. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Xiao, W.; Mu, Q.; Li, D.; Chen, X.; Wu, H.; et al. How does nitrate regulate plant senescence? Plant Physiol. Biochem. 2020, 157, 60–69. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Hao, Y.; Su, W.; Liu, H.; Sun, G.; et al. Ammonium transporter (BcAMT1.2) mediates the interaction of ammonium and nitrate in Brassica campestris. Front. Plant Sci. 2020, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Liu, X.; Fan, J. Study on key enzyme activity in nitrogen metabolism and the content of molybdenum and iron in alfalfa under different NO3--N/NH4+-N ratio. Agr. Res. Arid Areas. 2017, 35, 190–197. [Google Scholar] [CrossRef]
- Zhao, C.; Cao, X; Niu, J. Effects of Melatonin on Morphological Characteristics, Mineral Nutrition, Nitrogen Metabolism, and Energy Status in Alfalfa Under High-Nitrate Stress. Front. Plant Sci. 2021, 12, 694179. [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M. N.; Wani, A. S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: a review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Cao, Y.; Tanaka, K.; Nguyen, C. T.; Stacey, G. Extracellular ATP is a central signaling molecule in plant stress responses. Curr. Opin. Plant. Biol. 2014, 20, 82–87. [Google Scholar] [CrossRef] [PubMed]
- De Col, V.; Fuchs, P.; Nietzel, T.; Elsässer, M.; Voon, C. P.; Candeo, A.; et al. ATP sensing in living plant cells reveals tissue gradients and stress dynamics ofenergy physiology. eLife. 2017, 6, e26770. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Zhang, T.; Koo, A. J.; Stacey, G.; Tanaka, K. Extracellular ATP acts on jasmonate signaling to reinforce plant defense. Plant Physiol. 2018, 176, 511–523. [Google Scholar] [CrossRef]
- Erdal, S.; Turk, H. Cysteine-induced upregulation of nitrogen metabolism-related genes and enzyme activities enhance tolerance of maize seedlings to cadmium stress. Environ. and Exper. Bot. 2016, 132, 92–99. [Google Scholar] [CrossRef]
- Duan, W.; Wang, Q.; Zhang, H.; Xie, B.; Li, A.; Hou, F.; et al. Comparative study on carbon–nitrogen metabolism and endogenous hormone contents in normal and overgrown sweetpotato. S. Afr. J. Bot. 2018, 115, 199–207. [Google Scholar] [CrossRef]
- Jabeen, N.; Ahmad, R. Growth response and nitrogen metabolism of sunflower (Helianthus annuus L.) to vermicompost and biogas slurry under salinity stress. J. Plant Nutr. 2017, 40, 104–114. [Google Scholar] [CrossRef]
- Lin, Y. C.; Zhang, J.; Gao, W. C.; Chen, Y.; Li, H. X.; Lawlor, D. W.; et al. Exogenous trehalose improves growth under limiting nitrogen through upregulation of nitrogen metabolism. BMC Plant Biol. 2017, 17, 247. [Google Scholar] [CrossRef] [PubMed]
- Serkan, E. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Reports. 2019, 38, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. M.; Sun, Y. K.; Liu, Z. Y.; Jin, W.; Sun, Y. Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress. J. Pineal Res. 2017, 62(4), 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liang, B. W.; Ma, C. Q.; Zhang, Z. J.; Wei, Z. W.; Gao, T. T.; Zhao, Q.; et al. Long-term exogenous application of melatonin improves nutrient uptake fuxes in apple plants under moderate drought stress. Environ. Exper. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Arnao, M. B.; Hernández-Ruiz, J. Melatonin: a new plant hormone and/or a plant master regulator? Trends in Plant Science. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M. B.; Hernández-Ruiz, J. Melatonin against environmental plant stressors: a review. Current Prot. and Pept. Sci. 2021, 22, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. U.; Mun, B. G.; Bae, E. K.; Kim, J. Y.; Kim, H. H.; Shahid, M.; Choi, Y. I.; Hussain, A.; Yun, B. W. Drought Stress-Mediated Transcriptome Profile Reveals NCED as a Key Player Modulating Drought Tolerance in Populus davidiana. Front Plant Sci. 2021, 12, 755539. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M. B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Annals of Botany. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Nakashima K, Yamaguchi-Shinozaki K. 2013. ABA signaling in stressresponse and seed development. Plant Cell Report. 2013, 32, 959–970. [CrossRef] [PubMed]
- Léo, G.; Caroline, B.; Stéphane, G. Polyamines: double agents in disease and plant immunity. Trends in Plant Science. 2021, 26, 1061–1071. [Google Scholar] [CrossRef]
- Lee, H. Y.; Back, K. Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. J. Pineal Res. 2016, 60, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Y.; Back, K. Melatonin is required for H2O2-and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana. J. Pineal Res. 2017, 62, e12379. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Y.; Back, K. Melatonin plays a pivotal role in conferring tolerance against endoplasmic reticulum stress via mitogen-activated protein kinases and bZIP60 in Arabidopsis thaliana. Melatonin Res. 2018, 1, 93–107. [Google Scholar] [CrossRef]
- Shiu, S. H.; Karlowski, W. M.; Pan, R.; Tzeng, Y. H.; Mayer, K .F. X.; Li, W. H. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 1220, 16, 1220–1234. [CrossRef] [PubMed]
- Asal, T.; Tena, G.; Plotnikova, J.; Willmann, M. R.; Chiu, W. L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F. M.; Sheen, J. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature. 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.; Michalska, K.; Sikorski, M.; Jaskolski, M. Structural and functional aspects of PR-10 proteins. FEBS J. 2013, 280, 1169–1199. [Google Scholar] [CrossRef] [PubMed]
- Sliwiak, J.; Dauter, Z.; Jaskolski, M. Crystal structure of Hyp-1, a Hypericum perforatum PR-10 protein, in complex with melatonin. Front. Plant Sci. 2016, 7, 668. [Google Scholar] [CrossRef] [PubMed]
- Sliwiak, J.; Sikorski, M.; Jaskolski, M. PR-10 proteins as potential mediators of melatonin-cytokinin cross-talk in plants: crystallographic studies of LIPR-10.2B isoform from yellow lupine. FEBS J. 2018, 285, 1907–1922. [Google Scholar] [CrossRef] [PubMed]
- Liu, G. T.; Wang, B. B.; Lecourieux, D.; Li, M. J.; Liu, M. B.; Liu, R. Q.; Shang, B. X.; Yin, X.; Wang, L. J.; Lecourieux, F.; Xu, Y. Proteomic analysis of early-stage incompatible and compatible interactions between grapevine and P. viticola. Hortic Res. 2021, 8, 100. [Google Scholar] [CrossRef]
- Rao S., S.; Huntley M., H.; Durand N., C.; Stamenova E., K.; Bochkov I., D.; Robinson J., T.; Sanborn A., L.; Machol, I.; Omer A., D.; Lander E., S.; Aiden E., L. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014, 159(7), 1665–80. [Google Scholar] [CrossRef] [PubMed]
- Xia K.; Sun H. X.; Li J.; Li J.; Zhao Y.; Chen L.; Qin C.; Chen R.; Chen Z.; Liu G.; Yin R.; Mu B.; Wang X.; Xu M.; Li X.; Yuan P.; Qiao Y.; Hao S.; Wang J.; Xie Q.; Xu J.; Liu S.; Li Y.; Chen A.; Liu L.; Yin Y.; Yang H.; Wang J.; Gu Y.; Xu X. The single-cell stereo-seq reveals region-specific cell subtypes and transcriptome profiling in Arabidopsis leaves. Dev Cell. 2022, 23, 57(10), 1299-1310.e4. [CrossRef]
- Sutton G., J.; Poppe, D.; Simmons R., K.; Walsh, K.; Nawaz, U.; Lister, R.; Gagnon-Bartsch J., A.; Voineagu, I. Comprehensive evaluation of deconvolution methods for human brain gene expression. Nat Commun. 2022, 13(1), 1358. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).