Submitted:
03 June 2024
Posted:
04 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
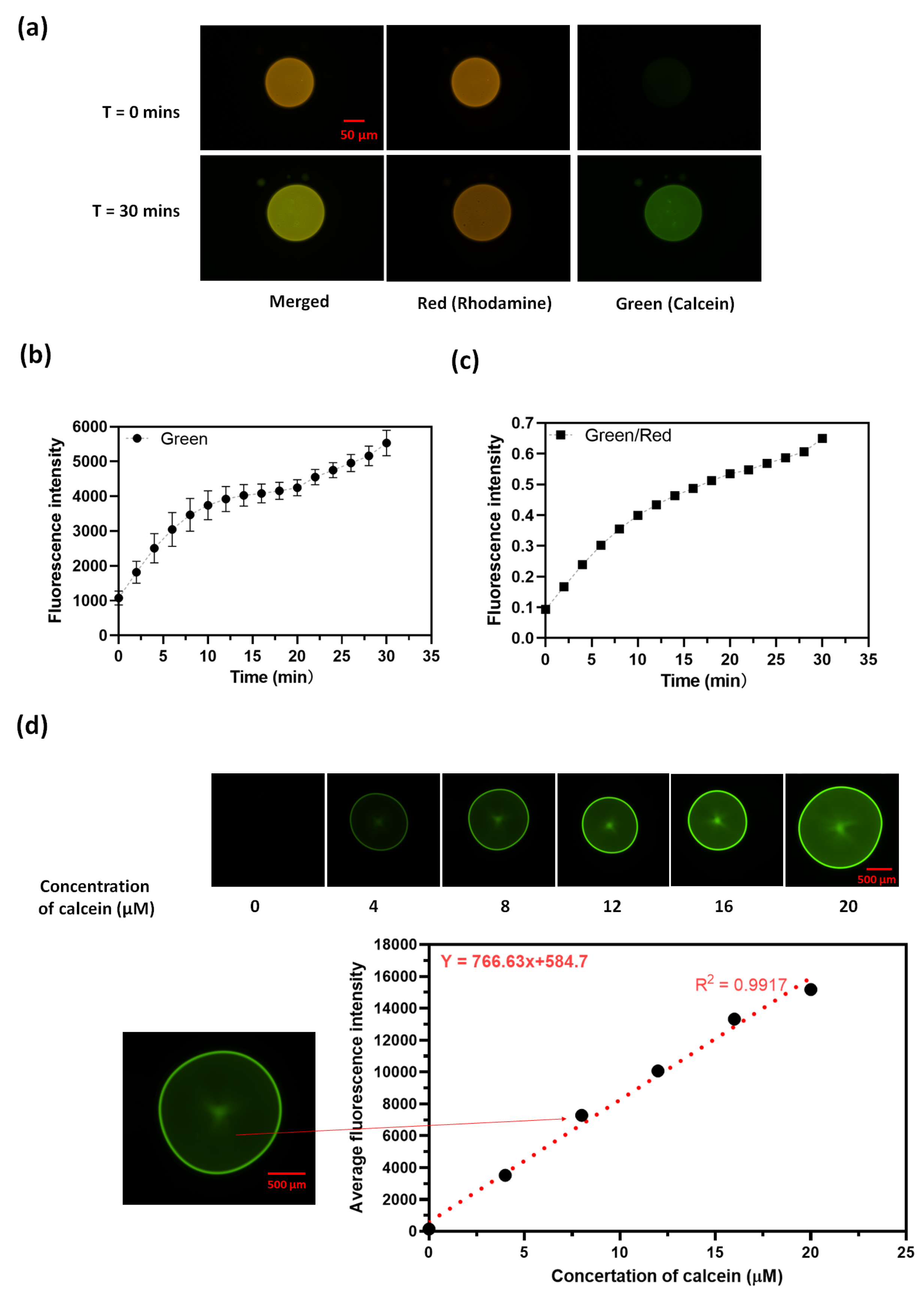
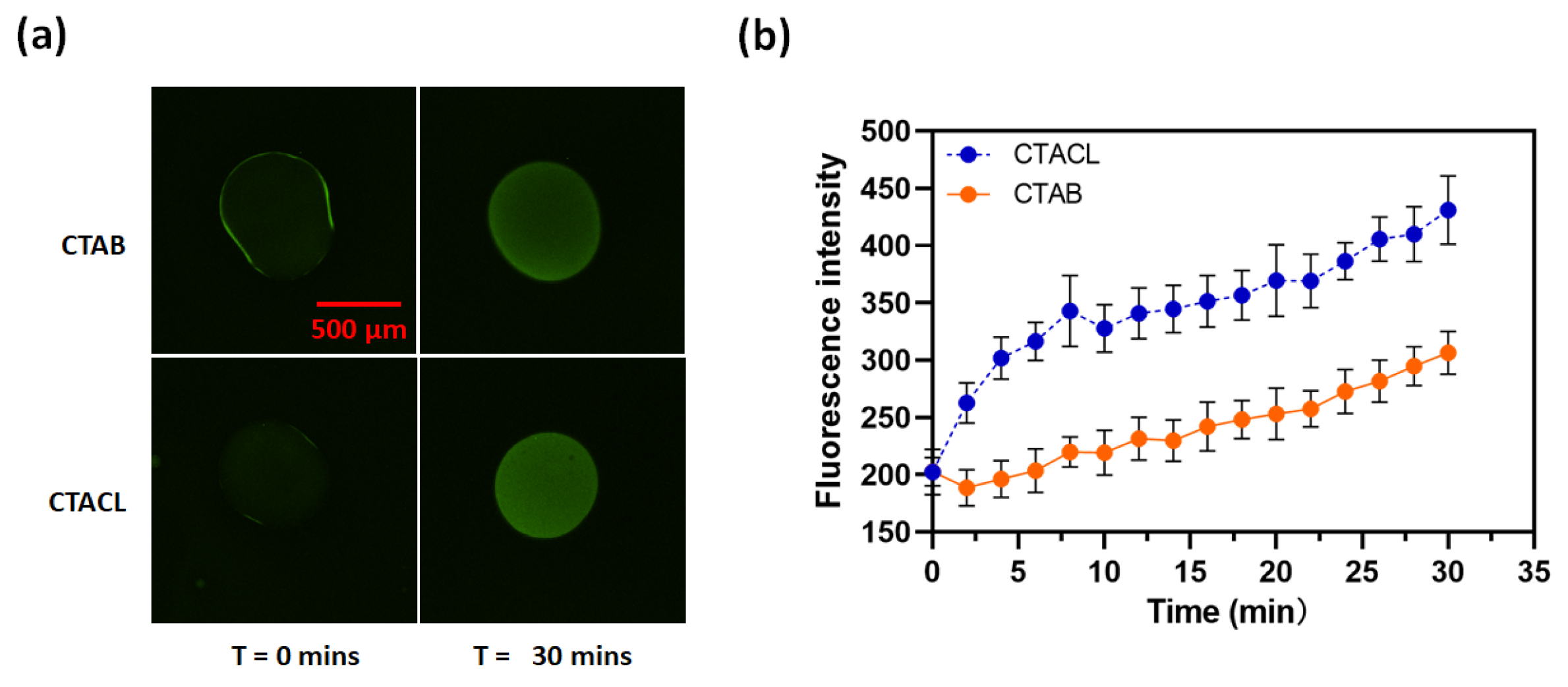
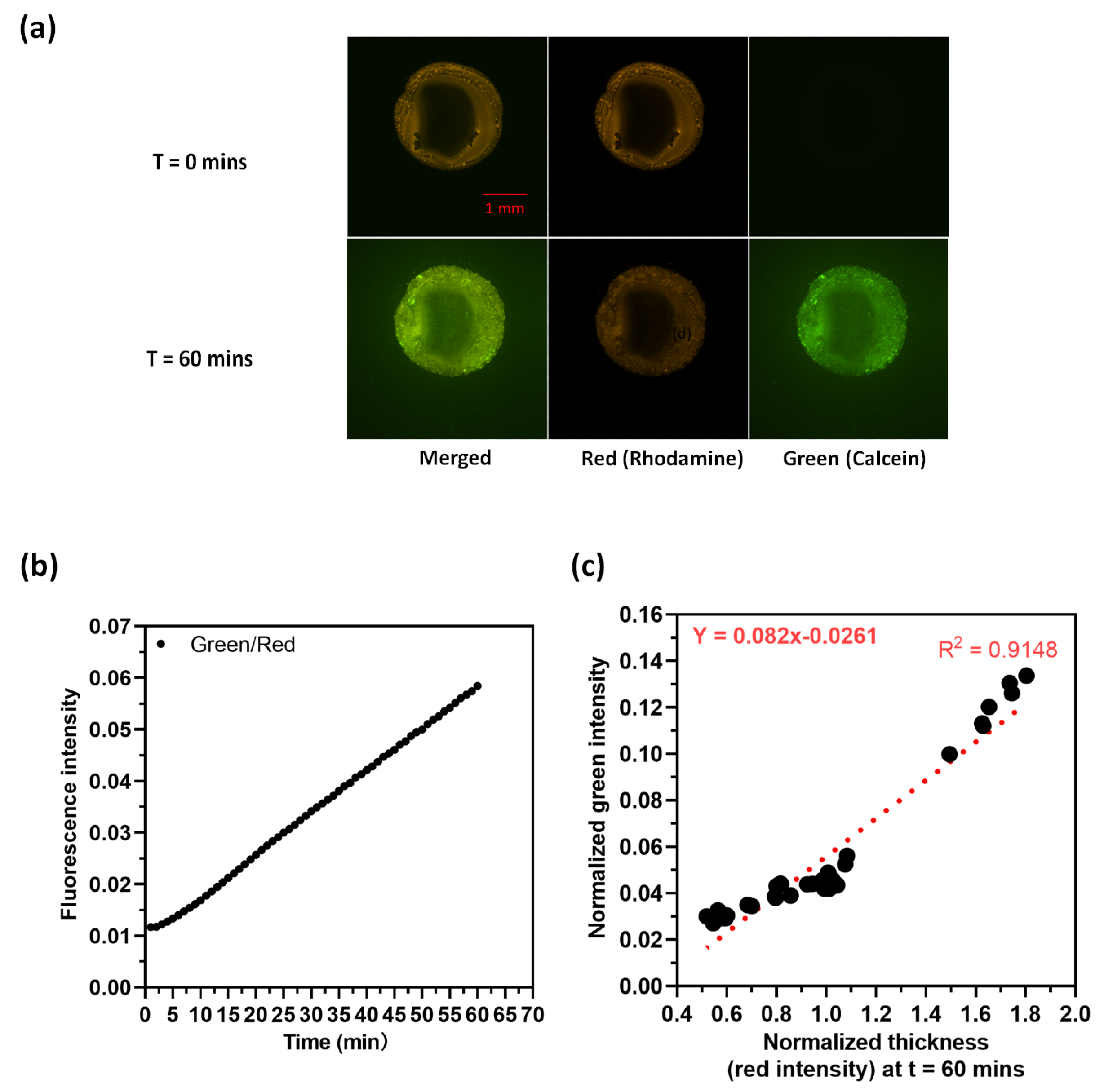
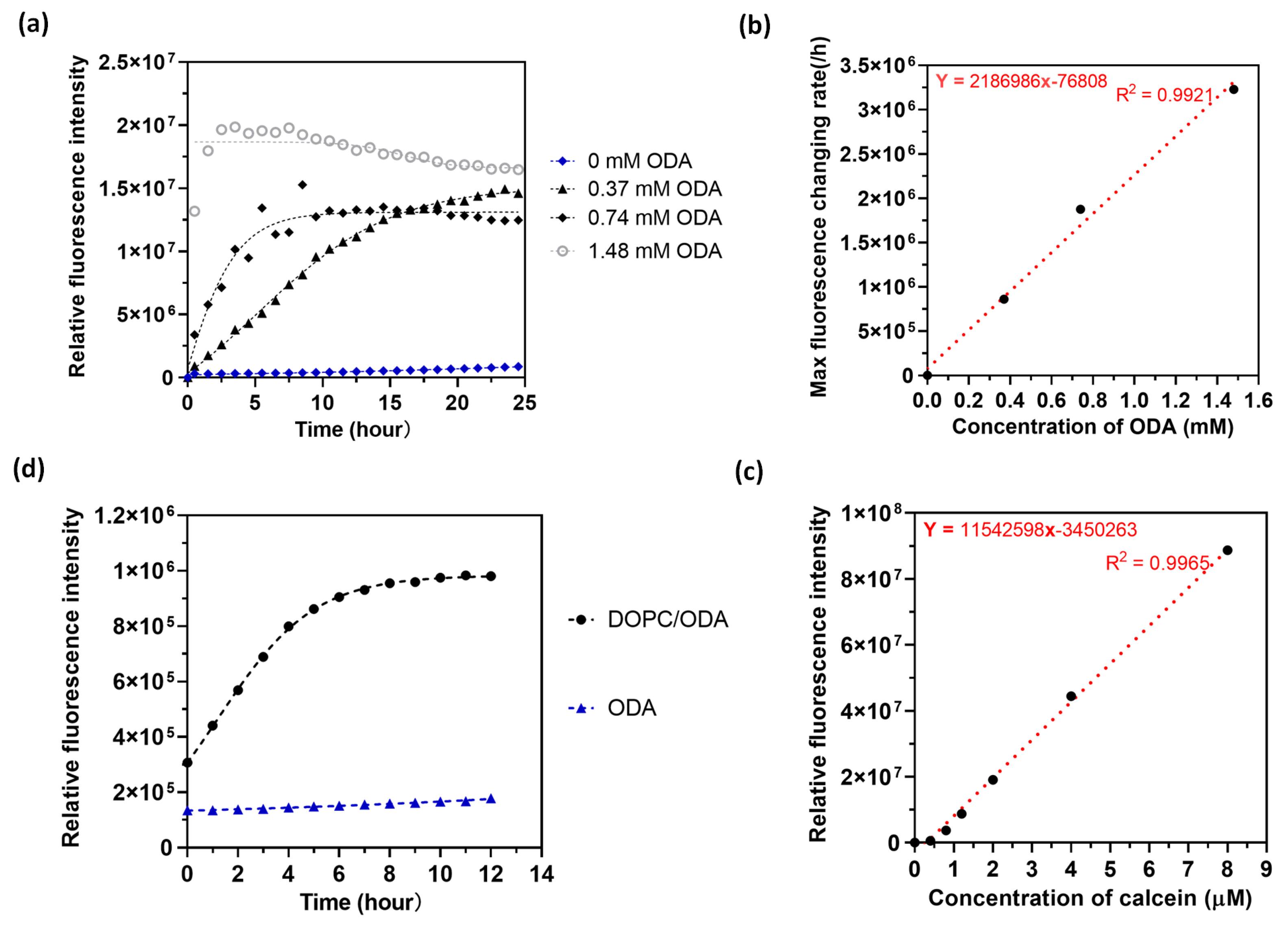
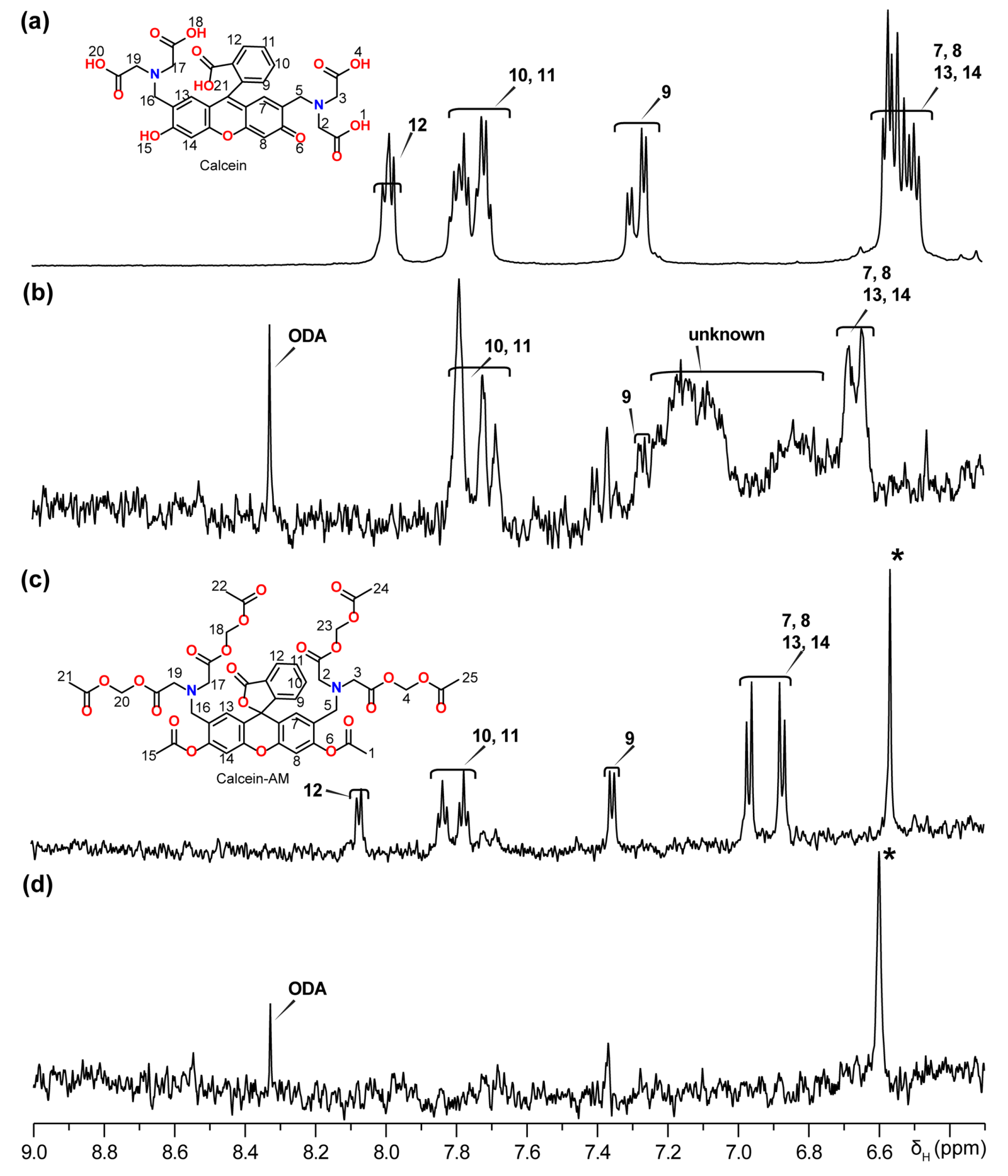
2. Results
3. Discussion
4. Methods
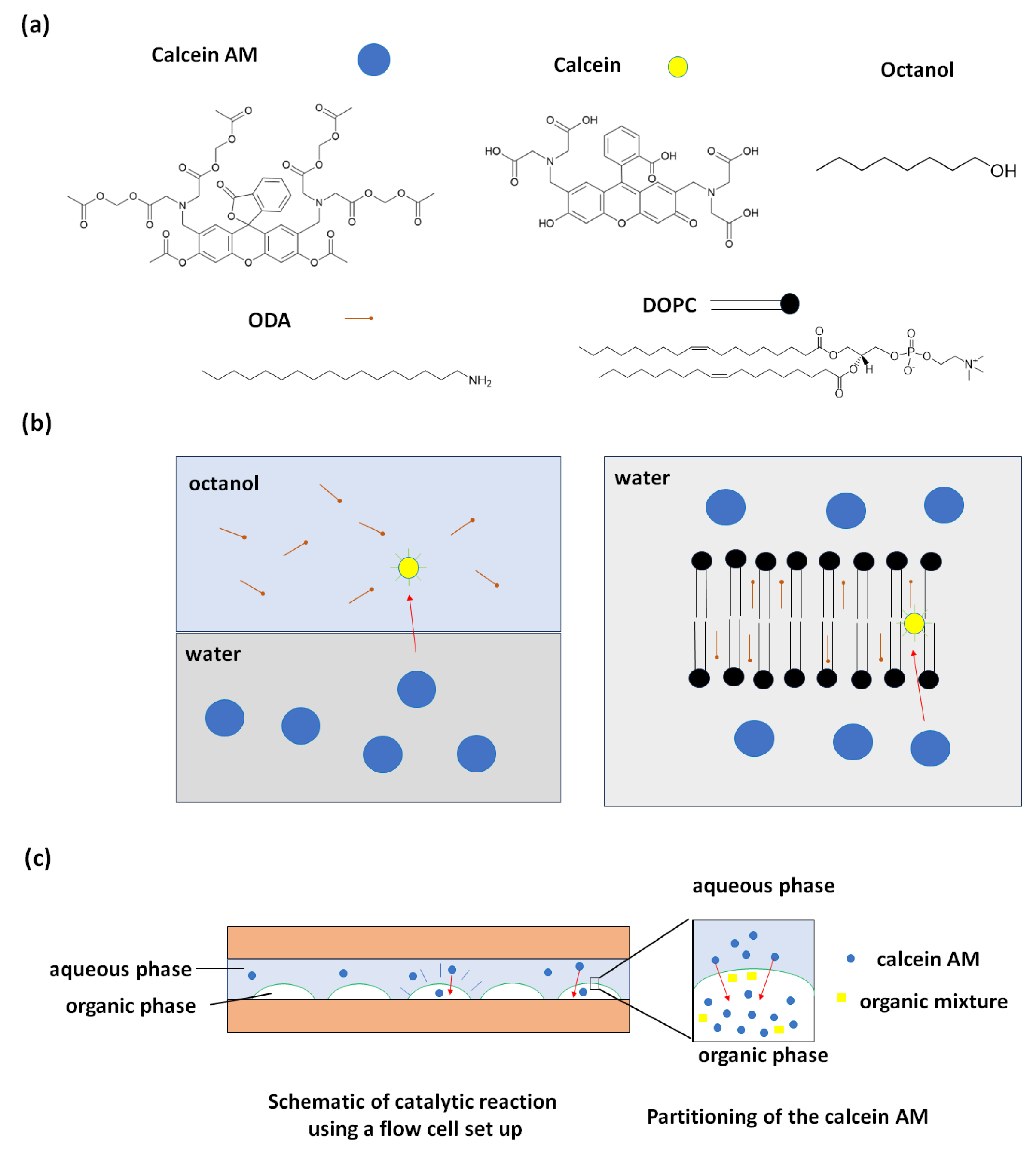
4.1. Materials
4.2. Vesicle Preparation
4.3. Fluorescence Microscopy
4.4. Fluorescence Characterization
4.5. Flow Cell Setup
4.6. NMR
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Supuran, C.T.; Winum, J.Y. Introduction to zinc enzymes as drug targets; Wiley, Hoboken, 2009.
- Robertson, J.G. Mechanistic basis of enzyme-targeted drugs. Biochemistry 2005, 44, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A.; Harpel, M.R.; Tummino, P.J. Targeting enzyme inhibitors in drug discovery. Expert opinion on therapeutic targets 2007, 11, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Dodson, G.; Wlodawer, A. Catalytic triads and their relatives. Trends in biochemical sciences 1998, 23, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Schramm, V.L. Transition states and transition state analogue interactions with enzymes. Accounts of Chemical Research 2015, 48, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Koshland Jr, D.E. Application of a theory of enzyme specificity to protein synthesis. Proceedings of the National Academy of Sciences 1958, 44, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Koshland Jr, D.E. The key–lock theory and the induced fit theory. Angewandte Chemie International Edition in English 1995, 33, 2375–2378. [Google Scholar] [CrossRef]
- Rebek Jr, J. Molecular recognition with model systems. Angewandte Chemie International Edition in English 1990, 29, 245–255. [Google Scholar] [CrossRef]
- Bosshard, H.R. Molecular recognition by induced fit: how fit is the concept? Physiology 2001, 16, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Boehr, D.D.; Nussinov, R.; Wright, P.E. The role of dynamic conformational ensembles in biomolecular recognition. Nature chemical biology 2009, 5, 789–796. [Google Scholar] [CrossRef]
- Ballester, P.; Scarso, A. Supramolecular Aspects in Catalysis, 2019.
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chemical Society Reviews 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Steed, J.W.; Atwood, J.L. Supramolecular chemistry; John Wiley & Sons, 2022.
- Raynal, M.; Ballester, P.; Vidal-Ferran, A.; Van Leeuwen, P.W. Supramolecular catalysis. Part 2: artificial enzyme mimics. Chemical Society Reviews 2014, 43, 1734–1787. [Google Scholar] [CrossRef] [PubMed]
- Kahana, A.; Lancet, D. Self-reproducing catalytic micelles as nanoscopic protocell precursors. Nature Reviews Chemistry 2021, 5, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Doherty, E.A.; Doudna, J.A. Ribozyme structures and mechanisms. Annual review of biochemistry 2000, 69, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Lorsch, J.R. Ribozyme catalysis: not different, just worse. Nature structural & molecular biology 2005, 12, 395–402. [Google Scholar]
- Chen, X.; Li, N.; Ellington, A.D. Ribozyme catalysis of metabolism in the RNA world. Chemistry & biodiversity 2007, 4, 633–655. [Google Scholar]
- Segré, D.; Ben-Eli, D.; Deamer, D.W.; Lancet, D. The lipid world. Origins of Life and Evolution of the Biosphere 2001, 31, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Lancet, D.; Segrè, D.; Kahana, A. Twenty years of “lipid world”: a fertile partnership with David Deamer. Life 2019, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Fendler, J.H. Interactions and reactions in reversed micellar systems. Accounts of Chemical Research 1976, 9, 153–161. [Google Scholar] [CrossRef]
- Cuccovia, I.M.; Quina, F.H.; Chaimovich, H. A remarkable enhancement of the rate of ester thiolysis by synthetic amphiphile vesicles. Tetrahedron 1982, 38, 917–920. [Google Scholar] [CrossRef]
- Kust, P.R.; Rathman, J.F. Synthesis of surfactants by micellar autocatalysis: N, N-dimethyldodecylamine N-oxide. Langmuir 1995, 11, 3007–3012. [Google Scholar] [CrossRef]
- Bell, T.N.; Feng, K.; Calvin, G.; Van Winkle, D.H.; Lenhert, S. Organic composomes as supramolecular aptamers. ACS omega 2020, 5, 27393–27400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shiel, E.; Bell, T.; Lin, S.; Lenhert, S. Kinetic Mechanism of Surfactant-Based Molecular Recognition: Selective Permeability across an Oil–Water Interface Regulated by Supramolecular Aggregates. The Journal of Physical Chemistry B 2023, 127, 10201–10214. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, A.T.; Wang, H.; Van Winkle, D.; Lenhert, S. Combinatorial mixtures of organic solutes for improved liquid/liquid extraction of ions. Soft Matter 2023, 19, 6903–6910. [Google Scholar] [CrossRef] [PubMed]
- Lowry, T.W.; Kusi-Appiah, A.E.; Fadool, D.A.; Lenhert, S. Odor discrimination by lipid membranes. Membranes 2023, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Yotsumoto, M.; Matsuo, M.; Kitahata, H.; Nakanishi, S.; Denda, M.; Nagayama, M.; Nakata, S. Phospholipid Molecular Layer that Enhances Distinction of Odors Based on Artificial Sniffing. ACS sensors 2023, 8, 4494–4503. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, H.; Hussain, A.; Shabbir, S.; Ali, S.; Bilal, M.; Sher, F.; Iqbal, H.M. Esterases as emerging biocatalysts: Mechanistic insights, genomic and metagenomic, immobilization, and biotechnological applications. Biotechnology and applied biochemistry 2022, 69, 2176–2194. [Google Scholar] [CrossRef] [PubMed]
- Barzkar, N.; Sohail, M.; Tamadoni Jahromi, S.; Gozari, M.; Poormozaffar, S.; Nahavandi, R.; Hafezieh, M. Marine bacterial esterases: Emerging biocatalysts for industrial applications. Applied Biochemistry and Biotechnology 2021, 193, 1187–1214. [Google Scholar] [CrossRef] [PubMed]
- Bratosin, D.; Mitrofan, L.; Palii, C.; Estaquier, J.; Montreuil, J. Novel fluorescence assay using calcein-AM for the determination of human erythrocyte viability and aging. Cytometry Part A: the journal of the International Society for Analytical Cytology 2005, 66, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Uggeri, J.; Gatti, R.; Belletti, S.; Scandroglio, R.; Corradini, R.; Rotoli, B.M.; Orlandini, G. Calcein-AM is a detector of intracellular oxidative activity. Histochemistry and cell biology 2000, 122, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Klonis, N.; Sawyer, W.H. Spectral properties of the prototropic forms of fluorescein in aqueous solution. Journal of fluorescence 1996, 6, 147–157. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced drug delivery reviews 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Boudart, M. Turnover rates in heterogeneous catalysis. Chemical reviews 1995, 95, 661–666. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-dependent catalytic activity and dynamics of gold nanoparticles at the single-molecule level. Journal of the American Chemical Society 2010, 132, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Nafday, O.A.; Lenhert, S. High-throughput optical quality control of lipid multilayers fabricated by dip-pen nanolithography. Nanotechnology 2011, 22, 225301. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.E.; MacMillan, D.W. Synergistic catalysis: a powerful synthetic strategy for new reaction development. Chemical science 2012, 3, 633–658. [Google Scholar] [CrossRef] [PubMed]
- Costentin, C.; Drouet, S.; Robert, M.; Saveant, J.M. Turnover numbers, turnover frequencies, and overpotential in molecular catalysis of electrochemical reactions. Cyclic voltammetry and preparative-scale electrolysis. Journal of the American Chemical Society 2012, 134, 11235–11242. [Google Scholar] [CrossRef] [PubMed]
- Pegis, M.L.; McKeown, B.A.; Kumar, N.; Lang, K.; Wasylenko, D.J.; Zhang, X.P.; Raugei, S.; Mayer, J.M. Homogenous electrocatalytic oxygen reduction rates correlate with reaction overpotential in acidic organic solutions. ACS Central Science 2016, 2, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Okuom, M.O.; Wilson, M.V.; Jackson, A.; Holmes, A.E.; et al. Intermolecular Interactions between Eosin Y and Caffeine Using 1 H-NMR Spectroscopy. International journal of spectroscopy 2013, 2013. [Google Scholar] [CrossRef]
- Herriott, A.W.; Picker, D. Phase transfer catalysis. Evaluation of catalysis. Journal of the American chemical Society 1975, 97, 2345–2349. [Google Scholar] [CrossRef]
- Starks, C.M.; Halper, M. Phase-transfer catalysis: fundamentals, applications, and industrial perspectives; Springer Science & Business Media, 2012.
- Ooi, T.; Maruoka, K. Recent advances in asymmetric phase-transfer catalysis. Angewandte Chemie International Edition 2007, 46, 4222–4266. [Google Scholar] [CrossRef]
- Godha, A.K.; Thiruvengadam, J.; Abhilash, V.; Balgi, P.; Narayanareddy, A.; Vignesh, K.; Gadakh, A.V.; Sathiyanarayanan, A.; Ganesh, S. Environmentally benign nucleophilic substitution reaction of arylalkyl halides in water using CTAB as the inverse phase transfer catalyst. New Journal of Chemistry 2019, 43, 16041–16045. [Google Scholar] [CrossRef]
- Kumar, A.; Joshi, H.; Pasricha, R.; Mandale, A.; Sastry, M. Phase transfer of silver nanoparticles from aqueous to organic solutions using fatty amine molecules. Journal of colloid and interface science 2003, 264, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Wikander, K.; Petit, C.; Holmberg, K.; Pileni, M.P. Size control and growth process of alkylamine-stabilized platinum nanocrystals: a comparison between the phase transfer and reverse micelles methods. Langmuir 2006, 22, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Starks, C.M. Phase-transfer catalysis. I. Heterogeneous reactions involving anion transfer by quaternary ammonium and phosphonium salts. Journal of the American Chemical Society 1971, 93, 195–199. [Google Scholar] [CrossRef]
- Kumar, A.; Joshi, H.M.; Mandale, A.B.; Srivastava, R.; Adyanthaya, S.D.; Pasricha, R.; Sastry, M. Phase transfer of platinum nanoparticles from aqueous to organic solutions using fatty amine molecules. Journal of Chemical Sciences 2004, 116, 293–300. [Google Scholar] [CrossRef]
- Xu, D.Q.; Pan, Z.W. Phase-transfer catalysis of a new cationic gemini surfactant with ester groups for nucleophilic substitution reaction. Chinese Chemical Letters 2014, 25, 1169–1173. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, X.; Kong, W.; Yang, X.L.; Molnár, G.; Vondráková, Z.; Filepová, R.; Petrášek, J.; Friml, J.; Xue, H.W. The lipid code-dependent phosphoswitch PDK1–D6PK activates PIN-mediated auxin efflux in Arabidopsis. Nature Plants 2020, 6, 556–569. [Google Scholar] [CrossRef]
- Turunen, M.; Olsson, J.; Dallner, G. Metabolism and function of coenzyme Q. Biochimica et Biophysica Acta (BBA)-Biomembranes 2004, 1660, 171–199. [Google Scholar] [CrossRef]
- Stafford, D. The vitamin K cycle. Journal of Thrombosis and Haemostasis 2005, 3, 1873–1878. [Google Scholar] [CrossRef]
- Lokugamage, M.P.; Sago, C.D.; Gan, Z.; Krupczak, B.R.; Dahlman, J.E. Constrained nanoparticles deliver siRNA and sgRNA to T cells in vivo without targeting ligands. Advanced Materials 2019, 31, 1902251. [Google Scholar] [CrossRef]
- Lian, T.; Ho, R.J. Trends and developments in liposome drug delivery systems. Journal of pharmaceutical sciences 2001, 90, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: where they are and how they behave. Nature reviews Molecular cell biology 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




