Submitted:
04 June 2024
Posted:
05 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Existence and Uniqueness of the Global Solution
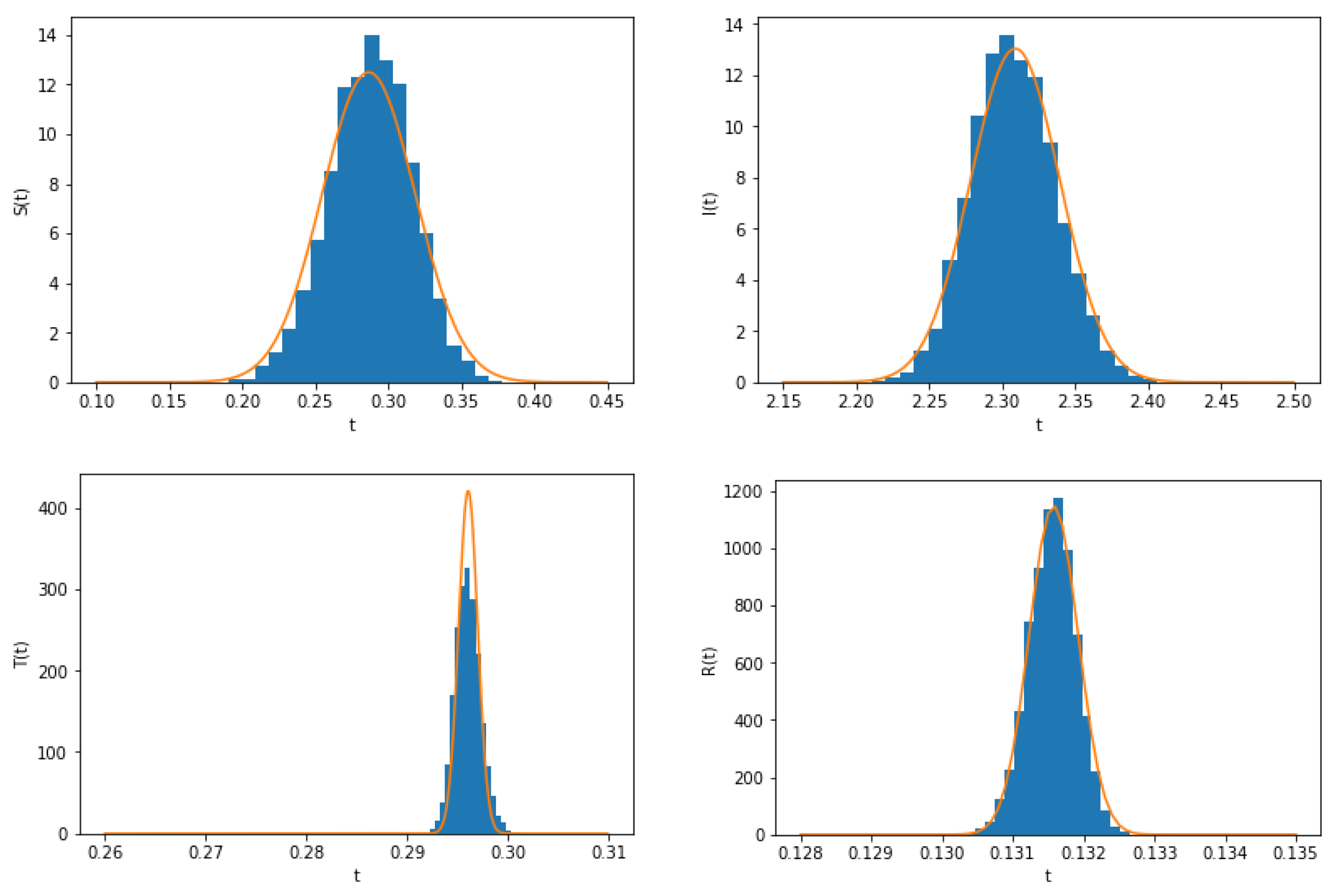
3. Stationary Distribution
4. Density Function
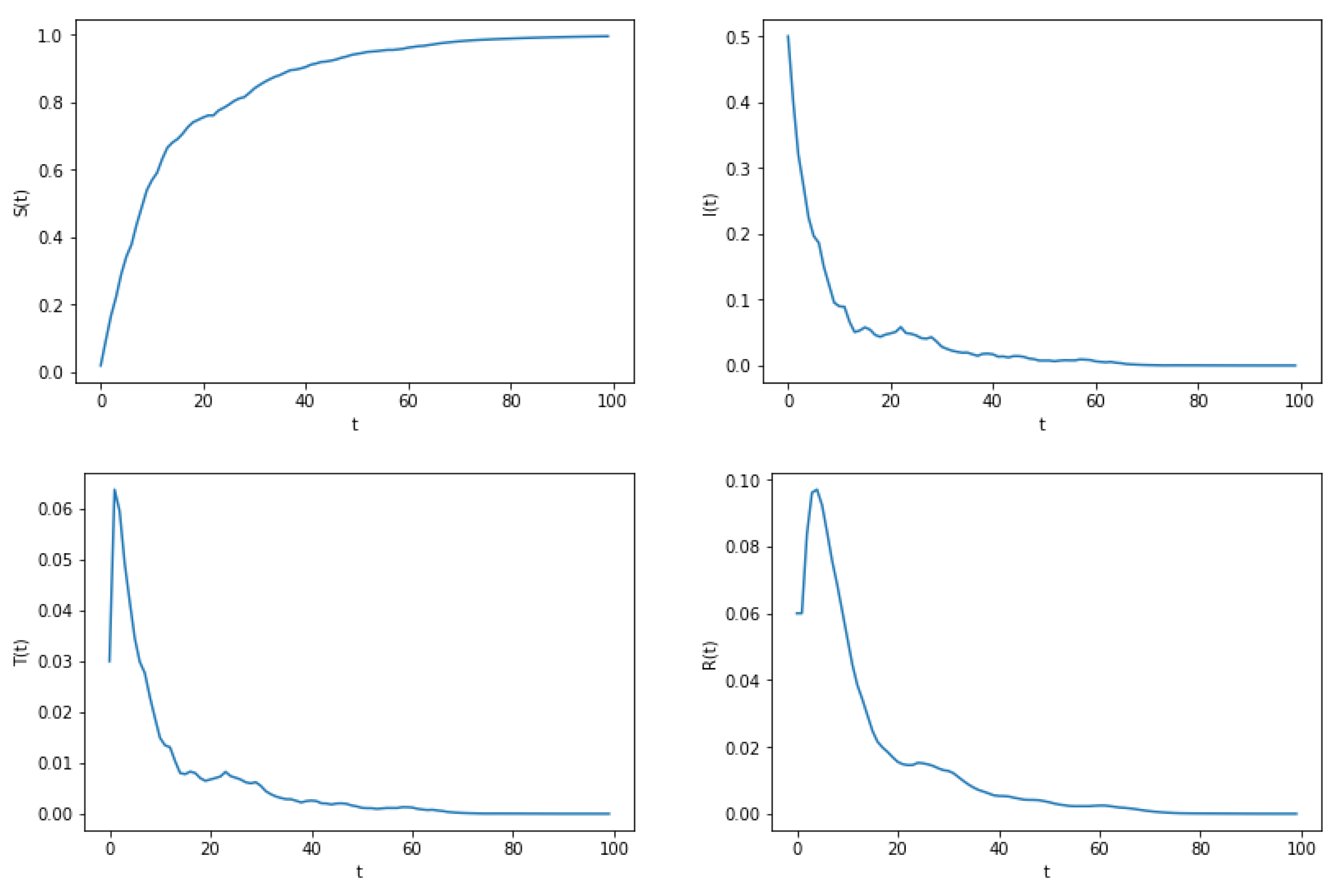
5. Extinction
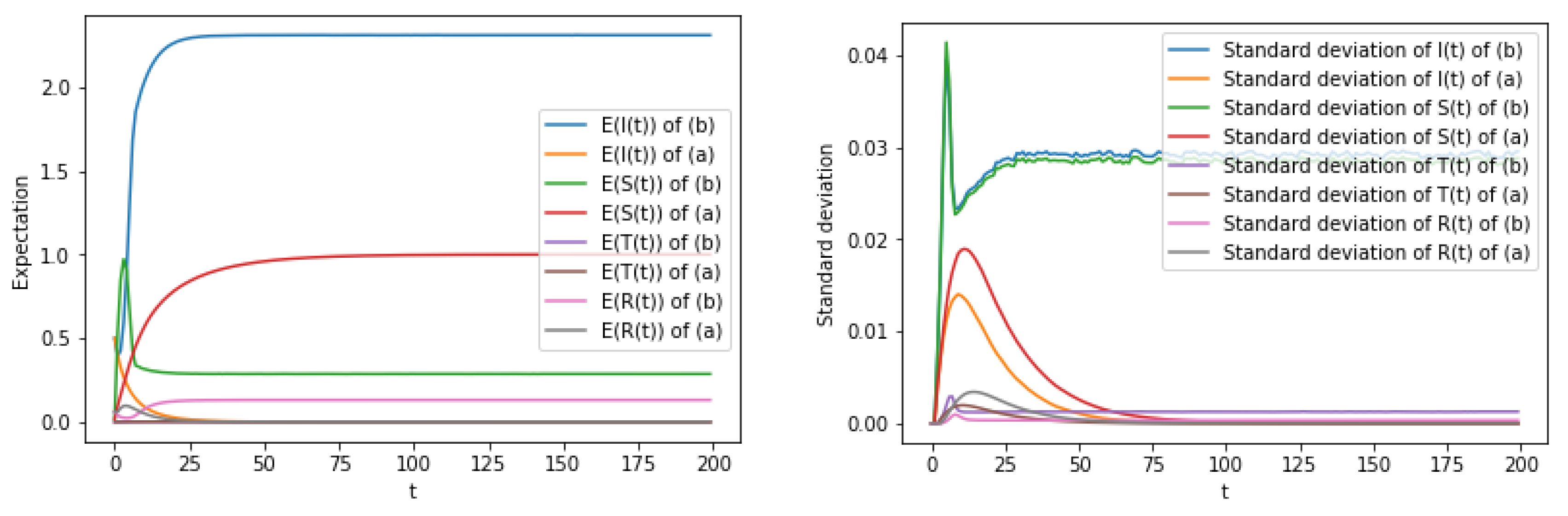
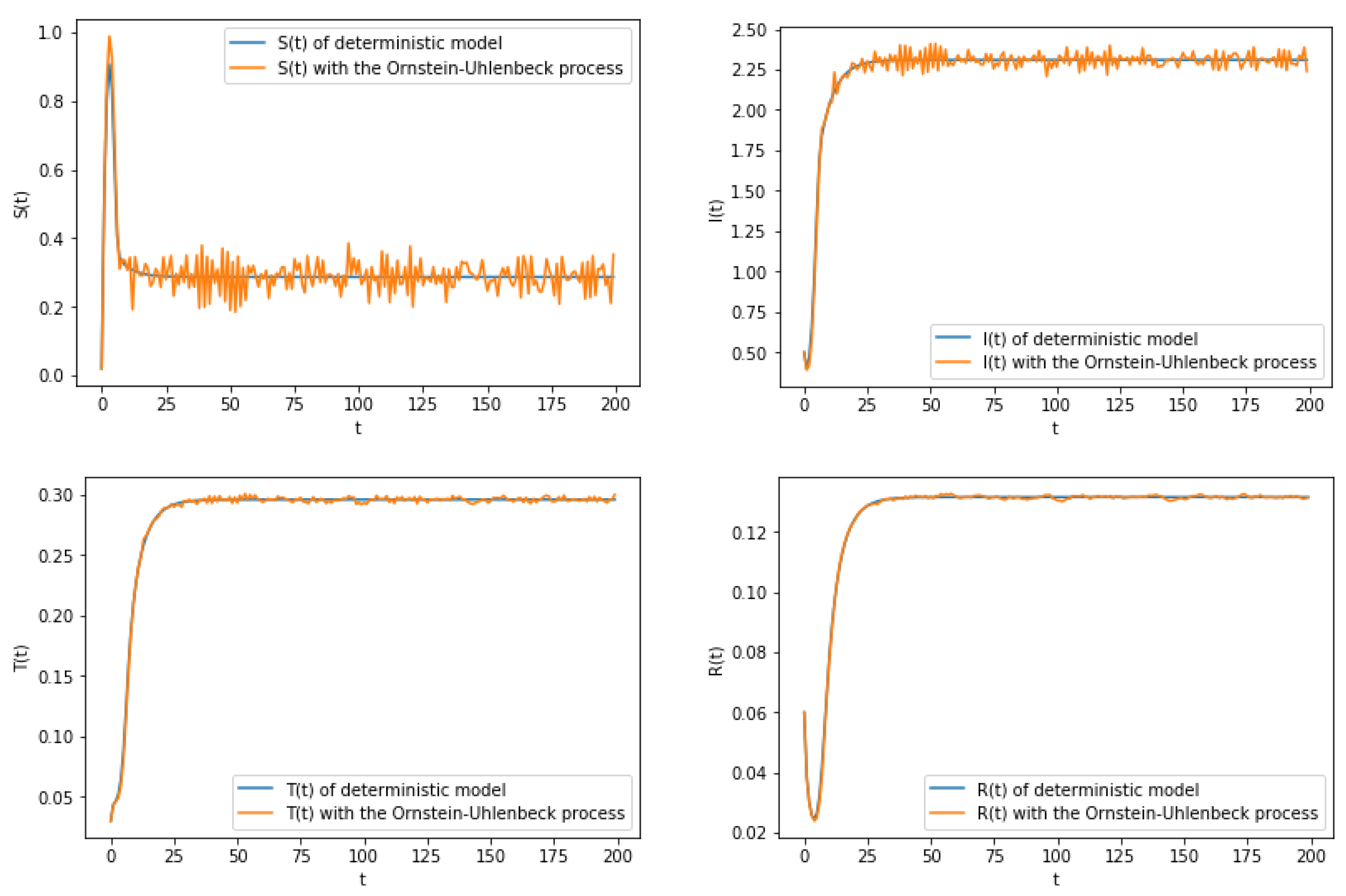
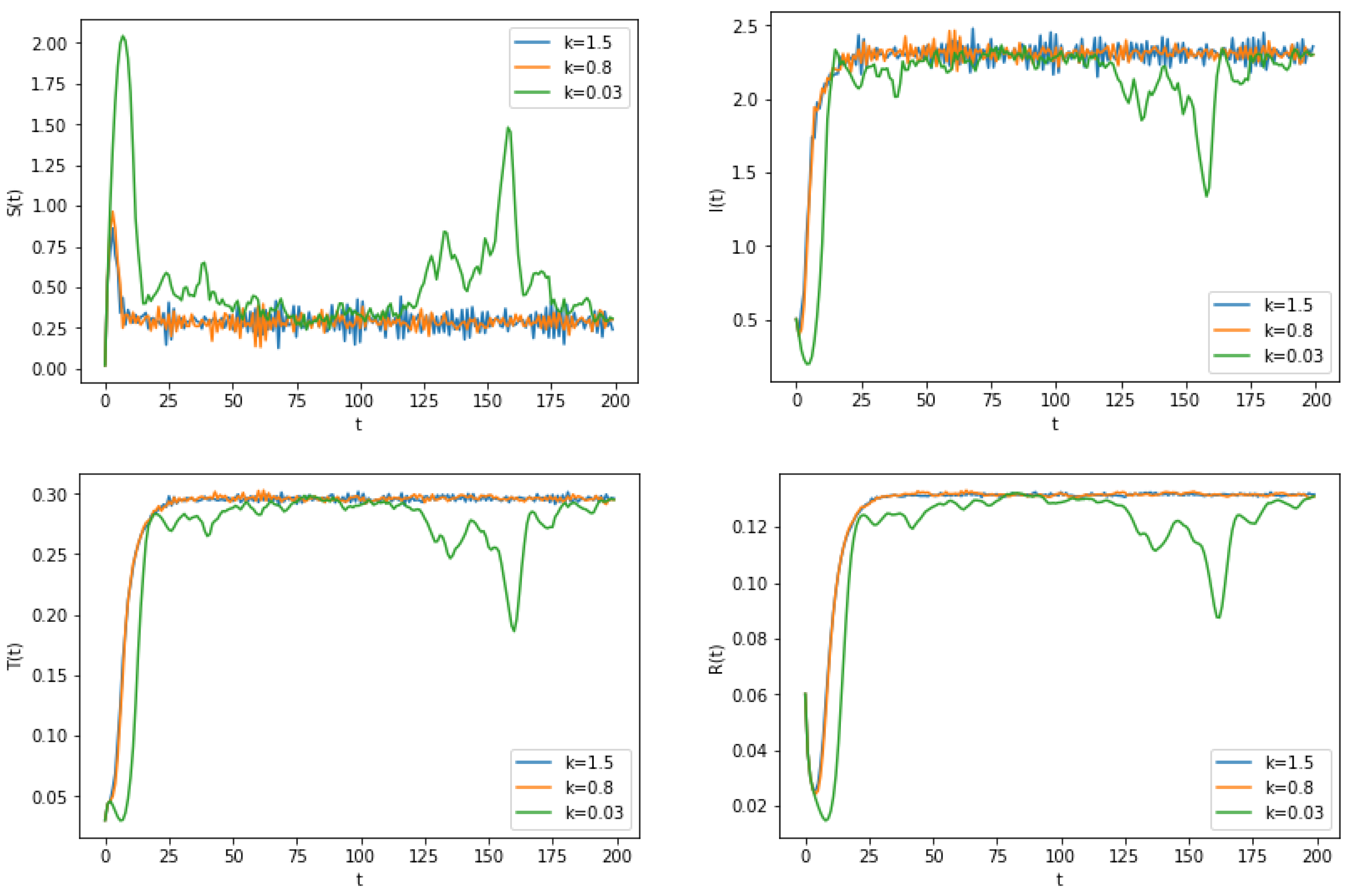
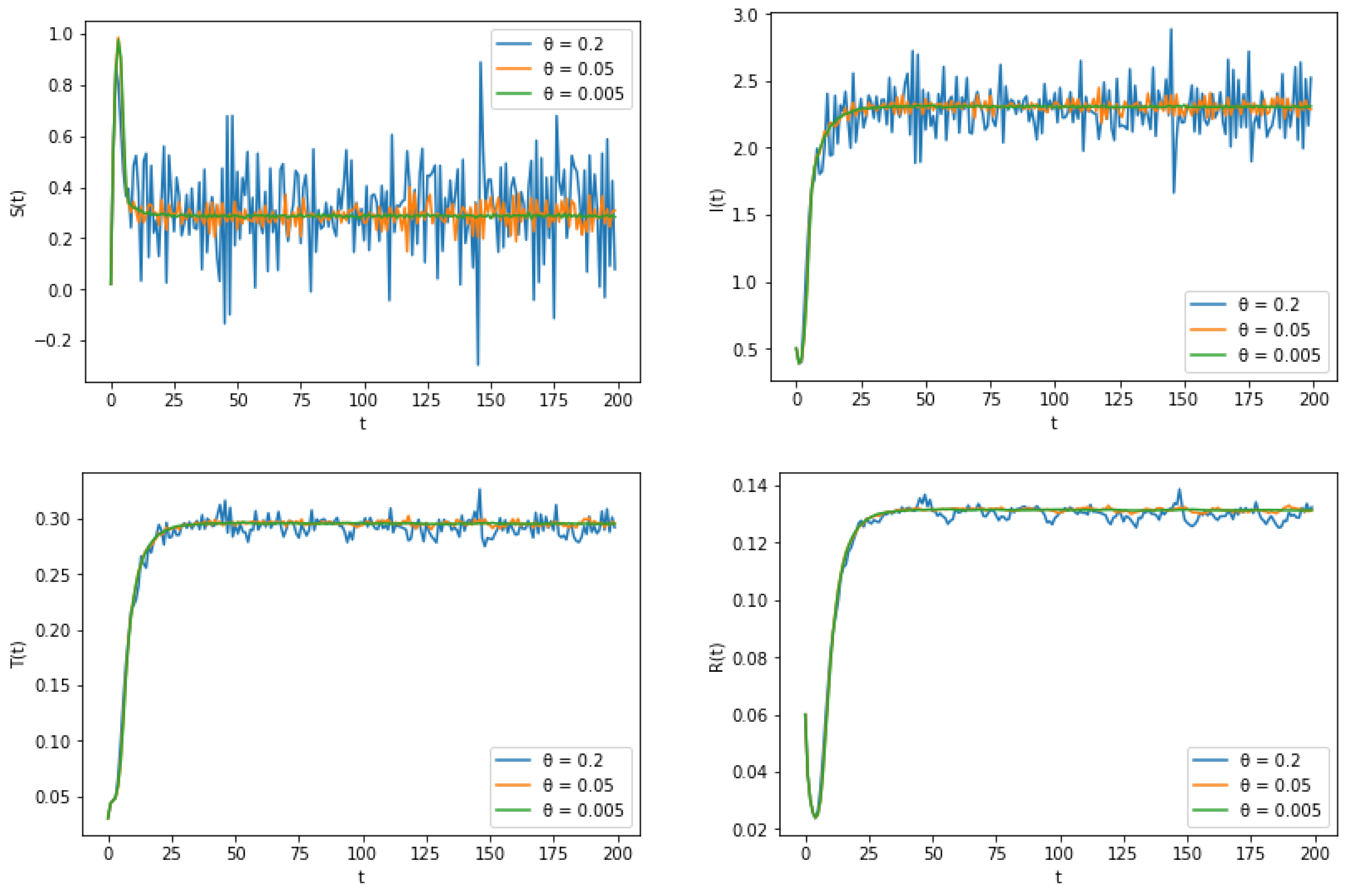
6. Numerical Simulation
7. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Z.; Zhou, Y.; Wu, J. Modeling and Dynamics of Infectious Diseases.; 1st ed.; Higher Education Press: Beijing, 2009; pp. 1–35. [Google Scholar]
- Cai, Y.; Kang, Y.; Banerjee, M.; Wang, W. A stochastic epidemic model incorporating media coverage. Commun. Math. Sci. 2016, 14, 893–910. [Google Scholar] [CrossRef]
- World Health Organization (WHO), Cholera-Global situation. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON437 (accessed on 11 February 2023).
- Beryl, M. O.; George, L. O.; Fredrick, N. O. Mathematical analysis of a cholera transmission model incorporating media coverage. Int. J. Pure. Appl. Math. 2016, 111, 219–231. [Google Scholar] [CrossRef]
- Fatima, S.; Krishnarajah, I.; Jaffar, M. Z. A. M.; Adam, M. B. A mathematical model for the control of cholera in Nigeria. Res. J. Environ. Earth Sci. 2014, 6, 321–325. [Google Scholar] [CrossRef]
- Nelson, E. J.; Harris, J. B.; Glenn M., J.; Galderwood, S. B.; Camilli, A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 2009, 7, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Zhang, X. Stationary distribution and extinction of a stochastic generalized SEI epidemic model with Ornstein-Uhlenbeck process. Appl. Math. Lett. 2023, 143, 108690. [Google Scholar] [CrossRef]
- He, J.; Bai, Z. Global hopf bifurcation of a cholera model with media coverage. Math. Biosci. Eng. 2023, 20, 18468–18490. [Google Scholar] [CrossRef]
- Lemos-Paiao, A. P.; Silva, C. J.; Torres, D. F. M. A cholera mathematical model with vaccination and the biggest outbreak of world’s history. AIMS Mathematics 2018, 3, 448–463. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, X.; Wei, M. Dynamical behavior and optimal control of a stochastic mathematical model for cholera. Chaos Solitons Fractals 2022, 156, 111854. [Google Scholar] [CrossRef]
- Hartley, D. M.; Jr, J. G. M.; Smith, D. L. Hyperinfectivity: A critical element in the ability of V. cholerae to cause epidemics? PLoS Med. 2005, 3, e7. [Google Scholar] [CrossRef]
- Lemos-Paiao, A. P.; silva, C. J.; Torres D. F., M. An epidemic model for cholera with optimal control treatment. J. Comput. Appl. Math. 2017, 318, 168–180. [Google Scholar] [CrossRef]
- Mwasa, A.; Tchuenche, J. M. Mathematical analysis of a cholera model with public health interventions. Biosystems 2011, 105, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Han, Y. Threshold dynamics of a chronological age and infection age structured cholera model with Neumann boundary condition. Z. Angew. Math. Phys. 2023, 74, 170. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, J. Modeling and analysis of a diffusive cholera model with seasonally forced intrinsic incubation period and bacterial hyperinfectivity. J. Math. Anal. Appl. 2023, 527, 127414. [Google Scholar] [CrossRef]
- Tilahun, G. T.; Woldegerima, W. A. Wondifraw, A. Stochastic and deterministic mathematical model of cholera disease dynamics with direct transmission. Adv. Differ. Equ. 2020, 2020, 670. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, D. Dynamical behavior of a stochastic SIQR epidemic model with Ornstein-Uhlenbeck process and standard incidence rate after dimensionality reduction. Commun. Nonlinear Sci. Numer. Simul. 2023, 116, 106878. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X. Threshold behaviors and density function of a stochastic parasite-host epidemic model with Ornstein–Uhlenbeck process. Appl. Math. Lett. 2024, 153, 109079. [Google Scholar] [CrossRef]
- Bao, K.; Rong, L.; Zhang, Q. Analysis of a stochastic SIRS model with interval parameters. Discrete Contin. Dyn. Syst. Ser. B 2019, 24, 4827–4849. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, D.; Hayat, T.; Alsaedi, A. Dynamical behavior of a stochastic epidemic model for cholera. J. Franklin Inst. 2019, 356, 7486–7514. [Google Scholar] [CrossRef]
- Zaho, Y.; Lin, Y.; Jiang, D.; Mao, X.; Li, Y. Stationary distribution of stochastic SIRS epidemic model with standard incidence. Discrete Contin. Dyn. Syst. Ser. B 2016, 21, 2363–2378. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, R. A stochastic chemostat model with mean-reverting Ornstein-Uhlenbeck process and Monod-Haldane response function. Appl. Math. Comput. 2021, 394, 125833. [Google Scholar] [CrossRef]
- Allen, E. ; Environmental variability and mean-reverting processes. Discrete Contin. Dyn. Syst. Ser. B 2016, 21, 2073–2089. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, X.; Jiang, D.; Dai, Y. Dynamics and density function analysis of a stochastic SVI epidemic model with half saturated incidence rate. Chaos Solitons Fractals 2020, 137, 109865. [Google Scholar] [CrossRef]
- Alshammari, F. S.; Akyildiz, F. T. Epidemic waves in a stochastic SIRVI epidemic model incorporating the Ornstein-Uhlenbeck process. Mathematics 2023, 11, 3876. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, D.; Hayat, T. Analysis of a stochastic population model with mean-reverting Ornstein-Uhlenbeck process and Allee effects. Commun. Nonlinear Sci. Numer. Simul. 2022, 111, 106450. [Google Scholar] [CrossRef]
- Wu, S.; Yuan, S.; Lan, G.; Zhang, T. Understanding the dynamics of hepatitis B transmission: A stochastic model with vaccination and Ornstein-Uhlenbeck process. Appl. Math. Comput. 2024, 476, 128766. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, D.; Han, B.; Hayat, T. Threshold dynamics and density function of a stochastic epidemic model with media coverage and mean-reverting Ornstein–Uhlenbeck process. Math. Comput. Simul. 2022, 196, 15–44. [Google Scholar] [CrossRef]
- Liu, Q. ; A stochastic predator-prey model with two competitive preys and Ornstein-Uhlenbeck process. J. Biol. Dyn. 2023, 17, 2193211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liang, G.; Chang, K. Stationary distribution of a reaction-diffusion hepatitis B virus infection model driven by the Ornstein-Uhlenbeck process. PLoS One 2023, 18, e0292073. [Google Scholar] [CrossRef] [PubMed]
- Mao, X. Stochastic Differential Equations and Applications 2nd ed.; Horwood, Chichester, UK, 2008; pp. 149-155.
- Khasminskii, R. Stochastic Stability of Differential Equations 2nd ed.; Springer-Verlag, Berlin Heidelberg, 2012; pp. 59-93.
- Meyn, S. P.; Tweedie, R. L. Stability of Markovian processes III: Foster-Lyapunov criteria for continuous-time processes. Adv. Appl. Probab. 1993, 25, 518–548. [Google Scholar] [CrossRef]
- Roozen, H. An asymptotic solution to a two-dimensional exit problem arising in population dynamics. SIAM J. Appl. Math. 1989, 49, 1793–1810. [Google Scholar] [CrossRef]
- Zhang, X.; Su, T. Jiang, D. Dynamics of a stochastic SVEIR epidemic model incorporating general incidence rate and Ornstein-Uhlenbeck process. J. Nonlinear Sci. 2023, 33, 76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).