Submitted:
03 June 2024
Posted:
04 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dispersions of Cellulose Nanocrystals
2.3. Preparation of Suspensions of Solid Particles in CNC Dispersion
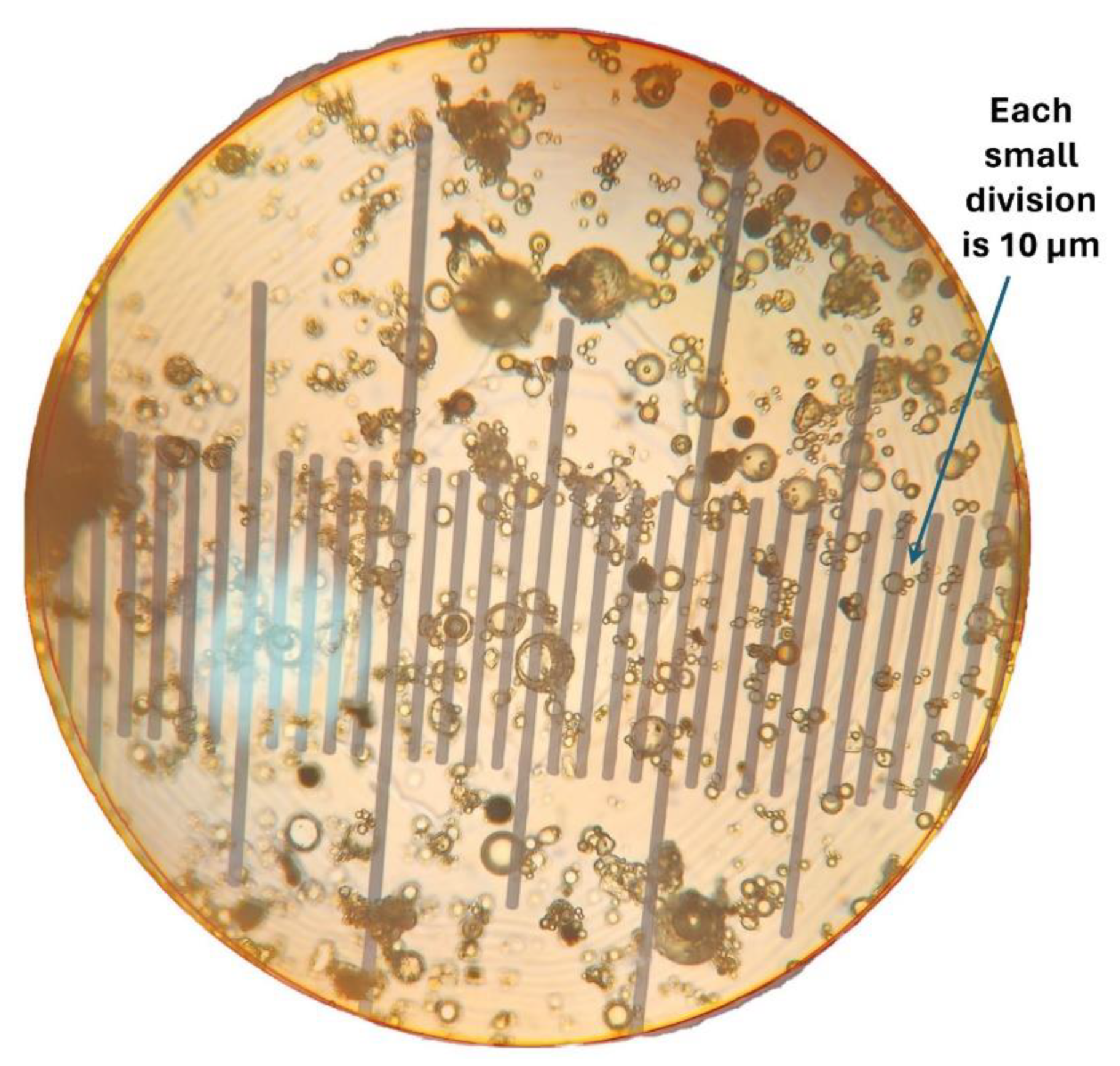
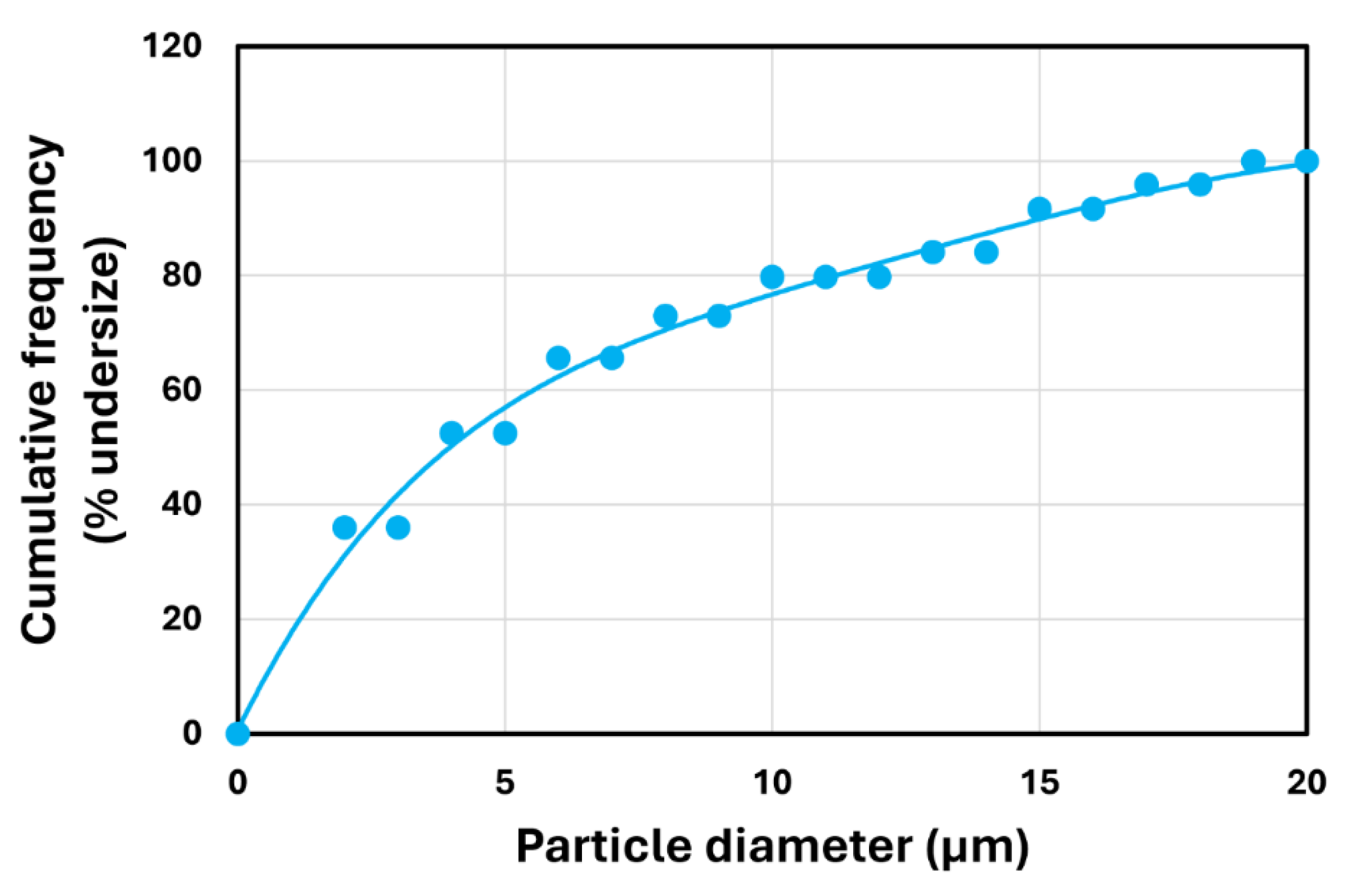
2.4. Measurements
3. Results and Discussion
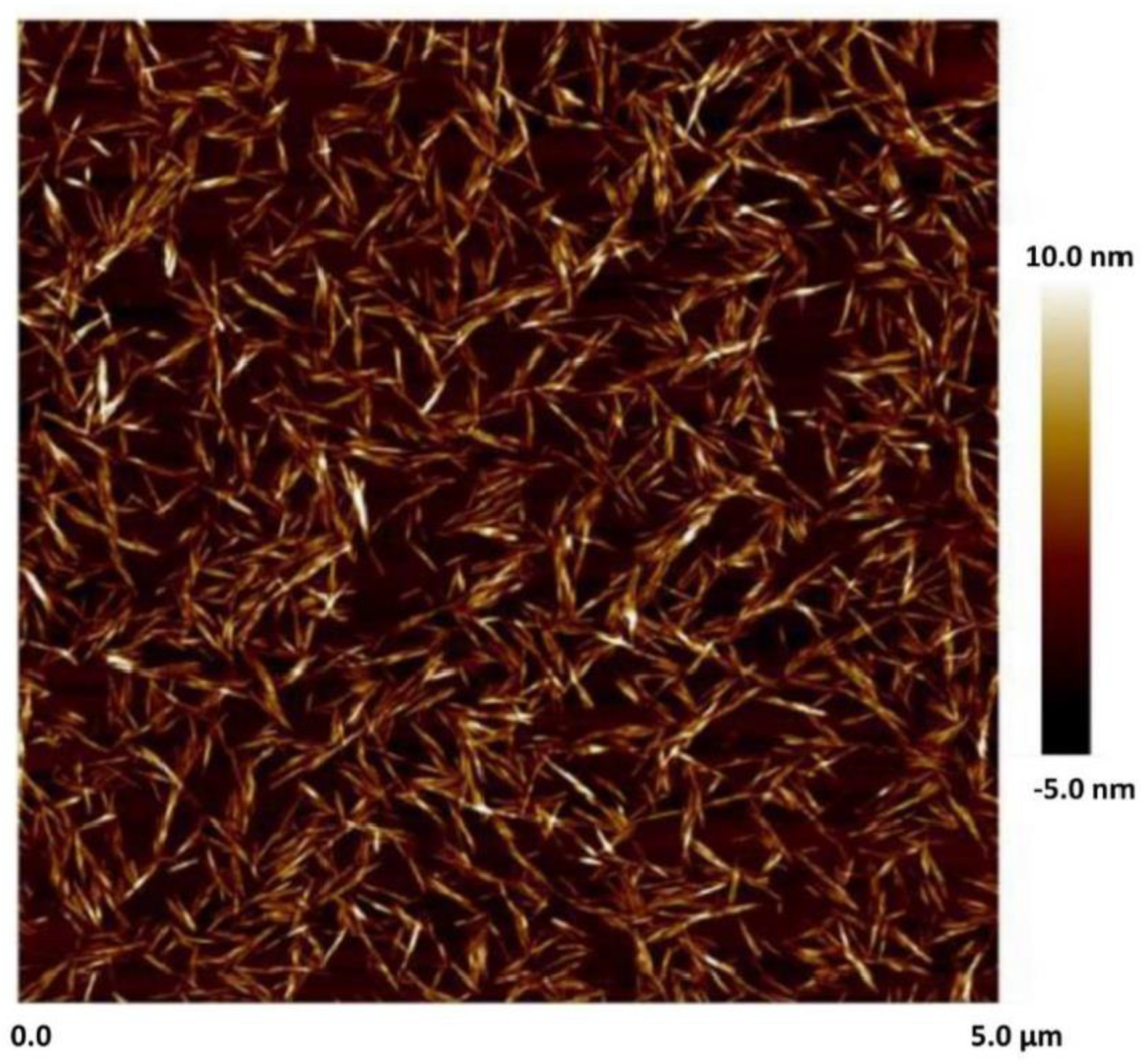
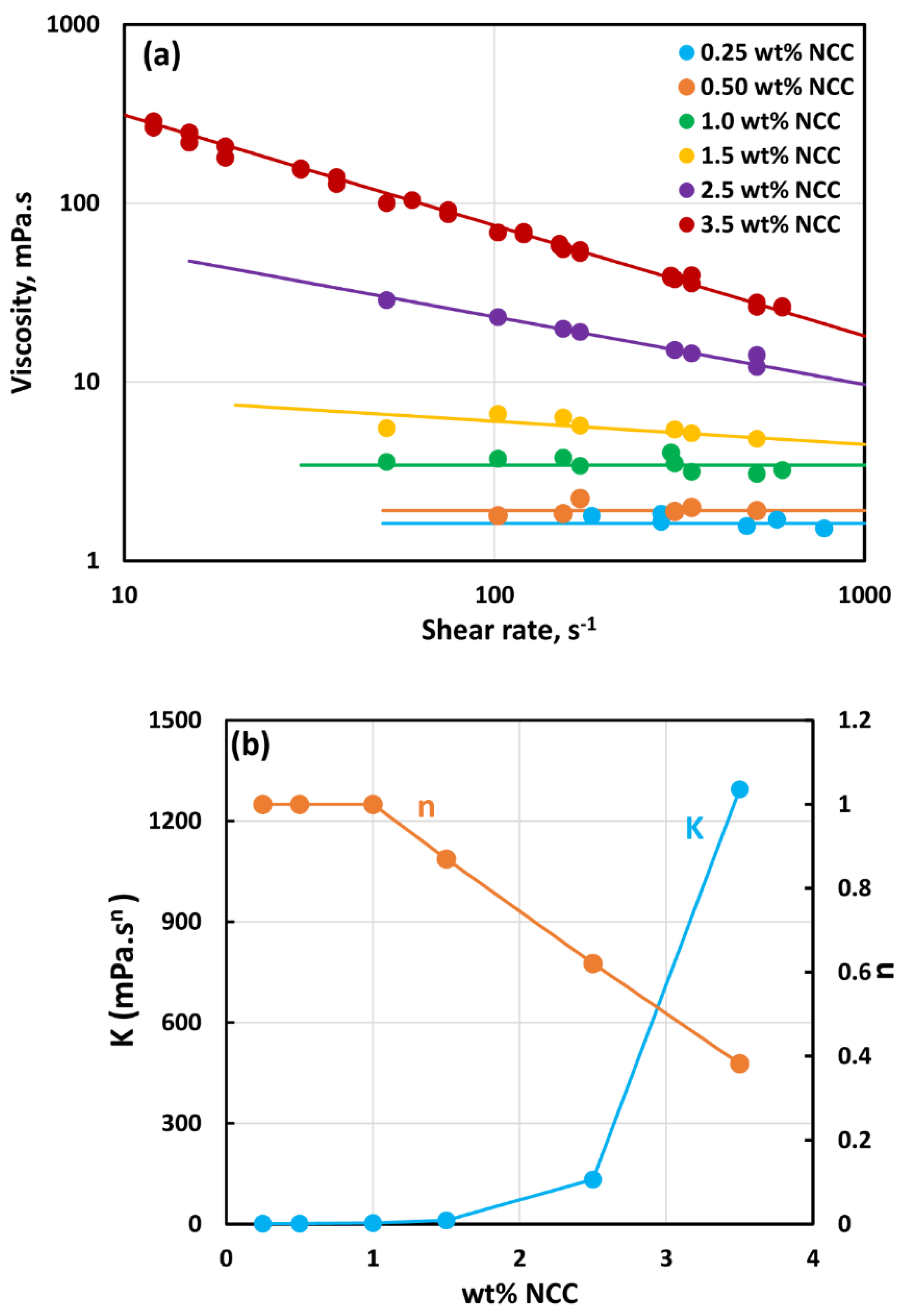
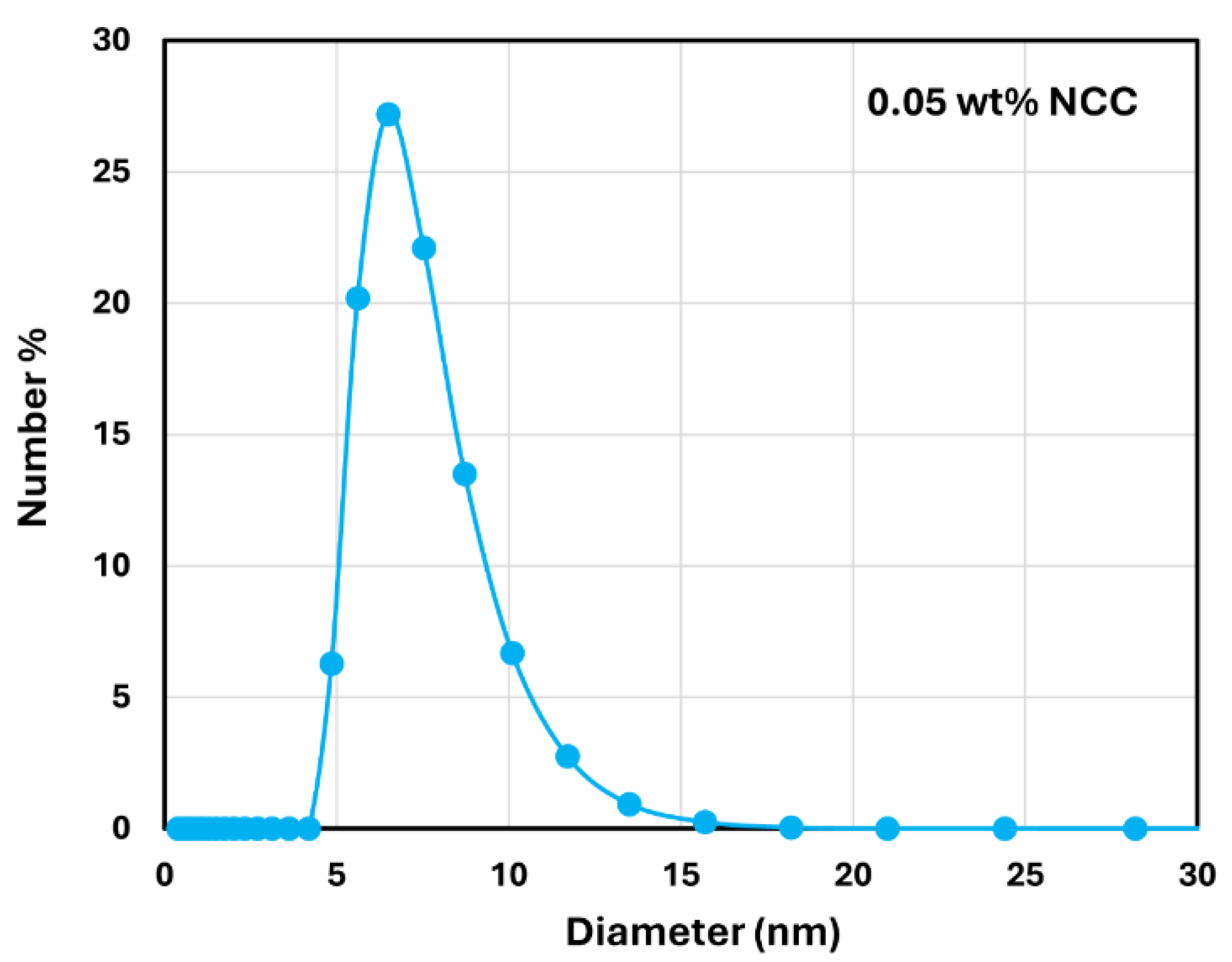
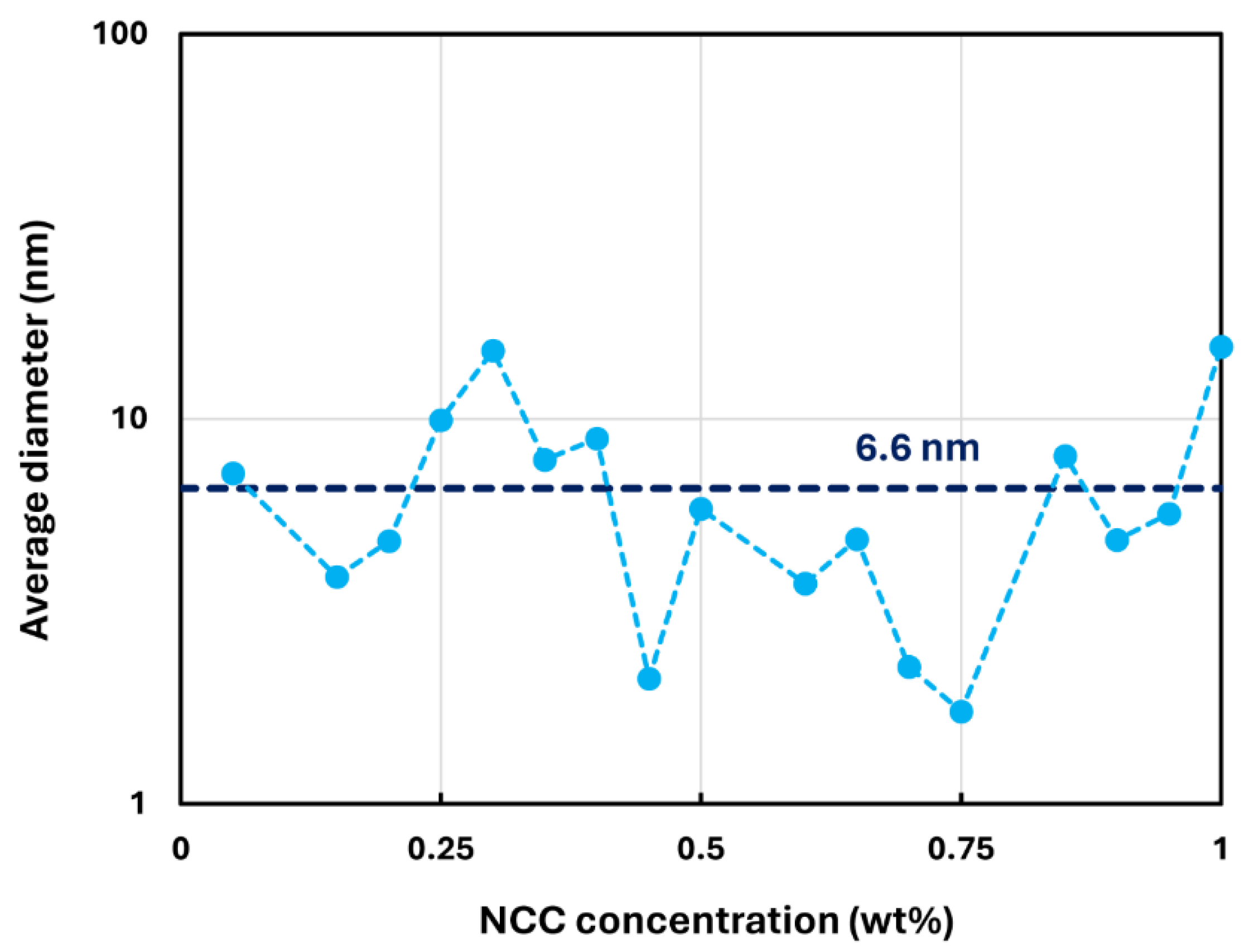
3.1. Rheology of CNC Dispersions
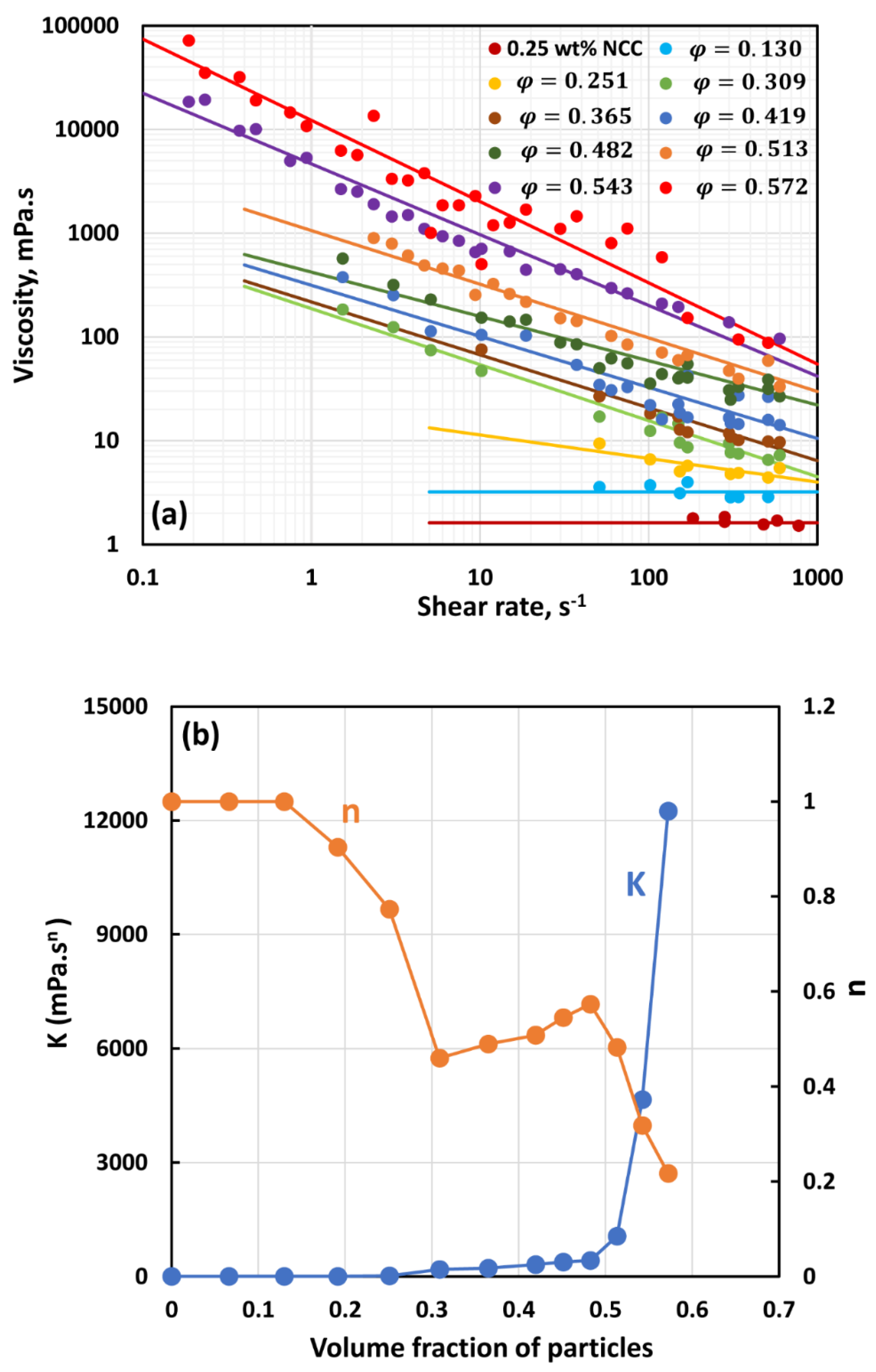
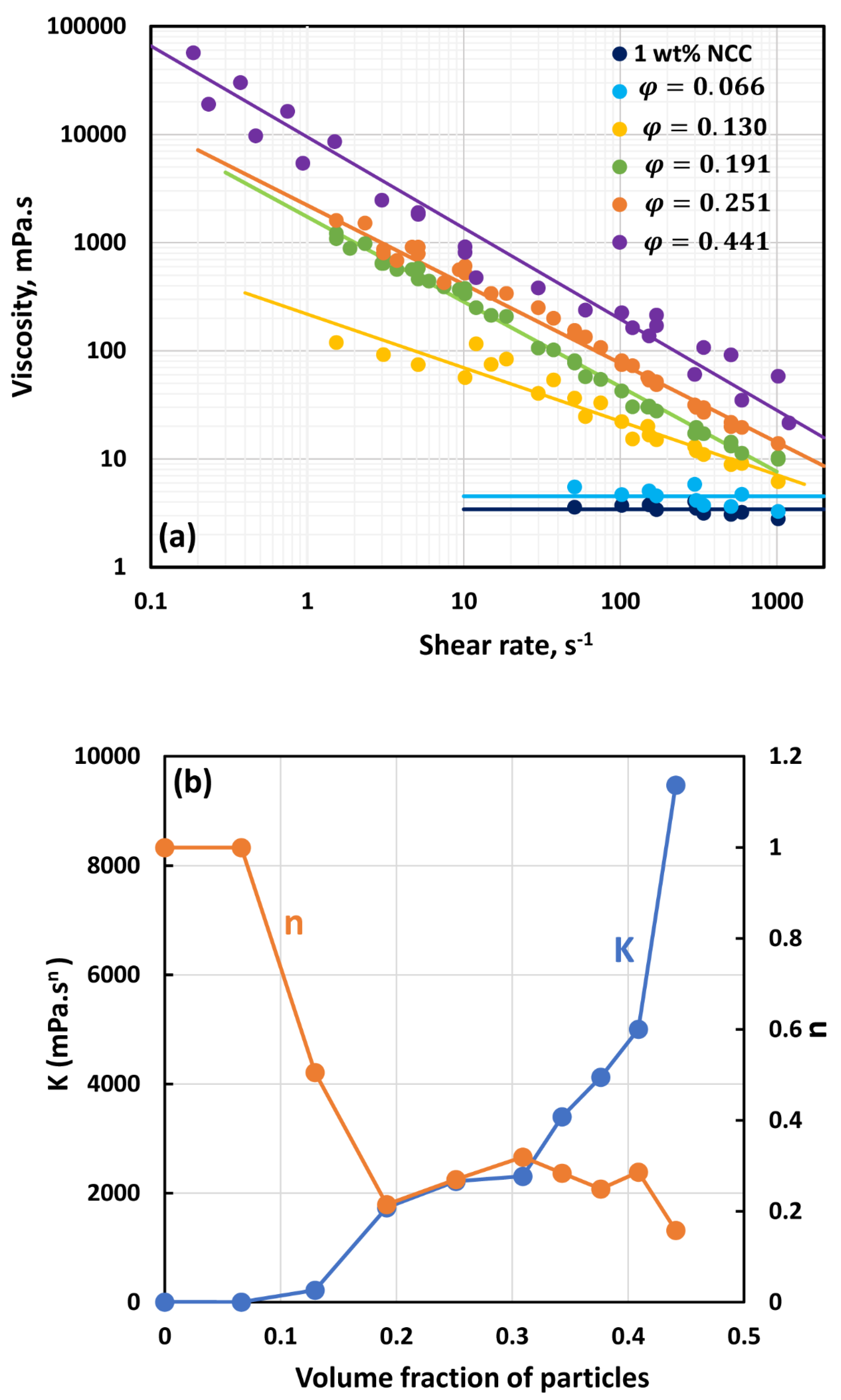
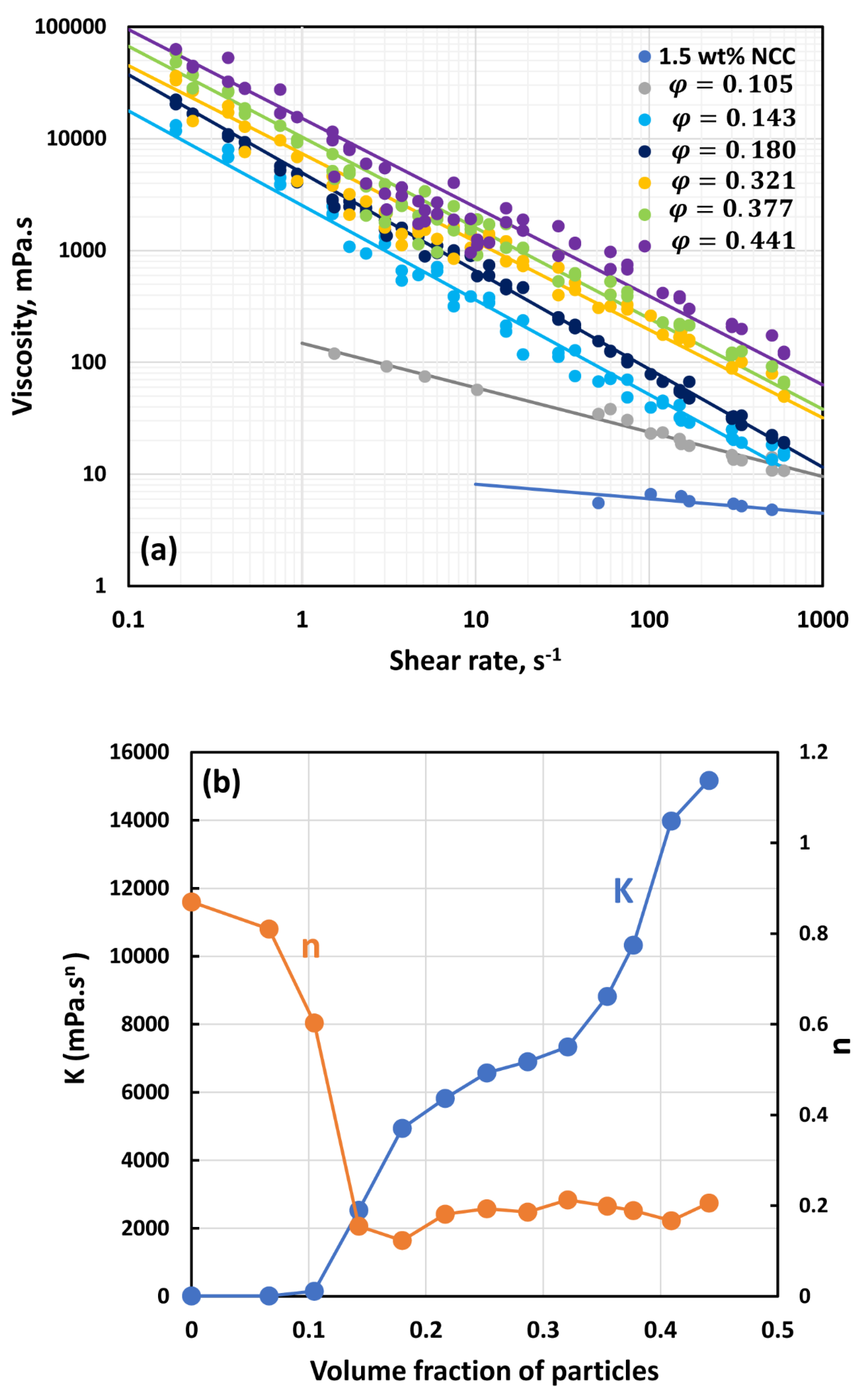
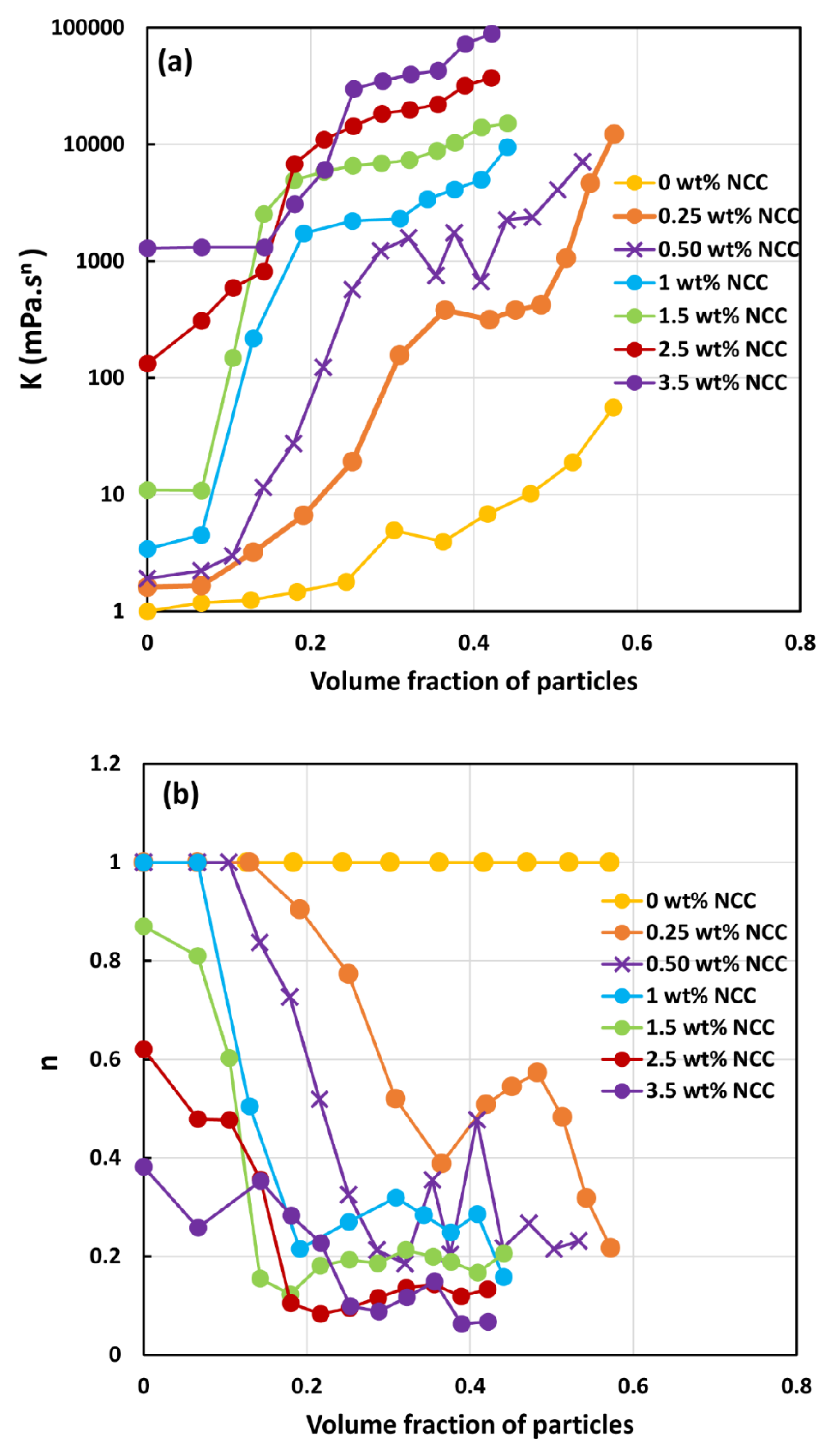
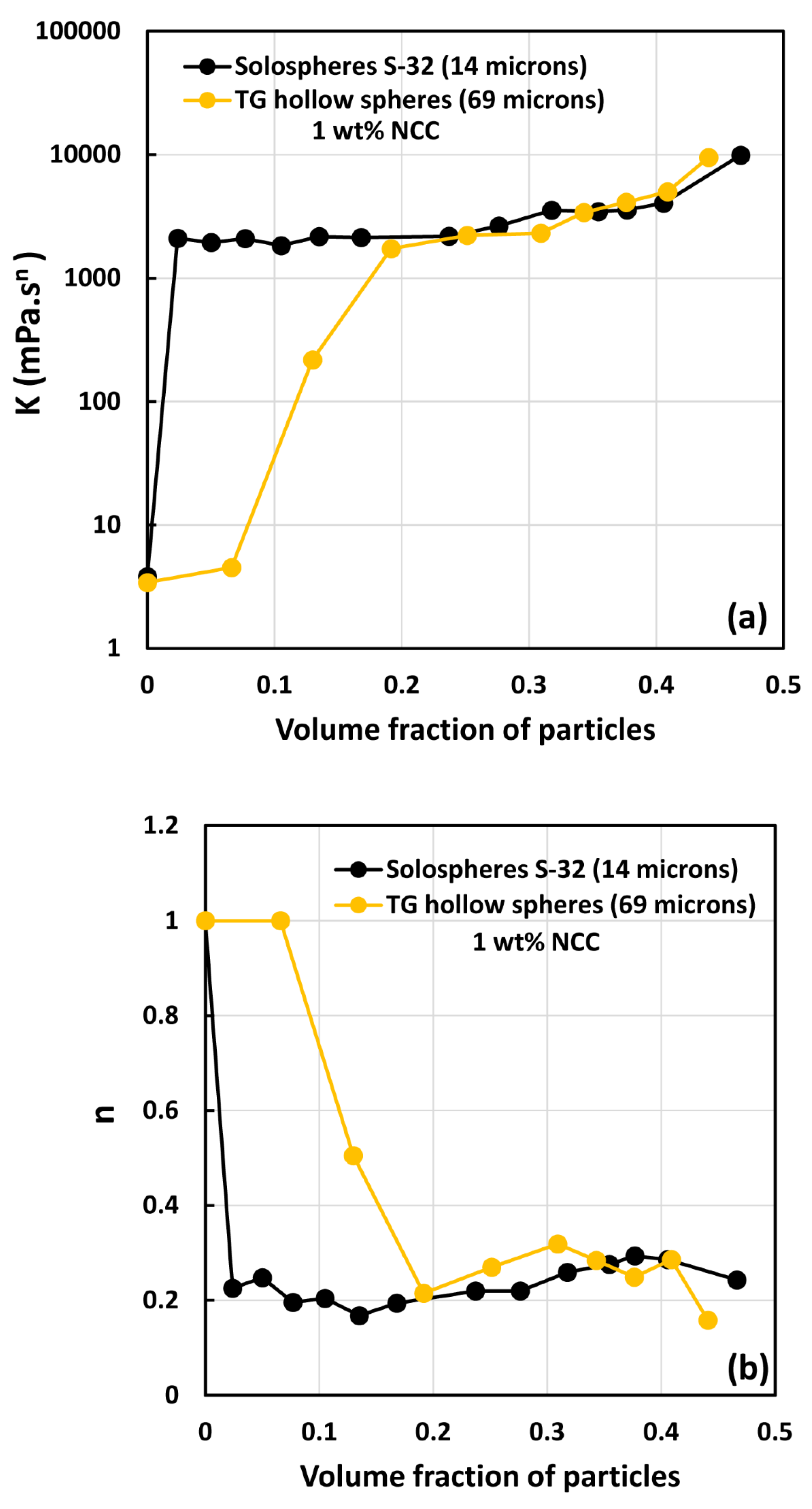
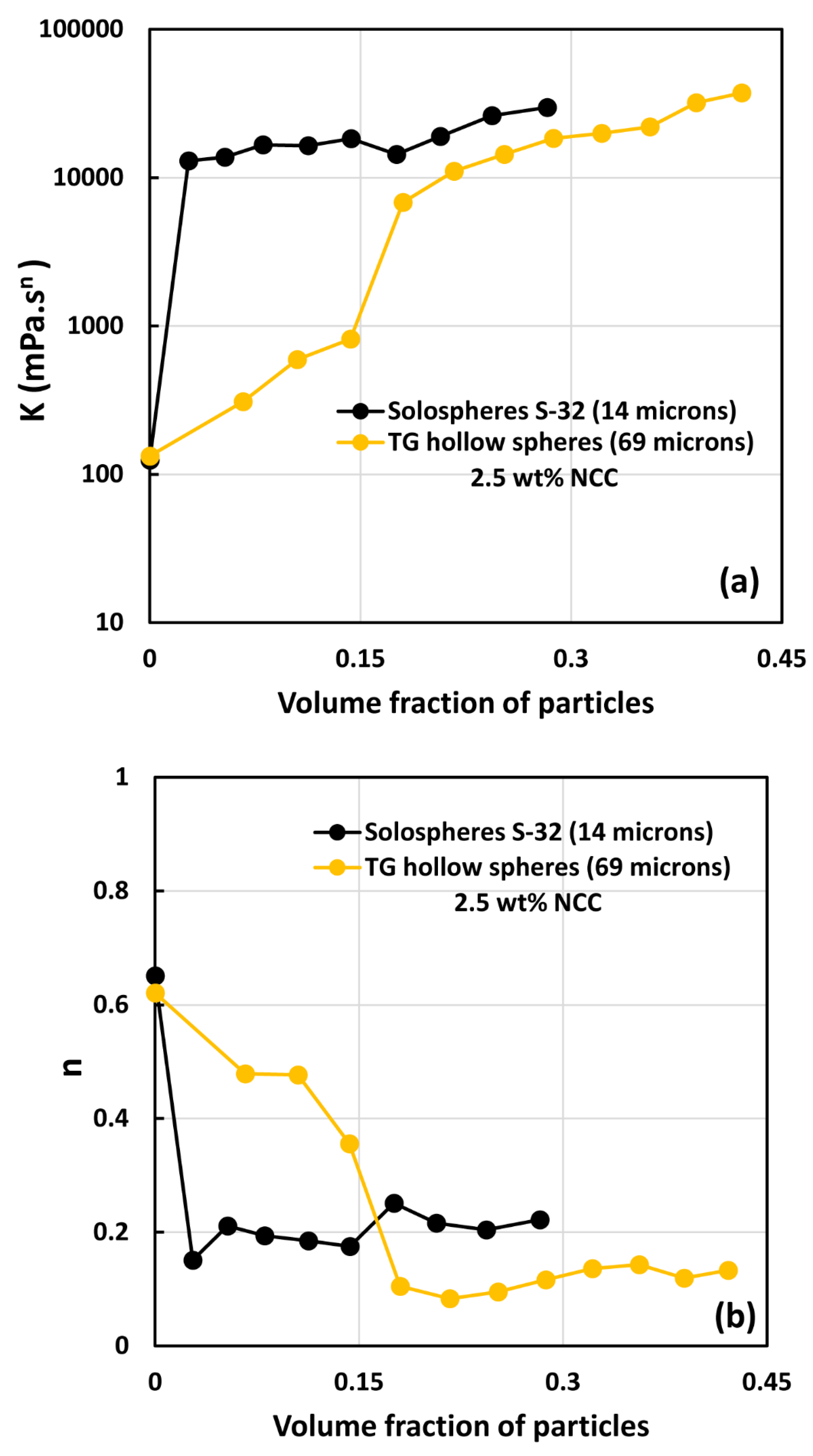
3.2. Rheology of Suspensions of Large Particles (TG Hollow Spheres)
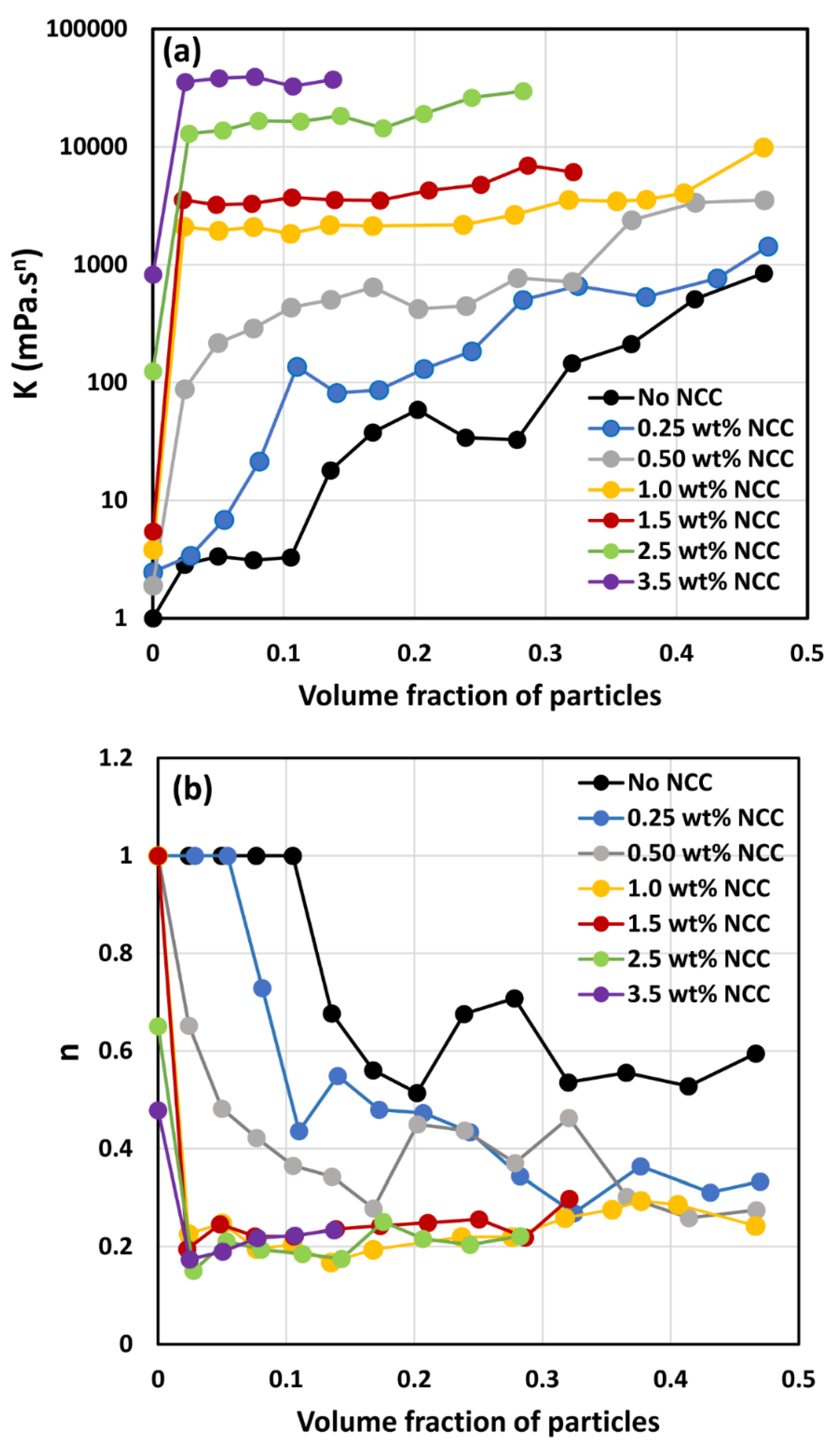
- At low NCC concentrations (1 wt%), the suspensions are Newtonian at low volume fractions of particles. At high volume fractions of particles, shear-thinning is observed.
- At high NCC concentrations (1.5 wt%), the suspensions are non-Newtonian shear thinning at all volume fractions of particles.
- The degree of shear thinning increases with the increase in particle volume fraction at any given NCC concentration.
- All suspensions at different particle volume fractions and different NCC concentrations follow the power-law behavior (see Equations (2) and (3)). The plots of viscosity versus shear rate are linear on a log-log scale.
- At a given NCC concentration, the consistency of suspensions as measured by consistency index increases with the increase in particle volume fraction.
- At a fixed particle volume fraction, the consistency of suspension generally increases with the increase in NCC concentration.
- The suspensions containing NCC are shear-thinning () at high volume fraction of particles. The flow behavior index depends on both NCC and particle concentrations. It generally decreases with the increase in NCC and particle concentrations.
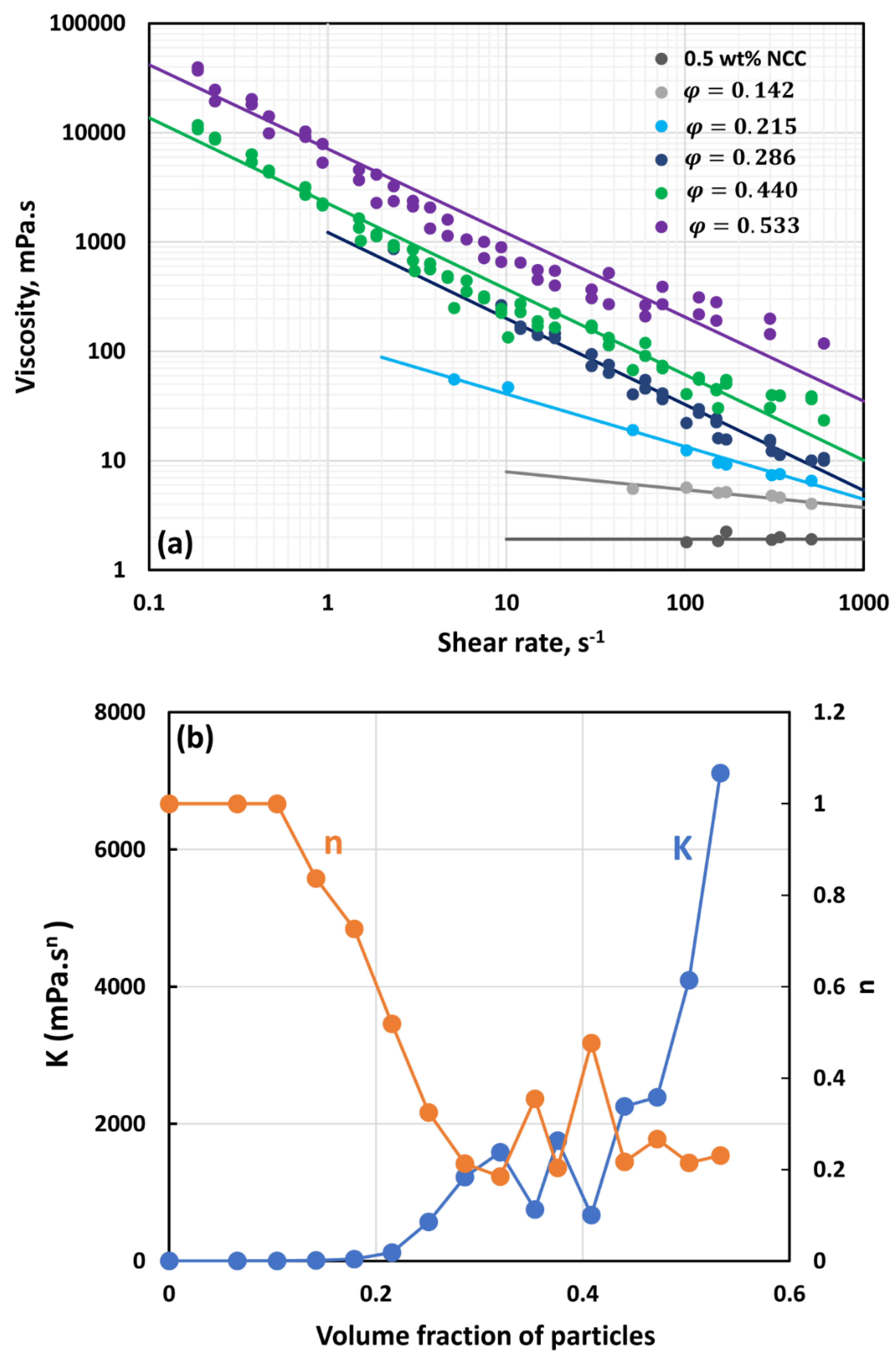
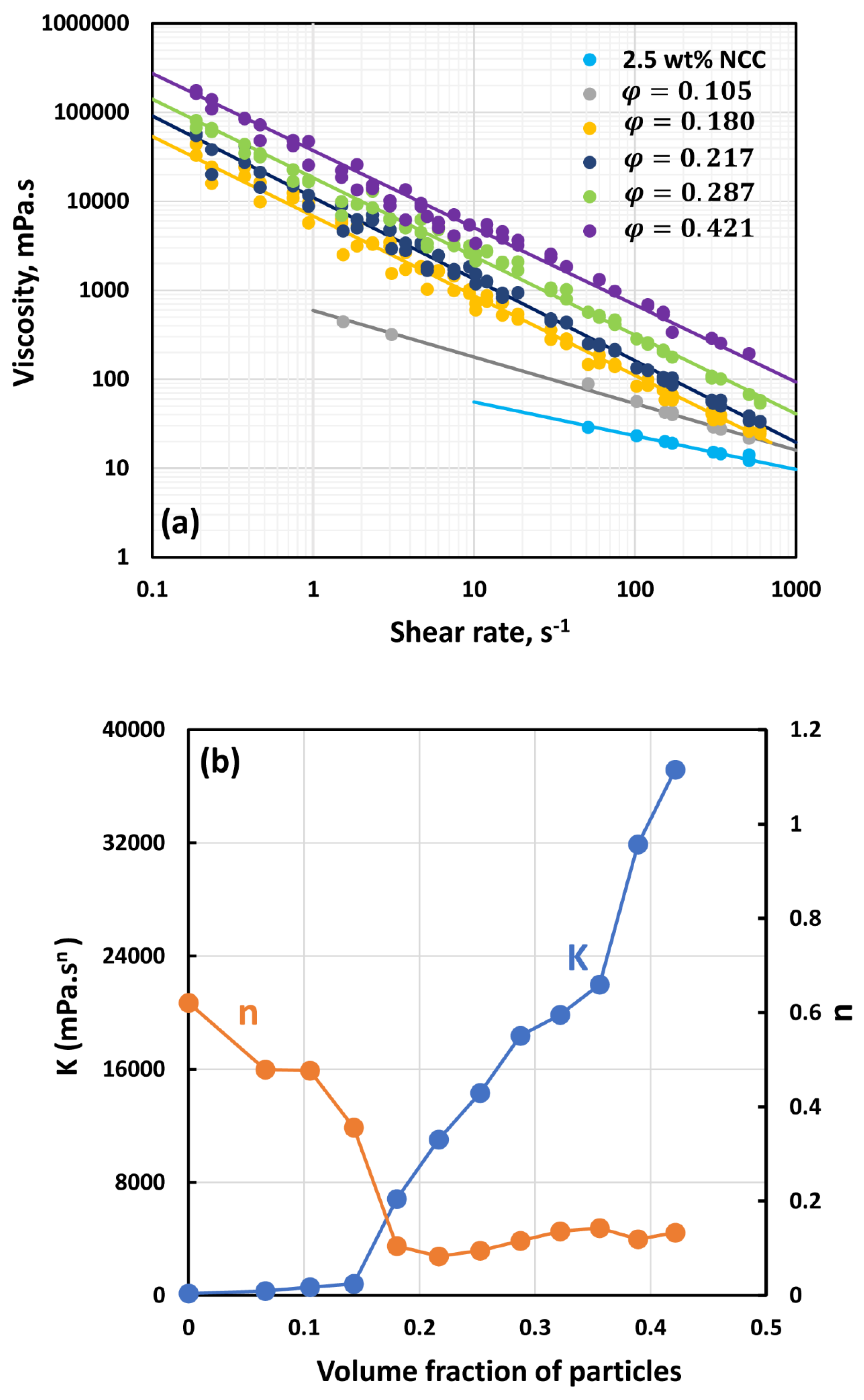
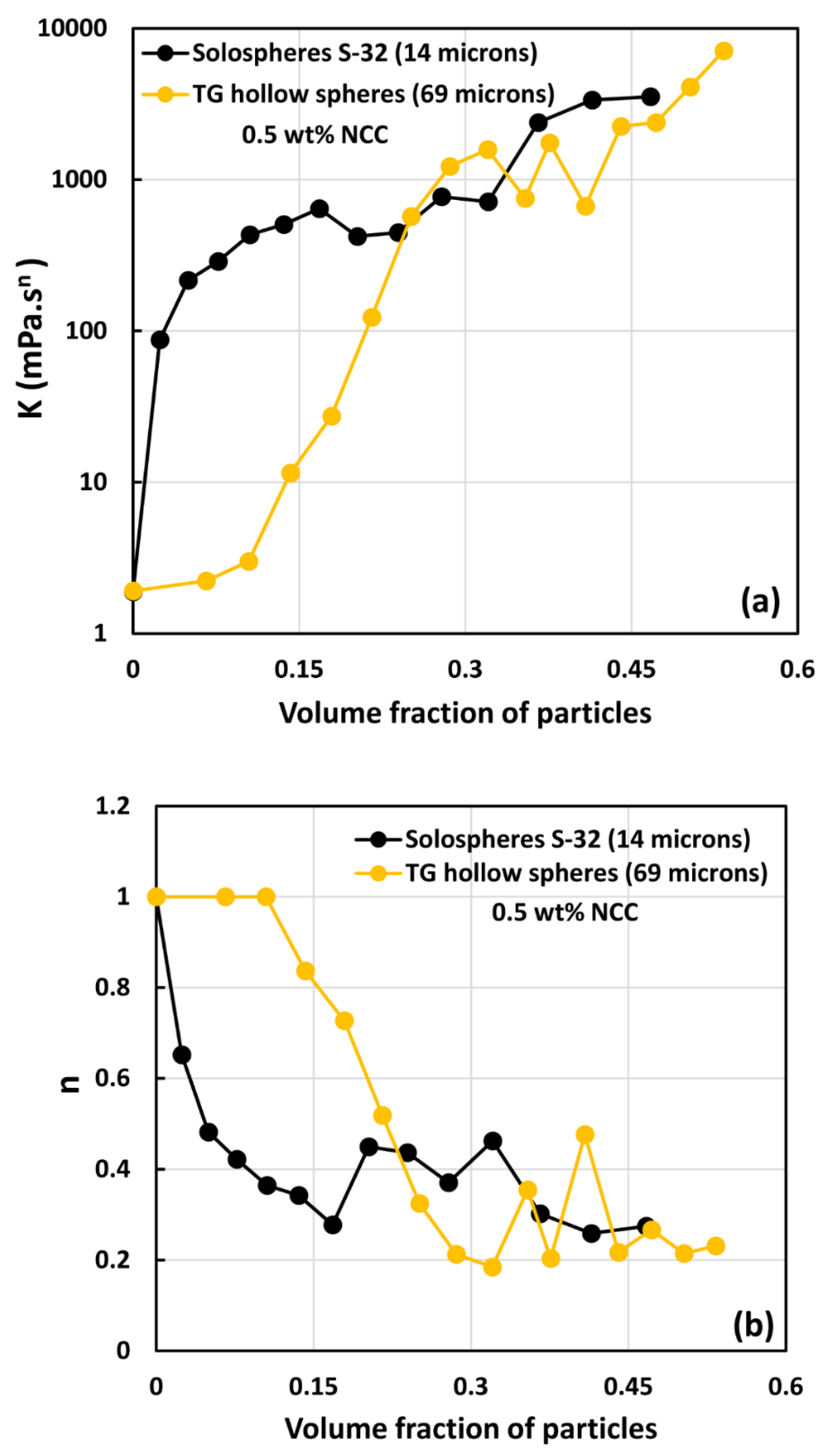
3.3. Rheology of Suspensions of Small Particles (Solospheres S-32)
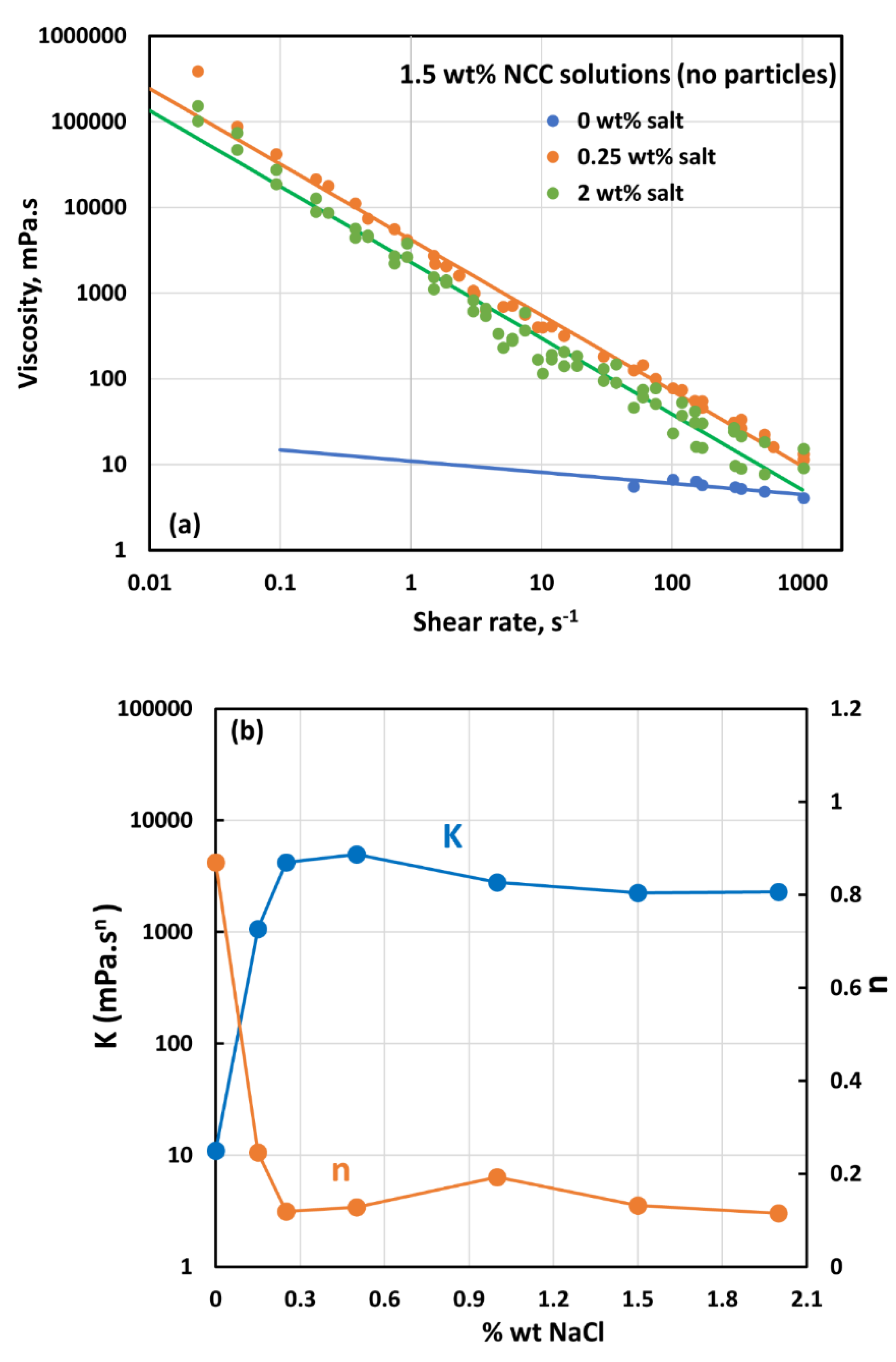
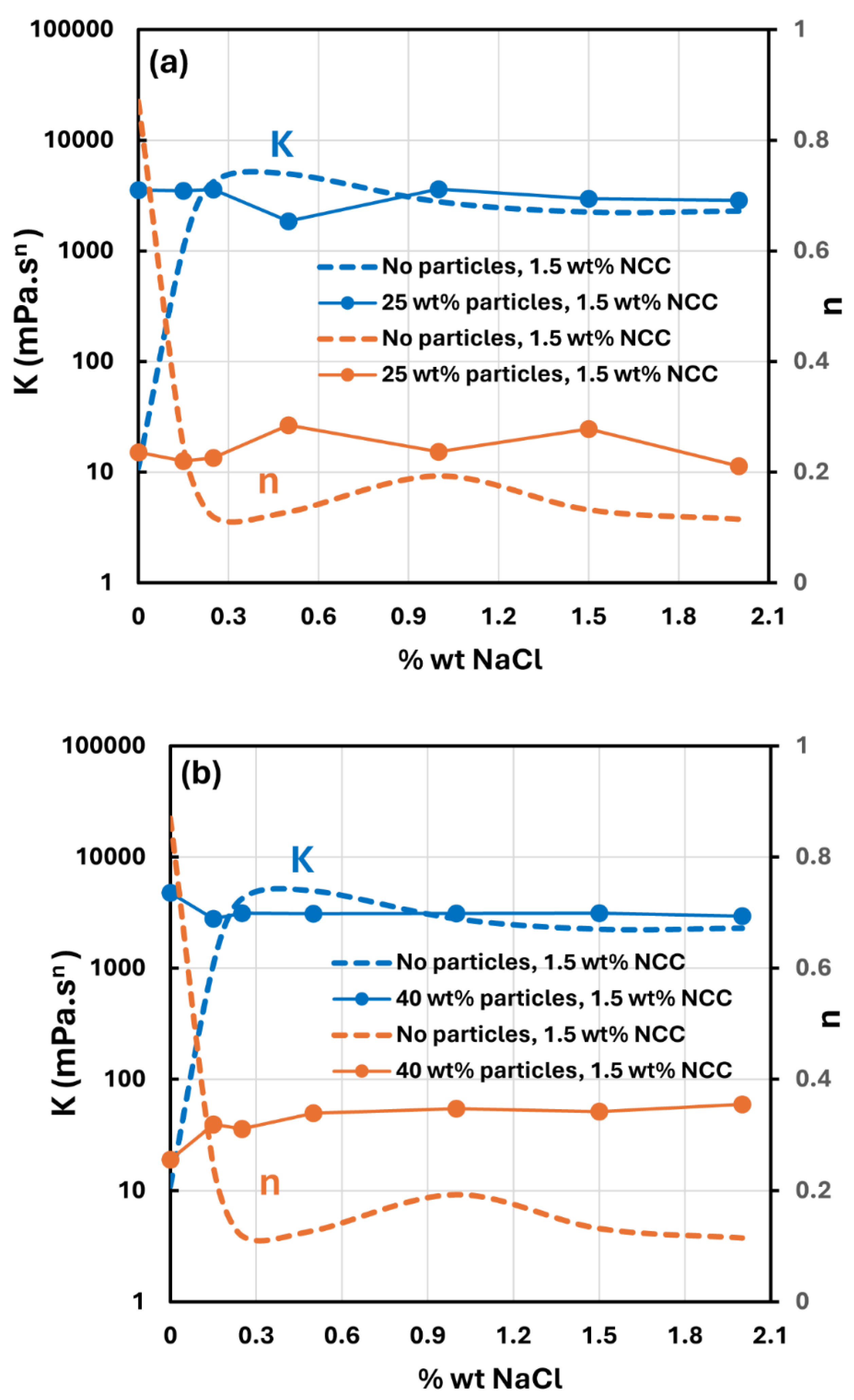
3.4. Effect of Salt on the Rheology of Suspensions
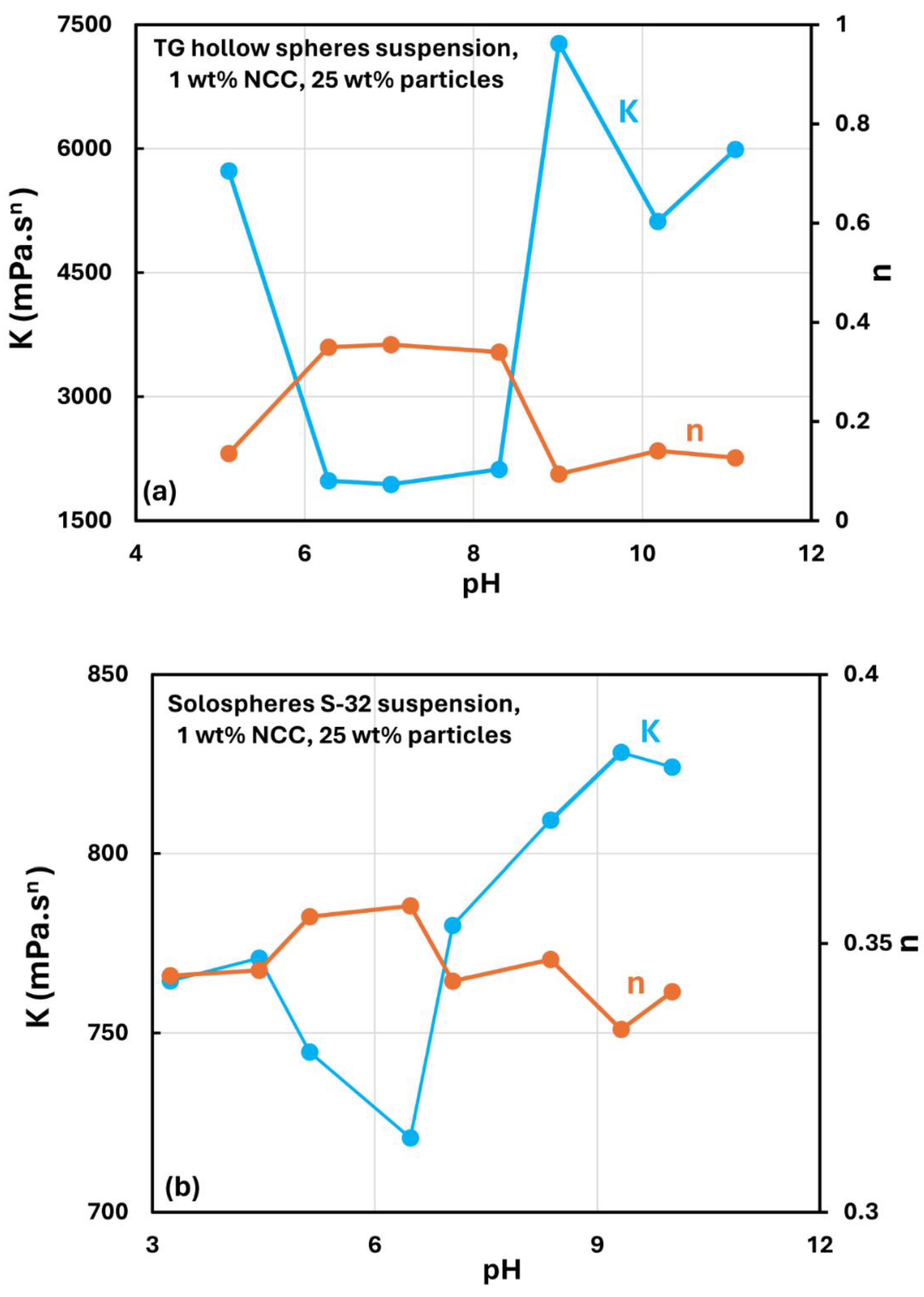
3.5. Effect of pH on the Rheology of Suspensions
4. Conclusions
- The dispersions of cellulose nanocrystals at NCC 1 wt% are shear-thinning due to disaggregation and orientation of nanocrystals with shear.
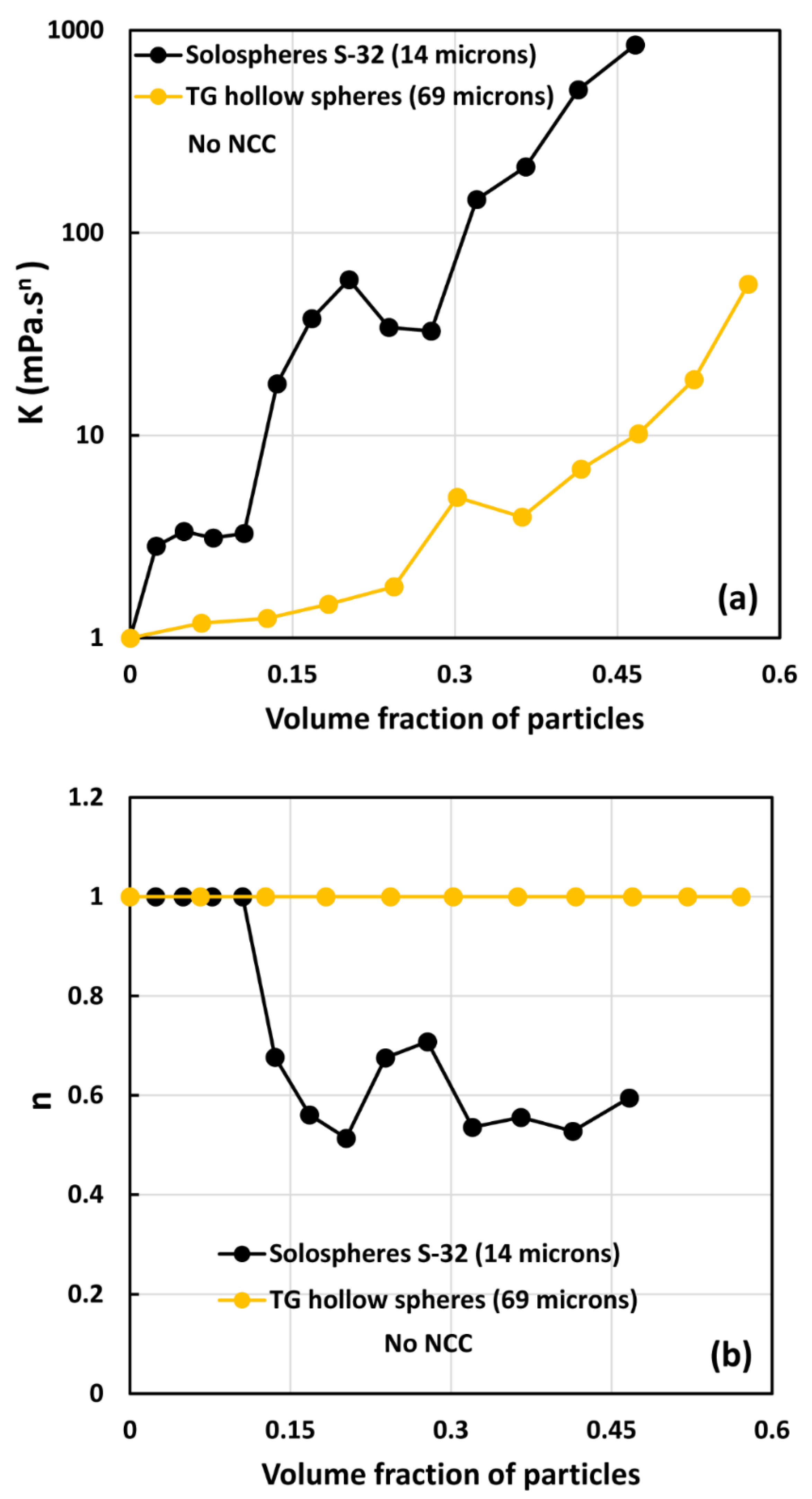
- The suspensions of large size particles (TG hollow spheres, Sauter mean diameter 69 µm) are Newtonian in the absence of cellulose nanocrystals. However, the addition of nanocrystals makes them shear-thinning and more viscous. The degree of shear-thinning increases with the increases in NCC and particle concentrations.
- The suspensions of small size particles (Solospheres S-32, Sauter mean diameter 14 µm) are shear-thinning at particle volume fractions 0.1 even in the absence of any nanocrystals. The addition of nanocrystals makes them more shear-thinning and viscous.
- The addition of salt has a strong influence on the rheology of nanocrystal dispersions and nanocrystal-thickened suspensions. A sharp rise in the consistency index and a large drop in the flow behavior index are observed with the addition of salt.
- The nanocrystal-thickened suspensions show a minimum in consistency index under neutral condition (pH7). The consistency rises substantially with the decrease in pH below 7 and with the increase in pH above 7.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tadros, T.F. Suspension Concentrates: Preparation, Stability and Industrial Applications; De Gruyter, Berlin, Boston, 2017.
- Schramm, L.L. Emulsions, Foams, Suspensions, and Aerosols: Microscience and Applications; Wiley-VCH Verlag, Weinheim, 2014.
- Kulshreshtha, A.; Wall, M. Pharmaceutical Suspensions: From formulation development to manufacturing; Springer, London, 2010.
- Pal, R. Rheology of Particulate Dispersions and Composites; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Pal, R. Electromagnetic, Mechanical, and Transport Properties of Composite Materials; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Stickel, J.J.; Powell, R.L. Fluid mechanics and rheology of dense suspensions. Annu. Rev. Fluid Mech. 2005, 37, 127–149. [Google Scholar] [CrossRef]
- Halloran, J.W.; Tomeckova, V. Flow behavior of polymerizable ceramic suspensions as function of ceramic volume fraction and temperature. J. European Ceramic Soc. 2011, 31, 2535–2542. [Google Scholar]
- Camargo, I.L.; Morais, M.M.; Fortulan, C.A.; Branciforti, M.C. A review on the rheological behavior and formulations of ceramic suspensions for vat polymerization. Ceramics International 2021, 47, 11906–11921. [Google Scholar] [CrossRef]
- Zhou, W.; Li, D.; Wang, H. A novel aqueous ceramic suspension for ceramic stereolithography. Rapid Prototyping Journal 2010, 16, 29–35. [Google Scholar] [CrossRef]
- Denn, M.M.; Morris, J.F. Rheology of non-Brownian suspensions. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 203–228. [Google Scholar] [CrossRef] [PubMed]
- Guazzelli, E.; Pouliquen, O. Rheology of dense granular suspensions. J. Fluid. Mech. 2018, 852, 1–73. [Google Scholar] [CrossRef]
- Pal, R. Modeling of Sedimentation and creaming in suspensions and Pickering emulsions. Fluids 2019, 4, 186. [Google Scholar] [CrossRef]
- Pal, R. Rheology of emulsions containing polymeric liquids. In Encyclopedia of Emulsion Technology, Becher, P., Ed.; Marcel Dekker: New York, USA, 1996; Volume 4, pp. 93–263. [Google Scholar]
- Prathapan, R.; Thapa, R.; Garnier, G.; Tabor, R.F. Modulating the zeta potential of cellulose nanocrystals using salts and surfactants. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2016, 509, 11–18. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y. Preparation and properties of cellulose nanocrystals: rods, spheres, and network. Carbohydrate Polymers 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Girard, M.; Vidal, D.; Bertrand, F.; Tavares, J.R.; Heuzey, M. Evidence-based guidelines for the ultrasonic dispersion of cellulose nanocrystals. Ultrasonics Sonochemistry 2021, 71, 105378. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose nanocrystal-based composites: A review. Composites Part C: Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Yang, X.; Biswas, S.K.; Han, J.; Tanpichai, S.; Li, M.; Chen, C.; Zhu, S.; Das, A.K.; Yano, H. Surface and interface engineering for nanocellulosic advanced materials. Advanced Materials 2021, 33, 2002264. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Fan, H.; Zhang, X.; Haq, A.; Ullah, R.; Khan, F.U.; Iqbal, M. Advance study of cellulose nanocrystals properties and applications. J. Polymers and Environment 2020, 28, 1117–1128. [Google Scholar] [CrossRef]
- Aziz, T.; Ullah, A.; Fan, H.; Ullah, R.; Haq, F.; Khan, F.U.; Iqbal, M.; Wei, J. Cellulose nanocrystals applications in health, medicine, and catalysis. J. Polymers and Environment 2021, 29, 2062–2071. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose processing properties and potential applications. Current Forestry Reports 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nature Reviews/Materials 2021, 6, 124–144. [Google Scholar] [CrossRef]
- Panchal, P.; Ogunsona, E.; Mekonnen, T. Trends in advanced functional material applications of nanocellulose. Processes 2019, 7, 1–27. [Google Scholar] [CrossRef]
- Zhang, H.; Dou, C.; Pal, L.; Hubbe, M.A. Review of electrically conductive composites and films containing cellulosic fibers or nanocellulose. BioResources 2019, 14, 7494–7542. [Google Scholar] [CrossRef]
- Gupta, A.; Mekonnen, T.H. Cellulose nanocrystals enabled sustainable polycaprolactone based shape memory polyurethane bionanocomposites. J.Colloid Interface Sci. 2022, 611, 726–738. [Google Scholar] [CrossRef]
- Kinra, S.; Pal, R. Rheology of Pickering emulsions stabilized and thickened by cellulose nanocrystals over broad ranges of oil and nanocrystal concentrations. Colloids Interfaces 2023, 7, 36. [Google Scholar] [CrossRef]
- Shafiei-Sabet, S.; Hamad, W.Y.; Hatzikiriakos, S.G. Rheology of nanocrystalline cellulose aqueous suspensions. Langmuir 2012, 28, 17124–17133. [Google Scholar] [CrossRef] [PubMed]
- Kadar, R.; Fazilati, M.; Nypelo, T. Unexpected microphase transitions in flow towards nematic order of cellulose nanocrystals. Cellulose 2020, 27, 2003–2014. [Google Scholar] [CrossRef]
- Pignon, F.; Challamel, M.; De Geyer, A.; Elchamaa, M.; Semeraro, E.F.; Hengl, N.; Jean, B.; Putaux, J.; Gicquel, E.; Bras, J.; Prevost, S.; Sztucki, M.; Narayanan, T.; Djeridi, H. Breakdown and buildup mechanisms of cellulose nanocrystal suspensions under shear and upon relaxation probed by SAXS and SALS. Carbohydrate Polymers 2021, 260, 117751. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Meng, Y.; Wang, S.; Li, Y.; Fu, S.; Ma, L.; Harper, D. Rheological behavior of cellulose nanocrystal suspension: influence of concentration and aspect ratio. J. Appl. Poly. Sci. 2014, 131, 40525. [Google Scholar] [CrossRef]
- Qi, W.; Yu, J.; Zhang, Z.; Xu, H.N. Effect of pH on the aggregation behavior of cellulose nanocrystals in aqueous medium. Mater. Res. Express 2019, 6, 125078. [Google Scholar] [CrossRef]
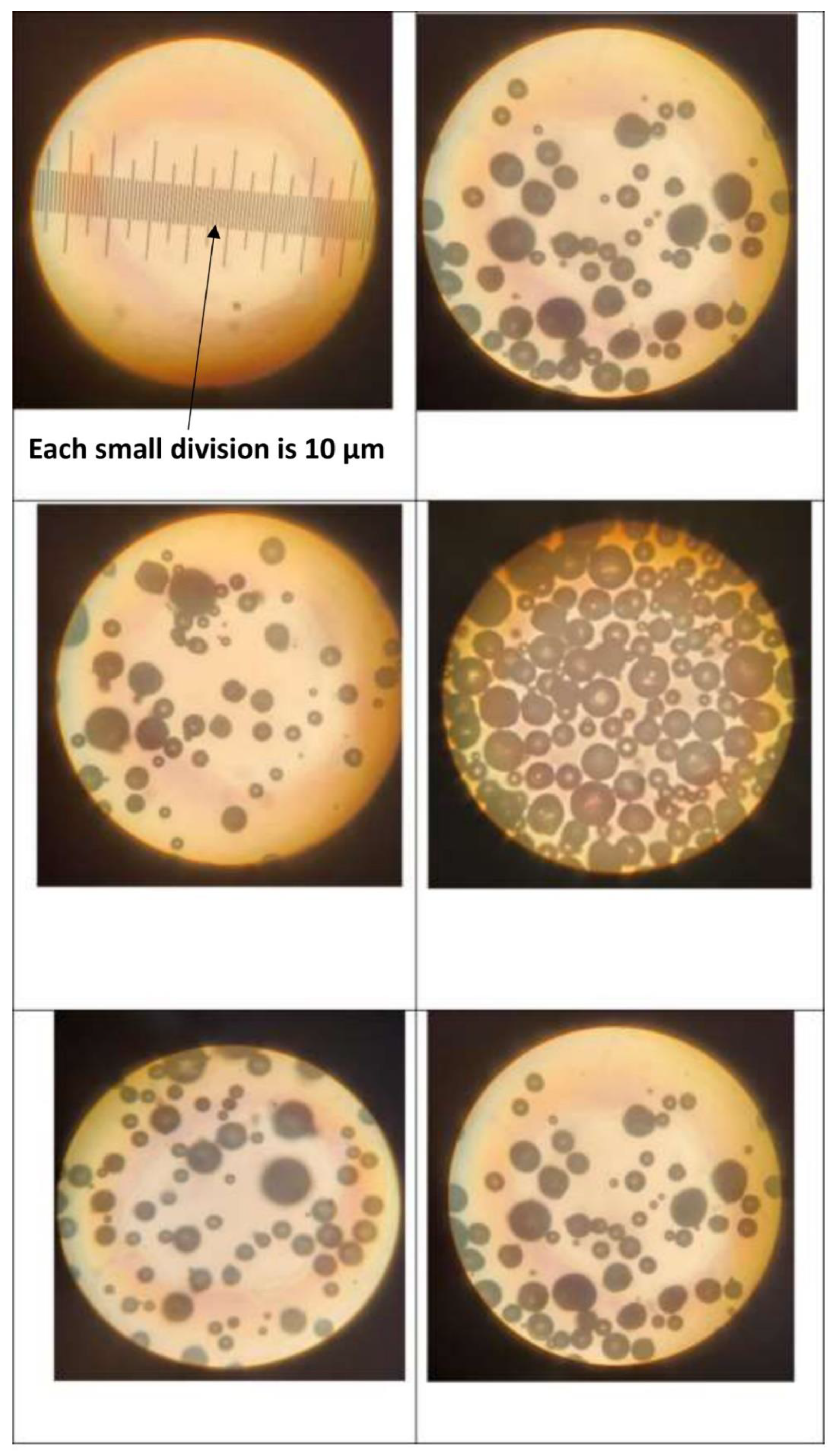
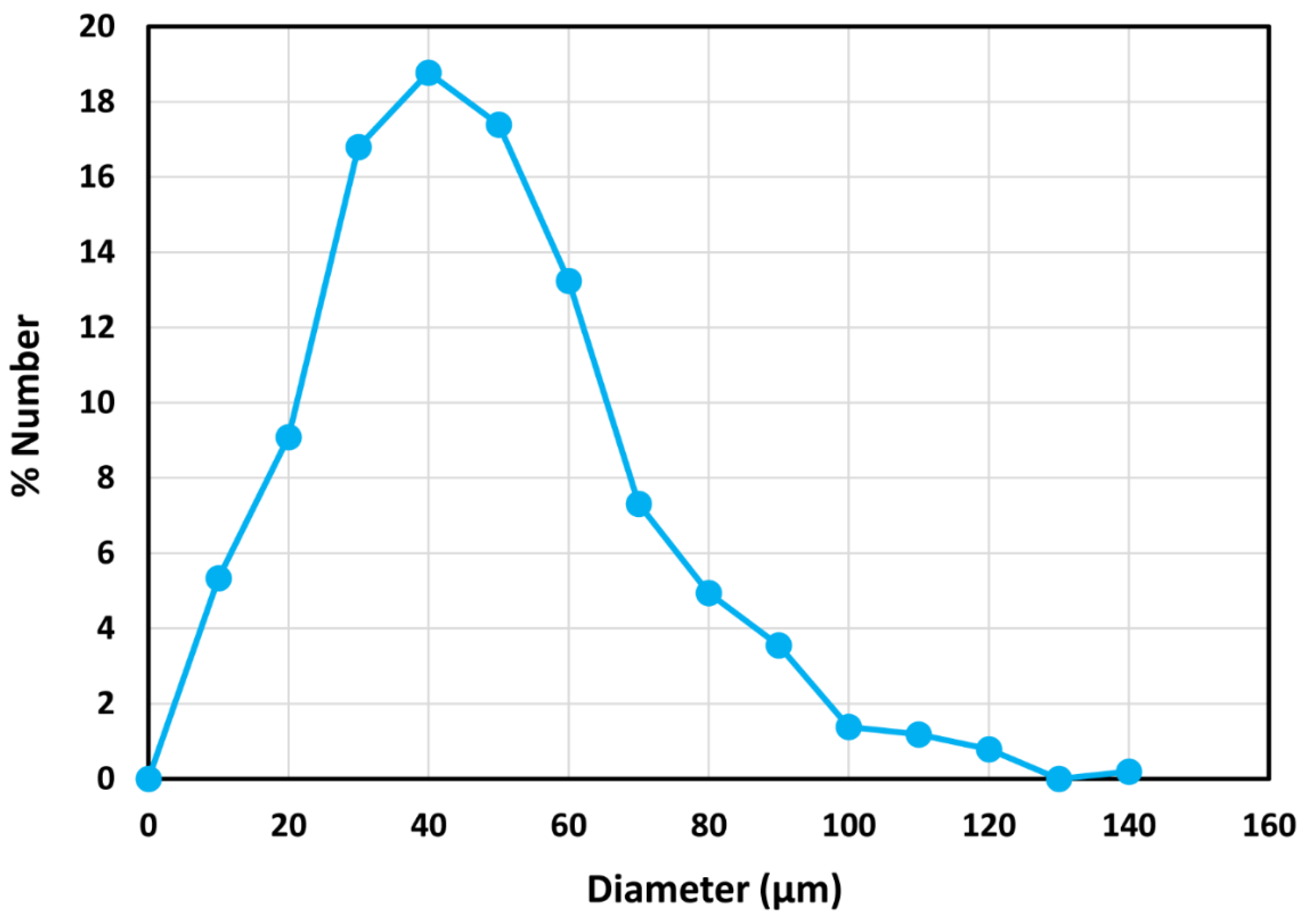



| Viscometer | Length of inner cylinder | Gap-width | ||
|---|---|---|---|---|
| Fann 35A/SR-12 (low torsion spring constant) | 1.72 cm | 1.84 cm | 3.8 cm | 0.12 cm |
| Fann 35A (high torsion spring constant) | 1.72 cm | 1.84 cm | 3.8 cm | 0.12 cm |
| Haake Roto- visco RV 12 with MV I | 2.00 cm | 2.1 cm | 6.0 cm | 0.10 cm |
| Haake Roto- visco RV 12 with MV II | 1.84 cm | 2.1 cm | 6.0 cm | 0.26 cm |
| CNC Concentration of Matrix Phase (wt%) | Solids Concentration of Suspension (wt%) | Solids Concentration of Suspension (vol%) |
|---|---|---|
| 0 0.25 |
Ten concentrations: 5, 9.8, 14.4, 19.4, 24.4, 29.8, 34.8, 39.8, 44.8, 49.8 Twelve concentrations: 5, 10, 15, 20, 25, 30, 35, 38, 41, 44, 47, 50 |
Ten concentrations: 6.6, 12.7, 18.3, 24.4, 30.2, 36.2, 41.7, 47.0, 52.1, 57.1 Twelve concentrations: 6.6, 13.0, 19.1, 25.1, 30.9, 36.5, 41.9, 45.1, 48.2, 51.3, 54.3, 57.2 |
| 0.50 | Fifteen concentrations: 5, 8, 11, 14, 17, 20, 23, 26, 29, 31, 34, 37, 40, 43,46 |
Fifteen concentrations: 6.6, 10.4, 14.2, 17.9, 21.5, 25.1, 28.6, 32, 35.4, 37.6, 40.8, 44, 47.2, 50.3, 53.3 |
| 1.0 | Nine concentrations: 5, 10, 15, 20, 25, 28, 31, 34, 37 |
Nine concentrations: 6.6, 13.0, 19.1, 25.1, 30.9, 34.3, 37.6, 40.9, 44.1 |
| 1.5 | Twelve concentrations: 5, 8, 11, 14, 17, 20, 23, 26, 29, 31, 34, 37 |
Twelve concentrations: 6.6, 10.5, 14.3, 18.0, 21.6, 25.2, 28.7, 32.1, 35.5, 37.7, 40.9, 44.1 |
| 2.5 | Eleven concentrations: 5, 8, 11, 14, 17, 20, 23, 26, 29.1, 32.1, 35 |
Eleven concentrations: 6.6, 10.5, 14.3, 18.0, 21.7, 25.2, 28.7, 32.2, 35.6, 38.9, 42.1 |
| 3.5 | Ten concentrations: 5, 11, 14, 17, 20, 23, 26, 29, 32, 35 |
Ten concentrations: 6.7, 14.3, 18.1, 21.7, 25.3, 28.8, 32.3, 35.6, 38.9, 42.2 |
| CNC Concentration of Matrix Phase (wt%) | Solids Concentration of Suspension (wt%) | Solids Concentration of Suspension (vol%) |
|---|---|---|
| 0 0.25 |
Thirteen concentrations: 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65 Thirteen concentrations: 5.9, 10.9, 15.8, 20.8, 25.7, 30.7, 35.6, 40.6, 45.5, 50.5, 56.2, 61.6, 65.3 |
Thirteen concentrations: 2.4, 5.0, 7.7, 10.5, 13.6, 16.8, 20.2, 23.9, 27.8, 32, 36.5, 41.4, 46.6 Thirteen concentrations: 2.9, 5.4, 8.1, 11.0, 14.0, 17.3, 20.7, 24.4, 28.3, 32.5, 37.7, 43.1, 47 |
| 0.50 | Thirteen concentrations: 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65 |
Thirteen concentrations: 2.4, 5.0, 7.7, 10.5, 13.6, 16.8, 20.3, 23.9, 27.8, 32.0, 36.6, 41.4, 46.7 |
| 1.0 | Thirteen concentrations: 4.9, 10, 15, 19.9, 24.8, 29.9, 39.7, 44.7, 49.6, 53.7, 56.1, 59.1, 64.9 |
Thirteen concentrations: 2.4, 5, 7.7, 10.5, 13.5, 16.8, 23.7, 27.6, 31.8, 35.4, 37.7, 40.6, 46.6 |
| 1.5 | Ten concentrations: 4.7, 9.7, 14.7, 20.1, 25.4, 30.7, 36, 41.4, 45.9, 50.1 | Ten concentrations: 2.3, 4.8, 7.6, 10.6, 13.9, 17.3, 21.1, 25, 28.6, 32.1 |
| 2.5 |
Nine concentrations: 5.6, 10.6, 15.6, 21.1, 26, 31, 35.4, 40.4, 45.4 |
Nine concentrations: 2.8, 5.3, 8.1, 11.3, 14.3, 17.6, 20.7, 24.4, 28.3 |
| 3.5 |
Five concentrations: 5.1, 10.1, 15, 20.1, 25.1 |
Five concentrations: 2.5, 5.1, 7.8, 10.7, 13.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





