Submitted:
03 June 2024
Posted:
04 June 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
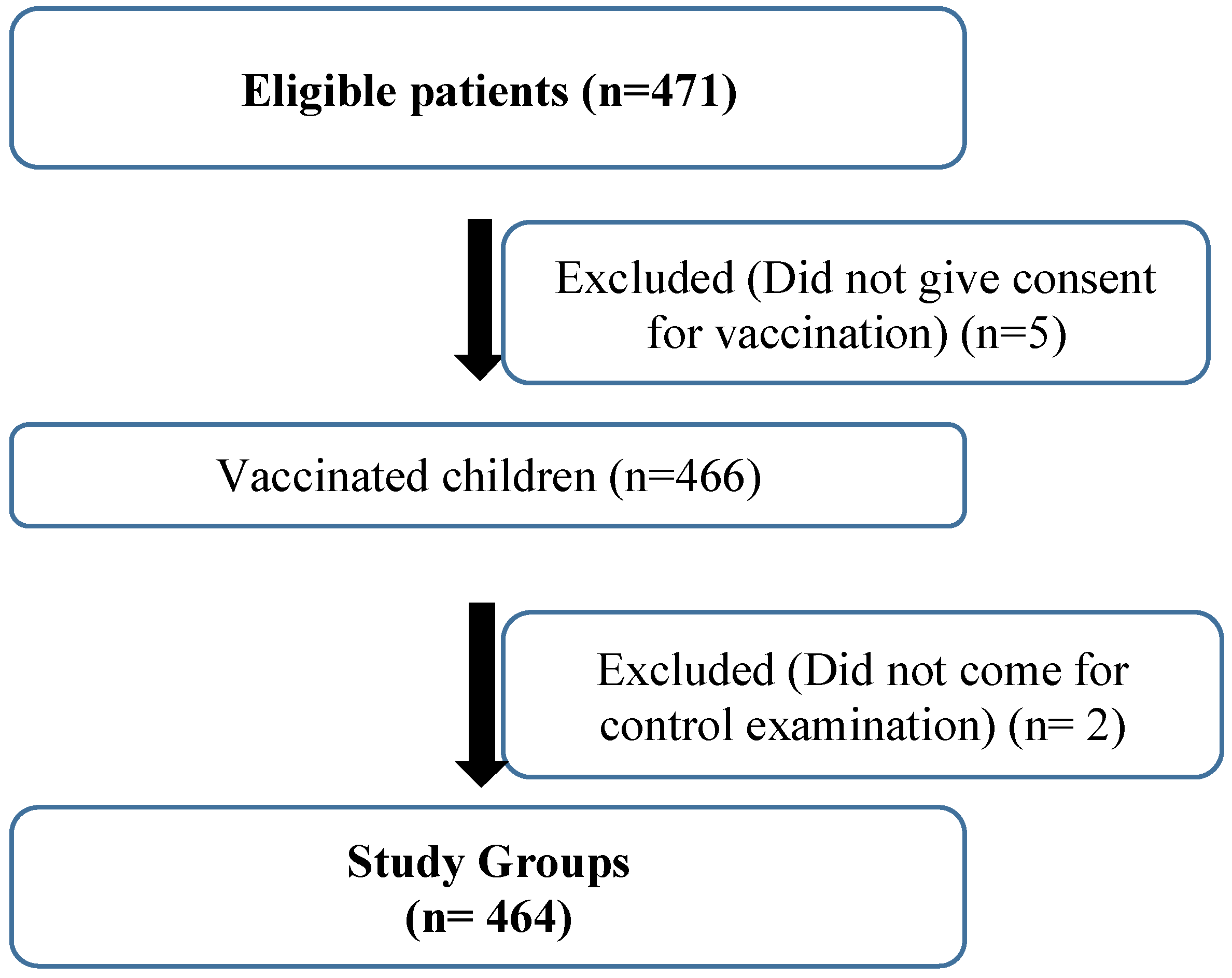
Inclusion Criteria
Exclusion Criteria
Laboratory Assessments
Statistical Methods
Results
Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nadel, S.; Ninis, N. Invasive Meningococcal Disease in the Vaccine Era. Front. Pediatr. 2018, 6, 321. [Google Scholar] [CrossRef]
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1-20.
- T.C Başbakanlık Türkiye İstatistik Kurumu Ölüm İstatistikleri. Ölüm nedenlerinin cinsiyete göre dağılımı, 27/04/2017 tarihi itibariyle, 2009-2016. http:// www.tuik.gov.tr/PreTablo.do? 1083.
- Banzhoff, A. Multicomponent meningococcal B vaccination (4CMenB) of adolescents and college students in the United States. Ther. Adv. Vaccines 2017, 5, 3–14. [Google Scholar] [CrossRef]
- Bagwe, P.; Bajaj, L.; Gala, R.; Zughaier, S.M.; Uddin, M.N.; D’souza, M.J. Meningococcal Vaccines: Challenges and Prospects. Vaccines 2020, 8, 738. [Google Scholar] [CrossRef]
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378-1388.
- Di Pietro, G.M.; Biffi, G.; Castellazzi, M.L.; Tagliabue, C.; Pinzani, R.; Bosis, S.; Marchisio, P.G. Meningococcal Disease in Pediatric Age: A Focus on Epidemiology and Prevention. Int. J. Environ. Res. Public Heal. 2022, 19, 4035. [Google Scholar] [CrossRef]
- Millar BC, Banks L, Bourke TW, et al. Meningococcal Disease Section 3: Diagnosis and Management: MeningoNI Forum. Ulster Med J. 2018;87:94-98.
- Runde TJ, Anjum F, Hafner JW. Bacterial Meningitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih. 4703.
- Hrishi, A.P.; Sethuraman, M. Cerebrospinal Fluid (CSF) Analysis and Interpretation in Neurocritical Care for Acute Neurological Conditions. Indian J. Crit. Care Med. 2019, 23, 115–119. [Google Scholar] [CrossRef]
- Gioia, S.; Piazze, J.; Anceschi, M.M.; Cerekja, A.; Alberini, A.; Giancotti, A.; Larciprete, G.; Cosmi, E.V. Mean platelet volume: Association with adverse neonatal outcome. Platelets 2007, 18, 284–288. [Google Scholar] [CrossRef]
- Kim, N.Y.; Chun, D.-H.; Kim, S.Y.; Kim, N.K.; Baik, S.H.; Hong, J.H.; Kim, K.S.; Shin, C.-S. Prognostic Value of Systemic Inflammatory Indices, NLR, PLR, and MPV, for Predicting 1-Year Survival of Patients Undergoing Cytoreductive Surgery with HIPEC. J. Clin. Med. 2019, 8, 589. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper-Lenkiewicz, O.M.; Kamińska, J.; Kemona, H.; Dymicka-Piekarska, V. Mean Platelet Volume (MPV): New Perspectives for an Old Marker in the Course and Prognosis of Inflammatory Conditions. Mediat. Inflamm. 2018, 2019, 1–14. [Google Scholar] [CrossRef]
- Mihai A, Caruntu A, Opris-Belinski D, et al. The Predictive Role of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), Monocytes-to-Lymphocyte Ratio (MLR) and Gammaglobulins for the Development of Cutaneous Vasculitis Lesions in Primary Sjögren's Syndrome. J Clin Med. 2022;11:5525.
- Lee, J.S.; Kim, N.Y.; Na, S.H.; Youn, Y.H.; Shin, C.S. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 2018, 97, e11138. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Meningococcal infections. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL: American Academy of Pediatrics; 2018: p.550-561.
- Robinson, J.L. Update on invasive meningococcal vaccination for Canadian children and youth. Paediatr. Child Heal. 2018, 23, e1–e4. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Immunization Schedules. For Health Care Providers. Child and Adolescent Immunization Schedule(birth through 18 years) (Accessed , 2019, available at: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html). 12 November.
- European Centre for Disease Prevention and Control. Vaccine Scheduler. (Accessed , 2019, available at: https://vaccine-schedule.ecdc.europa.eu/). 12 November.
- Wilkins, A. ; Snape Emerging clinical experience with vaccines against group B meningococcal disease. Vaccine 2018, 36, 5470–5476. [Google Scholar] [CrossRef]
- Ladhani SN, Giuliani MM, Biolchi A, Pizza M, Beebeejaun K, Lucidarme J, Findlow J, Ramsay ME, Borrow R. Effectiveness of Meningococcal B Vaccine against Endemic Hypervirulent Neisseria meningitidis W Strain, England. Emerg Infect Dis 2016 Feb;22(2):309-11.
- Baxter, R.; Baine, Y.; Ensor, K.; Bianco, V.; Friedland, L.R.; Miller, J.M. Immunogenicity and Safety of an Investigational Quadrivalent Meningococcal ACWY Tetanus Toxoid Conjugate Vaccine in Healthy Adolescents and Young Adults 10 to 25 Years of Age. Pediatr. Infect. Dis. J. 2011, 30, e41–e48. [Google Scholar] [CrossRef]
- Dull PM, McIntosh ED. Meningococcal vaccine development from glycoconjugates against MenACYW to proteins against MenB-potential for broad protection against meningococcal disease. Vaccine 2012; 30: 18-25.
- Nadel, S. Prospects for eradication of meningococcal disease. Arch. Dis. Child. 2012, 97, 993–998. [Google Scholar] [CrossRef]
- McQuaid, F.; Snape, M.D.; John, T.M.; Kelly, S.; Robinson, H.; Yu, L.-M.; Toneatto, D.; D’agostino, D.; Dull, P.M.; Pollard, A.J. Persistence of specific bactericidal antibodies at 5 years of age after vaccination against serogroup B meningococcus in infancy and at 40 months. Can. Med Assoc. J. 2015, 187, E215–E223. [Google Scholar] [CrossRef]
- Stefanizzi, P.; Di Lorenzo, A.; Martinelli, A.; Moscara, L.; Stella, P.; Ancona, D.; Tafuri, S. Adverse events following immunization (AEFIs) with anti-meningococcus type B vaccine (4CMenB): Data of post-marketing active surveillance program. Apulia Region (Italy), 2019–2023. Vaccine 2023, 41, 7096–7102. [Google Scholar] [CrossRef]
- Stefanizzi, P.; Bianchi, F.P.; Spinelli, G.; Amoruso, F.; Ancona, D.; Stella, P.; Tafuri, S. Postmarketing surveillance of adverse events following meningococcal B vaccination: data from Apulia Region, 2014–19. Hum. Vaccines Immunother. 2021, 18, 1–6. [Google Scholar] [CrossRef]
- Prymula, R.; Esposito, S.; Zuccotti, G.V.; Xie, F.; Toneatto, D.; Kohl, I.; Dull, P.M. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine (I). Hum. Vaccines Immunother. 2014, 10, 1993–2004. [Google Scholar] [CrossRef]
- Imran MM, Ahmad U, Usman U, Ali M, Shaukat A, Gul N. Neutrophil/lymphocyte ratio-A marker of COVID-19 pneumonia severity [retracted in: Int J Clin Pract. 2021 Dec;75(12):e14927]. Int J Clin Pract. 2021;75(4):e13698.
- Parthasarathi, A.; Padukudru, S.; Arunachal, S.; Basavaraj, C.K.; Krishna, M.T.; Ganguly, K.; Upadhyay, S.; Anand, M.P. The Role of Neutrophil-to-Lymphocyte Ratio in Risk Stratification and Prognostication of COVID-19: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1233. [Google Scholar] [CrossRef]
- Shusterman, E.; Prozan, L.; Ablin, J.N.; Weiss-Meilik, A.; Adler, A.; Choshen, G.; Kehat, O. Neutrophil-to-lymphocyte ratio trend at admission predicts adverse outcome in hospitalized respiratory syncytial virus patients. Heliyon 2023, 9, e16482. [Google Scholar] [CrossRef]
- Terradas, R.; Grau, S.; Blanch, J.; Riu, M.; Saballs, P.; Castells, X.; Horcajada, J.P.; Knobel, H. Eosinophil Count and Neutrophil-Lymphocyte Count Ratio as Prognostic Markers in Patients with Bacteremia: A Retrospective Cohort Study. PLOS ONE 2012, 7, e42860. [Google Scholar] [CrossRef]
- Mediu, R.; Rama, A.; Puca, E. Evaluation of neutrophil-to-lymphocyte ratio and immune response in patients vaccinated with Pfizer-Biontech vaccine. J. Infect. Dev. Ctries. 2022, 16, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-H.; Lin, S.-Y.; Liou, H.-H.; Chen, C.-C.; Shu, C.-C.; Lee, C.-Y.; Tsai, M.-K.; Yu, C.-J. Protective Effect of BCG and Neutrophil-to-Lymphocyte Ratio on Latent Tuberculosis in End Stage Renal Disease. Infect. Dis. Ther. 2023, 12, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wan, Y.; Zhang, W. The Clinical Value of Systemic Immune Inflammation Index (SII) in Predicting the Severity of Hospitalized Children with Mycoplasma Pneumoniae Pneumonia: A Retrospective Study. Int. J. Gen. Med. 2024, ume 17, 935–942. [Google Scholar] [CrossRef]
- Okuyan, O.; Elgormus, Y.; Sayili, U.; Dumur, S.; Isık, O.E.; Uzun, H. The Effect of Virus-Specific Vaccination on Laboratory Infection Markers of Children with Acute Rotavirus-Associated Acute Gastroenteritis. Vaccines 2023, 11, 580. [Google Scholar] [CrossRef]
- McDade, T.W.; Borja, J.B.; Kuzawa, C.W.; Perez, T.L.L.; Adair, L.S. C-reactive protein response to influenza vaccination as a model of mild inflammatory stimulation in the Philippines. Vaccine 2015, 33, 2004–2008. [Google Scholar] [CrossRef] [PubMed]
| Vaccine Type | ||||||
|---|---|---|---|---|---|---|
| Nimenrix | Bexsero | |||||
| Mean±Std | Median(25p-75p) | Mean±Std | Median(25p-75p) | p value | ||
| Before Vaccination | WBC (103/µL) | 7918±1401 | 8030(6820-9000) | 7970±1445 | 8080(6910-9200) | 0,565* |
| HGB (g/dL) | 11.58±1.83 | 11.1(10.6-12.2) | 11.45±1.81 | 11.1(10.5-12.2) | 0,530* | |
| HCT (%) | 34.4±4.64 | 33.3(31.8-35.9) | 33.88±4.72 | 33.1(31.2-35.3) | 0,223* | |
| PLT (103/µL) | 316721±59404 | 318000(271000-363000) | 316836±65539 | 318000(267000-361000) | 0.984† | |
| LYM (103/µL) | 4.67±1.38 | 4.86(3.5-5.64) | 4.7±1.52 | 4.65(3.65-5.48) | 0.833† | |
| NEUTROPHIL (103/µL) | 2.55±1.1 | 2.34(1.75-3.2) | 2.63±1.22 | 2.43(1.7-3.32) | 0.477† | |
| MONOCYTE (103/µL) | 0.99±0.32 | 0.96(0.73-1.25) | 0.99±0.42 | 0.9(0.65-1.25) | 0,298* | |
| PLR | 74.09±28.03 | 69.01(54.78-84.47) | 74.67±31.55 | 68.46(54.41-84.35) | 0,855* | |
| NLR | 0.63±0.39 | 0.55(0.33-0.82) | 0.63±0.4 | 0.57(0.33-0.83) | 0,801* | |
| SII | 196.68±124.75 | 171.28(107.19-245.07) | 197.62±128.2 | 173.71(108.16-251.85) | 0,938* | |
| dNLR | 0.56±0.43 | 0.48(0.28-0.67) | 0.61±0.53 | 0.47(0.28-0.7) | 0,893* | |
| SIR-I | 0.57±0.33 | 0.48(0.34-0.74) | 0.63±0.5 | 0.47(0.29-0.84) | 0,761* | |
| CRP (mg/L) | 1.85±1.37 | 1.25(0.76-2.76) | 1.81±1.28 | 1.25(0.85-2.55) | 0,955* | |
| After Vaccination | WBC (103/µL) | 7775±1298 | 7680(6720-8830) | 7800±1423 | 7880(6590-8920) | 0,804* |
| HGB (g/dL) | 11.53±1.21 | 11.4(10.8-12.2) | 11.52±1.13 | 11.4(10.8-12.2) | 0,858* | |
| HCT (%) | 34.41±3.03 | 34.1(32.5-36.3) | 34.32±2.99 | 34(32.4-36.3) | 0,998* | |
| PLT (103/µL) | 312886±62948 | 306000(272000-361000) | 309030±67329 | 299000(262000-355000) | 0.524† | |
| LYM (103/µL) | 4.52±1.26 | 4.46(3.53-5.31) | 4.64±1.65 | 4.51(3.34-5.73) | 0.366† | |
| NEUTROPHIL (103/µL) | 2.67±1.09 | 2.4(1.86-3.38) | 2.51±1.04 | 2.39(1.68-3.28) | 0.122† | |
| MONOCYTE (103/µL) | 0.94±0.47 | 0.76(0.62-1.09) | 0.95±0.43 | 0.81(0.63-1.25) | 0,387* | |
| PLR | 73.41±21.03 | 69.57(57.12-83.53) | 74.63±30.49 | 69.17(53.87-86.06) | 0,522* | |
| NLR | 0.66±0.39 | 0.57(0.36-0.81) | 0.64±0.44 | 0.52(0.34-0.74) | 0,162* | |
| SII | 201.47±112.97 | 178.07(116.04-269.14) | 192.51±129 | 154.93(103.77-256.46) | 0,106* | |
| dNLR | 0.6±0.4 | 0.5(0.34-0.75) | 0.57±0.44 | 0.43(0.29-0.73) | 0,095* | |
| SIR-I | 0.63±0.46 | 0.52(0.26-0.9) | 0.63±0.52 | 0.47(0.23-0.9) | 0,538* | |
| CRP (mg/L) | 1.69±1.03 | 1.35(0.95-2.35) | 1.88±1.25 | 1.45(0.95-2.61) | 0,264* | |
| Vaccine Type | Two-way repeated measures ANOVA p value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nimenrix | Bexsero | |||||||||
| Before Vaccination | After Vaccination | p value | Before Vaccination | After Vaccination | p value | Time | Vaccine Type | Time*Vaccine Type | ||
| WBC (103/µL) | Mean±Std | 7918±1401 | 7775±1298 | 0.186* | 7970±1445 | 7800±1423 | 0.102* | 0,091 | 0,671 | 0,88 |
| Median(25p-75p) | 8030(6820-9000) | 7680(6720-8830) | 8080(6910-9200) | 7880(6590-8920) | ||||||
| HGB (g/dL) | Mean±Std | 11.58±1.83 | 11.53±1.21 | 0.113* | 11.45±1.81 | 11.52±1.13 | 0.015* | 0,951 | 0,47 | 0,54 |
| Median(25p-75p) | 11.1(10.6-12.2) | 11.4(10.8-12.2) | 11.1(10.5-12.2) | 11.4(10.8-12.2) | ||||||
| HCT (%) | Mean±Std | 34.4±4.64 | 34.41±3.03 | 0.058* | 33.88±4.72 | 34.32±2.99 | 0.001* | 0,374 | 0,248 | 0,389 |
| Median(25p-75p) | 33.3(31.8-35.9) | 34.1(32.5-36.3) | 33.1(31.2-35.3) | 34(32.4-36.3) | ||||||
| PLT (103/µL) | Mean±Std | 316721±59404 | 312886±62948 | 0.478† | 316836±65539 | 309030±67329 | 0.186† | 0,146 | 0,67 | 0,619 |
| Median(25p-75p) | 318000(271000-363000) | 306000(272000-361000) | 318000(267000-361000) | 299000(262000-355000) | ||||||
| LYM (103/µL) | Mean±Std | 4.67±1.38 | 4.52±1.26 | 0.156† | 4.7±1.52 | 4.64±1.65 | 0.680† | 0,24 | 0,448 | 0,607 |
| Median(25p-75p) | 4.86(3.5-5.64) | 4.46(3.53-5.31) | 4.65(3.65-5.48) | 4.51(3.34-5.73) | ||||||
| NEUTROPHIL (103/µL) | Mean±Std | 2.55±1.1 | 2.67±1.09 | 0.290† | 2.63±1.22 | 2.51±1.04 | 0.263† | 0,973 | 0,592 | 0,124 |
| Median(25p-75p) | 2.34(1.75-3.2) | 2.4(1.86-3.38) | 2.43(1.7-3.32) | 2.39(1.68-3.28) | ||||||
| MONOCYTE (103/µL) | Mean±Std | 0.99±0.32 | 0.94±0.47 | 0.004* | 0.99±0.42 | 0.95±0.43 | 0.17* | 0,067 | 0,859 | 0,796 |
| Median(25p-75p) | 0.96(0.73-1.25) | 0.76(0.62-1.09) | 0.9(0.65-1.25) | 0.81(0.63-1.25) | ||||||
| PLR | Mean±Std | 74.09±28.03 | 73.41±21.03 | 0.672* | 74.67±31.55 | 74.63±30.49 | 0.862* | 0,847 | 0,627 | 0,864 |
| Median(25p-75p) | 69.01(54.78-84.47) | 69.57(57.12-83.53) | 68.46(54.41-84.35) | 69.17(53.87-86.06) | ||||||
| NLR | Mean±Std | 0.63±0.39 | 0.66±0.39 | 0.374* | 0.63±0.4 | 0.64±0.44 | 0.739* | 0,479 | 0,705 | 0,567 |
| Median(25p-75p) | 0.55(0.33-0.82) | 0.57(0.36-0.81) | 0.57(0.33-0.83) | 0.52(0.34-0.74) | ||||||
| SII | Mean±Std | 196.68±124.75 | 201.47±112.97 | 0.403* | 197.62±128.2 | 192.51±129 | 0.405* | 0,985 | 0,616 | 0,551 |
| Median(25p-75p) | 171.28(107.19-245.07) | 178.07(116.04-269.14) | 173.71(108.16-251.85) | 154.93(103.77-256.46) | ||||||
| dNLR | Mean±Std | 0.56±0.43 | 0.6±0.4 | 0.118* | 0.61±0.53 | 0.57±0.44 | 0.748* | 0,94 | 0,769 | 0,215 |
| Median(25p-75p) | 0.48(0.28-0.67) | 0.5(0.34-0.75) | 0.47(0.28-0.7) | 0.43(0.29-0.73) | ||||||
| SIR-I | Mean±Std | 0.57±0.33 | 0.63±0.46 | 0.244* | 0.63±0.5 | 0.63±0.52 | 0.859* | 0,32 | 0,295 | 0,4 |
| Median(25p-75p) | 0.48(0.34-0.74) | 0.52(0.26-0.9) | 0.47(0.29-0.84) | 0.47(0.23-0.9) | ||||||
| CRP (mg/L) | Mean±Std | 1.85±1.37 | 1.69±1.03 | 0.297* | 1.81±1.28 | 1.88±1.25 | 0.458* | 0,566 | 0,353 | 0,135 |
| Median(25p-75p) | 1.25(0.76-2.76) | 1.35(0.95-2.35) | 1.25(0.85-2.55) | 1.45(0.95-2.61) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





