Submitted:
04 June 2024
Posted:
04 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
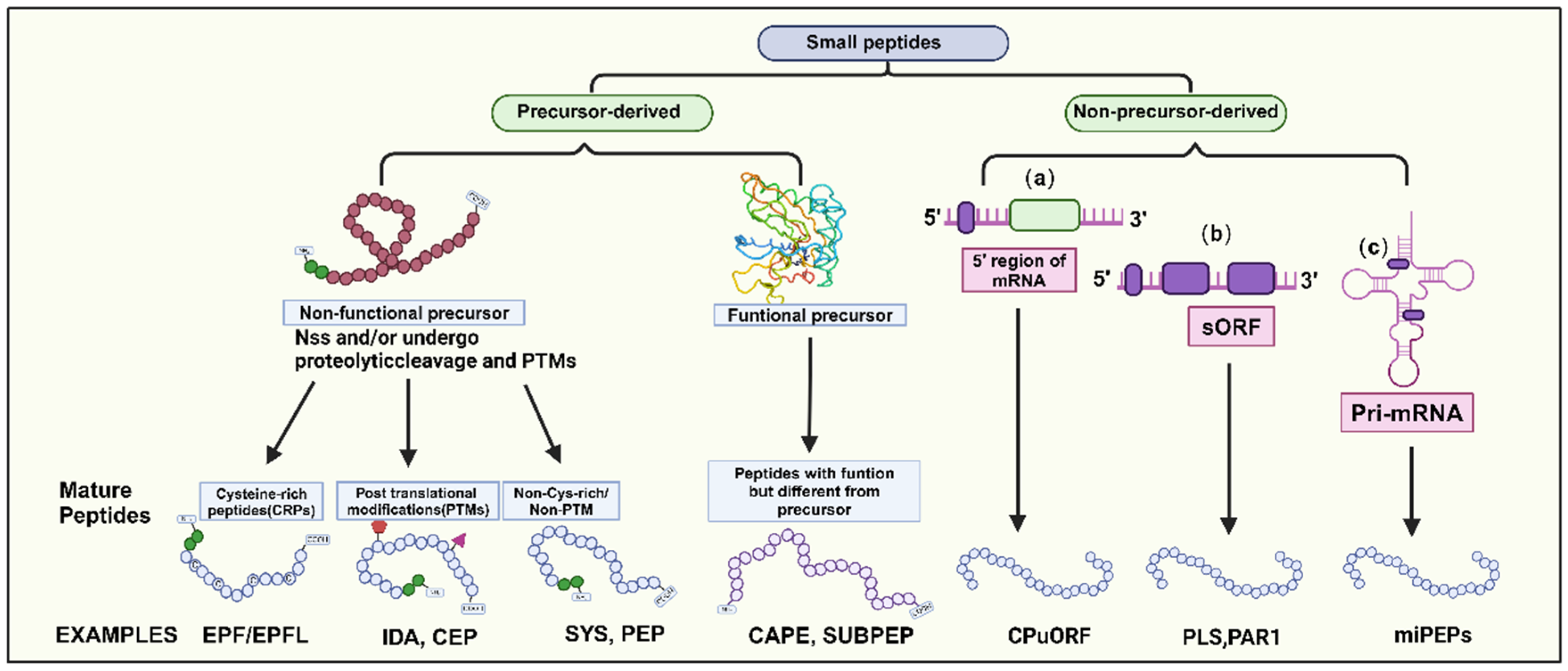
1.1. Classification & Identification of SPs
1.2. Classification Based on Origin
1.3. Based on the N-Terminal Sequence
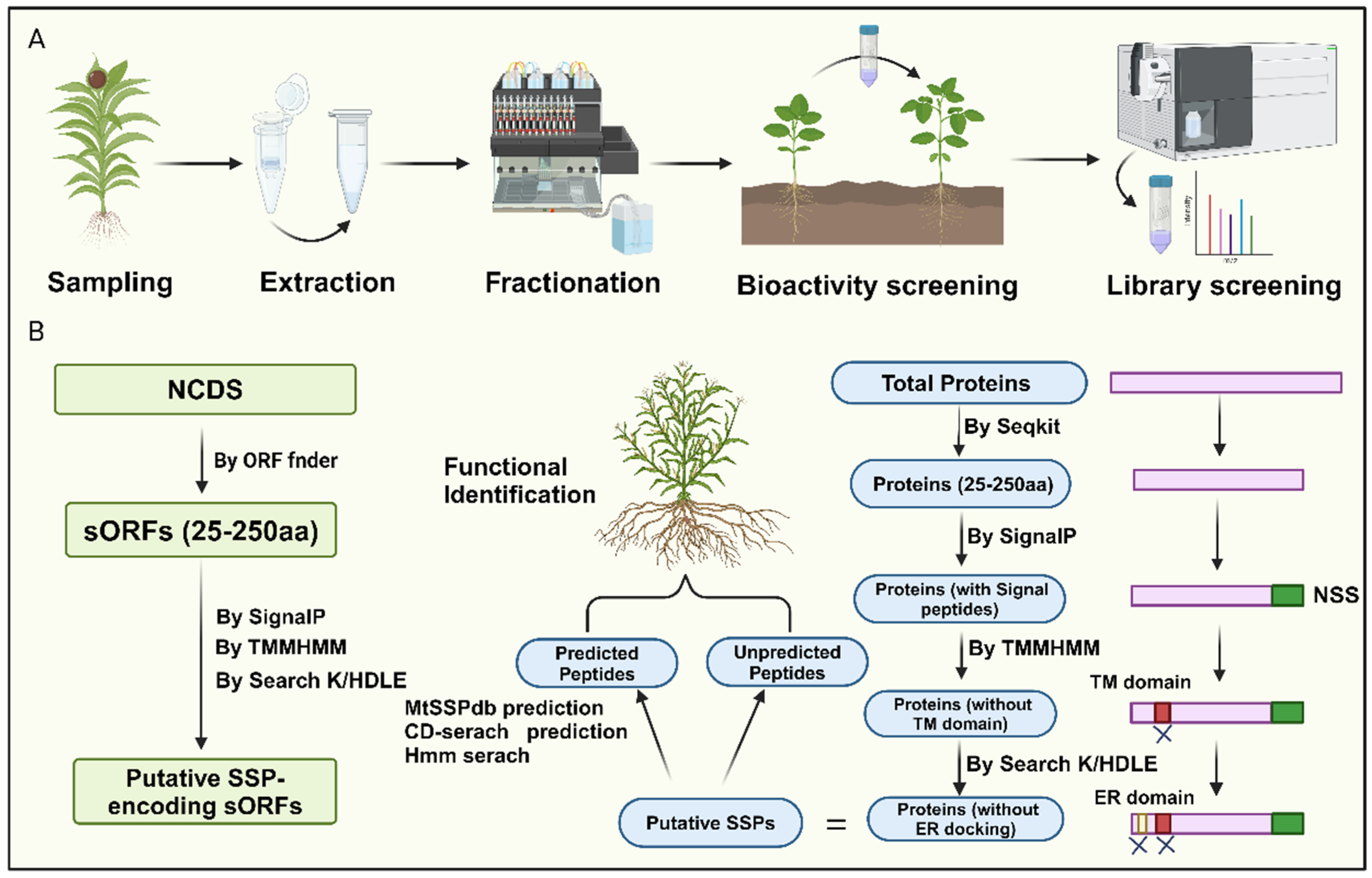
1.4. Identification Methods of SPs
1.5. Tyrosine-Sulfated Peptides
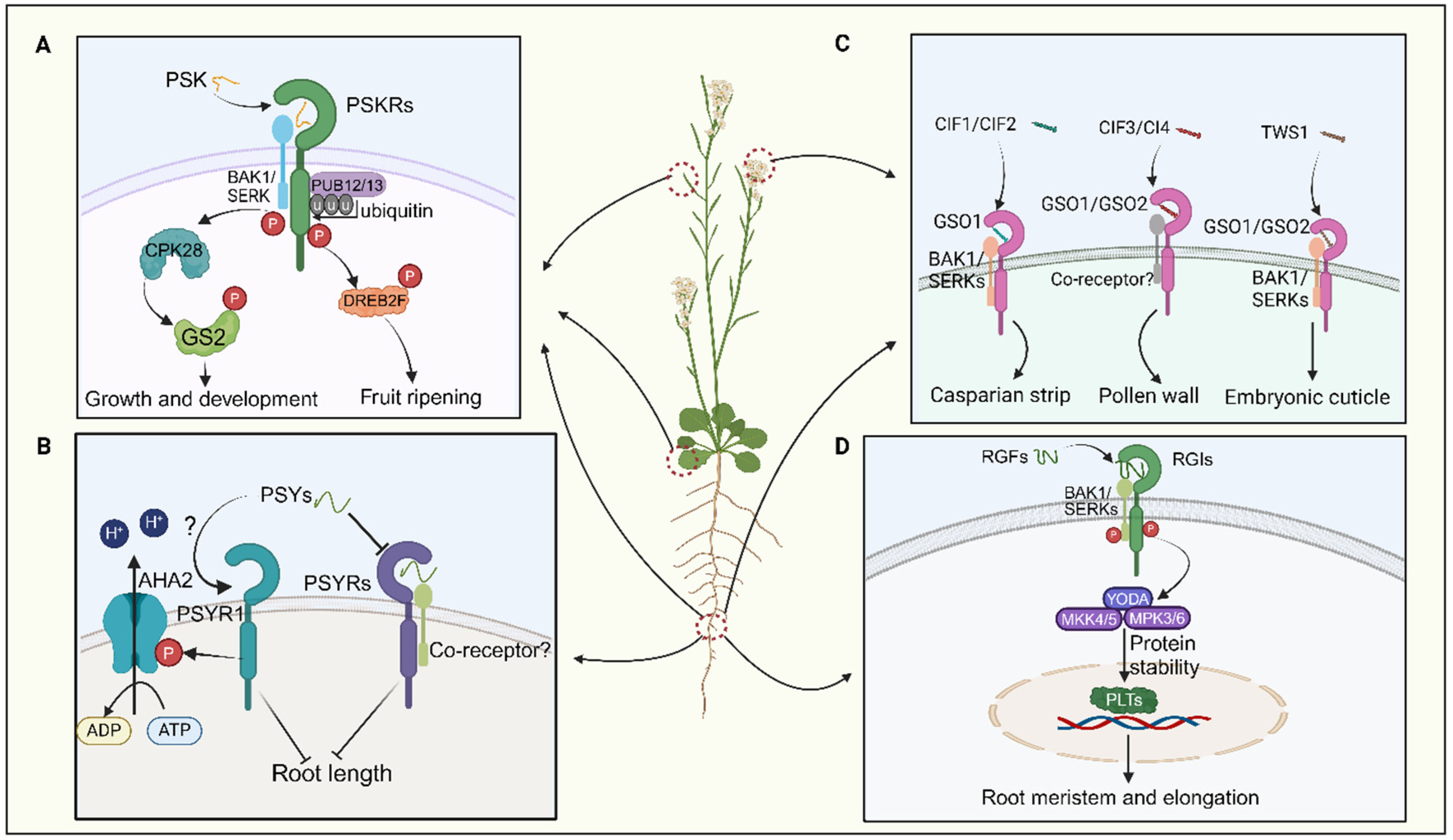
1.6. PSK Peptides
1.7. PSY Peptides
1.8. CIFs and TWS1
1.9. RGF/GLV/CLEL Peptides
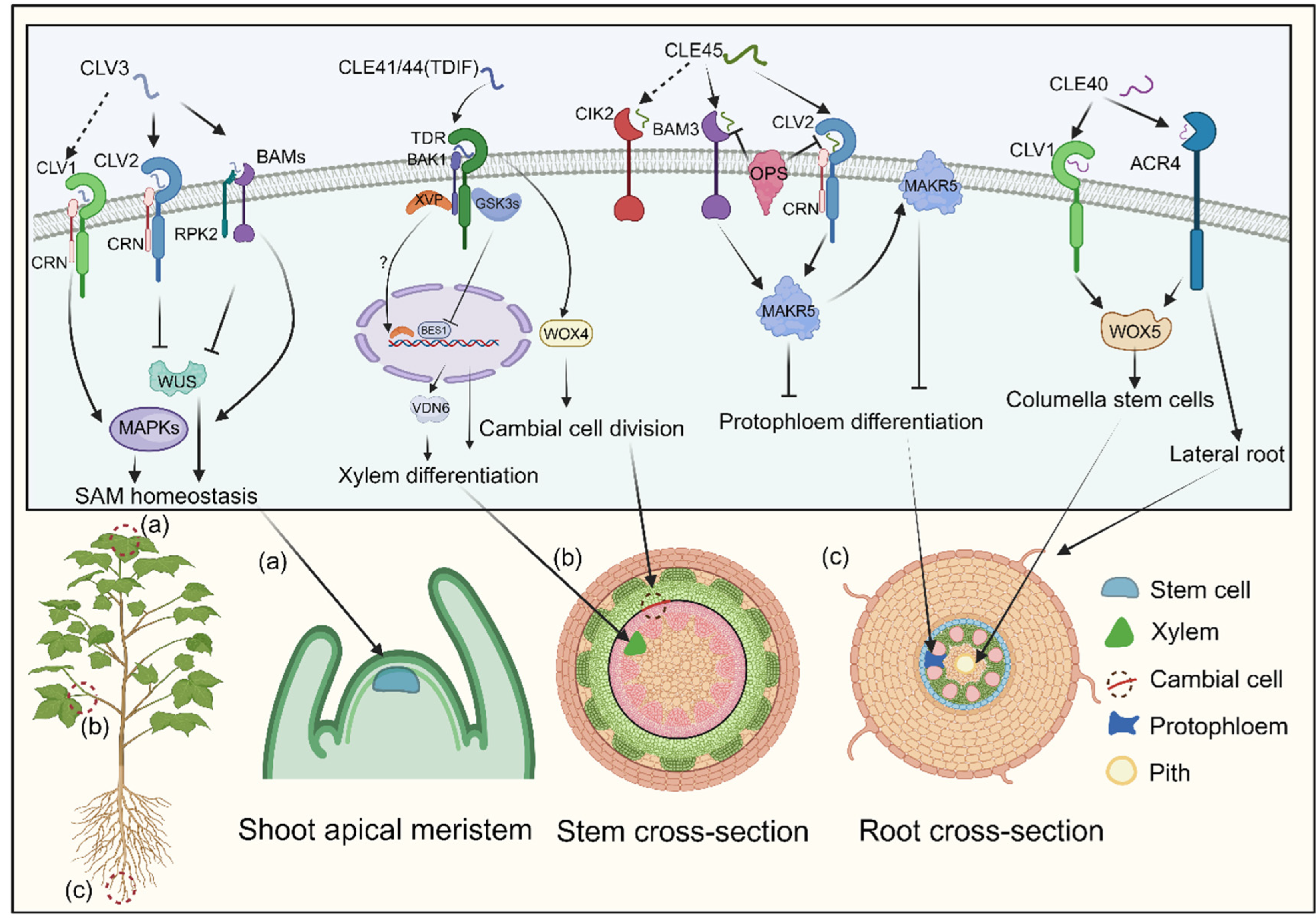
1.10. CLE Peptides
1.11. CLV3
1.12. TDIF
1.13. CLE40
1.14. CLE45
1.15. Other SPs
1.15.1. EPF/EPFL Peptides
1.15.2. LURE Peptides
1.15.3. RALF Peptides
1.15.4. CEP Peptides
1.15.5. IDA Peptides
2. Conclusions and Perspectives
Author Contributions
Acknowledgments
References
- Abarca A, Franck CM, Zipfel C (2021) Family-wide evaluation of RAPID ALKALINIZATION FACTOR peptides. Plant Physiol 187 (2):996-1010.
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh Y-S, Amasino R, Scheres B (2004) The PLETHORA Genes Mediate Patterning of the Arabidopsis Root Stem Cell Niche. Cell 119 (1):109-120.
- Amano Y, Tsubouchi H, Shinohara H, Ogawa M, Matsubayashi Y (2007) Tyrosine-sulfated glycopeptide involved in cellular proliferation and expansion in Arabidopsis. Proc Natl Acad Sci USA 104 (46):18333-18338.
- Anne P, Amiguet-Vercher A, Brandt B, Kalmbach L, Geldner N, Hothorn M, Hardtke CS (2018) CLERK is a novel receptor kinase required for sensing of root-active CLE peptides in Arabidopsis. Development 145 (10):dev162354.
- Anne P, Azzopardi M, Gissot L, Beaubiat S, Hématy K, Palauqui JC (2015) OCTOPUS Negatively Regulates BIN2 to Control Phloem Differentiation in Arabidopsis thaliana. Curr Biol 25 (19):2584-2590.
- Araya T, Miyamoto M, Wibowo J, Suzuki A, Kojima S, Tsuchiya YN, Sawa S, Fukuda H, von Wirén N, Takahashi H (2014) CLE-CLAVATA1 peptide-receptor signaling module regulates the expansion of plant root systems in a nitrogen-dependent manner. Proc Natl Acad Sci USA 111 (5):2029-2034.
- Bellegarde F, Gojon A, Martin A (2017) Signals and players in the transcriptional regulation of root responses by local and systemic N signaling in Arabidopsis thaliana. J Exp Bot 68 (10):2553-2565.
- Bendahmane M, Stührwohldt N, Dahlke RI, Steffens B, Johnson A, Sauter M (2011) Phytosulfokine-α Controls Hypocotyl Length and Cell Expansion in Arabidopsis thaliana through Phytosulfokine Receptor 1. PLoS One, 6(6), e21054.
- Berckmans B, Kirschner G, Gerlitz N, Stadler R, Simon R (2020) CLE40 Signaling Regulates Root Stem Cell Fate. Plant Physiol 182 (4):1776-1792.
- Bessho-Uehara K, Wang DR, Furuta T, Minami A, Nagai K, Gamuyao R, Asano K, Angeles-Shim RB, Shimizu Y, Ayano M, Komeda N, Doi K, Miura K, Toda Y, Kinoshita T, Okuda S, Higashiyama T, Nomoto M, Tada Y, Shinohara H, Matsubayashi Y, Greenberg A, Wu J, Yasui H, Yoshimura A, Mori H, McCouch SR, Ashikari M (2016) Loss of function at RAE2, a previously unidentified EPFL, is required for awnlessness in cultivated Asian rice. Proc Natl Acad Sci USA 113 (32):8969-8974.
- Campbell L, Turner SR (2017) A Comprehensive Analysis of RALF Proteins in Green Plants Suggests There Are Two Distinct Functional Groups. Front Plant Sci 8: 240798.
- Chapman K, Taleski M, Ogilvie HA, Imin N, Djordjevic MA (2019) CEP-CEPR1 signalling inhibits the sucrose-dependent enhancement of lateral root growth. J Exp Bot 70 (15):3955-3967.
- Chen YF, Matsubayashi Y, Sakagami Y (2000) Peptide growth factor phytosulfokine-alpha contributes to the pollen population effect. Planta 211 (5):752-755.
- Chien P-S, Nam HG, Chen Y-R (2015) A salt-regulated peptide derived from the CAP superfamily protein negatively regulates salt-stress tolerance in Arabidopsis. J Exp Bot 66 (17):5301-5313.
- Czyzewicz N, Shi CL, Vu LD, Van De Cotte B, Hodgman C, Butenko MA, De Smet I (2015) Modulation of Arabidopsis and monocot root architecture by CLAVATA3/EMBRYO SURROUNDING REGION 26 peptide. J Exp Bot 66 (17):5229-5243.
- Datta T, Kumar RS, Sinha H, Trivedi PK (2024) Small but mighty: Peptides regulating abiotic stress responses in plants. Plant Cell Environ 47 (4):1207-1223.
- De Smet I, Vassileva V, De Rybel B, Levesque MP, Grunewald W, Van Damme D, Van Noorden G, Naudts M, Van Isterdael G, De Clercq R, Wang JY, Meuli N, Vanneste S, Friml J, Hilson P, Jürgens G, Ingram GC, Inzé D, Benfey PN, Beeckman T (2008) Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 322 (5901):594-597.
- Ding S, Lv J, Hu Z, Wang J, Wang P, Yu J, Foyer CH, Shi K (2023) Phytosulfokine peptide optimizes plant growth and defense via glutamine synthetase GS2 phosphorylation in tomato. EMBO J 42 (6):e111858.
- Doll NM, Royek S, Fujita S, Okuda S, Chamot S, Stintzi A, Widiez T, Hothorn M, Schaller A, Geldner N, Ingram G (2020) A two-way molecular dialogue between embryo and endosperm is required for seed development. Science 367 (6476):431-435.
- Dong Q, Wang G, Iqbal A, Muhammad N, Wang X, Gui H, Zhang H, Kayoumu M, Li X, Zhang X (2022) Identification and expression analysis of the NPF genes in cotton. Int J Mol Sci 23 (22):14262.
- Fang H, Zuo J, Ma Q, Zhang X, Xu Y, Ding S, Wang J, Luo Q, Li Y, Wu C, Lv J, Yu J, Shi K (2024) Phytosulfokine promotes fruit ripening and quality via phosphorylation of transcription factor DREB2F in tomato. Plant Physiol 194 (4):2739-2754.
- Feng YZ, Zhu QF, Xue J, Chen P, Yu Y (2023) Shining in the dark: the big world of small peptides in plants. aBIOTECH 4 (3):238-256.
- Fletcher JC (2020) Recent Advances in Arabidopsis CLE Peptide Signaling. Trends Plant Sci 25 (10):1005-1016.
- Franck CM, Westermann J, Bürssner S, Lentz R, Lituiev DS, Boisson-Dernier A (2018) The Protein Phosphatases ATUNIS1 and ATUNIS2 Regulate Cell Wall Integrity in Tip-Growing Cells. Plant Cell 30 (8):1906-1923.
- Fuglsang AT, Kristensen A, Cuin TA, Schulze WX, Persson J, Thuesen KH, Ytting CK, Oehlenschlæger CB, Mahmood K, Sondergaard TE, Shabala S, Palmgren MG (2014) Receptor kinase-mediated control of primary active proton pumping at the plasma membrane. Plant J 80 (6):951-964.
- Fujita S (2021) CASPARIAN STRIP INTEGRITY FACTOR (CIF) family peptides - regulator of plant extracellular barriers. Peptides 143:170599.
- Galindo-Trigo S, Blanco-Touriñán N, DeFalco TA, Wells ES, Gray JE, Zipfel C, Smith LM (2020) CrRLK1L receptor-like kinases HERK1 and ANJEA are female determinants of pollen tube reception. EMBO Rep 21 (2):e48466.
- Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B (2007) PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449 (7165):1053-1057.
- Gao Q, Wang C, Xi Y, Shao Q, Hou C, Li L, Luan S (2023) RALF signaling pathway activates MLO calcium channels to maintain pollen tube integrity. Cell Res 33 (1):71-79.
- Ge Z, Bergonci T, Zhao Y, Zou Y, Du S, Liu M-C, Luo X, Ruan H, García-Valencia LE, Zhong S, Hou S, Huang Q, Lai L, Moura DS, Gu H, Dong J, Wu H-M, Dresselhaus T, Xiao J, Cheung AY, Qu L-J (2017) Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358 (6370):1596-1600.
- Ge Z, Zhao Y, Liu MC, Zhou LZ, Wang L, Zhong S, Hou S, Jiang J, Liu T, Huang Q, Xiao J, Gu H, Wu HM, Dong J, Dresselhaus T, Cheung AY, Qu LJ (2019) LLG2/3 Are Co-receptors in BUPS/ANX-RALF Signaling to Regulate Arabidopsis Pollen Tube Integrity. Curr Biol 29 (19):3256-3265.e3255.
- Geng R, Shan Y, Li L, Shi CL, Zhang W, Wang J, Sarwar R, Xue YX, Li YL, Zhu KM, Wang Z, Xu LZ, Aalen RB, Tan XL (2022) CRISPR-mediated BnaIDA editing prevents silique shattering, floral organ abscission, and spreading of Sclerotinia sclerotiorum in Brassica napus. Plant Commun 3 (6):100452.
- Geng Y, Jian C, Xu W, Liu H, Hao C, Hou J, Liu H, Zhang X, Li T (2020) miR164-targeted TaPSK5 encodes a phytosulfokine precursor that regulates root growth and yield traits in common wheat (Triticum aestivum L.). Plant Mol Biol 104 (6):615-628.
- Germain H, Chevalier E, Caron S, Matton DP (2005) Characterization of five RALF-like genes from Solanum chacoense provides support for a developmental role in plants. Planta 220 (3):447-454.
- Goad DM, Zhu C, Kellogg EA (2016) Comprehensive identification and clustering of CLV3/ESR-related (De Smet et al.,) genes in plants finds groups with potentially shared function. New Phytol 216 (2):605-616.
- Hake S, Hirakawa Y, Uchida N, Yamaguchi YL, Tabata R, Ishida S, Ishizaki K, Nishihama R, Kohchi T, Sawa S, Bowman JL (2019) Control of proliferation in the haploid meristem by CLE peptide signaling in Marchantia polymorpha. PLOS Genetics 15 (3).
- Han H, Liu X, Zhou Y (2020) Transcriptional circuits in control of shoot stem cell homeostasis. Curr Opin Plant Biol 53:50-56.
- Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T (2007) The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev 21 (14):1720-1725.
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A Peptide Hormone and Its Receptor Protein Kinase Regulate Plant Cell Expansion. Science 343 (6169):408-411.
- Hazak O, Brandt B, Cattaneo P, Santiago J, Rodriguez-Villalon A, Hothorn M, Hardtke CS (2017) Perception of root-active CLE peptides requires CORYNE function in the phloem vasculature. EMBO Rep 18 (8):1367-1381.
- He L, Wu L, Li J (2024) Sulfated peptides and their receptors: Key regulators of plant development and stress adaptation. Plant Commun:100918.
- He Y, He X, Wang X, Hao M, Gao J, Wang Y, Yang ZN, Meng X (2023) An EPFL peptide signaling pathway promotes stamen elongation via enhancing filament cell proliferation to ensure successful self-pollination in Arabidopsis thaliana. New Phytol 238 (3):1045-1058.
- Hellens RP, Brown CM, Chisnall MAW, Waterhouse PM, Macknight RC (2016) The Emerging World of Small ORFs. Trends Plant Sci 21 (4):317-328.
- Hirakawa Y, Kondo Y, Fukuda H (2010) TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 22 (8):2618-2629.
- Hirakawa Y, Shinohara H, Kondo Y, Inoue A, Nakanomyo I, Ogawa M, Sawa S, Ohashi-Ito K, Matsubayashi Y, Fukuda H (2008) Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc Natl Acad Sci USA 105 (39):15208-15213.
- Hobe M, Müller R, Grünewald M, Brand U, Simon R (2003) Loss of CLE40, a protein functionally equivalent to the stem cell restricting signal CLV3, enhances root waving in Arabidopsis. Dev Genes Evol 213 (8):371-381.
- Hohmann U, Ramakrishna P, Wang K, Lorenzo-Orts L, Nicolet J, Henschen A, Barberon M, Bayer M, Hothorn M (2020) Constitutive Activation of Leucine-Rich Repeat Receptor Kinase Signaling Pathways by BAK1-INTERACTING RECEPTOR-LIKE KINASE3 Chimera. Plant Cell 32 (10):3311-3323.
- Holzwart E, Huerta AI, Glöckner N, Garnelo Gómez B, Wanke F, Augustin S, Askani JC, Schürholz AK, Harter K, Wolf S (2018) BRI1 controls vascular cell fate in the Arabidopsis root through RLP44 and phytosulfokine signaling. Proc Natl Acad Sci USA 115 (46):11838-11843.
- Hsiao Y C, Shiue S Y, Yen M R(2024). RGF1 controls PLT2 protein stability through ROS-dependent regulation of a cysteine residue in root meristem development. bioRxiv, 2024: 2024.04. 08.588570.
- Hsiao YC, Yamada M (2020) The Roles of Peptide Hormones and Their Receptors during Plant Root Development. Genes, 12(1), 22.
- Hu X-L, Lu H, Hassan MM, Zhang J, Yuan G, Abraham PE, Shrestha HK, Villalobos Solis MI, Chen J-G, Tschaplinski TJ (2021) Advances and perspectives in discovery and functional analysis of small secreted proteins in plants. Hortic, 8(1), 130.
- Hu Z, Fang H, Zhu C, Gu S, Ding S, Yu J, Shi K (2023) Ubiquitylation of PHYTOSULFOKINE RECEPTOR 1 modulates the defense response in tomato. Plant Physiol 192 (3):2507-2522.
- Huang CH, Sun R, Hu Y, Zeng L, Zhang N, Cai L, Zhang Q, Koch MA, Al-Shehbaz I, Edger PP, Pires JC, Tan DY, Zhong Y, Ma H (2016) Resolution of Brassicaceae Phylogeny Using Nuclear Genes Uncovers Nested Radiations and Supports Convergent Morphological Evolution. Mol Biol Evol 33 (2):394-412.
- Huang WJ, Liu HK, McCormick S, Tang WH (2014) Tomato Pistil Factor STIG1 Promotes in Vivo Pollen Tube Growth by Binding to Phosphatidylinositol 3-Phosphate and the Extracellular Domain of the Pollen Receptor Kinase LePRK2. Plant Cell 26 (6):2505-2523.
- Imin N, Mohd-Radzman NA, Ogilvie HA, Djordjevic MA (2013) The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J Exp Bot 64 (17):5395-5409.
- Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H (2006) Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313 (5788):842-845.
- Je BI, Xu F, Wu Q, Liu L, Meeley R, Gallagher JP, Corcilius L, Payne RJ, Bartlett ME, Jackson D (2018) The CLAVATA receptor FASCIATED EAR2 responds to distinct CLE peptides by signaling through two downstream effectors. Elife, 7, e35673.
- Jeon BW, Kim MJ, Pandey SK, Oh E, Seo PJ, Kim J (2021) Recent advances in peptide signaling during Arabidopsis root development. J Exp Bot 72(8):2889-2902.
- Jing Y, Zhao F, Lai K, Sun F, Sun C, Zou X, Xu M, Fu A, Sharifi R, Chen J (2024) Plant elicitor Peptides regulate root hair development in Arabidopsis. Frontiers in Plant Sci 15:1336129.
- Jones DS, John A, VanDerMolen KR, Nimchuk ZL (2021) CLAVATA Signaling Ensures Reproductive Development in Plants across Thermal Environments. Curr Biol 31 (1):220-227.e225.
- Jourquin J, Fernandez AI, Wang Q, Xu K, Chen J, Šimura J, Ljung K, Vanneste S, Beeckman T (2023) GOLVEN peptides regulate lateral root spacing as part of a negative feedback loop on the establishment of auxin maxima. J Exp Bot 74 (14):4031-4049.
- Katharina Schardon (2016) Precursor processing for plant peptide hormone maturation by subtilisin-like serine proteinases. Science 354(6319), 1594–1597.
- Kang YH, Hardtke CS (2016) Arabidopsis MAKR5 is a positive effector of BAM3-dependent CLE45 signaling. EMBO Rep 17 (8):1145-1154.
- Komori R, Amano Y, Ogawa-Ohnishi M, Matsubayashi Y (2009) Identification of tyrosylprotein sulfotransferase in Arabidopsis. Proc Natl Acad Sci USA 106 (35):15067-15072.
- Kosentka PZ, Overholt A, Maradiaga R, Mitoubsi O, Shpak ED (2019) EPFL Signals in the Boundary Region of the SAM Restrict Its Size and Promote Leaf Initiation. Plant Physiol 179 (1):265-279.
- Kou X, Liu Q, Sun Y, (2020) The peptide PbrPSK2 from phytosulfokine family induces reactive oxygen species (ROS) production to regulate pear pollen tube growth [J]. Frontiers in Plant Sci 11: 601993.
- Kutschmar A, Rzewuski G, Stührwohldt N, Beemster GTS, Inzé D, Sauter M (2008) PSK-α promotes root growth in Arabidopsis. New Phytol 181 (4):820-831.
- Lee H, Jun YS, Cha O-K, Sheen J (2019) Mitogen-activated protein kinases MPK3 and MPK6 are required for stem cell maintenance in the Arabidopsis shoot apical meristem. Plant Cell Rep 38 (3):311-319.
- Lee KP, Liu K, Kim EY, Medina-Puche L, Dong H, Duan J, Li M, Dogra V, Li Y, Lv R, Li Z, Lozano-Duran R, Kim C (2020) PLANT NATRIURETIC PEPTIDE A and Its Putative Receptor PNP-R2 Antagonize Salicylic Acid-Mediated Signaling and Cell Death. Plant Cell 32 (7):2237-2250.
- Li J, Wen J, Lease K.A, Doke J.T, Tax F.E, Walker, J.C (2002). BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110:213–222.
- Li Z, Liu D, Xia Y, Li Z, Niu N, Ma S, Wang J, Song Y, Zhang G (2019) Identification and Functional Analysis of the CLAVATA3/EMBRYO SURROUNDING REGION. Int J Mol Sci 20(17), 4319.
- Lin G, Zhang L, Han Z, Yang X, Liu W, Li E, Chang J, Qi Y, Shpak ED, Chai J (2017) A receptor-like protein acts as a specificity switch for the regulation of stomatal development. Genes Dev 31 (9):927-938.
- Lu X, Shi H, Ou Y, Cui Y, Chang J, Peng L, Gou X, He K, Li J. RGF1-RGI1(2020) a Peptide-Receptor Complex, Regulates Arabidopsis Root Meristem Development via a MAPK Signaling Cascade. Mol Plant 13(11):1594-1607.
- Lu Q, Li H, Hong Y, Zhang G, Wen S, Li X, Zhou G, Li S, Liu H, Liu H, Liu Z, Varshney RK, Chen X, Liang X (2018) Genome Sequencing and Analysis of the Peanut B-Genome Progenitor (Arachis ipaensis). Front Plant Sci 9:604.
- Maruyama D, Higashiyama T (2016) The end of temptation: the elimination of persistent synergid cell identity. Curr Opin Plant Biol 34:122-126.
- Matos JL, Fiori CS, Silva-Filho MC, Moura DS (2008) A conserved dibasic site is essential for correct processing of the peptide hormone AtRALF1 in Arabidopsis thaliana. FEBS Lett 582 (23-24):3343-3347.
- Matsubayashi Y, Ogawa M, Kihara H, Niwa M, Sakagami Y (2006) Disruption and overexpression of Arabidopsis phytosulfokine receptor gene affects cellular longevity and potential for growth. Plant Physiol 142 (1):45-53.
- Matsubayashi Y, Sakagami Y (1996) Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cells of Asparagus officinalis L. Proc Natl Acad Sci USA 93 (15):7623-7627.
- Matsuzaki Y, Ogawa-Ohnishi M, Mori A, Matsubayashi Y (2010) Secreted peptide signals required for maintenance of root stem cell niche in Arabidopsis. Science 329 (5995):1065-1067.
- Mei Z, Li B, Zhu S, Li Y, Yao J, Pan J, Zhang Y, Chen W (2024) A Genome-Wide Analysis of the CEP Gene Family in Cotton and a Functional Study of GhCEP46-D05 in Plant Development. Int J Mol Sci 25 (8).
- Meng L, Buchanan BB, Feldman LJ, Luan S (2012) CLE-like (CLEL) peptides control the pattern of root growth and lateral root development in Arabidopsis. Proc Natl Acad Sci USA 109 (5):1760-1765.
- Morita J, Kato K, Nakane T, Kondo Y, Fukuda H, Nishimasu H, Ishitani R, Nureki O (2016) Crystal structure of the plant receptor-like kinase TDR in complex with the TDIF peptide. Nat Commun 7:12383.
- Moussu S, Doll NM, Chamot S, Brocard L, Creff A, Fourquin C, Widiez T, Nimchuk ZL, Ingram G (2017) ZHOUPI and KERBEROS Mediate Embryo/Endosperm Separation by Promoting the Formation of an Extracuticular Sheath at the Embryo Surface. Plant Cell 29 (7):1642-1656.
- Ogawa-Ohnishi M, Yamashita T, Kakita M, Nakayama T, Ohkubo Y, Hayashi Y, Yamashita Y, Nomura T, Noda S, Shinohara H, Matsubayashi Y (2022) Peptide ligand-mediated trade-off between plant growth and stress response. Science 378 (6616):175-180.
- Ohyama K, Ogawa M, Matsubayashi Y (2008) Identification of a biologically active, small, secreted peptide in Arabidopsis by in silico gene screening, followed by LC-MS-based structure analysis. Plant J 55 (1):152-160.
- Okamoto S, Kawasaki A, Makino Y, Ishida T, Sawa S (2022) Long-distance translocation of CLAVATA3/ESR-related 2 peptide and its positive effect on roots sucrose status. Plant Physiol 189 (4):2357-2367.
- Okuda S, Fujita S, Moretti A, Hohmann U, Doblas VG, Ma Y, Pfister A, Brandt B, Geldner N, Hothorn M (2020) Molecular mechanism for the recognition of sequence-divergent CIF peptides by the plant receptor kinases GSO1/SGN3 and GSO2. Proc Natl Acad Sci USA 117 (5):2693-2703.
- Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, Kawano N, Sakakibara T, Namiki S, Itoh K, Otsuka K, Matsuzaki M, Nozaki H, Kuroiwa T, Nakano A, Kanaoka MM, Dresselhaus T, Sasaki N, Higashiyama T (2009) Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458 (7236):357-361.
- Olsson V, Joos L, Zhu S, Gevaert K, Butenko MA, De Smet I (2019) Look Closely, the Beautiful May Be Small: Precursor-Derived Peptides in Plants. Annu Rev Plant Biol 70:153-186.
- Ong SN, Tan BC, Al-Idrus A, Teo CH (2022) Small open reading frames in plant research: from prediction to functional characterization. 3 Biotech 12 (3):76.
- Ota R, Ohkubo Y, Yamashita Y, Ogawa-Ohnishi M, Matsubayashi Y (2020) Shoot-to-root mobile CEPD-like 2 integrates shoot nitrogen status to systemically regulate nitrate uptake in Arabidopsis. Nat Commun 11 (1):641.
- Ou Y, Lu X, Zi Q, Xun Q, Zhang J, Wu Y, Shi H, Wei Z, Zhao B, Zhang X, He K, Gou X, Li C, Li J (2016) RGF1 INSENSITIVE 1 to 5, a group of LRR receptor-like kinases, are essential for the perception of root meristem growth factor 1 in Arabidopsis thaliana. Cell Res 26 (6):686-698.
- Ou Y, Tao B, Wu Y, Cai Z, Li H, Li M, He K, Gou X, Li J (2022) Essential roles of SERKs in the ROOT MERISTEM GROWTH FACTOR-mediated signaling pathway. Plant Physiol 189 (1):165-177.
- Pearce G, Moura DS, Stratmann J, Ryan CA (2001) RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc Natl. Acad. Sci. USA, 98, 12 843–12 847.
- Pearce G, Munske G, Yamaguchi Y, Ryan CA (2010) Structure-activity studies of GmSubPep, a soybean peptide defense signal derived from an extracellular protease. Peptides 31 (12):2159-2164.
- Pei D, Hua D, Deng J, Wang Z, Gong Z (2022). Phosphorylation of the plasma membrane H+-ATPase AHA2 by BAK1 is required for ABA-induced stomatal closure in Arabidopsis. Plant Cell 34(7), 2708-2729.
- Qin Y, Yang L, Sun Z, Wang X, Wang Y, Zhang J, Rehman AU, Chen Z, Qi J, Wang B, Song C, Yang S, Gong Z (2019) Redox-Mediated Endocytosis of a Receptor-Like Kinase during Distal Stem Cell Differentiation Depends on Its Tumor Necrosis Factor Receptor Domain. Plant Physiol 181 (3):1075-1095.
- Reichardt S, Piepho H-P, Stintzi A, Schaller A (2020) Peptide signaling for drought-induced tomato flower drop. Science 367 (6485):1482-1485.
- Richards S, Wink RH, Simon R (2015) Mathematical modelling of WOX5- and CLE40-mediated columella stem cell homeostasis in Arabidopsis. J Exp Bot 66 (17):5375-5384.
- Roberts I, Smith S, Stes E, De Rybel B, Staes A, van de Cotte B, Njo MF, Dedeyne L, Demol H, Lavenus J, Audenaert D, Gevaert K, Beeckman T, De Smet I (2016) CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis. J Exp Bot 67 (16):4889-4899.
- Rodriguez-Villalon A, Gujas B, Kang YH, Breda AS, Cattaneo P, Depuydt S, Hardtke CS (2014) Molecular genetic framework for protophloem formation. Proc Natl Acad Sci USA 111 (31):11551-11556.
- Royek S, Bayer M, Pfannstiel J, Pleiss J, Ingram G, Stintzi A, Schaller A (2022) Processing of a plant peptide hormone precursor facilitated by posttranslational tyrosine sulfation. Proc Natl Acad Sci USA 119 (16):e2201195119.
- Ryan CA, Pearce G (1998) Systemin: a polypeptide signal for plant defensive genes. Annu Rev Cell Dev Biol 14:1-17.
- Rzemieniewski J, Leicher H, Lee H K(2022) CEP signaling coordinates plant immunity with nitrogen status. bioRxiv, 2022-12.
- Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T (2007) Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446 (7137):811-814.
- Sauter M (2015) Phytosulfokine peptide signalling. J Exp Bot 66 (17):5161-5169.
- Shao Y, Yu X, Xu X, Li Y, Yuan W, Xu Y, Mao C, Zhang S, Xu J(2020). The YDA-MKK4/MKK5-MPK3/MPK6 Cascade Functions Downstream of the RGF1-RGI Ligand-Receptor Pair in Regulating Mitotic Activity in Root Apical Meristem. Mol Plant.13(11):1608-1623.
- Shen W, Zhang X, Liu J, Tao K, Li C, Xiao S, Zhang W, Li JF (2022) Plant elicitor peptide signalling confers rice resistance to piercing-sucking insect herbivores and pathogens. Plant Biotechnol J 20 (5):991-1005.
- Shinohara H (2021) Root meristem growth factor RGF, a sulfated peptide hormone in plants. Peptides 142, 170556.
- Shinohara H, Mori A, Yasue N, Sumida K, Matsubayashi Y (2016) Identification of three LRR-RKs involved in perception of root meristem growth factor in Arabidopsis. Proc Natl Acad Sci USA 113 (14):3897-3902.
- Smit ME, McGregor SR, Sun H, Gough C, Bågman AM, Soyars CL, Kroon JT, Gaudinier A, Williams CJ, Yang X, Nimchuk ZL, Weijers D, Turner SR, Brady SM, Etchells JP (2020) A PXY-Mediated Transcriptional Network Integrates Signaling Mechanisms to Control Vascular Development in Arabidopsis. Plant Cell 32 (2):319-335.
- Somssich M, Ma Q, Weidtkamp-Peters S, Stahl Y, Felekyan S, Bleckmann A, Seidel CA, Simon R (2015) Real-time dynamics of peptide ligand-dependent receptor complex formation in planta. Sci Signal 8(388), ra76-ra76.
- Song W, Liu L, Wang J, Wu Z, Zhang H, Tang J, Lin G, Wang Y, Wen X, Li W, Han Z, Guo H, Chai J (2016) Signature motif-guided identification of receptors for peptide hormones essential for root meristem growth. Cell Res 26 (6):674-685.
- Soyano T, Hirakawa H, Sato S, Hayashi M, Kawaguchi M (2014) Nodule Inception creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production. Proc Natl Acad Sci USA 111 (40):14607-14612.
- Stahl Y, Grabowski S, Bleckmann A, Kühnemuth R, Weidtkamp-Peters S, Pinto KG, Kirschner GK, Schmid JB, Wink RH, Hülsewede A, Felekyan S, Seidel CA, Simon R (2013) Moderation of Arabidopsis root stemness by CLAVATA1 and ARABIDOPSIS CRINKLY4 receptor kinase complexes. Curr Biol 23 (5):362-371.
- Stegmann M, Monaghan J, Smakowska-Luzan E, Rovenich H, Lehner A, Holton N, Belkhadir Y, Zipfel C (2017) The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355 (6322):287-289.
- Stenvik GE, Tandstad NM, Guo Y, Shi CL, Kristiansen W, Holmgren A, Clark SE, Aalen RB, Butenko MA (2008) The EPIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is sufficient to induce abscission in arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell 20 (7):1805-1817.
- Stintzi A, Schaller A (2022) Biogenesis of post-translationally modified peptide signals for plant reproductive development. Curr Opin Plant Biol 69:102274.
- Stührwohldt N, Bühler E, Sauter M, Schaller A, Bozhkov P (2021) Phytosulfokine (PSK) precursor processing by subtilase SBT3.8 and PSK signaling improve drought stress tolerance in Arabidopsis. J Exp Bot 72 (9):3427-3440.
- Takahashi, F., Suzuki, T., Osakabe, Y. (2018). A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556, 235–238.
- Takeuchi H (2021) The role of diverse LURE-type cysteine-rich peptides as signaling molecules in plant reproduction. Peptides 142:170572.
- Takeuchi H, Higashiyama T (2016) Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531 (7593):245-248.
- Taleski M, Chapman K, Novák O, Schmülling T, Frank M, Djordjevic MA (2023) CEP peptide and cytokinin pathways converge on CEPD glutaredoxins to inhibit root growth. Nature Communications 14 (1):1683.
- Taleski M, Imin N, Djordjevic MA (2018) CEP peptide hormones: key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. J Exp Bot 69 (8):1829-1836.
- Tavormina P, De Coninck B, Nikonorova N, De Smet I, Cammue BP (2015) The Plant Peptidome: An Expanding Repertoire of Structural Features and Biological Functions. Plant Cell 27 (8):2095-2118.
- Taylor I, Baer J, Calcutt R, Walker JC (2019) Hypermorphic SERK1 Mutations Function via a SOBIR1 Pathway to Activate Floral Abscission Signaling. Plant Physiol 180 (2):1219-1229.
- Tian D, Xie Q, Deng Z, Xue J, Li W, Zhang Z, Dai Y, Zheng B, Lu T, De Smet I, Guo Y (2022) Small secreted peptides encoded on the wheat (triticum aestivum L.) genome and their potential roles in stress responses. Front Plant Sci 13:1000297.
- Tost AS, Kristensen A, Olsen LI, Axelsen KB, Fuglsang AT (2021) The PSY Peptide Family-Expression, Modification and Physiological Implications. Genes, 12(2), 218.
- Truskina J, Brück S, Stintzi A, Boeuf S, Doll NM, Fujita S, Geldner N, Schaller A, Ingram GC (2022) A peptide-mediated, multilateral molecular dialogue for the coordination of pollen wall formation. Proc Natl Acad Sci USA 119 (22):e2201446119.
- Tsuwamoto R, Fukuoka H, Takahata Y (2008) GASSHO1 and GASSHO2 encoding a putative leucine-rich repeat transmembrane-type receptor kinase are essential for the normal development of the epidermal surface in Arabidopsis embryos. The Plant Journal 54 (1):30-42.
- Uchida N, Lee JS, Horst RJ, Lai HH, Kajita R, Kakimoto T, Tasaka M, Torii KU (2012) Regulation of inflorescence architecture by intertissue layer ligand-receptor communication between endodermis and phloem. Proc Natl Acad Sci USA 109 (16):6337-6342.
- Vie AK, Najafi J, Liu B, Winge P, Butenko MA, Hornslien KS, Kumpf R, Aalen RB, Bones AM, Brembu T (2015) The IDA/IDA-LIKE and PIP/PIP-LIKE gene families in Arabidopsis: phylogenetic relationship, expression patterns, and transcriptional effect of the PIPL3 peptide. J Exp Bot 66 (17):5351-5365.
- Vie AK, Najafi J, Winge P, Cattan E, Wrzaczek M, Kangasjärvi J, Miller G, Brembu T, Bones AM (2017) The IDA-LIKE peptides IDL6 and IDL7 are negative modulators of stress responses in Arabidopsis thaliana. J Exp Bot 68 (13):3557-3571.
- Wang J, Li H, Han Z, Zhang H, Wang T, Lin G, Chang J, Yang W, Chai J (2015) Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature 525 (7568):265-268.
- Wang P, Yao S, Kosami K.I, Guo T, Li J, Zhang Y, Fukao Y, Kaneko-Kawano T, Zhang H, She Y.M, Wang P, Xing W, Hanada K, Liu R, and Kawano Y. (2020). Identification of endogenous small peptides involved in rice immunity through transcriptomics- and proteomics-based screening. Plant Biotechnol J 18, 415-428.
- Wang P, Wu T, Jiang C, Huang B, Li Z (2023) Brt9SIDA/IDALs as peptide signals mediate diverse biological pathways in plants. Plant Science 330:111642.
- Wang Y, Shirakawa M, Ito T (2022) Dynamic Changes in Reactive Oxygen Species in the Shoot Apex Contribute to Stem Cell Death in Arabidopsis thaliana. Int J Mol Sci 23 (7).
- Whitford R, Fernandez A, De Groodt R, Ortega E, Hilson P (2008) Plant CLE peptides from two distinct functional classes synergistically induce division of vascular cells. Proc Natl Acad Sci USA 105 (47):18625-18630.
- Willoughby AC, Nimchuk ZL (2021) WOX going on: CLE peptides in plant development. Curr Opin Plant Biol 63:102056.
- Xiao F, Zhou H (2023) Plant salt response: Perception, signaling, and tolerance. Front Plant Sci 13:1053699.
- Xiao Y, Stegmann M, Han Z, DeFalco TA, Parys K, Xu L, Belkhadir Y, Zipfel C, Chai J (2019) Mechanisms of RALF peptide perception by a heterotypic receptor complex. Nature 572 (7768):270-274.
- Xie M, Zhao C, Song M, Xiang Y, Tong C (2022) Genome-wide identification and comparative analysis of CLE family in rapeseed and its diploid progenitors. Front Plant Sci 13:998082.
- Xu C, Xiang L, Huang W, Zhang X, Mao C, Wu S, Li T, Wang S, Wang S(2024). Unraveling a Small Secreted Peptide SUBPEP3 That Positively Regulates Salt-Stress Tolerance in Pyrus betulifolia. Int J Mol Sci.25(9):4612.
- Xu K, Tian D, Wang T, Zhang A, Elsadek MAY, Liu W, Chen L, Guo Y (2023) Small secreted peptides (SSPs) in tomato and their potential roles in drought stress response. Mol Hortic 3 (1):17.
- Yamaguchi YL, Ishida T, Sawa S (2016) CLE peptides and their signaling pathways in plant development. J Exp Bot 67 (16):4813-4826.
- Yamada M, Han X, Benfey P.N (2020). RGF1 controls root meristem size through ROS signalling. Nature 577, 85–88.
- Yang JH, Lee KH, Du Q, Yang S, Yuan B, Qi L, Wang H (2020) A membrane-associated NAC domain transcription factor XVP interacts with TDIF co-receptor and regulates vascular meristem activity. New Phytol 226 (1):59-74.
- Yang W, Zhai H, Wu F, Deng L, Chao Y, Meng X, Chen Q, Liu C, Bie X, Sun C, Yu Y, Zhang X, Zhang X, Chang Z, Xue M, Zhao Y, Meng X, Li B, Zhang X, Zhang D, Zhao X, Gao C, Li J, Li C (2024) Peptide REF1 is a local wound signal promoting plant regeneration. Cell, S0092-8674(24)00466-5.
- Yang Y, Niu Y, Chen T, Zhang H, Zhang J, Qian D, Bi M, Fan Y, An L, Xiang Y (2022) The phospholipid flippase ALA3 regulates pollen tube growth and guidance in Arabidopsis. Plant Cell 34 (10):3718-3736.
- Yin X, Biswal AK, Dionora J, Perdigon KM, Balahadia CP, Mazumdar S, Chater C, Lin H-C, Coe RA, Kretzschmar T, Gray JE, Quick PW, Bandyopadhyay A (2017) CRISPR-Cas9 and CRISPR-Cpf1 mediated targeting of a stomatal developmental gene EPFL9 in rice. Plant Cell Rep 36 (5):745-757.
- Ying P, Li C, Liu X, Xia R, Zhao M, Li J (2016) Identification and molecular characterization of an IDA-like gene from litchi, LcIDL1, whose ectopic expression promotes floral organ abscission in Arabidopsis. Scientific Reports 6 (1):37135.
- Yu L, Liu Y, Zeng S, Yan J, Wang E, Luo L (2019a) Expression of a novel PSK-encoding gene from soybean improves seed growth and yield in transgenic plants. Planta 249 (4):1239-1250.
- Yu Z, Xu Y, Liu L, Guo Y, Yuan X, Man X, Liu C, Yang G, Huang J, Yan K, Zheng C, Wu C, Zhang S (2019b) The Importance of Conserved Serine for C-Terminally Encoded Peptides Function Exertion in Apple. Int J Mol Sci, 20(3), 775.
- Yuan B, Wang H (2021) Peptide signaling pathways regulate plant vascular development. Front Plant Sci 12:719606.
- Zelman AK, Berkowitz GA(2023). Plant Elicitor Peptide (Pep) Signaling and Pathogen Defense in Tomato. Plants 12(15):2856.
- Zeng J, Dong Z, Wu H, Tian Z, Zhao Z (2017) Redox regulation of plant stem cell fate.EMBO J 36(19): 2844–2855.
- Zhang H, Hu Z, Lei C, Zheng C, Wang J, Shao S, Li X, Xia X, Cai X, Zhou J, Zhou Y, Yu J, Foyer CH, Shi K (2018) A Plant Phytosulfokine Peptide Initiates Auxin-Dependent Immunity through Cytosolic Ca2+ Signaling in Tomato. Plant Cell 30 (3):652-667.
- Zhang H, Li X, Wang W, Li H, Cui Y, Zhu Y, Kui H, Yi J, Li J, Gou X (2022) SERKs regulate embryonic cuticle integrity through the TWS1-GSO1/2 signaling pathway in Arabidopsis. New Phytol 233 (1):313-328.
- Zhang H, Lin X, Han Z, Qu LJ, Chai J (2016) Crystal structure of PXY-TDIF complex reveals a conserved recognition mechanism among CLE peptide-receptor pairs. Cell Res 26 (5):543-555.
- Zhang M, Zhang S (2022) Mitogen-activated protein kinase cascades in plant signaling. J Integr Plant Biol 64 (2):301-341.
- Zhou H, Xia F, Zheng Y, Liu G, Zhuang Y, Wang Z, Zhang Y, He J, Fu C, and Lin H.(2022). PAMP-INDUCED SECRETED PEPTIDE 3 modulates salt tolerance through RECEPTOR-LIKE KINASE 7 in plants. Plant Cell 34, 927-944.
- Zhu Y, Hu C, Cui Y, Zeng L, Li S, Zhu M, Gou, X. (2021). Conserved and differentiated functions of CIK receptor kinases in modulating stem cell signaling in Arabidopsis. Mol Plant 14(7): 1119-1134.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



