1. Introduction
The global prevalence of obesity has increased incidences of related comorbidities, such as osteoarthritis, cardiovascular diseases and cancer[
1,
2]. The imbalance of Firmicutes-to-Bacteroidetes ratio (F/B) is regarded as a significant characteristic in obese patients[
3,
4]. While fecal microbial transplantation from obese individuals can induce adiposity phenotypes in mice[
5], suggesting that microbial dysbiosis plays a crucial role in obesity. In a cohort study, the supplementation of
Bifidobacterium and
Lactobacillus at 50 billion CFU/day for 6 months resulted in significant decreases in body weight, serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels[
6]. In addition,
Limosilactobacillus reuteri GMNL263 exhibited the ability to inhibit weight gain and reduce insulin resistance in obese rats induced by high-fat diet (HFD)[
7,
8]. Similarly,
Lactiplantibacillus plantarum Y44 not only alleviated lipid metabolism disorder by decreasing TC and LDL-C levels, but also reduced serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in obese mice[
9]. Thus, probiotic interventions have proven to be an effective strategy in alleviating obesity.
The anti-obesity effect of probiotics are attributed to specific metabolites or enzymes, such as bile salt hydrolase (BSH), short-chain fatty acids (SCFAs), and conjugated linoleic acid (CLA) isomerase[
10,
11,
12,
13]. Recent studies have highlighted the potential role of BSH in regulating lipid metabolism and ameliorating obesity-related syndrome.
Lp. plantarum H-87 with a distinguished BSH activity exhibited a significant inhibitory effect on weight gain and lipid accumulation in HFD-fed mice[
14]. Furthermore, the recombinant
Escherichia coli MG1655 with
bsh1 from
Lactobacillus salivarius JCM1046 significantly reduced weight gain, LDL-C and triglyceride (TG) levels in obese mice[
15].
Bile acids (BAs), synthesized in the liver, are stored within the gallbladder and released into the gastrointestinal tract following food ingestion[
16]. BSH deconjugated tauro-conjugated or glycol-conjugated BAs in the small intestine, generating their corresponding free bile acids[
17]. The reduced solubility of these free bile acids increases bile excretion, promoting the conversion of cholesterol to BAs. This effect contributes to the reduction of cholesterol levels and lipid absorption in the small intestine[
18,
19,
20]. Due to this gateway efficacy, primary BAs can be further transformed into secondary bile acids (sBAs) by cecal and colonic microbiota[
21,
22]. Certain sBAs serve as ligands for multiple BA receptors, including the farnesoid X receptor (FXR) and Takeda G protein-coupled receptor TGR5[
23]. Activation of FXR by deoxycholic acid (DCA) inhibits SREBP1c production, thereby reducing triglyceride synthesis and ameliorating hepatic steatosis[
24,
25,
26]. As potent TGR5 agonists, lithocholic acid (LCA) and deoxycholic acid (DCA) stimulate intestinal GLP-1 secretion, thereby enhancing insulin sensitivity and glucose homeostasis in obese individuals[
24].
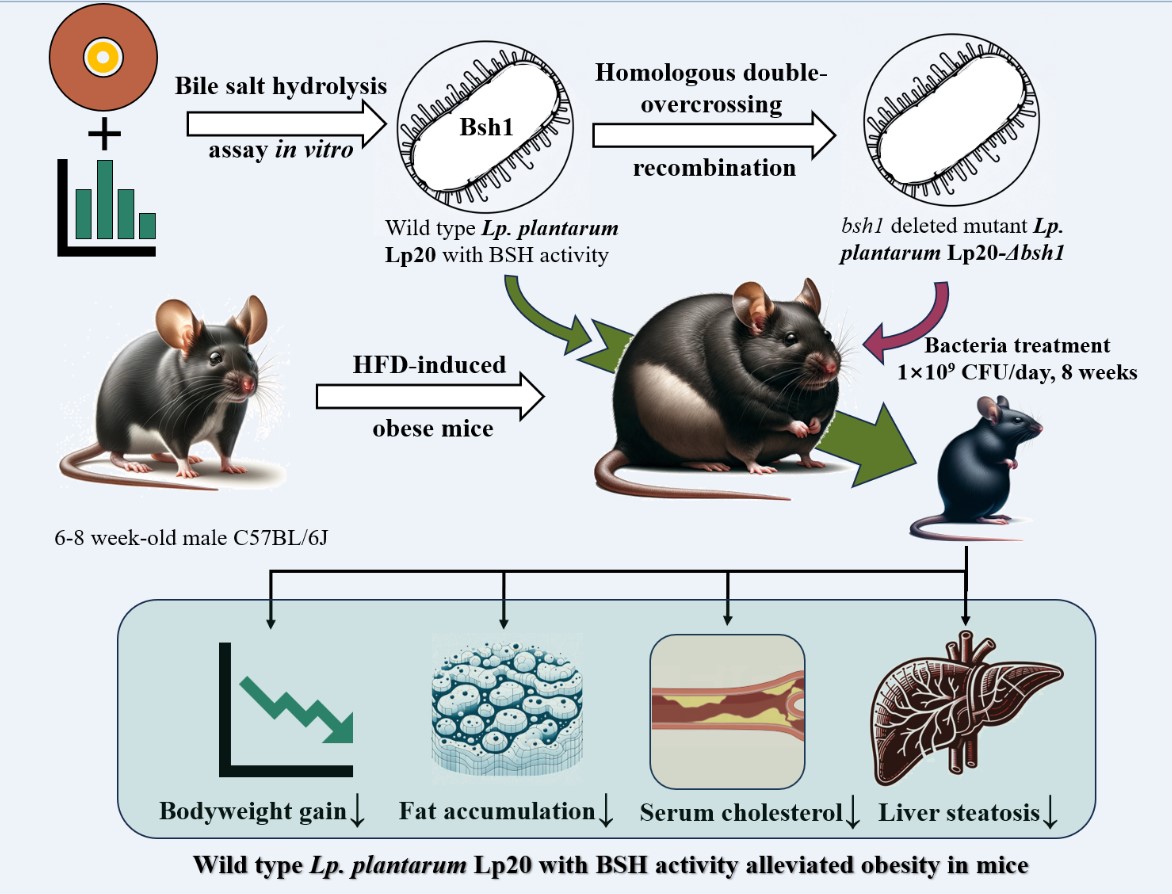
In this study, Lp. plantarum Lp20, exhibiting the highest BSH activity, was selected from a pool of 23 BSH-positive Lp. plantarum strains. Genomic sequencing and bioinformatics analysis indicated that gene bsh1 was associated with BSH activity of Lp. plantarum Lp20. Subsequently, a bsh1 mutant strain of Lp. plantarum Lp20 was constructed by homologous double-overcrossing recombination. The bsh1 mutant strain was used as the negative control for Lp. plantarum Lp20 to investigate the correlation between probiotic BSH activity and its efficacy in reducing fat in the high fat diet-induced obese mice.
2. Materials and Methods
2.1. Bacterial Strains and Culture Condition
The bacterial strains and plasmids used in this study are listed in
Table S1.
Lp. plantarum were cultured at 37°C in de Man, Rogosa, and Sharpe (MRS) medium.
Escherichia coli DH5α was grown at 37 °C in Luria−Bertani (LB) broth for 12 h with shaking at 220 rpm.
Lactococcus lactis NZ9000 was cultured at 30°C in M17 medium (Oxoid, Unipath, Basingstoke, UK) containing 0.5% wt/vol glucose (GM17) for 14 h. When required, the media were supplemented with the appropriate antibiotics at the following concentrations: 100 µg/mL ampicillin for
E. coli, 10 µg/mL chloramphenicol for
L. lactis and 10 µg/mL erythromycin for
Lp.
plantarum.
2.2. BSH Activity Assays of Lp. Plantarum
For the qualitative assay of BSH activity, the bile salt plate method was employed in this study[
27,
28]. Briefly, MRS agar plate was supplemented with 0.1% wt/vol glycodeoxycholic acid (GDCA, Sigma Aldrich) and 0.1% wt/vol taurodeoxycholic acid (TDCA, Sigma Aldrich), respectively. Bacterial cells were streaked onto these plates and incubated anaerobically for 48 h to observe deoxycholic acid precipitated in the agar medium below and around a colony (precipitation halo).
BSH activity was also analyzed quantitatively by the ninhydrin assay, which was a colorimetric test to determine the amount of amino acids released from individual bile acids through BSH deconjugation[
29,
30,
31]. Bacterial cells in the stationary phase were collected by centrifugation, washed with sodium phosphate buffer, and resuspended to OD
600 nm=5.0. A 50 μL cell suspension was mixed with an equal volume of 20 mM bile salt substrate and incubated at 37°C for 1 h. The reaction was terminated by the addition of 100 μL trichloroacetic acid. Then the mixture was centrifuged to collect 100 μL supernatant, which was then mixed with 900 μL ninhydrin. The reaction system was carried out in boiling water bath for 14 min and immediately transferred to an ice bath for color stabilization. Based on a standard curve, the OD at 570 nm was measured to determine the amino acid concentration. The bile salt hydrolysis rate was calculated using the following formula:
5 stands for the initial concentration of bile salt substrates in the reaction system is 5mM, c is the concentration of amino acids after substrate hydrolysis.
2.3. Draft Genome Sequencing and Comparative Analysis
The genome of Lp20 was extracted using the Bacterial Genome Extraction Kit (Tiangen, Beijing, China). Purified genome was sequenced and assembled by Beijing Novogene Bioinformatics Technology Co., Ltd. Genomic DNA samples were fragmented using the Covaris ultrasonic disruptor, followed by the construction of a 350 bp short fragment library. Sequencing was performed on the Illumina NovaSeq PE150 platform. The raw data were processed using genome assembly tools including SOAP denovo, SPAdes and ABySS, and then was integrated with CISA. The assembly was further refined with GapCloser, filtering out fragments below 500 bp, resulting in a draft genomic scaffold sequence. GeneMarkS 4.17 was employed for the prediction of coding genes. Subsequently, the protein sequences of structural genes were functionally annotated through Diamond alignment against universal databases including GO (Gene Ontology), KEGG (Kyoto Encyclopedia of Genes and Genomes), COG (Clusters of Orthologous Groups), NR (Non-Redundant Protein Database), Pfam, TCDB (Transporter Classification Database), and Swiss-Prot[
32,
33,
34,
35,
36,
37,
38,
39]. The alignment results with the highest score (minimum identity ≥ 40%, coverage ≥ 40%) were selected for annotation.
Genome comparisons were conducted using BLAST. Circular genome maps were generated using the BRIG JAVA script based on CGView[
40]. Intergenic regions between
Lp. plantarum Lp20 and
Lp. plantarum WCFS1 were compared using SnapGene 6.0.2.
2.4. Construction of bsh1 Deletion Mutant and Complemented Strains
The entire open reading frame of the
bsh1 gene was deleted by the homologous double-overcrossing recombination. A schematic diagram of the suicide plasmid construction and
bsh1 deletion is shown in
Figure S2 and S3. Primer pairs used in this study are listed in
Table S2. Homologous arms were amplified using two primers pairs (Lp20_GM1654L-F/Lp20_GM1654L-R and Lp20_GM1654R-F/Lp20_GM1654R-R), and then cloned into pUC19E by seamless cloning (CWBIO, Beijing, China) to obtain pUCbsh1. This suicide plasmid was then transformed into
Lp. plantarum Lp20 cells by electroporation. MRS agar plates containing erythromycin were used to screen for positive colonies, which were then continuous cultivation in antibiotic-free MRS for 20 passages to select for double-overcrossing transformants. The replica plating method was performed to screen for colonies sensitive to erythromycin, and meanwhile hydrolase precipitation zones of this positive colonies disappeared (
Figure S3C). Subsequently, the
Δbsh1 gene knockout mutants were confirmed by PCR with primers pairs Up-F/Up-R (UF/UR) and Down-F/Down/R (DF/DR).
To generate a gene complementation, the bsh1 gene from Lp. plantarum Lp20 was amplified with primers (NcoI-bsh1/SacI-bsh1) and then cloned into pNZ11. Then, the recombinant pNZbsh1 plasmid was electroporated into the Lp. plantarum Lp20-Δbsh1 gene knockout mutants to obtain the complementation strain, which was designated as Lp. plantarum Lp20-Δbsh1 +.
2.5. Animal Experiment
Specific pathogen-free C57BL/6J mice (male, 6-8 weeks) were purchased from Beijing Huafukang Bioscience Co. Ltd. (Beijing, China) amd maintained in a barrier facility conditions (22 ± 2°C, 50 ± 5% humidity, 12-hour light/dark cycle and free access to food and water). After a 7-day acclimation, mice were divided into four groups: the low-fat diet group (LFD, n=6) was fed a normal diet with 10% of energy from fat (D12450J, Research Diets,
Table 1) for 12 weeks; the high-fat diet groups were given a diet with 60% of energy from fat (D12492, Research Diets,
Table 1) for 12 weeks to induce obesity. The successful establishment of the obesity model was defined as a twenty percent increase in the mean body weight of all HFD-fed mice compared to that of LFD group[
41]. Subsequently, obese mice were treated with bacteria strains (n=6): (1) HFD group, fed with HFD and PBS; (2) HFD + Lp20-WT group, fed with HFD and 1×10
9 CFU wild-type
Lp. plantarum Lp20 with BSH activity; (3) HFD + Lp20-
Δbsh1 group, fed with HFD and 1×10
9 CFU
Lp. plantarum Lp20-
Δbsh1 mutant. PBS or bacterial suspension was daily administered by oral gavage at a volume 0.2 mL for additional 8 weeks.
Body weight of each group mice was recorded weekly, and the average daily energy intake was calculated according to energy density of diets in
Table 1 (
Table S3 and S4). Before sacrificing, mice have been fasted overnight, anesthetized with tribromoethanol (TargetMol, Boston, MA). Body composition, including fat mass and lean mass, was analyzed before sacrifice using a MiniQMR23-060H-I type Body Composition Analyzer (Shanghai Niumag Corporation, Shanghai, China). Blood serum was collected after euthanasia by cervical dislocation. Organs and adipose tissues, including epididymal (eWAT), inguinal white adipose tissue (iWAT), and mesenteric fat were collected and weighed immediately. The experimental design was evaluated and approved by the Institutional Animal Care and Use Committee of the China Agricultural University, Beijing, China (No. AW81503202-5-2). All procedures were performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
2.6. Biochemical Assay of Blood Serum
Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum were measured using the Hitachi Automatic Analyzer 3100 (Hitachi, Japan), which was performed by biomedical assay kits (Maccura Biotechnology Ltd., Chengdu, China).
2.7. Liver Histological Analysis
Liver tissues were fixed in 4% paraformaldehyde, followed by progressively dehydrated and embedded in paraffin wax. Then, the liver tissues were cut into 4 μm sections and stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS). The fields were randomly selected and observed under a light microscope (Leica, Germany).
2.8. Statistical Analysis
The statistical differences among experimental groups were determined by one-way ANOVA, followed by Tukey’s post hoc test using GraphPad Prism software (version 9.0.0). The data were presented as mean ± standard deviation (SD) in this study. The P value < 0.05 was considered as statistically significant.
3. Results and Discussion
3.1. Lp. Plantarum Lp20 Exhibited the Highest GDCA Hydrolase Activity
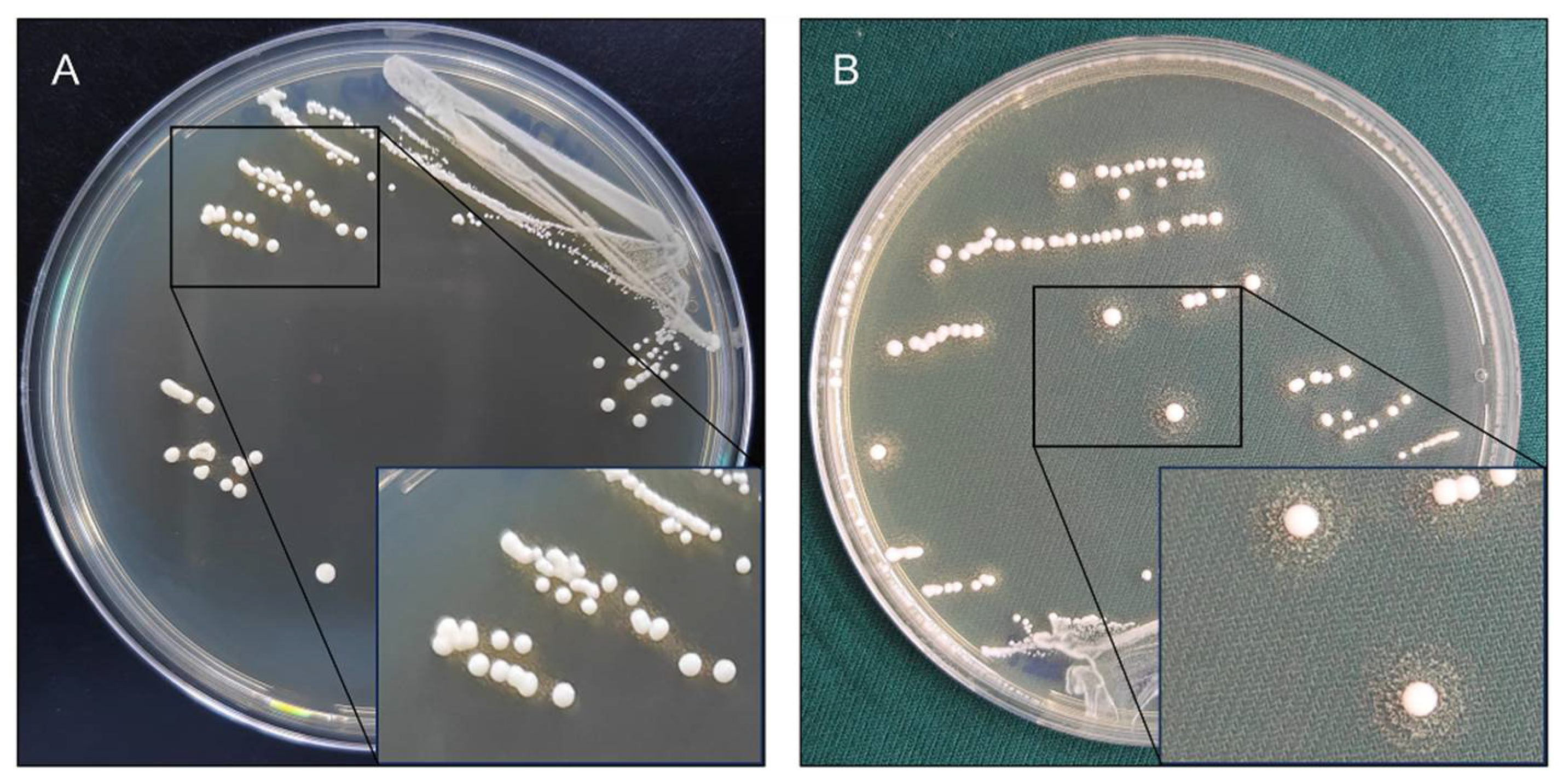
Twenty-eight
Lp. plantarum strains were investigated to produce BSH activity by bile salt plate method. Of these strains, 23 were able to produce precipitation halos on GDCA plates. Notably,
Lp. plantarum Lp20 produced the most prominent precipitation halos on GDCA plate. The reference strain
Lp. plantarum WCFS1 only produced white colonies with opaque granular precipitate halos (
Figure 1). However, none of these strains formed precipitation halos on TDCA plates (
Figure S1).
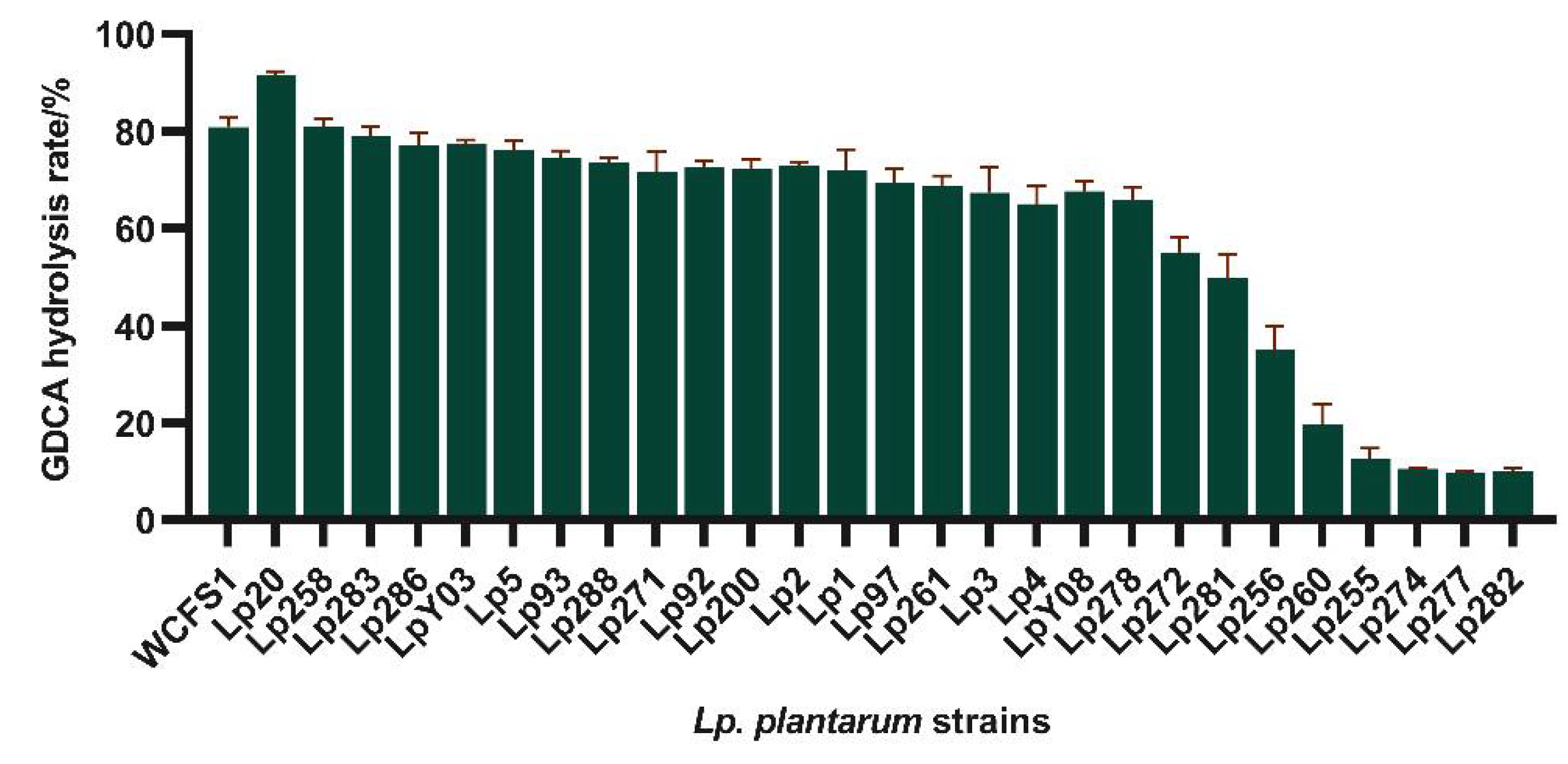
The BSH activity of 28
Lp. plantarum strains was further measured using the ninhydrin assay. Most results were consistent with those of plate assay.
Lp. plantarum Lp20 exhibited the highest GDCA hydrolysis activity, with a hydrolysis rate of 91.62% (
Figure 2). In addition,
Lp. plantarum Lp255, 260, 274, 277, and 282 produced no deconjugation precipitation halos and showed GDCA hydrolysis rate below 15%. The bile salt plate method proves to be an effective and rapid screening method for identifying BSH-active strains. Consequently, Lp20 was selected to investigate the correlation between its BSH activity and the presence of bile salt hydrolase genes.
BSH is prevalent within
Lactiplantibacillus genus[
42], in particular, the
bsh1 gene contributes significantly to the activity. Here we found that most
Lactiplantibacillus plantarum strains have a strong specificity to glycine-conjugated bile salts, which is likely because
Lp. plantarum is one of the prevalent strains in the human intestinal microbiota. The existence of BSH activity is considered a conservative microbial adaptation to the human intestinal environment[
43,
44]. Given that more than 60% of bile acids in the human bile acid pool are glycine-conjugated, the general deconjugating activity and preference for glycine-conjugated bile salts by
Lactiplantibacillus likely result from the evolutionary conservation of the
bsh1 gene[
45,
46].
3.2. BSH-Like Genes Were Located in the Chromosome of Lp. Plantarum Lp20
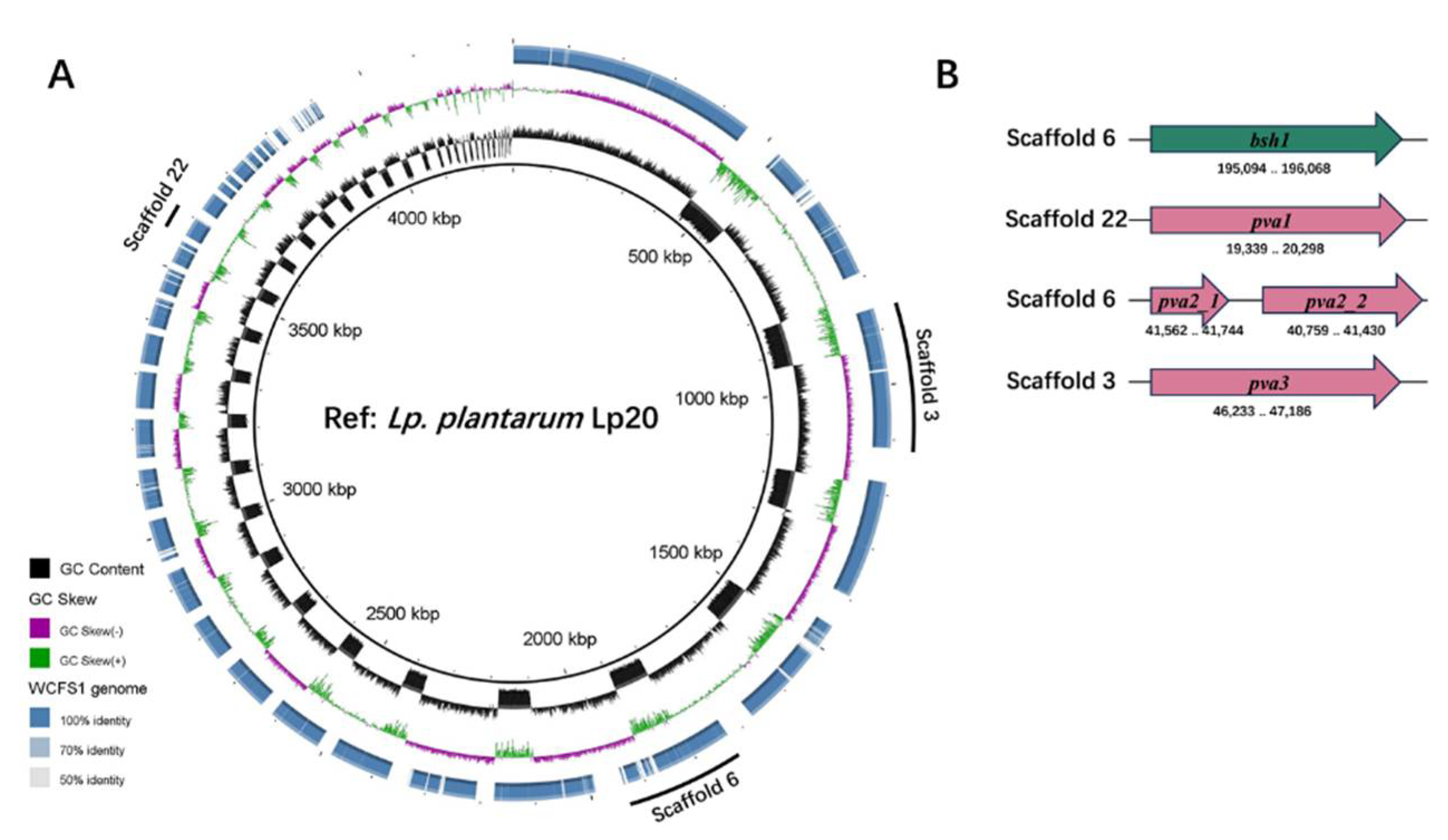
Draft genome sequencing demonstrated that
Lp. plantarum Lp20 contains 4 choloylglycine hydrolase (CGH) family genes, identified as
bsh1,
pva1,
pva2 and
pva3. However,
pva2 gene appears to be a pseudogene due to a frame-shift at 109 bp downstream the start codon in
Lp. plantarum Lp20 (
Figure 3). When compared to the amino acid sequences of Bsh1, Pva1 and Pva3 in
Lp. plantarum WCFS1, the corresponding sequences in
Lp. plantarum Lp20 showed similarities of 99.69%, 99.69%, and 97.48%, respectively. It was found that
pva2 and
pva4 were conservative for penicillin acylase activity, while
bsh1 contributed the major BSH activity in
Lp. plantarum WCFS1[
27,
47]. Therefore, the high BSH activity in
Lp. plantarum Lp20 might be attributed to the gene
bsh1.
3.3. Bsh1 Played a Key Role in the GDCA Hydrolysis Activity of Lp. Plantarum Lp20
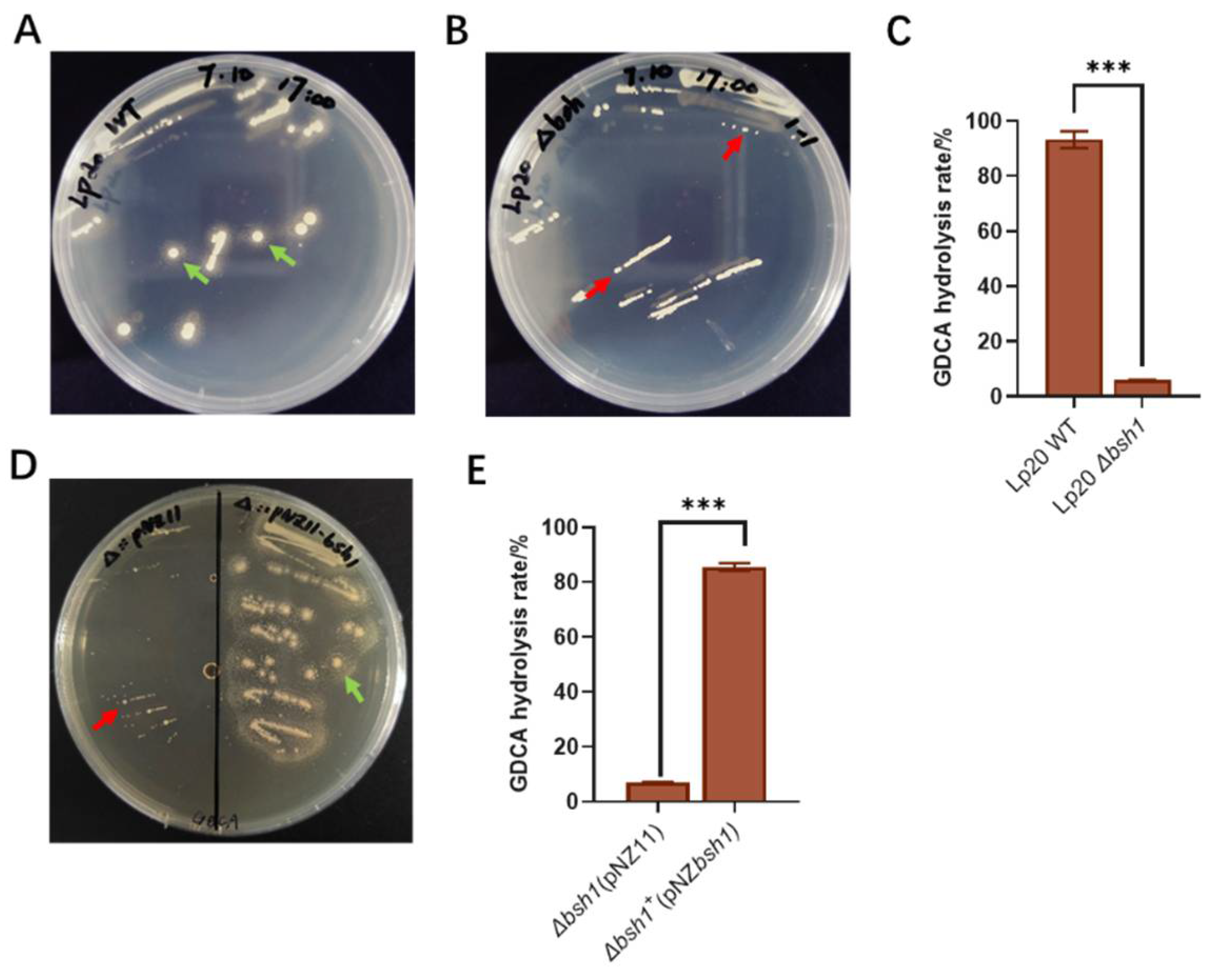
The
bsh1-deleted strain,
Lp. plantarum Lp20-
Δbsh1, and complementary strain,
Lp. plantarum Lp20-
Δbsh1+, were constructed in this study (
Figure S2 and S3). The results revealed the absence of precipitation halo formation in the
Lp. plantarum Lp20-
Δbsh1 mutant on GDCA plate (
Figure 4A and B). Meanwhile, the rate of bile salt hydrolysis was only 5.86% (
Figure 4C). The complementation of the
bsh1 gene into the
Lp. plantarum Lp20-
Δbsh1 strain restored its hydrolysis activity (
Figure 4D and E). These results confirm that the bsh1 gene confers GDCA hydrolysis activity to
Lp. plantarum Lp20. Our findings are consistent with previous studies on
Lp. plantarum WCFS1, where the knockout of
bsh1 led to the loss of GDCA hydrolysis activity[
47]. Subsequently,
Lp. plantarum Lp20 and
Δbsh1 mutant strain were used to investigate the relationship between bile salt hydrolase activity and obesity amelioration.
It is well known that bacteria with high BSH-producing ability could affect host energy harvesting and body weight gain.
Lactiplantibacillus plantarum H-87 with excellent bile salts hydrolysis ability could inhibit HFD-induced body weight gain, fat accumulation and liver lipogenesis in C57BL/6J mice[
14]. Bsh1 from
Ligilactobacillus salivarius strains significantly reduces weight gain in mice fed normal or high-fat diets and also reduces serum LDL cholesterol and liver triglycerides in mice[
15]. The supplementation of BSH-producing bacteria may be an effective way to alleviate obesity achieved by regulating the bile acid related lipid metabolism pathway to influence host triglyceride homeostasis, energy storage and fat digestion. Accordingly, to correlate Bsh1 from
Lp. plantarum Lp20 with weight gain in animals,
Lp. plantarum Lp20 and
Δbsh1 mutant strain were administered daily for 8 weeks to diet-induced obese mice, respectively.
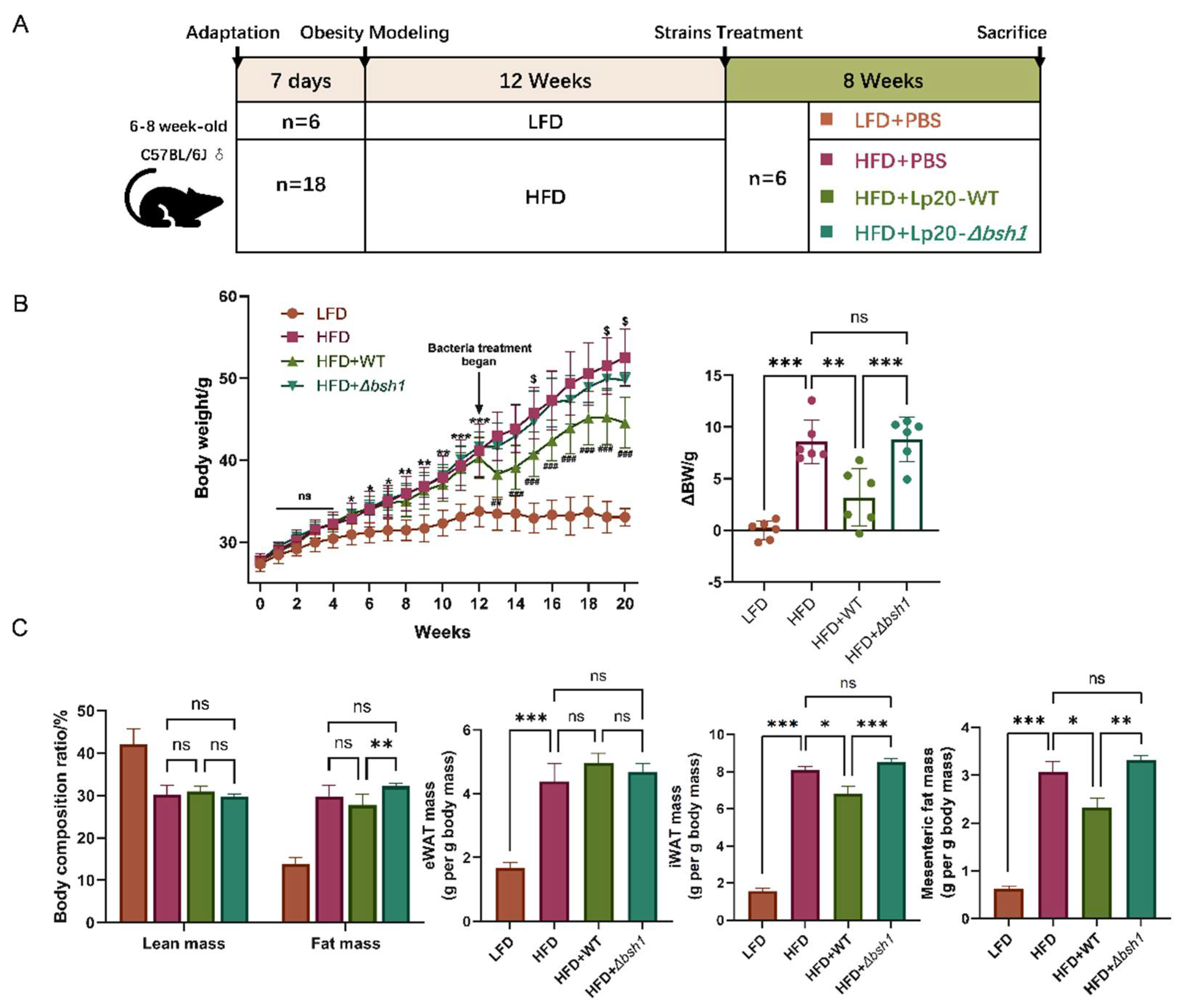
3.4. Lp. Plantarum Lp20 Decreased Weight Gain and Fat Accumulation in Obese Mice
The obesity induced by high fat diet in mice and the grouping intervention scheme are shown in
Figure 5A. The bodyweight of HFD group mice reached 41.03 g after 12 weeks, which was 1.21-fold higher than that of LFD group mice, indicating the successful establishment of an obese mouse model (
Figure 5B). After 8 weeks
Lp. plantarum intervention, the net bodyweight gains in HFD, HFD + Lp20-WT and HFD + Lp20-
Δbsh1 group mice were 8.58 g, 3.18 g and 8.81 g, respectively. These results showed that wild type
Lp. plantarum Lp20 with BSH enzyme activity significantly reduced a net bodyweight gain compared with that of HFD group, while no difference was observed between the mutant strain group and HFD group (
Figure 5B). Moreover,
Lp. plantarum Lp20 treatment decreased fat mass ratio in body composition compared with the
Lp. plantarum Lp20-
Δbsh1 mutant treatment group, without affecting the lean mass (
Figure 5C). Furthermore, there is no difference on the mass of eWAT among the three groups, but the mass of iWAT and mesenteric fat in wild-type
Lp. plantarum Lp20 treated mice was significantly reduced than both the mutant strain group and HFD group (
Figure 5C). However, no difference was observed between the mutant stain group and HFD group. Taken together, the bile salt hydrolase Bsh1 of
Lp. plantarum Lp20 contributes to ameliorating obese-related syndromes by reducing weight gain and body fat accumulation.
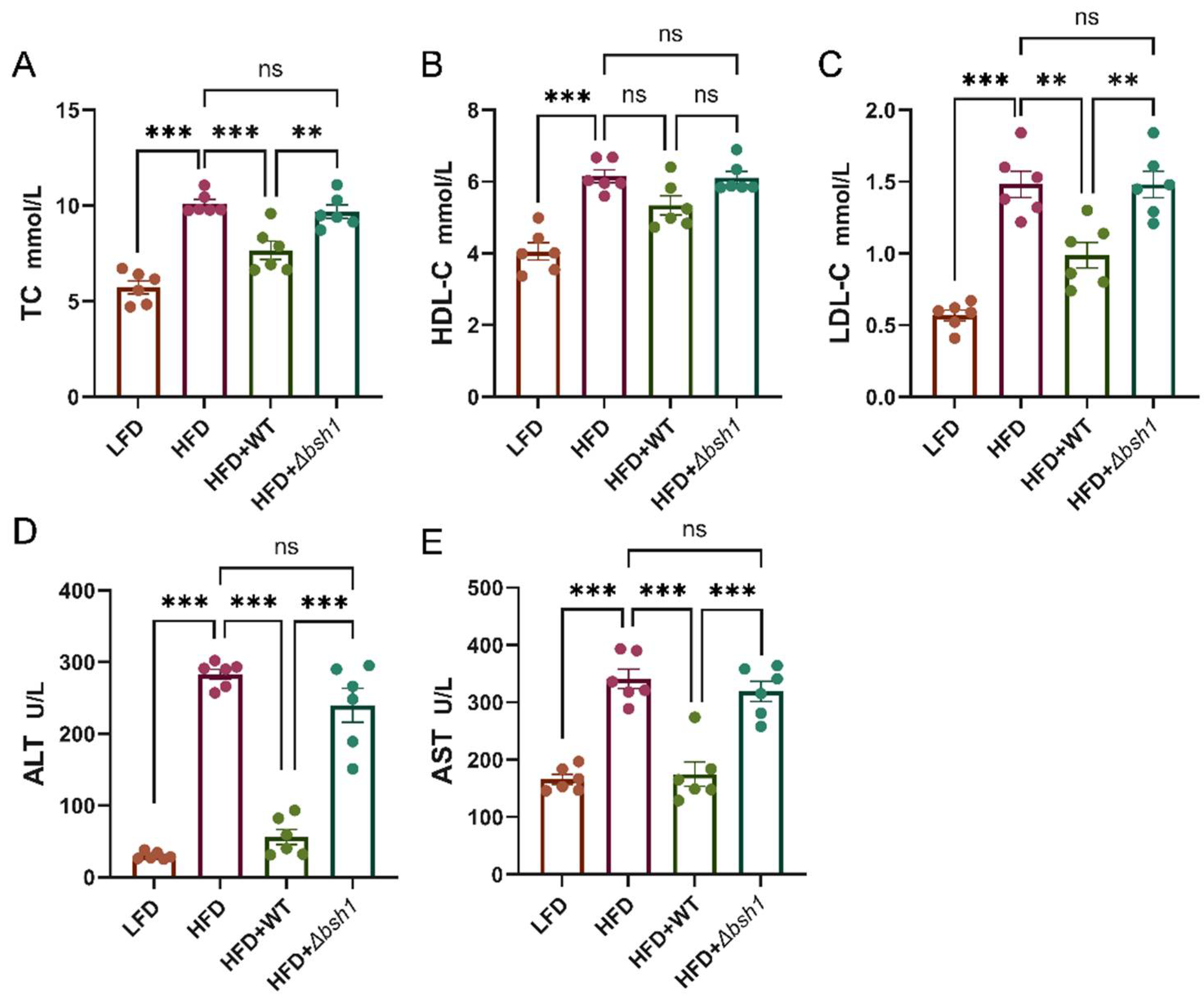
3.5. Bsh1 Decreased Serum Cholesterol in Obese Mice
The changes of serum cholesterol levels in four experimental groups are shown in
Figure 6. After 8 weeks feeding with HFD diet, the TC levels of HFD group increased to 9.15 mmol/L, which was 1.76-fold higher than that in LFD group mice. Notably, only wild-type
Lp. plantarum Lp20 treatment significantly reduced the total serum cholesterol compared with HFD group, while no significant difference was observed between mutant and HFD group (
Figure 6A). Further analysis revealed that wild-type
Lp. plantarum Lp20 primarily decreased serum LDL-C levels, with no significant impact on serum HDL-C levels (
Figure 6B and C). Therefore, the bile salt hydrolase Bsh1 of the
Lp. plantarum Lp20 appears to play a role in mitigating abnormal blood lipid levels by regulating serum cholesterol levels in obese mice. Furthermore, in comparison to the HFD + Lp20-
Δbsh1 group, the
Lp. plantarum Lp20 group exhibited a 76.6% reduction in serum ALT levels and a 45.3% reduction in AST levels, respectively (
Figure 6D and E).
Increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in the bloodstream are typically indicative of liver injury or steatosis[
48]. Elevated ALT and AST levels may reflect inflammation and damage to liver cells. Therefore, it can be inferred that BSH activity might play a protective role against liver injury.
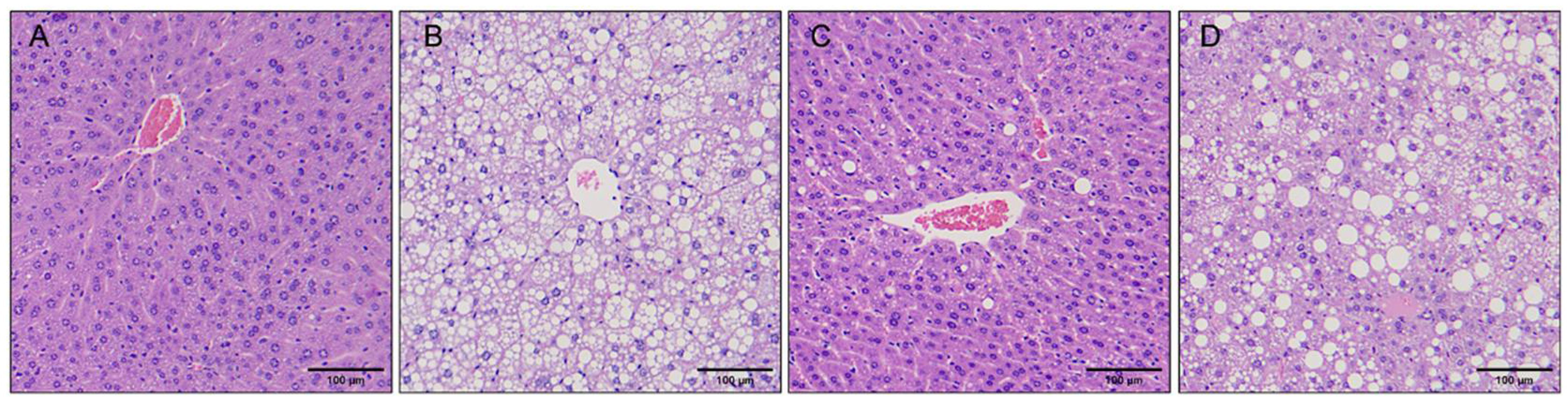
3.6. Bsh1 Alleviated Liver Steatosis in Obese Mice
Due to the effect of Bsh1 on ALT and AST in serum, a histological analysis of the liver was carried out. In this study, hepatic steatosis with enlarged, yellowish liver cells were obviously observed in the HFD and HFD + Lp20-
Δbsh1 groups. In contrast, a healthier and reddish liver was observed in the
Lp. plantarum Lp20 and LFD groups (
Figure S4). The liver weight in the HFD + Lp20-WT group was 1.20 g, which was lower than that in the HFD + Lp20-
Δbsh1 group (
Table S5). Additionally, there was no significantly difference in liver weight between the HFD + Lp20-WT group and LFD group. All these findings collectively suggest that BSH activity plays a protective role against liver injury.
Hepatocytes in LFD group mice displayed a purple-red cytoplasm with uniformly stained chromatin, indicating an absence of significant fat accumulation or steatosis (
Figure 7). In contrast, varying degrees of vacuolation, indicative of lipid accumulation within hepatocytes, were observed in HFD-fed mice. This result was also confirmed by PAS staining, which distinguished steatosis from glycogen or other polysaccharides accumulation (
Figure S5). It is worth noting that the HFD + Lp20-WT group exhibited a darker cytoplasm and a substantial reduction in lipid droplets. However, the
Δbsh1 group exhibited a same level of steatosis as the HFD group in both droplet size and quantity.
HFD-induced liver steatosis has been reported in previous studies[
49,
50]. Treatment with
Lactobacillus strains has shown significant improvement for steatosis[
51,
52]. However, there are few studies which confirmed the anti-liver steatosis effect of Bsh1 in
Lactobacillus strains. The reduction in serum ALT and AST levels and the histopathological improvements observed through H&E and PAS staining provide strong evidence for the liver-protective role of Bsh1 from
Lp. plantarum Lp20. Consequently, these results indicate that wild-type
Lp. plantarum Lp20 with
bsh1 significantly alleviates liver lipid accumulation, suggesting a potential therapeutic benefit of BSH in Non-Alcoholic Fatty Liver Disease (NAFLD). Given the close relationship between the enterohepatic circulation of bile acids and host metabolism, further analysis of the host's bile acid pool is crucial for understanding the mechanism through which Bsh1 participates in metabolic regulation. Additionally, recent studies have revealed that BSH not only deconjugates amide bonds but also possesses acyltransferase activity, broadening our understanding of BSH functionality[
53]. The modification of the bile pool composition by BSH may significantly impact the role of these strains in their ecological niche, highlighting the need for further research to elucidate these effects.
4. Conclusions
The BSH is recognized as a metabolic marker for probiotics with the ability to reduce lipid accumulation. In this study, Lp. plantarum Lp20 was screened out for its highest BSH activity among 28 candidate strains. To further validate the relationship between BSH and its anti-obesity effects, a bsh1 knock-out mutant confirmed that the anti-obesity effects of Lp. plantarum Lp20 are BSH-dependent. In obese mice, the wild-type strain significantly inhibited body weight gain, reduced serum cholesterol, and improved liver steatosis, whereas no identical results were observed in BSH-deficient mutant strain treated mice. These findings highlight the crucial role of the BSH gene and povide direct evidence that BSH activity in the obesity-ameliorating effects of Lactiplantibacillus strains.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Bacteria strains and plasmids used in this study; Table S2: Primers used in this study; Table S3: Mice average daily energy intake during the modeling period; Table S4: Mice average daily energy intake during treatment; Table S5: Liver weight and liver index of mice after 8 weeks of intervention; Figure S1: Negative results in precipitation halo assay for Lactiplantibacillus BSH activity on GDCA plates; Figure S2: A schematic representing the generation of a markerless bsh1 deletion in Lp. plantarum Lp20 using the pUCbsh1arm suicide plasmid; Figure S3: Illustrations of the PCR detection of bsh1 single crossover insertion vectors using primer pairs DF/UF and DR/UR; Figure S4: Fat distribution in abdominal cavity and schematic diagram of liver in mice; Figure S5: The impact of a high-fat diet and Lp. plantarum Lp20 bile salt hydrolase on liver damage and steatosis.
Author Contributions
ZZY and HYL conceived and designed this study. LFZ wrote the manuscript. LFZ conducted major experiments, analyzed the data and perfected the animal-related experiments design. WH and QHD contributed to critically revising the manuscript. All authors read and approved the final version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (Grant number: 2022YFF1100100).
Institutional Review Board Statement
This study was approved by the Institutional Animal Care and Use Committee of the China Agricultural University, Beijing, China (No. AW81503202-5-2). All of the animal handling and experimental procedures were performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this study is available from the corresponding author upon reasonable request. The draft genome and assembly of Lactiplantibacillus plantarum Lp20 strain was deposited to GenBank (Accession munber: JBAJNI000000000).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R.; Beck, M.A.; Alwarawrah, Y.; MacIver, N.J. Emerging Mechanisms of Obesity-Associated Immune Dysfunction. Nat. Rev. Endocrinol. 2023, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The Critical Role of Gut Microbiota in Obesity. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Michael, D.R.; Jack, A.A.; Masetti, G.; Davies, T.S.; Loxley, K.E.; Kerry-Smith, J.; Plummer, J.F.; Marchesi, J.R.; Mullish, B.H.; McDonald, J. a. K.; et al. A Randomised Controlled Study Shows Supplementation of Overweight and Obese Adults with Lactobacilli and Bifidobacteria Reduces Bodyweight and Improves Well-Being. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.-C.; Lan, C.-C.E.; Huang, T.-Y.; Chen, K.-W.; Chai, C.-Y.; Chen, W.-T.; Fang, A.-H.; Chen, Y.-H.; Wu, C.-S. Heat-Killed and Live Lactobacillus Reuteri GMNL-263 Exhibit Similar Effects on Improving Metabolic Functions in High-Fat Diet-Induced Obese Rats. Food Funct. 2016, 7, 2374–2388. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.-C.; Lee, C.-L.; Chai, C.-Y.; Chen, W.-T.; Lu, Y.-C.; Wu, C.-S. Oral Administration of Lactobacillus Reuteri GMNL-263 Improves Insulin Resistance and Ameliorates Hepatic Steatosis in High Fructose-Fed Rats. Nutr. Metab. 2013, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, Y.; Ma, F.; Sun, M.; Mu, G.; Tuo, Y. The Ameliorative Effect of Lactobacillus Plantarum Y44 Oral Administration on Inflammation and Lipid Metabolism in Obese Mice Fed with a High Fat Diet. Food Funct. 2020, 11, 5024–5039. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Golubeva, A.V.; Zhdanov, A.V.; Wallace, S.; Arboleya, S.; Papkovsky, D.B.; El Aidy, S.; Ross, P.; Roy, B.L.; Stanton, C.; et al. Short-Chain Fatty Acids and Microbiota Metabolites Attenuate Ghrelin Receptor Signaling. FASEB J. 2019, 33, 13546–13559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, H.; Zhou, X.; Sun, J.; Liang, X.; Lv, Y.; Bai, L.; Zhang, J.; Gong, P.; Liu, T.; et al. Lactobacillus Casei YRL577 Ameliorates Markers of Non-Alcoholic Fatty Liver and Alters Expression of Genes within the Intestinal Bile Acid Pathway. Br. J. Nutr. 2021, 125, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, T.E.; da Silva, M.R.; Camacho, A.; Marcadenti, A.; Lehnen, A.M. A Review on Effects of Conjugated Linoleic Fatty Acid (CLA) upon Body Composition and Energetic Metabolism. J. Int. Soc. Sports Nutr. 2015, 12, 36. [Google Scholar] [CrossRef]
- Liang, C.; Zhou, X.-H.; Gong, P.-M.; Niu, H.-Y.; Lyu, L.-Z.; Wu, Y.-F.; Han, X.; Zhang, L.-W. Lactiplantibacillus Plantarum H-87 Prevents High-Fat Diet-Induced Obesity by Regulating Bile Acid Metabolism in C57BL/6J Mice. Food Funct. 2021, 12, 4315–4324. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G.M. Regulation of Host Weight Gain and Lipid Metabolism by Bacterial Bile Acid Modification in the Gut. Proc. Natl. Acad. Sci. 2014, 111, 7421–7426. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Zou, Y.; Han, X.; Bae, J.-W.; Jeon, C.O. Gut Microbiome-Mediated Mechanisms for Reducing Cholesterol Levels: Implications for Ameliorating Cardiovascular Disease. Trends Microbiol. 2023, 31, 76–91. [Google Scholar] [CrossRef]
- Joyce, S.A.; Shanahan, F.; Hill, C.; Gahan, C.G.M. Bacterial Bile Salt Hydrolase in Host Metabolism: Potential for Influencing Gastrointestinal Microbe-Host Crosstalk. Gut Microbes 2014, 5, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Degirolamo, C.; Rainaldi, S.; Bovenga, F.; Murzilli, S.; Moschetta, A. Microbiota Modification with Probiotics Induces Hepatic Bile Acid Synthesis via Downregulation of the Fxr-Fgf15 Axis in Mice. Cell Rep. 2014, 7, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-B.; Lew, L.-C.; Yeo, S.-K.; Nair Parvathy, S.; Liong, M.-T. Probiotics and the BSH-Related Cholesterol Lowering Mechanism: A Jekyll and Hyde Scenario. Crit. Rev. Biotechnol. 2015, 35, 392–401. [Google Scholar] [CrossRef]
- Xie, C.; Huang, W.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Role of Bile Acids in the Regulation of Food Intake, and Their Dysregulation in Metabolic Disease. Nutrients 2021, 13, 1104. [Google Scholar] [CrossRef]
- Foley, M.H.; O’Flaherty, S.; Barrangou, R.; Theriot, C.M. Bile Salt Hydrolases: Gatekeepers of Bile Acid Metabolism and Host-Microbiome Crosstalk in the Gastrointestinal Tract. PLOS Pathog. 2019, 15, e1007581. [Google Scholar] [CrossRef] [PubMed]
- Karlov, D.S.; Long, S.L.; Zeng, X.; Xu, F.; Lal, K.; Cao, L.; Hayoun, K.; Lin, J.; Joyce, S.A.; Tikhonova, I.G. Characterization of the Mechanism of Bile Salt Hydrolase Substrate Specificity by Experimental and Computational Analyses. Structure 2023, 31, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Rg, P.; S, A.; Kg, J.; Ja, L. Circulating Bile Acids as a Link between the Gut Microbiota and Cardiovascular Health: Impact of Prebiotics, Probiotics and Polyphenol-Rich Foods. Nutr. Res. Rev. 2022, 35. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile Acids and the Gut Microbiota: Metabolic Interactions and Impacts on Disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gege, C.; Hambruch, E.; Hambruch, N.; Kinzel, O.; Kremoser, C. Nonsteroidal FXR Ligands: Current Status and Clinical Applications. Handb. Exp. Pharmacol. 2019, 256, 167–205. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sun, K.; Wu, Z.; Yao, J.; Guo, B. All 4 Bile Salt Hydrolase Proteins Are Responsible for the Hydrolysis Activity in Lactobacillus Plantarum ST-III. J. Food Sci. 2011, 76. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Grover, S.; Batish, V.K. Bile Salt Hydrolase (Bsh) Activity Screening of Lactobacilli: In Vitro Selection of Indigenous Lactobacillus Strains with Potential Bile Salt Hydrolysing and Cholesterol-Lowering Ability. Probiotics Antimicrob. Proteins 2012, 4, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.T.; Shah, N.P. Bile Salt Deconjugation Ability, Bile Salt Hydrolase Activity and Cholesterol Co-Precipitation Ability of Lactobacilli Strains. Int. Dairy J. 2005, 15, 391–398. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, F.; Zhang, W.; Xia, W.; Chen, Y.; Liu, Y.; Fan, Z.; Zhang, Y.; Yang, Y. Cholesterol-Lowering Effect of Bile Salt Hydrolase from a Lactobacillus Johnsonii Strain Mediated by FXR Pathway Regulation. Food Funct. 2022, 13, 725–736. [Google Scholar] [CrossRef]
- Zhao, M.; Kuang, W.; Yang, J.; Liu, Y.; Yang, M.; Chen, Y.; Zhu, H.; Yang, Y. Cholesterol Lowering in Diet-Induced Hypercholesterolemic Mice Using Lactobacillus Bile Salt Hydrolases with Different Substrate Specificities. Food Funct. 0487. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes Database (CAZy): An Expert Resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–238. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG Resource for Deciphering the Genome. Nucleic Acids Res. 2004, 32, D277–280. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Hattori, M.; Aoki-Kinoshita, K.F.; Itoh, M.; Kawashima, S.; Katayama, T.; Araki, M.; Hirakawa, M. From Genomics to Chemical Genomics: New Developments in KEGG. Nucleic Acids Res. 2006, 34, D354–357. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Expanded Microbial Genome Coverage and Improved Protein Family Annotation in the COG Database. Nucleic Acids Res. 2015, 43, D261–269. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jaroszewski, L.; Godzik, A. Tolerating Some Redundancy Significantly Speeds up Clustering of Large Protein Databases. Bioinforma. Oxf. Engl. 2002, 18, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Saier, M.H.; Reddy, V.S.; Moreno-Hagelsieb, G.; Hendargo, K.J.; Zhang, Y.; Iddamsetty, V.; Lam, K.J.K.; Tian, N.; Russum, S.; Wang, J.; et al. The Transporter Classification Database (TCDB): 2021 Update. Nucleic Acids Res. 2021, 49, D461–D467. [Google Scholar] [CrossRef]
- Bairoch, A.; Apweiler, R. The SWISS-PROT Protein Sequence Database and Its Supplement TrEMBL in 2000. Nucleic Acids Res. 2000, 28, 45–48. [Google Scholar] [CrossRef]
- Alikhan, N.-F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple Prokaryote Genome Comparisons. BMC Genomics 2011, 12, 402. [Google Scholar] [CrossRef]
- Huo, Y.; Lu, X.; Wang, X.; Wang, X.; Chen, L.; Guo, H.; Zhang, M.; Li, Y. Bifidobacterium Animalis Subsp. Lactis A6 Alleviates Obesity Associated with Promoting Mitochondrial Biogenesis and Function of Adipose Tissue in Mice. Molecules 2020, 25, 1490. [Google Scholar] [CrossRef]
- Prete, R.; Long, S.L.; Gallardo, A.L.; Gahan, C.G.; Corsetti, A.; Joyce, S.A. Beneficial Bile Acid Metabolism from Lactobacillus Plantarum of Food Origin. Sci. Rep. 2020, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Pasolli, E.; De Filippis, F.; Mauriello, I.E.; Cumbo, F.; Walsh, A.M.; Leech, J.; Cotter, P.D.; Segata, N.; Ercolini, D. Large-Scale Genome-Wide Analysis Links Lactic Acid Bacteria from Food with the Gut Microbiome. Nat. Commun. 2020, 11, 2610. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.M.; Marchesi, J.R. Functional and Comparative Metagenomic Analysis of Bile Salt Hydrolase Activity in the Human Gut Microbiome. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 13580–13585. [Google Scholar] [CrossRef] [PubMed]
- Duar, R.M.; Lin, X.B.; Zheng, J.; Martino, M.E.; Grenier, T.; Pérez-Muñoz, M.E.; Leulier, F.; Gänzle, M.; Walter, J. Lifestyles in Transition: Evolution and Natural History of the Genus Lactobacillus. FEMS Microbiol. Rev. 2017, 41, S27–S48. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, H.; Feng, X.; Tang, H.; Xiong, Z.; Xia, Y.; Ai, L.; Song, X. Specific Bile Salt Hydrolase Genes in Lactobacillus Plantarum AR113 and Relationship with Bile Salt Resistance. LWT 2021, 145, 111208. [Google Scholar] [CrossRef]
- Lambert, J.M.; Bongers, R.S.; de Vos, W.M.; Kleerebezem, M. Functional Analysis of Four Bile Salt Hydrolase and Penicillin Acylase Family Members in Lactobacillus Plantarum WCFS1. Appl. Environ. Microbiol. 2008, 74, 4719–4726. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.S.; Kaplan, M.M. Evaluation of Abnormal Liver-Enzyme Results in Asymptomatic Patients. N. Engl. J. Med. 2000, 342, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The Role of the Gut Microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, I.; Solís-Muñoz, P.; Fernández-Moreira, D.; Grau, M.; Colina, F.; Muñoz-Yagüe, T.; Solís-Herruzo, J.A. High-Fat Diet Decreases Activity of the Oxidative Phosphorylation Complexes and Causes Nonalcoholic Steatohepatitis in Mice. Dis. Model. Mech. 2014, 7, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shen, Y.; Wang, Y.; Wang, L.; Zhang, L.; Zhao, Z.; Li, S. Lactobacillus Plantarum S9 Alleviates Lipid Profile, Insulin Resistance, and Inflammation in High-Fat Diet-Induced Metabolic Syndrome Rats. Sci. Rep. 2022, 12, 15490. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, C.; Zhang, L.; Zhao, Y.; Duan, C.; Zhang, X.; Gao, L.; Li, S. Lactobacillus Plantarum NA136 Improves the Non-Alcoholic Fatty Liver Disease by Modulating the AMPK/Nrf2 Pathway. Appl. Microbiol. Biotechnol. 2019, 103, 5843–5850. [Google Scholar] [CrossRef] [PubMed]
- Guzior, D.V.; Okros, M.; Shivel, M.; Armwald, B.; Bridges, C.; Fu, Y.; Martin, C.; Schilmiller, A.L.; Miller, W.M.; Ziegler, K.M.; et al. Bile Salt Hydrolase Acyltransferase Activity Expands Bile Acid Diversity. Nature 2024, 626, 852–858. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).