1. Introduction
With the development of the polymer industry, polymeric materials have found a wide range of applications, such as medicine, construction, automotive, electronics, etc. Nevertheless, most polymeric materials have the disadvantage of being flammable. Smoke and even toxic gases released during combustion are the main cause of the loss of human lives in case of a fire. Flame retardants are therefore introduced to limit the material's flammability and the flame spread of polymers [
1]. The main applications for flame retardant polymers are in the construction and electrical cable industries and the transportation sector, where electronics are expanding. Halogen-free flame-retardant (HFFR) polyolefin compounds, also named LZSH or LS0H (Low Smoke Zero Halogen), or even NHFR (Non-halogen flame retardant), have gained great popularity in the last two decades as the preferred materials in safe electrical cable applications, especially in Europe, Middle East, and Asia.
In HFFR compounds, the most used flame retardants (FRs) are metal hydroxides due to their combination of low cost, environmentally friendly nature, low smoke generation, and flame retardancy. However, the main disadvantage of these FRs is the high dosage required to achieve an appreciable improvement of flame retardancy (at least 40 - 50 % by weight, but in some applications up to 65 - 70 % by weight), which often leads to processing issues and deterioration in the mechanical properties of the compound [
2,
3]. The most commonly used are aluminium trihydroxide (ATH), Al(OH)
3, and magnesium hydroxide (MDH), Mg(OH)
2 [
4,
5]. ATH and MDH endothermically decompose at T > 200 °C and T > 300 °C respectively, with the release of water vapours, thus resulting in a fire retardancy effect due to cooling and dilution of the oxygen near the burning areas [
6].
In this work, a natural magnesium hydroxide, obtained by mining, selecting, and milling of natural Brucite, was used as flame retardant. During thermal decomposition, MDH releases MgO and water vapours (equation (1)), with the first that forms protective layers on the burning polymer surface, preventing heat and oxygen from reaching the polymer [
7,
8].
Polyolefins, with their many desirable properties such as excellent electrical properties, easy processability, and a wide range of mechanical properties, are among the most interesting polymer matrices for highly filled composites for insulation and sheathing of electrical cables. Polyolefin Elastomers (POE), such as copolymers of ethylene with 1-octene (C
8), were selected as the preferred polymeric matrix obtained by metallocene catalysis. The physical properties of C
8-POE, such as crystallinity, melting point and density, depend upon comonomer content [
9,
10] whose increasing leads to a decrease in the degree of crystallinity. This is because the short-chain branches interfere with the ability of the polymer backbone to organize into regular crystals. The advantages of C
8-POEs include good impact resistance, high flexibility, and recyclability [
11]. They can also withstand a large percentage of filler (up to over 65 % by weight) thanks to their low crystallinity [
12]. In addition, to comply with all the performance requirements of a thermoplastic HFFR compound for cable applications (i.e., elongation at break > 150 % and tensile strength > 10 - 12.5 MPa or 12.5 MPa for sheathing and insulating applications, respectively [
13]), C
8-POE is generally used in synergistic combination with linear low-density polyethylene (LLDPE). The LLDPE has the aim to increase the tensile strength and thermal stability up to 90 °C [
12] due to its high flexural modulus and melting point > 110 °C.
Moreover, the incorporation of a high amount of inorganic fillers leads to a substantial increase of nucleating sites, which influences the crystallization level and the internal morphology of the composite and mechanical properties [
14,
15]. This work aims to study the influence of the introduction of LLDPE and MDH fillers on the crystallization behaviour of C
8-POE. The variation in mechanical properties caused by the different compositions was also investigated.
2. Materials and Methods
Four grades of poly(ethylene-co-octene), supplied by Dow Chemical (Dow Europe GmbH, Horgen, Switzerland) under the trade name ENGAGE™ [
16,
17] and characterized by different contents of comonomer, were selected. As LLDPE, Dowlex 2045G was selected, a C
8-LLDPE ethylene-octene copolymer from Dow Chemical produced with metallocene catalysis in solution process. The characteristics of the polyolefins are summarized in
Table 1.
The used MDH was Ecopiren 3.5 (micronized brucite, n-MDH), supplied by Europiren (Rotterdam Science Tower, the Netherlands) at 92 % purity, D50 = 3.5 - 4 μm and a surface area of 11 m2/g.
Fusabond N525™ was used as coupling agent (maleic anhydride modified LLDPE), supplied by Dow Chemical, with density 0.880 g/cm3, MFI = 3.7 g/10min 2.16 kg@190°C and grafting level in the range 0.5 – 1 wt.%.
All blends and composites were prepared using a twin-roll mixer to get a good dispersion of MDH in the polymer at the temperature of 150-160 °C for 10 min.
The crystallinity of blends and composites was measured with a DSC 4000 Perkin Elmer, at a heating rate of 10 °C/min under a dry nitrogen atmosphere. About 5 mg of the sample was sealed in an aluminum pan for each measurement. A heat-cool-heat cycle was used, heating up from -65 to 160 °C, then cooling to -65 °C, followed by a second heating ramp to 160 °C. The heat flow versus temperature was recorded, and the peak melting temperatures of PEO and LLDPE were obtained from the heating scans. The degree of crystallinity (X
C, %) was finally calculated using equation (2).
where ΔH
fus is the melting enthalpy of the sample, ΔH
0 is the melting enthalpy of the 100 % crystalline polyethylene (292 J/g) [
9], ΔH
graft is the melting enthalpy of the Fusabond N525, x
mix is the mass fraction of polymer in the composites [
19] and x
graft is the mass fraction of Fusabond N525 in the composites. The equation's correction is because adding filler reduces the measured crystallinity.
Mechanical properties were measured with a Lloyd Instruments LS 500 dynamometer by using an elongation speed of 250 mm/min at 23 °C. The width and thickness of the specimens were 4.0 mm and 2.0 mm ± 0.2 mm, respectively, and the stretched length was 20 mm ± 0.5 mm, according to the standard ISO 37 [
20].
3. Results and Discussion
3.1. Crystallization Behavior of C8-POE/LLDPE Blends
Each grade of Engage™ was blended in a 3:1 (POE:LLDPE) ratio to produce four blends (
Table 2).
This is considered the optimum concentration in agreement with what is commonly used as the polymeric base in flame retardant compounds for electrical cable applications. Furthermore, it provides an adequately diluted concentration to investigate the impact of a predominantly amorphous matrix (C8-POE) on LLDPE crystallization, still maintaining a high sensitivity to detect the signal of LLDPE crystallization.
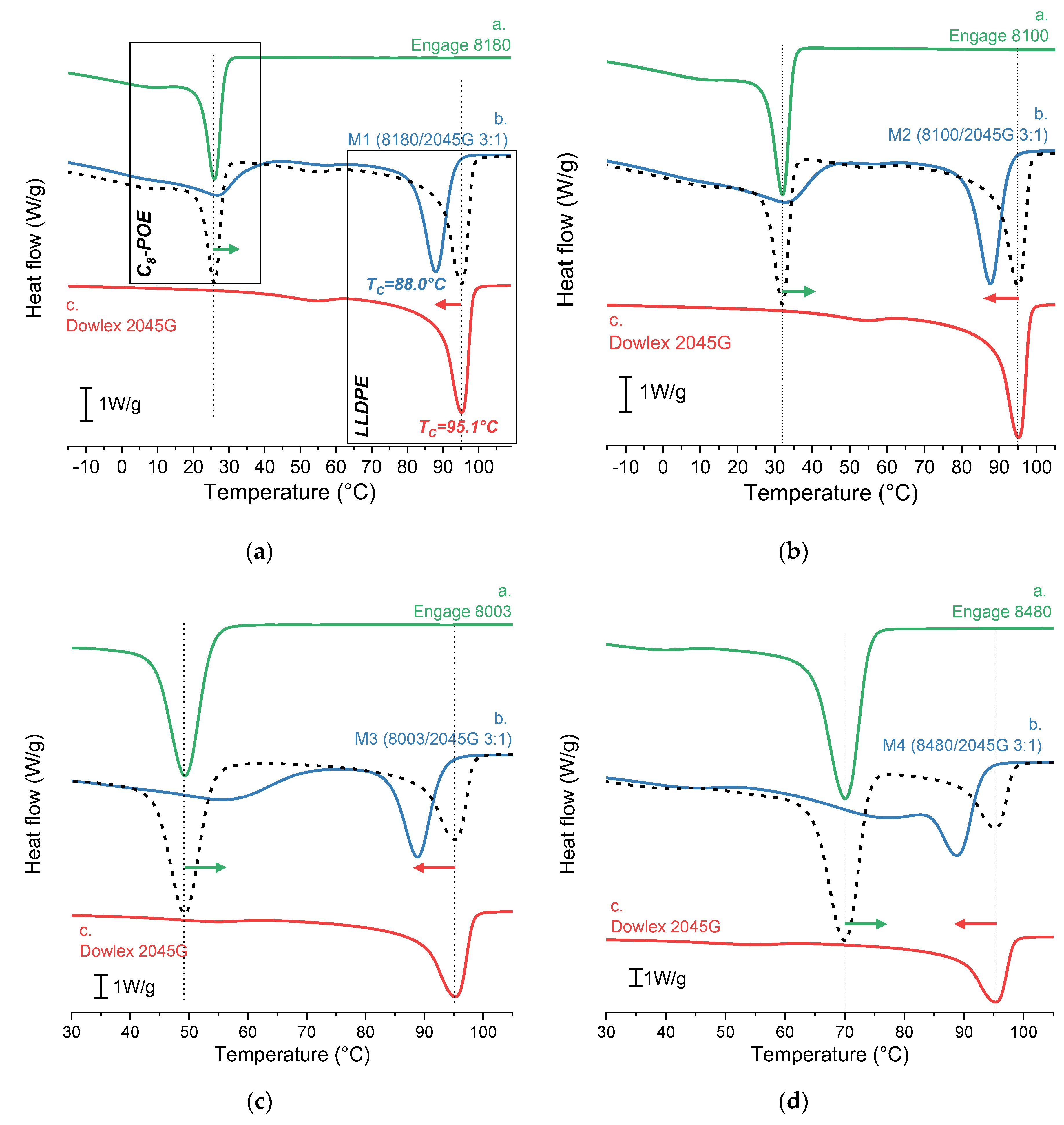
Figure 1 shows the cooling DSC curves of C
8-POE, LLDPE, and their 75:25 blend, whereas in
Table 3, the relative crystallization temperatures (T
C) are reported.
In all figures, it’s evident that the crystallization temperature (T
C) of LLDPE fraction blended with C
8-POE (peak at the higher temperature of blue line b.) shifts toward lower values compared to the T
C of pure LLDPE (red line c.), as evidenced by the red arrow. The shift at lower crystallization temperature is explained as a delay in the LLDPE crystallization because the concentration of LLDPE in the PEO-rich matrix is too low to form nuclei [
21]. No broadening of the LLDPE crystallization peak is observed.
On the contrary, for C
8-POE fraction within the blend, the crystallization temperature (peak at the lower temperature of line b.) is shifted to higher values than the T
C of pure C
8-POE (line a.). In this case, the crystallites of the already solidified LLDPE act as nucleating agents for the C
8-POE (less crystalline and with lower Tc), causing it to crystallize at a higher temperature [
22]. Furthermore, the C
8-POE signal in the blend is significantly broader than the calculated signal, indicating a strong influence of the intermixing with the LLDPE and the co-crystallization with the C
8-POE.
Additionally, it can be observed that the advance of the C8-POE crystallization temperature (ΔTPOE = TC (MX) - TC (MX calculated)) increases as the comonomer content decreases: for C8-POE copolymers with a lower comonomer concentration (=higher ethylene content) the co-crystallization with LLDPE is favourite, and the Tc is higher than for more amorphous C8-POE.
Similarly,
Table 4 shows the crystallization enthalpies for all C
8-POE/LLDPE blends.
In this case, it can be observed that the crystallization enthalpies of POE and LLPDE are slightly lower than those calculated as a linear combination of the two pure polymers, and there is no trend as the comonomer content changes.
3.2. Crystallization Behavior of C8-POE/LLDPE/n-MDH Composites
Mineral particles may trigger the nucleation and growth of C
8-POE crystals. This could also be the case of n-MDH, which is obtained by micronization of brucite and used as flame retardants in HFFR polyolefin compounds for electric cables. Twelve different formulations were prepared containing 36.8 wt.% C
8-POE+LLDPE, 60 wt.% MDH, 3 wt.% coupling agent, and 0.2 wt.% phenolic antioxidant stabilizer (
Table 5). Among these, the A series is based on neat C
8-POE, and the B and the C series has a POE/LLDPE ratio of 3:1 and 1:1, respectively. The latter ratio was investigated because a higher concentration of LLDPE gives both economic and performance benefits. The economic advantages are due to the lower cost of the polymer, while the performance benefits are associated with a higher tensile strength. These formulations were studied in a previous work [
23], but no studies about the contribution of the C
8-POE and LLDPE contents on the ultimate properties of the composites were investigated.
DSC thermograms were recorded for each C8-POE/LLDPE/n-MDH composite and compared with related C8-POE/LLDPE blends with the same C8-POE/LLDPE ratio and the pure polymers.
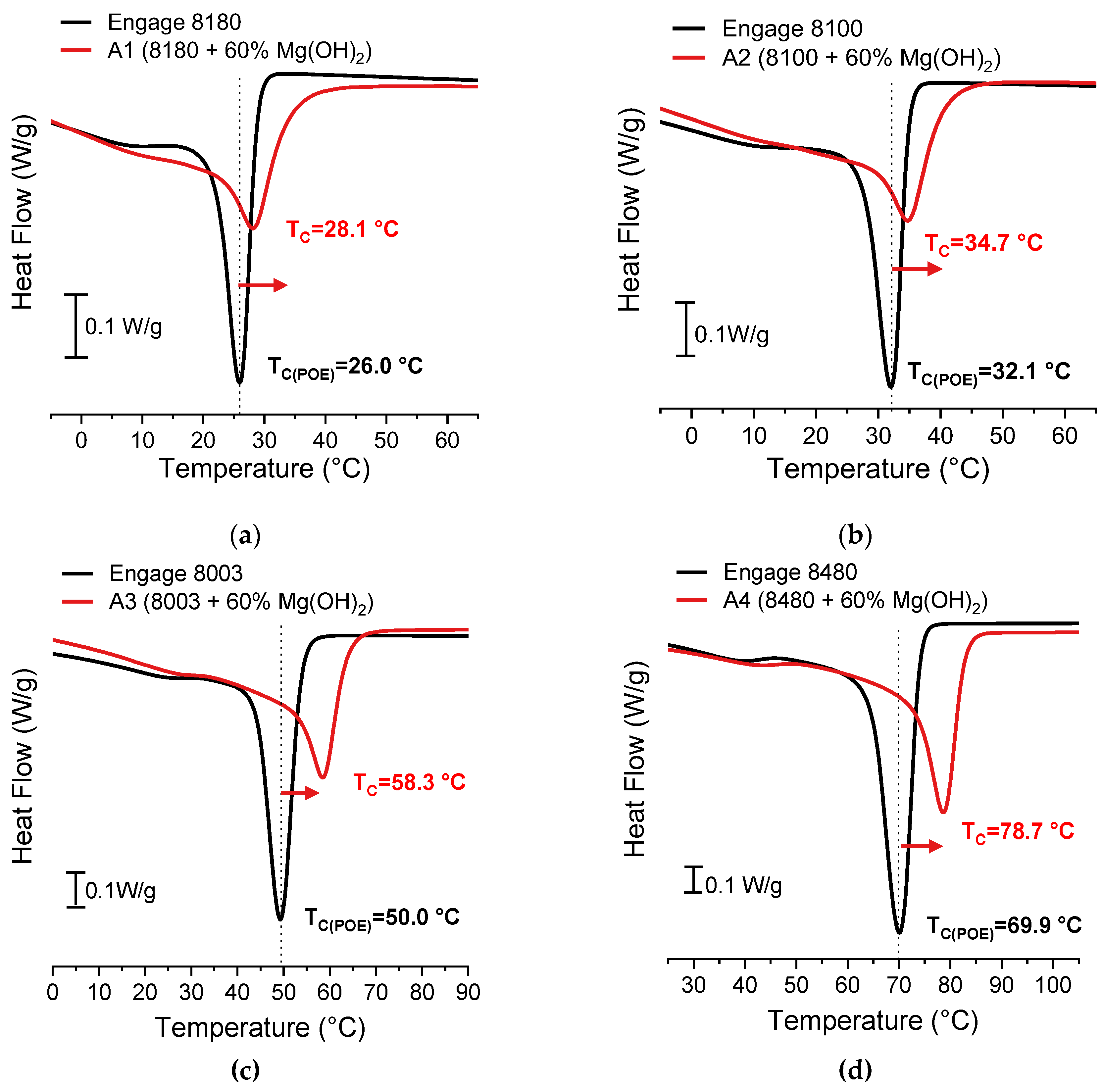
Figure 2 shows cooling DSC curves of the type “A” formulations based on C
8-POE versus the respective pure polymers.
As expected, an increase in crystallization temperature can be observed in the presence of MDH particles, which may be attributed to the heterogeneous nucleation caused by the fillers [
24].
Table 6 shows the T
C and ΔH
C values recorded for all C
8-POE+n-MDH composites compared to pure C
8-POE.
It can be observed that the addition of MDH increases the peak crystallization temperatures by 2.1 °C for the low crystallinity formulation (A1) and by 8.8 °C for the high crystallinity formulation (A4) by following a regular trend by moving from amorphous to more crystalline C8-POE grades. Similarly, the ΔHC of formulation “A” is influenced by the comonomer content. In particular, there is a greater deviation from the enthalpy of the pure polymer as the comonomer content decreases. However, enthalpy values lower than those of pure polymers are obtained in all cases.
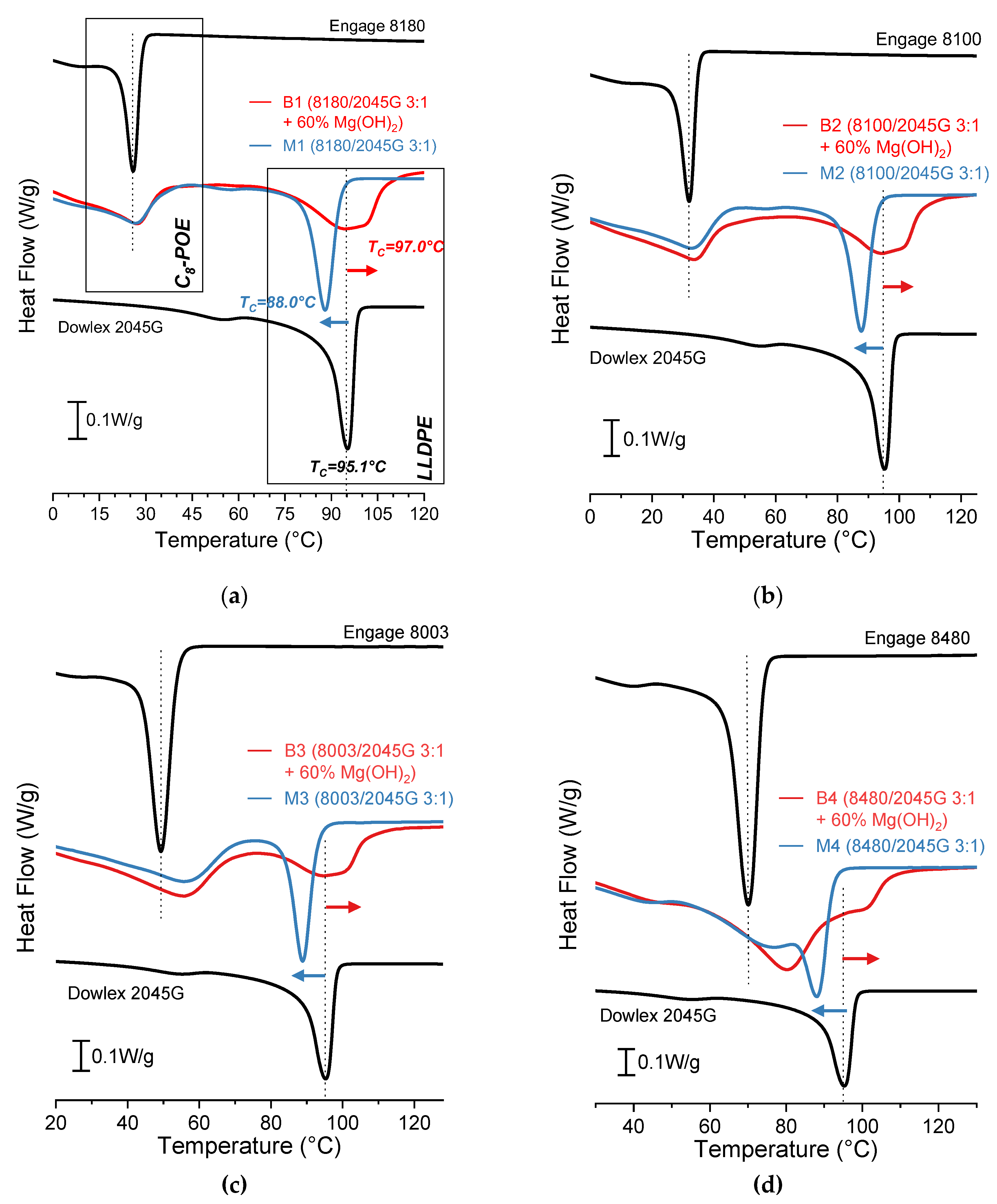
Figure 3 shows cooling DSC curves of the type “B” formulations (filled C
8-POE/LLDPE blends) and the respective pure polymers and polymer blends.
In the presence of n-MDH particles, only the LLDPE fraction into C
8-POE/LLDPE/n-MDH composite shows an increase in crystallization temperature. There is no significant effect on the T
C of the C
8-POE. The nucleating role of the additives is therefore evident only for the LLDPE, that is the polyolefin with the highest crystallinity [
25]. There is also a broadening of the crystallization peaks of the composites compared to pure polymers: the presence of the mineral particles leads to the formation of crystals of several different sizes, which is reflected on their different thermal stability. This results possibly indicates a certain interaction between polymeric matrix and the mineral triggered by the presence of the LLDPE-g-MAH coupling agent.
Table 7 and
Table 8 show the T
C and ΔH
C values of C
8-POE/LLDPE/n-MDH composites in comparison with pure C
8-POE and C
8-POE/LLDPE blends (M).
The TC of the C8-POE fraction in C8-POE+LLDPE+n-MDH composites and C8-POE+LLDPE blends (peaks at lower temperature of red and blue line, respectively) are similar and higher than that of pure C8-POE (upper black line). Therefore, the main evident nucleating effect is addressed to the already crystallized LLDPE rather than to mineral particles. Regarding the LLDPE fraction, it can be observed that the TC of C8-POE+LLDPE polymer blends (peak at higher temperature of blue line) is significantly lower than that of pure LLDPE (lower black line). However, the opposite behaviour occurs for C8-POE+LLDPE+n-MDH composites (peak at higher temperature of red line), with a TC higher than that of pure LLDPE (lower black line). This means that the nucleating effect of mineral particles of n-MDH (that is TC increasing) is more than enough to compensate the “dilution” effect of amorphous C8-POE (that is TC reducing, as demonstrated by that measured for C8-POE+LLDPE polymer blends). Furthermore, in all formulations (C8-POE+LLDPE+n-MDH composites and C8-POE+LLDPE blends) the crystallinity enthalpies of both C8-POE and LLDPE fractions are lower than that of the pure polymer.
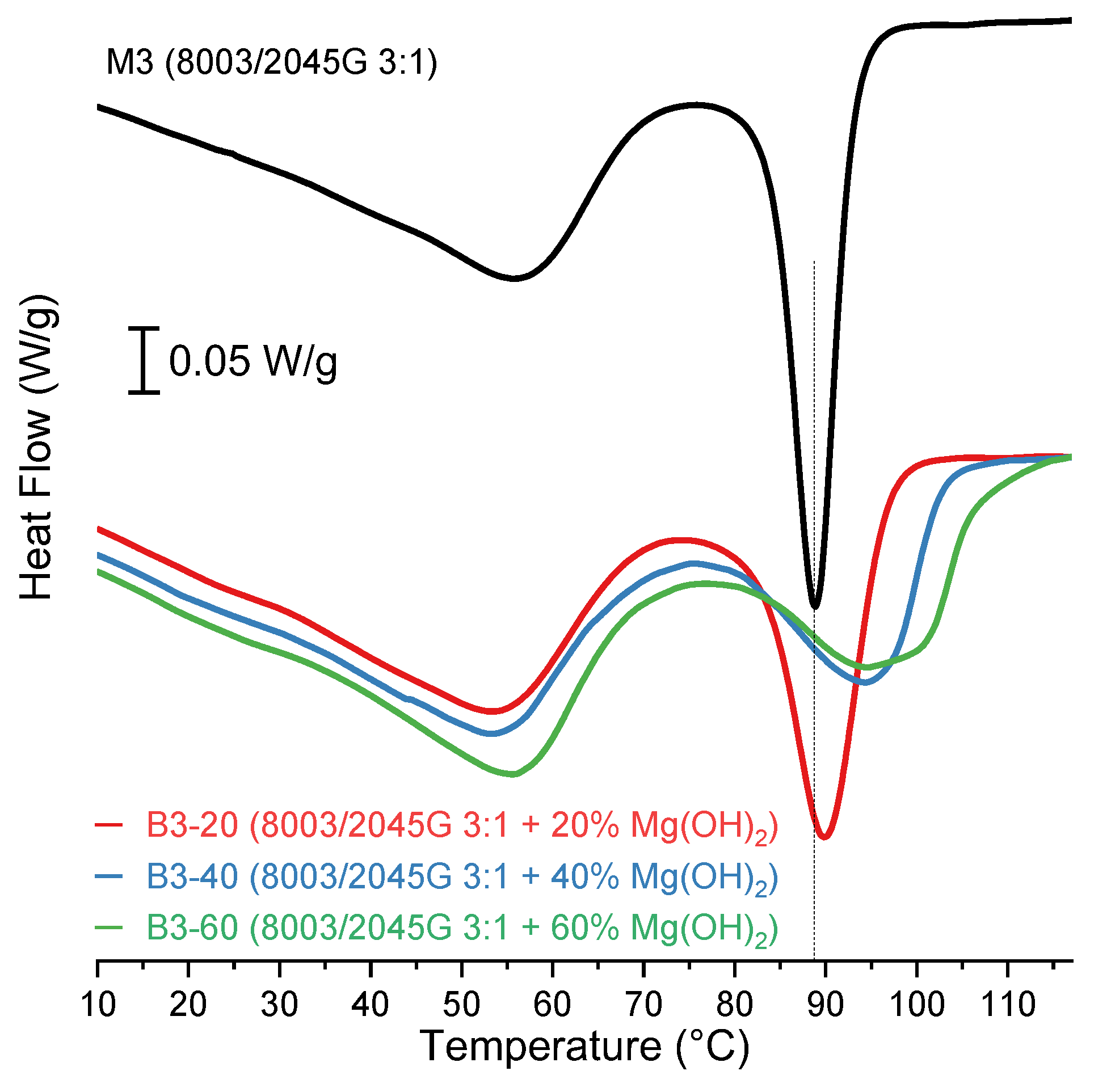
3.3. Influence of n-MDH Content on Composites TC
Two new formulations, reported in
Table 9, were produced and analysed by DSC. The values of T
C and crystallization enthalpy are shown in
Table 10.
The cooling thermograms of the B3-60, B3-20, and B3-40 formulations were reported in
Figure 4.
It can be observed that the T
C of the LLDPE fraction increases in agreement with the quantity of filler (T
C (B3-60) > T
C (B3-40) > T
C (B3-20)). Also, the broadening of the crystallization peak of LLDPE fraction is apparent as the filler fraction increases. This phenomenon may be ascribed to different heterogeneous nucleation mechanisms [
24] leading to the formation of LLDPE crystals of different sizes, and, as already noted, possibly addressed to the effective interaction between the polymeric matrix and the mineral provided by the LLDPE-g-MAH coupling agent. On the other hand, with increasing mineral content, there is no significant effect on the T
C of C
8-POE fraction and on the crystallization enthalpy of C
8-POE and LLDPE fractions.
3.4. Tensile Properties of C8-POE+LLDPE+n-MDH Composites
Depending on the degree of crystallinity, polymers have different mechanical properties. In the C8-POE+LLDPE+n-MDH composites, the content of mineral particles of n-MDH and the presence of the maleated LLDPE-g-MAH coupling agent influence the final mechanical behaviour.
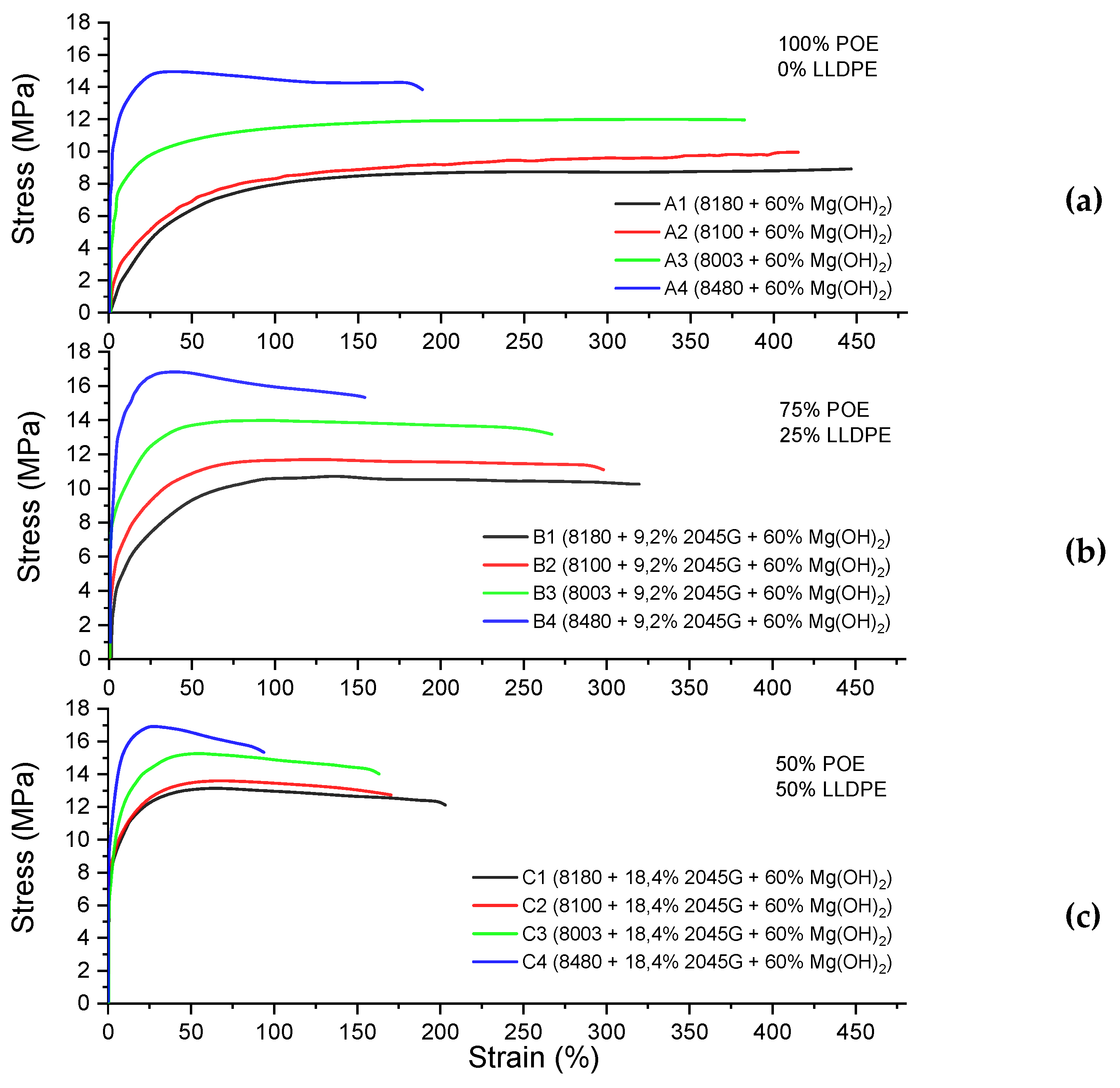
The C8-POE/LLDPE/n-MDH composites were tested via stress–strain experiments and the curves and the results values are shown in
Figure 5 and
Table 11, respectively.
As expected, the composites without LLDPE and based only on C
8-POE (A1, A2, A3, A4) show higher elongation at break in comparison to composites containing LLDPE, thanks to more amorphous polymeric matrix. For the same reason, by comparing the A1 vs A2 vs A3 vs A4 composites, we observe a clear increase in tensile properties and a decrease in elongation [
26]. As expected, the introduction of LLDPE leads to a decrease in E@B and an increase in tensile properties due to the higher crystallinity of the composite.
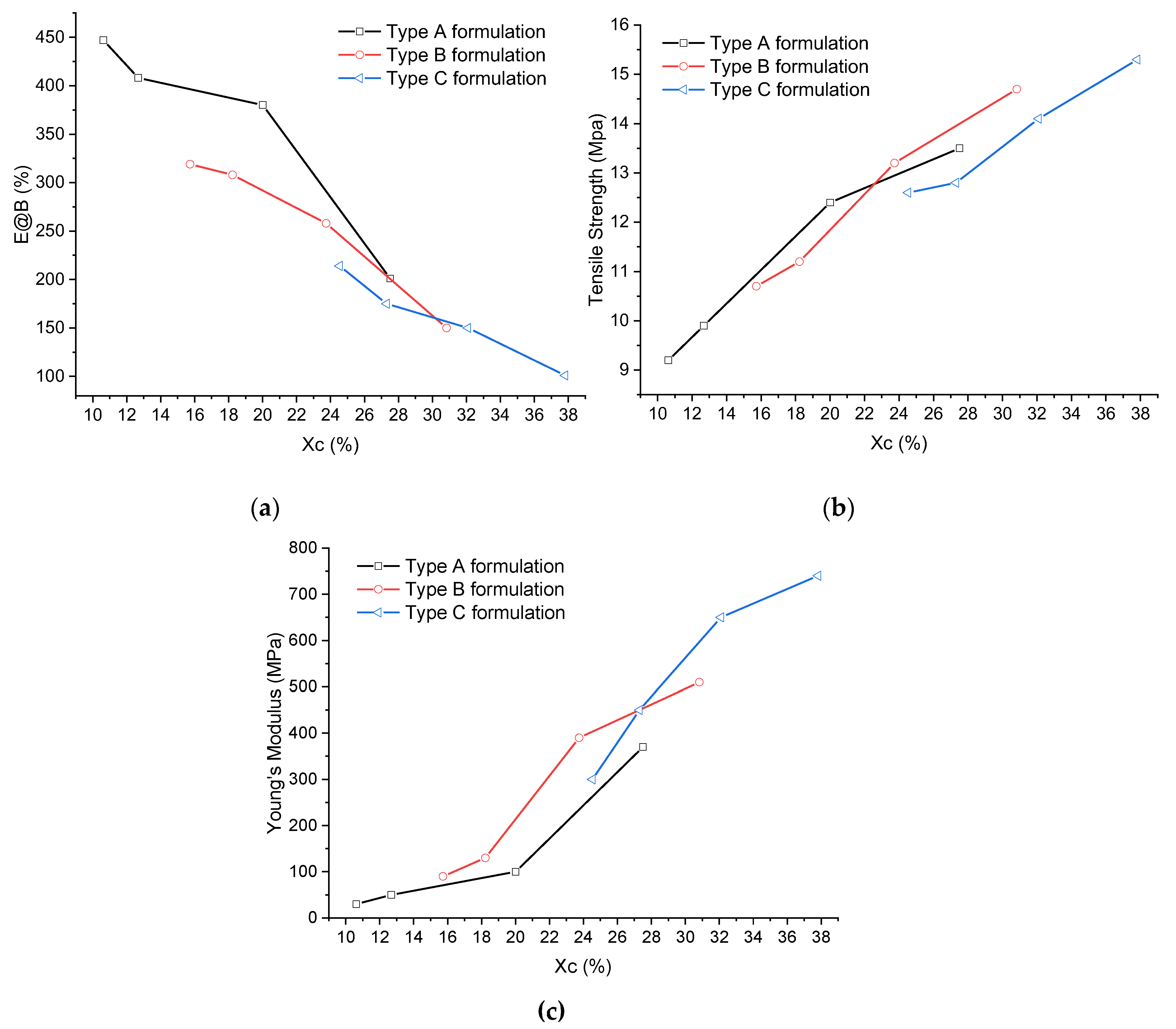
Figure 6 shows the mechanical properties of all the formulations as a function of the crystallinity.
An almost linear trend with increasing crystallinity can be observed for all the investigated mechanical properties. Notably, there is an increase in tensile strength and modulus and a decrease in elongation at break. The desired mechanical properties can be achieved by controlling the total crystallinity of the polymer matrix. This is independent from the method employed to achieve crystallinity: it is possible to combine the more crystalline POE with a smaller amount of LLDPE, or the more amorphous POE with a larger amount of LLDPE.
4. Conclusions
The introduction of n-MDH provided a strong nucleating effect, i.e. accelerating the crystallization process of the polyolefin matrices investigated in this work. For POE/n-MDH composites, it was observed that the lower the comonomer (1-octene) content in POE, the greater the increase in TC of C8-POE. Between the two opposite effects of C8-POE (reduction of TC) and n-MDH (increase of TC) on TC of LLDPE into C8-POE+LLDPE+n-MDH composites, the nucleating effect provided by the inorganic particles was predominant. Moreover, the LLDPE TC was also influenced by the amount of n-MDH since the lower the amount of n-MDH, the lower the measured TC. In terms of crystallization enthalpy, the blending of polymers and the introduction of n-MDH reduced the crystallization of pure polymers. As expected, the higher the crystallinity of the polymer matrix, the higher the tensile strength, and the lower the elongation at break.
Author Contributions
Conceptualization, V.M.; formal analysis, V.M. and M.M.; investigation, V.M.; data curation, S.H.; writing—original draft preparation, V.M.; writing—review and editing, A.P.; supervision, C.C.; project administration, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dara sharing not applicable.
Acknowledgments
Europiren B.V., and Dow Europe are kindly acknowledged for supplying the raw materials Ecopiren® and ENGAGE®, respectively, and related information.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hu, Y.; Qian, X.; Song, L.; Lu, H. Polymer/Layered compound nanocomposites: A way to improve fire safety of polymeric materials. Fire Saf. Sci. 2014, 11, 66–82. [Google Scholar] [CrossRef]
- PINFA. Flame Retardants in Electric and Electronic. 2017. https://www.pinfa.eu/wp-content/uploads/2018/05/PINFA_EE_brochure_Edition_2017-11.pdf.
- Haveriku, S.; Meucci, M.; Badalassi, M.; Cardelli, C.; Pucci, A. Rheological and Aesthetical Properties of Polyolefin Composites for Flame Retardant Cables with High Loading of Mineral Fillers. Micro 2022, 2, 524–540. [Google Scholar] [CrossRef]
- Gupta, V.; Jain, D. Optimization of Halogen Free Flame Retardant Wire and Cable Compounds. 2007.
- Haveriku, S.; Meucci, M.; Badalassi, M.; Cardelli, C.; Ruggeri, G.; Pucci, A. Optimization of the Mechanical Properties of Polyolefin Composites Loaded with Mineral Fillers for Flame Retardant Cables. Micro 2021, 1, 102–119. [Google Scholar] [CrossRef]
- Prabhakar, M.N.; Shah, A.U.R.; Song, J.-I. A Review on the Flammability and Flame Retardant Properties of Natural Fibers and Polymer Matrix Based Composites. Compos. Res. 2015, 28, 29–39. [Google Scholar] [CrossRef]
- Hornsby, P.R. Fire retardant fillers for polymers. Int. Mater. Rev. 2001, 46, 199–210. [Google Scholar] [CrossRef]
- Rothon, R.N.; Hornsby, P.R. Flame retardant effects of magnesium hydroxide. Polym. Degrad. Stab. 1996, 54(2-3 SPEC. ISS. 383–385. [CrossRef]
- Bamford, D.; Dlubek, G.; Lüpke, T.; Kilburn, D.; Stejnv, J.; Menke, T.J.; Alam, M.A. Free volume, glass transition and degree of branching in ethylene/α-olefin copolymers: Positron lifetime, differential scanning calorimetry, wide-angle X-ray scattering, and density studies. Macromol. Chem. Phys. 2006, 207, 492–502. [Google Scholar] [CrossRef]
- Quijada, R.; Dupont, J.; Miranda, M.S. L.; Scipioni, R.B.; Galland, G.B. Copolymerization of ethylene with 1-hexene and 1-octene: correlation between type of catalyst and comonomer incorporated. Macromol. Chem. Phys. 1995, 196, 3991–4000. [Google Scholar] [CrossRef]
- Simanke, A.G.; Galland, G.B.; Freitas, L.; Da Jornada, J.A. H.; Quijada, R.; Mauler, R.S. Influence of the comonomer content on the thermal and dynamic mechanical properties of metallocene ethylene/1-octene copolymers. Polymer (Guildf). 1999, 40, 5489–5495. [Google Scholar] [CrossRef]
- Dow chemical, Dow Wire and Cable. Cable Formulations ENGAGE Polyolefin Elastomers (POE) in Halogen Free Flame Retardant Cable Formulations.
- Camino, G.; Maffezzoli, A.; Braglia, M.; De Lazzaro, M.; Zammarano, M. Effect of hydroxides and hydroxycarbonate structure on fire retardant effectiveness and mechanical properties in ethylene-vinyl acetate copolymer. Polym. Degrad. Stab. 2001, 74, 457–464. [Google Scholar] [CrossRef]
- McGenity, P.M.; Hooper, J.J.; Paynter, C.D.; Riley, A.M.; Nutbeem, C.; Elton, N.J.; Adams, J.M. Nucleation and crystallization of polypropylene by mineral fillers: relationship to impact strength. Polymer 1992, 33, 5215–5224. [Google Scholar] [CrossRef]
- Rothon R., N. Particulate-Filled Polymer Composites Second Edition. Technology, 2003, 554.
- Batistini, A. New polyolefin plastomers and elastomers made with insiteTM technology: Structure - property relationship and benefits in flexible thermoplastic applications. Macromol. Symp. 1995, 100, 137–142. [Google Scholar] [CrossRef]
- Chum, P.S.; Swogger, K.W. Olefin polymer technologies-History and recent progress at The Dow Chemical Company. Prog. Polym. Sci. 2008, 33, 797–819. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Truss, R.W.; Laycock, B.; Weir, M.P.; Nicholson, T.M.; Garvey, C.J.; Halley, P.J. The effect of comonomer concentration and distribution on the photo-oxidative degradation of linear low density polyethylene films. Polymer (Guildf) 2017, 119, 66–75. [Google Scholar] [CrossRef]
- Kalantar Mehrjerdi, A.; Åkesson, D.; Skrifvars, M. Influence of talc fillers on bimodal polyethylene composites for ground heat exchangers. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- ISO 37:2017, INTERNATIONAL STANDARD tensile stress-strain properties. 2017.
- Li, J.; Shanks, R.A.; Olley, R.H.; Greenway, G.R. Miscibility and isothermal crystallisation of polypropylene in polyethylene melts. Polymer (Guildf). 2001, 42, 7685–7694. [Google Scholar] [CrossRef]
- Dikobe, D.G.; Luyt, A.S. Comparative study of the morphology and properties of PP/LLDPE/wood powder and MAPP/ LLDPE/wood powder polymer blend composites. Express Polym. Lett. 2010, 4, 729–741. [Google Scholar] [CrossRef]
- Meucci, M.; Haveriku, S.; Badalassi, M.; Cardelli, C.; Ruggeri, G.; Pucci, A. Effect of Polyolefin Elastomers’ Characteristics and Natural Magnesium Hydroxide Content on the Properties of Halogen-Free Flame-Retardant Polyolefin Composites. 2022, 2, 164–182. [CrossRef]
- Karrad, S.; Lopez Cuesta, J.M.; Crespy, A. Influence of a fine talc on the properties of composites with high density polyethylene and polyethylene/polystyrene blends. J. Mater. Sci. 1998, 33, 453–461. [Google Scholar] [CrossRef]
- Luyt, A.S.; Malik, S.S.; Gasmi, S.A.; Porfyris, A.; Andronopoulou, A.; Korres, D.; Vouyiouka, S.; Grosshauser, M.; Pfaendner, R.; Brüll, R. Halogen-free flame-retardant compounds. Thermal decomposition and flammability behavior for alternative polyethylene grades. Polymers (Basel). 2019, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- Li Pi Shan, C.; Soares, J.B. P.; Penlidis, A. Mechanical properties of ethylene/1-hexene copolymers with tailored short chain branching distributions. Polymer (Guildf). 2001, 43, 767–773. [Google Scholar] [CrossRef]
Figure 1.
DSC first cooling traces: curves a. (green line) and c. (red line) represent the pure polymers, curve b. (blue line) is the POE/LLDPE blend with 3:1 ratio, whereas the black dashed curve is purely theoretical, and it is calculated using Origin software as a linear combination by multiplying each point of the curve of pure polymers (a. and c.) by its weight fraction and then summing.
Figure 1.
DSC first cooling traces: curves a. (green line) and c. (red line) represent the pure polymers, curve b. (blue line) is the POE/LLDPE blend with 3:1 ratio, whereas the black dashed curve is purely theoretical, and it is calculated using Origin software as a linear combination by multiplying each point of the curve of pure polymers (a. and c.) by its weight fraction and then summing.
Figure 2.
Zoom of cooling scans of pure C
8-POE (black line) and Engage/60% MDH composites (red line, type “A” formulations as in
Table 5): (a) Engage 8180, (b) Engage 8100, (c) Engage 8003 and (d) Engage 8480.
Figure 2.
Zoom of cooling scans of pure C
8-POE (black line) and Engage/60% MDH composites (red line, type “A” formulations as in
Table 5): (a) Engage 8180, (b) Engage 8100, (c) Engage 8003 and (d) Engage 8480.
Figure 3.
DSC cooling scans of pure polymer (black line), C
8-POE/LLDPE blends 3/1 ratio (blue line), and C
8-POE/LLDPE/60% MDH compounds (red line), type “B” formulations as in
Table 5: (a) Engage 8180, (b) Engage 8100, (c) Engage 8003 and (d) Engage 8480.
Figure 3.
DSC cooling scans of pure polymer (black line), C
8-POE/LLDPE blends 3/1 ratio (blue line), and C
8-POE/LLDPE/60% MDH compounds (red line), type “B” formulations as in
Table 5: (a) Engage 8180, (b) Engage 8100, (c) Engage 8003 and (d) Engage 8480.
Figure 4.
Effect of MDH content on the crystallization temperature of the prepared formulations.
Figure 4.
Effect of MDH content on the crystallization temperature of the prepared formulations.
Figure 5.
Stress-strain curve of (a) type “A” formulation, (b) type “B” formulation, and (c) type “C” formulation.
Figure 5.
Stress-strain curve of (a) type “A” formulation, (b) type “B” formulation, and (c) type “C” formulation.
Figure 6.
Trend of mechanical properties as a function of crystallinity: (a) elongation at break, (b) tensile strength and (c) Young’s modulus.
Figure 6.
Trend of mechanical properties as a function of crystallinity: (a) elongation at break, (b) tensile strength and (c) Young’s modulus.
Table 1.
Characteristics of C8-POE samples.
Table 1.
Characteristics of C8-POE samples.
| |
Comonomer content
(wt.%) 1
|
Density
(g/cm³) 2
|
XC
(%) |
Tm
(°C) 3
|
MFI
(g/10min) 4 |
| Engage 8180 |
37.6 |
0.863 |
15.1 |
47.0 |
0.5 |
| Engage 8100 |
34.2 |
0.870 |
16.9 |
51.3 |
1.0 |
| Engage 8003 |
25.1 |
0.885 |
24.5 |
71.1 |
1.0 |
| Engage 8480 |
16.8 |
0.902 |
31.3 |
93.1 |
1.0 |
| Dowlex 2045G |
2.7 [18] |
0.920 |
45.5 |
114.8 |
1.0 |
Table 2.
Blends of C8-POE and LLDPE in 3/1 ratio with each Engage™.
Table 2.
Blends of C8-POE and LLDPE in 3/1 ratio with each Engage™.
| |
M1 |
M2 |
M3 |
M4 |
| Engage 8180 |
75 |
|
|
|
| Engage 8100 |
|
75 |
|
|
| Engage 8003 |
|
|
75 |
|
| Engage 8480 |
|
|
|
75 |
| Dowlex 2045G |
25 |
25 |
25 |
25 |
Table 3.
Temperature of crystallization peaks of C8-POE/LLDPE blends.
Table 3.
Temperature of crystallization peaks of C8-POE/LLDPE blends.
| Base polymer |
Blend |
TC C8-POE
(°C) |
ΔTPOE
(°C) |
TC LLDPE
(°C) |
ΔTLLDPE
(°C) |
| Engage 8180 |
M1 |
26.7 |
+0.8 |
88.0 |
-7.1 |
| Pure Polymer = calculated M1 |
25.9 |
95.1 |
| Engage 8100 |
M2 |
33.5 |
+1.4 |
87.8 |
-7.3 |
| Pure Polymer = calculated M1 |
32.1 |
95.1 |
| Engage 8003 |
M3 |
55.8 |
+6.6 |
88.4 |
-6.7 |
| Pure Polymer = calculated M1 |
49.2 |
95.1 |
| Engage 8480 |
M4 |
77.6 |
+7.6 |
88.0 |
-7.1 |
| Pure Polymer = calculated M1 |
70.0 |
95.1 |
Table 4.
Enthalpy of the crystallization peaks of C8-POE/LLDPE blends.
Table 4.
Enthalpy of the crystallization peaks of C8-POE/LLDPE blends.
| Base polymer |
Blend |
ΔHC C8-POE
(J/g) |
ΔHM -ΔCALC
POE
(J/g) |
ΔHC LLDPE
(J/g) |
ΔHM -ΔHCALC
LLDPE
(J/g) |
| Engage 8180 |
M1 |
24.1 |
-4.8 |
24.1 |
-4.7 |
| calculated M1 |
28.9 |
28.8 |
| Engage 8100 |
M2 |
30.4 |
-2.2 |
25.9 |
-2.9 |
| calculated M2 |
32.6 |
28.8 |
| Engage 8003 |
M3 |
48.9 |
-3.6 |
22.5 |
-6.3 |
| calculated M3 |
52.5 |
28.8 |
| Engage 8480 |
M4 |
68.3 |
-3.8 |
22.6 |
-6.2 |
| calculated M4 |
72.0 |
28.8 |
Table 5.
Compounds based on neat C8-POE (series A), C8-POE/LLDPE mix 3/1 ratio (series B), and C8-POE/LLDPE mix 1/1 ratio (series C), with the relative characteristics (density and MFI).
Table 5.
Compounds based on neat C8-POE (series A), C8-POE/LLDPE mix 3/1 ratio (series B), and C8-POE/LLDPE mix 1/1 ratio (series C), with the relative characteristics (density and MFI).
| |
A1
(%) |
A2
(%) |
A3
(%) |
A4
(%) |
B1
(%) |
B2
(%) |
B3
(%) |
B4
(%) |
C1
(%) |
C2
(%) |
C3
(%) |
C4
(%) |
| Engage 8180 |
36.8 |
|
|
|
27.6 |
|
|
|
18.4 |
|
|
|
| Engage 8100 |
|
36.8 |
|
|
|
27.6 |
|
|
|
18.4 |
|
|
| Engage 8003 |
|
|
36.8 |
|
|
|
27.6 |
|
|
|
18.4 |
|
| Engage 8480 |
|
|
|
36.8 |
|
|
|
27.6 |
|
|
|
18.4 |
| Dowlex 2045G |
- |
- |
- |
- |
9.2 |
9.2 |
9.2 |
9.2 |
18.4 |
18.4 |
18.4 |
18.4 |
| Fusabond N525 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
| Ecopiren 3.5 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
| Irganox 1010 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
| Properties |
|
|
|
|
|
|
|
|
|
|
|
|
| Density (g/cm3) |
1.402 |
1.404 |
1.414 |
1.435 |
1.413 |
1.414 |
1.424 |
1.436 |
1.419 |
1.426 |
1.433 |
1.443 |
| MFI (g/10min) 1
|
2.7 |
4.1 |
3.7 |
4.3 |
2.8 |
4.1 |
3.4 |
4.8 |
2.7 |
3.7 |
3.5 |
4.1 |
Table 6.
TC and ΔHC of “A” formulations and pure polymers and crystallinity (XC) of “A” formulations.
Table 6.
TC and ΔHC of “A” formulations and pure polymers and crystallinity (XC) of “A” formulations.
| Properties |
Engage 8180 |
A1 |
Engage 8100 |
A2 |
Engage 8003 |
A3 |
Engage 8480 |
A4 |
| TC (°C) |
26.0 |
28.1 |
32.1 |
34.7 |
50.0 |
58.3 |
69.9 |
78.7 |
| ΔT (°C) |
+2.1 |
+2.6 |
+8.3 |
+8.8 |
| ΔH (J/g) 1
|
38.6 |
32.3 |
43.4 |
38.3 |
70.0 |
59.7 |
96.1 |
81.6 |
| ΔHA-ΔHPOE
|
-6.3 |
-5.1 |
-10.3 |
-14.5 |
| XC (%) |
|
10.6 |
|
12.7 |
|
20.0 |
27.5 |
Table 7.
Crystallisation temperature of C8-POEs, LLDPE, C8-POE+LLDPE blends, and C8-POE+LLDPE+n-MDH composites.
Table 7.
Crystallisation temperature of C8-POEs, LLDPE, C8-POE+LLDPE blends, and C8-POE+LLDPE+n-MDH composites.
| Properties |
Engage
8180 |
M1 |
B1 |
Engage 8100 |
M2 |
B2 |
Engage
8003 |
M3 |
B3 |
Engage
8480 |
M4 |
B4 |
LLDPE |
| TC (POE) (°C) |
25.9 |
26.7 |
27.0 |
32.1 |
33.5 |
34.0 |
49.2 |
55.8 |
55.9 |
70.0 |
77.6 |
79.8 |
- |
| TM/B-TPOE
|
- |
+0.8 |
+1.1 |
- |
+1.4 |
+1.9 |
- |
+6.6 |
+6.7 |
- |
+7.6 |
+9.8 |
- |
| TC (LLDPE) (°C) |
- |
88.0 |
97.0 |
- |
87.8 |
96.9 |
- |
88.4 |
97.1 |
- |
88.0 |
98.7 |
95.1 |
| TM/B-TLLDPE
|
- |
-7.1 |
+1.9 |
- |
-7.3 |
+1.8 |
- |
-6.7 |
+2.0 |
- |
-7.1 |
+3.6 |
- |
Table 8.
Crystallisation enthalpy of C8-POEs, LLDPE, C8-POE+LLDPE blends, and C8-POE+LLDPE+n-MDH composites and crystallinity (XC) of “B” formulations.
Table 8.
Crystallisation enthalpy of C8-POEs, LLDPE, C8-POE+LLDPE blends, and C8-POE+LLDPE+n-MDH composites and crystallinity (XC) of “B” formulations.
| Properties |
Engage
8180 |
M1 |
B1 |
Engage 8100 |
M2 |
B2 |
Engage
8003 |
M3 |
B3 |
Engage
8480 |
M4 |
B4 |
LLDPE |
| ΔHC (POE) (J/g) |
28.9 |
24.1 |
23.4 |
32.6 |
30.4 |
29.7 |
52.5 |
48.9 |
48.6 |
72.0 |
68.3 |
68.9 |
- |
| ΔHM/B-ΔHPOE
|
- |
-4.8 |
-5.5 |
- |
-2.2 |
-2.9 |
- |
-3.6 |
-3.9 |
- |
-3.8 |
-3.1 |
- |
| ΔHC (LLDPE) (J/g) |
- |
24.1 |
23.7 |
- |
25.9 |
24.8 |
- |
22.5 |
22.0 |
- |
22.6 |
22.4 |
28.8 |
| ΔHM/B-ΔHLLDPE
|
- |
-4.7 |
-5.1 |
- |
-2.9 |
-4.0 |
- |
-6.3 |
-6.8 |
- |
-6.2 |
-6.4 |
- |
| XC (%) |
|
|
15.8 |
|
|
18.2 |
|
|
23.7 |
|
|
30.8 |
|
Table 9.
Formulations of C8-POE+LLDPE+n-MDH composites with C8-POE:LLDPE=3:1 and different n-MDH contents.
Table 9.
Formulations of C8-POE+LLDPE+n-MDH composites with C8-POE:LLDPE=3:1 and different n-MDH contents.
| |
B3-20
(%) |
B3-40
(%) |
B3-60
(%) |
M3
(%) |
| Engage 8003 |
57.6 |
42.6 |
36.8 |
75 |
| Dowlex 2045G |
19.2 |
14.2 |
9.2 |
25 |
| Fusabond N525 |
3 |
3 |
3 |
- |
| Ecopiren 3.5 |
20 |
40 |
60 |
- |
| Irganox 1010 |
0.2 |
0.2 |
0.2 |
- |
Table 10.
Crystallisation temperature and enthalpy of Engage 8003+LLDPE blends and Engage 8003+LLDPE+n-MDH composites with different MDH content (20 %, 40 %, and 60 %).
Table 10.
Crystallisation temperature and enthalpy of Engage 8003+LLDPE blends and Engage 8003+LLDPE+n-MDH composites with different MDH content (20 %, 40 %, and 60 %).
| Properties |
|
B3-20
(%) |
B3-40
(%) |
B3-60
(%) |
M3
(%) |
| TC (°C) |
POE
fraction |
54.2 |
53.9 |
55.9 |
55.8 |
| TB-TM
|
-1.6 |
-1.9 |
+0.1 |
- |
| ΔHC (J/g) |
46.2 |
47.1 |
48.6 |
48.9 |
| ΔHB-ΔHM
|
-2.7 |
-1.8 |
-0.3 |
- |
| TC (°C) |
LLDPE
fraction |
89.8 |
94.3 |
97.1 |
88.4 |
| TB-TM
|
+1.4 |
+5.9 |
+8.7 |
- |
| ΔHC (J/g) |
20.2 |
19.8 |
22.0 |
22.5 |
| ΔHB-ΔHM
|
-2.3 |
-2.7 |
-0.5 |
- |
Table 11.
Physical properties (DSC Crystallinity, Tensile Strength (TS), Elongation at break (E@B), Young’s modulus) of the C8-POE+LLDPE+n-MDH composites.
Table 11.
Physical properties (DSC Crystallinity, Tensile Strength (TS), Elongation at break (E@B), Young’s modulus) of the C8-POE+LLDPE+n-MDH composites.
| Base polymers |
Composite |
XC
(%) |
Tensile Strength
(MPa) |
Elong. @break
(%) |
Young’s mod.
(MPa) |
| Engage 8180 |
A1 (only C8-POE) |
10.6 |
9.2 ± 0.3 |
447 ± 36 |
30 |
| B1 (C8-POE:LLDPE 3:1) |
15.8 |
10.7 ± 0.5 |
319 ± 15 |
90 |
| C1 (C8-POE:LLDPE 1:1) |
24.5 |
12.6 ± 0.3 |
214 ± 29 |
300 |
| Engage 8100 |
A2 (only C8-POE) |
12.7 |
9.9 ± 0.1 |
408 ± 16 |
50 |
| B2 (C8-POE:LLDPE 3:1) |
18.2 |
11.2 ± 0.5 |
308 ± 26 |
130 |
| C2 (C8-POE:LLDPE 1:1) |
27.3 |
12.8 ± 0.2 |
175 ± 7 |
450 |
| Engage 8003 |
A3 (only C8-POE) |
20.2 |
12.4 ± 0.6 |
380 ± 43 |
100 |
| B3 (C8-POE:LLDPE 3:1) |
23.7 |
13.2 ± 0.3 |
258 ± 26 |
390 |
| C3 (C8-POE:LLDPE 1:1) |
32.1 |
14.1 ± 0.4 |
150 ± 35 |
650 |
| Engage 8480 |
A4 (only C8-POE) |
26.3 |
13.5 ± 0.3 |
201 ± 31 |
370 |
| B4 (C8-POE:LLDPE 3:1) |
30.8 |
14.7 ± 0.3 |
150 ± 28 |
510 |
| C4 (C8-POE:LLDPE 1:1) |
37.8 |
15.3 ± 0.4 |
101 ± 22 |
740 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).