1. Introduction
Sustained virologic response (SVR) after treatment of hepatitis C virus (HCV) infection is associated with improved patient survival rates [
1], with a reduction in the risk for liver decompensation and hepatocellular carcinoma (HCC). Achieving SVR is generally associated with several long-term changes in the liver: normalized hepatic enzyme levels; regression of hepatic liver necrosis, inflammation, and fibrosis; and improved hepatic function [
2]. In addition, portal venous pressure decreases after SVR with DAA therapy [
3,
4,
5,
6], and patients can be expected to avoid progression to portal hypertension. Portal hypertension is a major consequence of cirrhosis and is responsible for its most severe complications, including ascites, bleeding from esophagogastric varices (EGV), and portosystemic encephalopathy. Therefore, background liver management is necessary, and it is important for patients with HCV-related cirrhosis to achieve SVR.
However, when starting DAA therapy, if portal hypertension or EGV have already developed or if collateral vessels are dilated, symptoms associated with portal hypertension may be difficult to improve even if SVR is achieved. We have previously reported that patients with HCV-related cirrhosis who had already developed collateral vessels may experience aggravation of EGV or develop portosystemic encephalopathy even if they achieve SVR [
8,
9,
10].
On the other hand, risk factors for complications associated with worsening portal hypertension despite SVR remain unclear.
In portal hypertension, it is important to determine the status of portal venous pressure, and hepatic venous pressure gradient (HVPG) is used as an estimate [
11]. However, HVPG is an invasive examination. Therefore, we decided to use non-invasive testing to elucidate the risk factors for worsening portal hypertension after SVR with HCV-related cirrhosis. In addition to liver stiffness, platelet count, and diameter of portosystemic collateral vessels, we also analyzed autotaxin and bile acid levesl as liver fibrosis markers.
In this study, we retrospectively analyzed the risk of portal hypertension in patients with HCV-related cirrhosis after eradicating HCV.
2. Materials and Methods
We examined 167 patients with HCV-related cirrhosis who achieved SVR following DAA therapy at Hiroshima University Hospital between May 2010 and March 2020. Cirrhosis was assessed based on liver imaging tests or prior liver biopsy showing F4. Patients with Child-Pugh class A without a history of decompensated events were considered to have compensated cirrhosis, and patients with Child-Pugh class B or C or patients with a history of decompensated events were considered to have decompensated cirrhosis. All patients provided written informed consent to participate in the study in accordance with the ethical guidelines of the Declaration of Helsinki and with a program approved by the ethics committee of Hiroshima University Hospital.
2.1. Clinical Assessments
All patients underwent regular surveillance via liver function tests, ultrasonography, dynamic computed tomography (CT), and endoscopic examinations.Liver function tests were conducted before and at the end of the treatment (EOT) and 6 months and 1, 2, and 3 years after EOT.
2.2. Measurement of Liver Stiffness
We measured the severity of liver stiffness measurement (LSM) before treatment and 6 months and 1, 2, and 3 years after EOT using a FibroScan-502 (Echosens, Pari, France).
2.3. Endoscopic Examination for Assessing EGV
We evaluated the endoscopic findings of EGV based on the classification of the Japanese Society for Portal Hypertension and Esophageal Varices [
12]. The form (F) of EGV was classified as follows: F0 was treated and completely treated, with no varices; F1 was straight and relatively thin; F2 was beaded and moderately thick; and F3 was thick, nodular, or mass-like. There are three types of Red Color (RC) sign: red wale marking, cherry red spot, and hematocystic spot. RC1 is observed only in one-line varices, RC2 is observed between RC1 and RC3, and RC3 is observed in all circumferential varices. Endoscopy was performed within 6 months before starting antiviral therapy and was evaluated at least once each following year. Compared with baseline findings on follow-up endoscopy, worsening of F and RC signs was defined as aggravation of EGV. Endoscopy results were confirmed by two expert endoscopists.
2.4. CT Examination for Portal Hypertension
CT examination was performed in the high-quality scanning mode. We focused on the left gastric vein (LGV) and splenorenal shunt as portosystemic collateral vessels, and these vessels were evaluated by dynamic CT, measuring the vessel diameter and recording the widest part of the vessel in all cases in this study.
2.5. Statistical Analysis
Continuous variables were analyzed using the Mann-Whitney U-test. Aggravated EGV was calculated using the Kaplan–Meier method, and differences between groups were assessed using a log-rank test. Multivariate analysis was performed using a Cox proportional hazard model with a stepwise selection of variables or two logistic regression analyses. Receiver operating characteristic curves were used to determine the cutoff values for predicting the aggravated EGV-related events in the patients. All statistical analyses were performed using IBM SPSS version 23.0. P < 0.05 was considered significant.
3. Results
3.1. Baseline Characteristics of the Patients
The baseline characteristics of the 167 patients are shown in
Table 1. The present study included 82 men and 85 women, with a median age of 74 (range 48–90) years. The median FIB-4 index was 5.98 (range 3.27–26.09), the ALBI score was –2.56 (range –3.43 to –1.28), and the LSM was 18.9 (range 3.6–44.2) kPa. Before initiating DAA therapy, 51 of 167 (31%) patients had complications due to EGV, classified as F1 in 42 (25%) patients and F2 in 9 (5%) patients. The RC sign was not observed in any of the patients with EGV.
3.2. Aggravated EGV after Eradicating HCV
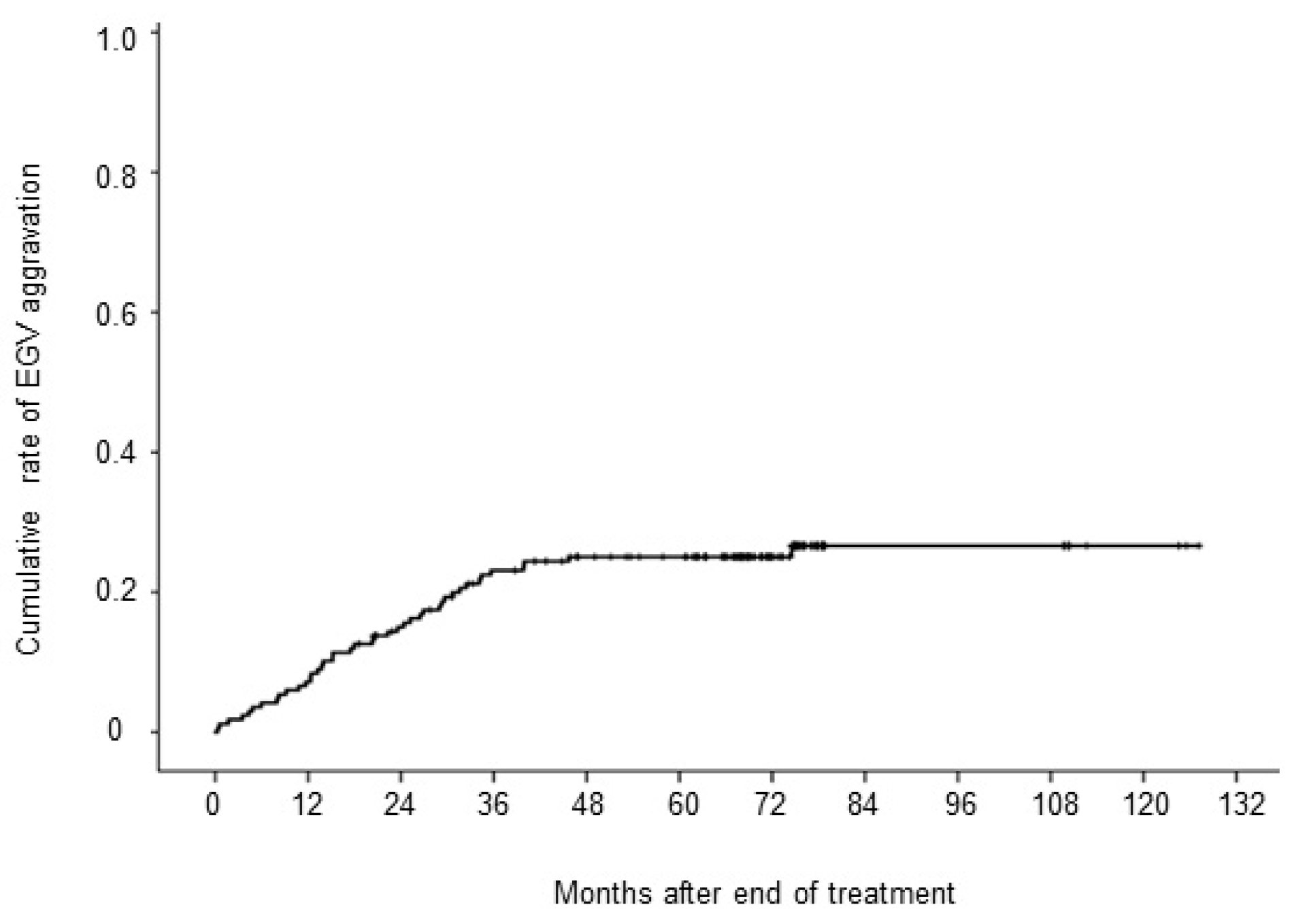
During the median follow-up period of 69 (range 3–127) months, EGV was aggravated in 42 (25%) patients despite achieving SVR. Twelve patients increased from F0 to F1, two patients from F0 to F3, 17 patients from F1 to F2 or appearance of the RC sign, 7 patients from F1 to F3 or appearance of the RC sign, and 4 patients from F2 to F3 or appearance of the RC sign. The cumulative 1, 3, 5, and 10-year aggravation rates of EGV were 7%, 23%, 25%, and 27%, respectively (
Figure 1). Although HCC recurred in 53 patients, portal vein tumor thrombosis was not observed in any of them.
3.3. Changes in Liver Function Test, Serum Fibrosis Markers and Liver Stiffness after Achieving SVR
Changes in liver function test, serum fibrosis markers, and liver stiffness after SVR depending on the presence or absence of EGV aggravation are shown in
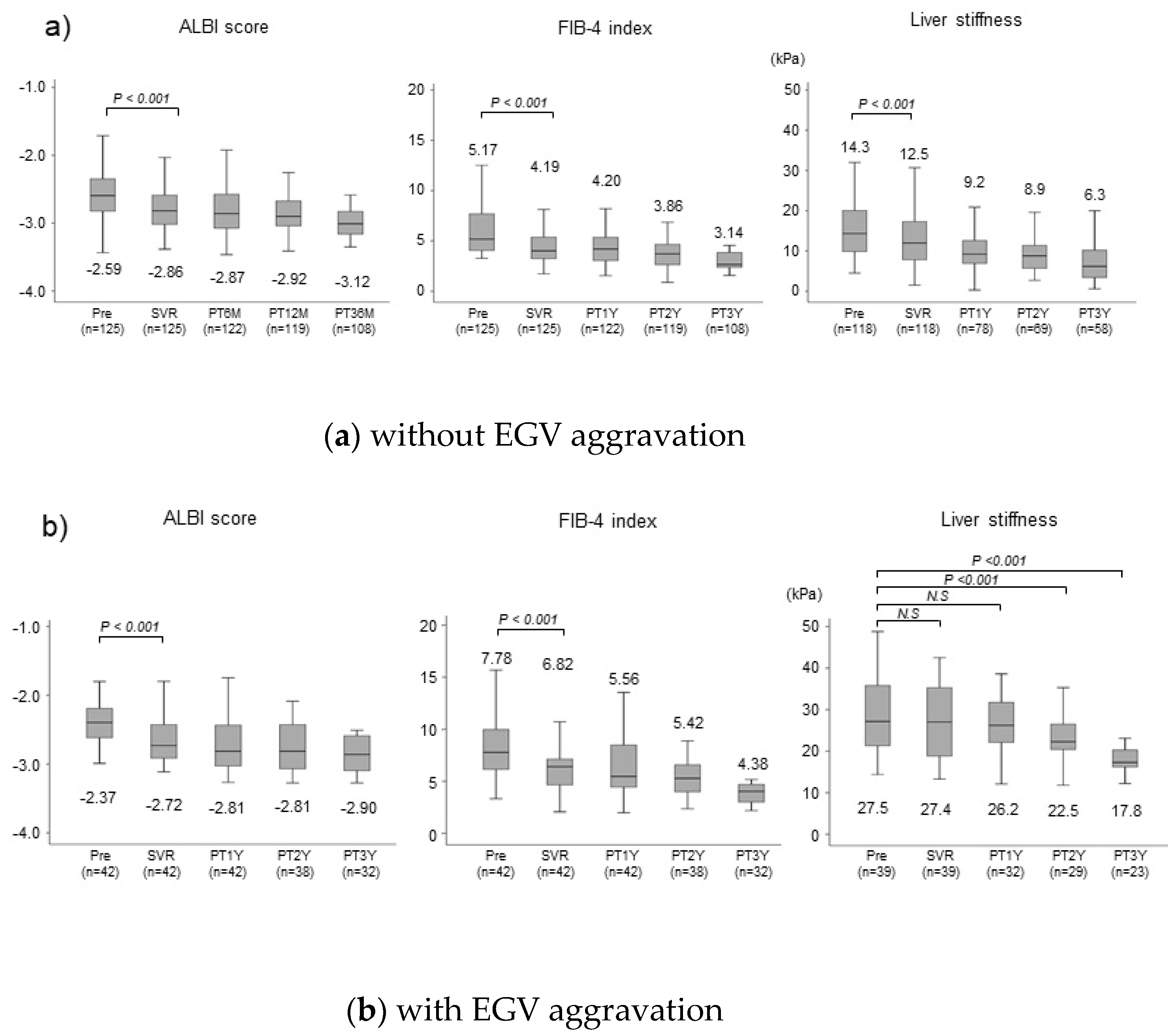
Figure 2. In patients without EGV aggravation, the median ALBI score decreased significantly (P<0.001) from -2.59 before treatment to –2.86 at the time of SVR achievement. One, two, and three years after EOT, the median ALBI score decreased to –2.87, –2.92, and –3.12, respectively, and the improvement in liver function was maintained. The median FIB-4 index decreased significantly (P<0.001) from 5.12 before treatment to 4.19 at the time of SVR. It decreased to 4.20, 3.86, and 3.14, one, two, and three years after EOT, respectively, and the improvement in liver function was also maintained. The median LSM decreased significantly (P<0.001) from 14.3kPa before treatment to 12.5kPa at the time of SVR, decreasing to 9.2kPa, 8.9kPa, and 6.3kPa, one, two, and three years after EOT, respectively, and the improvement in liver stiffness was maintained. In patients with aggravated EGV, the median ALBI score decreased significantly (P<0.001) from –2.37 before treatment to –2.72 at the time of SVR achievement, decreasing to –2.81, –2.81, and –3.12, one, two, and three years after EOT, respectively, and the improvement in liver function was maintained. The median FIB-4 index decreased significantly (P<0.001) from 7.78 before treatment to 6.82 at the time of SVR achievement and decreased to 5.56, 5.42, and 4.38, one, two, and three years after EOT, respectively. The improvement in liver function was maintained. On the other hand, no significant improvement in LSM was observed, decreasing only slightly from 27.5kPa before treatment to 27.4kPa at the time of SVR and 26.2kPa even one year after EOT. However, LSM decreased significantly to 22.5kPa two years after EOT (P<0.001).
3.4. Changes in Liver Stiffness after Achieving SVR and Aggravated EGV after Eradicating HCV by Pretreatment LSM
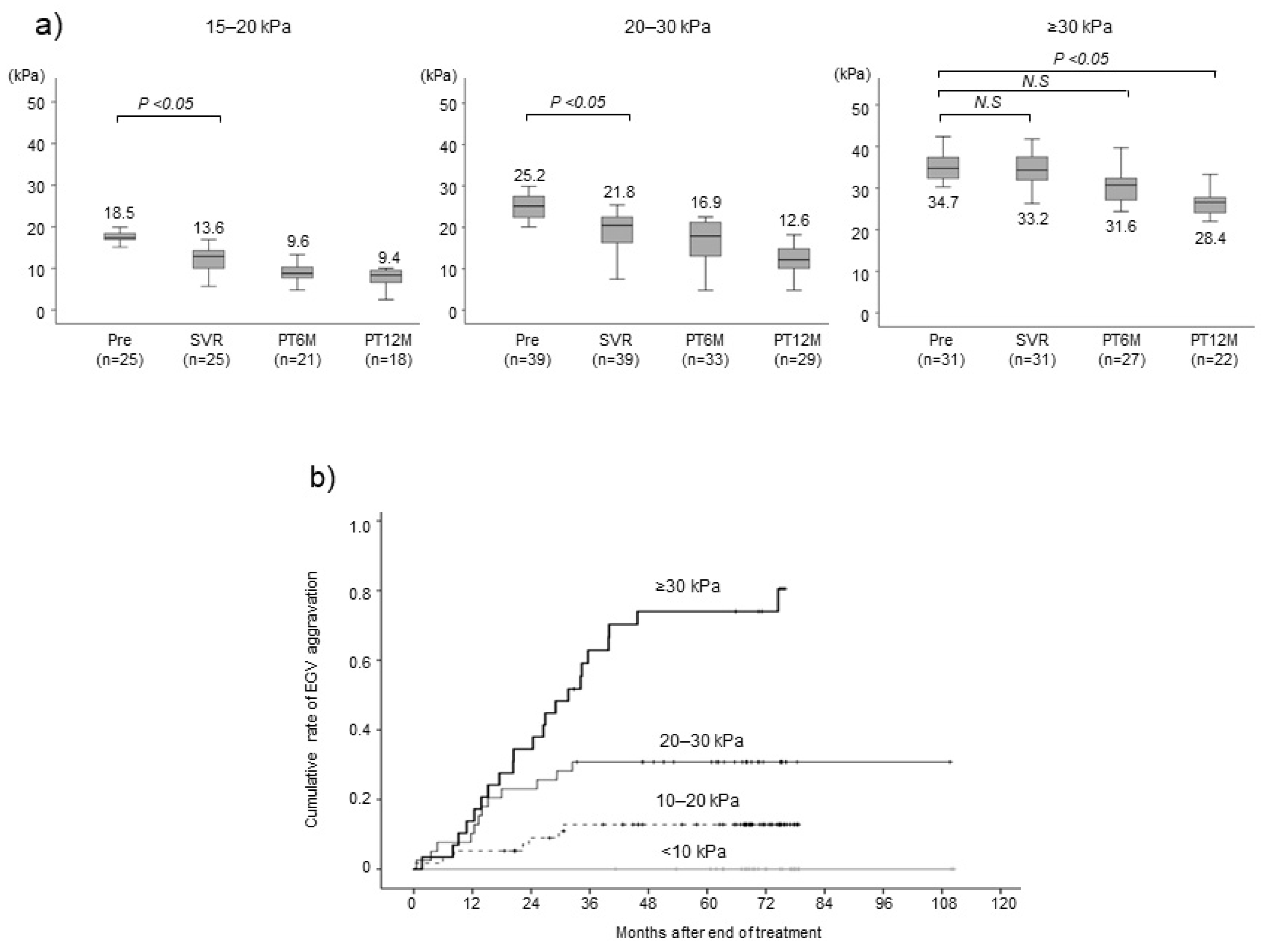
Because LSM seems to be associated with aggravated EGV, we analyzed changes in LSM after SVR based on pretreatment LSM level. In the group of patients with a pretreatment LSM of 15–20 kPa, the median LSM decreased significantly from 18.5 at pretreatment to 13.6 when SVR was achieved (P < 0.05) and further reduced to 9.6 and 9.4 kPa at 1 and 2 years after EOT, respectively (
Figure 3a). In the group with an LSM of 20–30 kPa, the median LSM decreased significantly from 25.2 at pretreatment to 21.8 when SVR was achieved (P < 0.05) and further reduced to 16.9 and 12.6 kPa at 1 and 2 years after EOT, respectively. By contrast, in the group with pretreatment LSM ≥30 kPa, the median LSM decreased only slightly from 34.7 at pretreatment to 33.2 when SVR was achieved and 31.6 kPa at 1 year after EOT. These findings indicate that LSM is less likely to improve despite eliminating HCV when pretreatment LSM ≥30 kPa. We analyzed the association between aggravated EGV and pretreatment LSM. The cumulative aggravated EGV rates at 1, 3, and 5 years were 14%, 63%, and 74% for the group with a pretreatment LSM ≥30 kPa, 10%, 31%, and 31% for the group with an LSM of 20–30 kPa, and 5%, 13%, and 13% for the group with an LSM of 10–20 kPa, respectively (
Figure 3b). By contrast, no patients with a pretreatment LSM <10 kPa had aggravated EGV (P < 0.001).
3.5. Serum Bile Acid and Predictive Factors Associated with Aggravated EGV
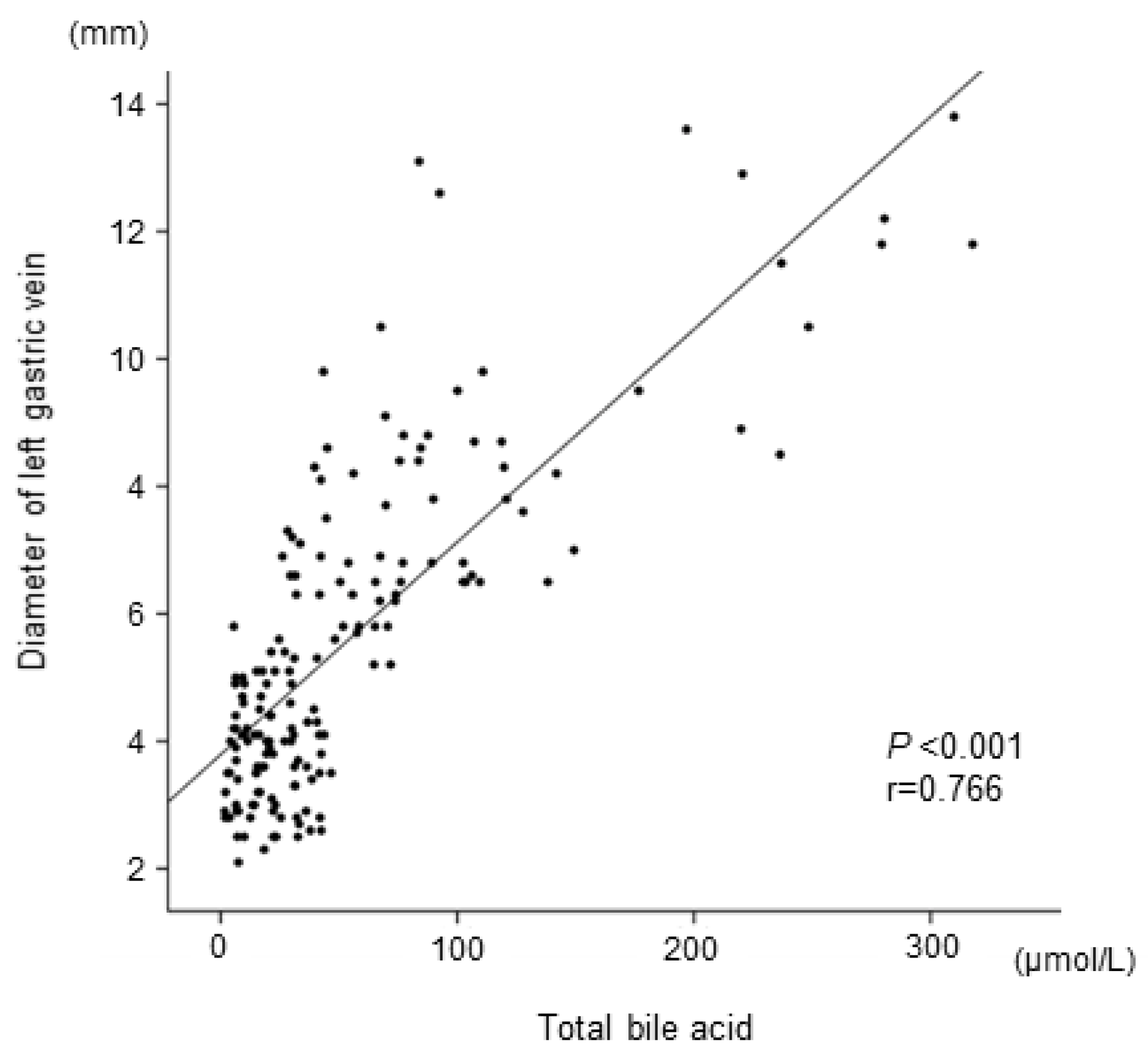
We focused on serum bile acid because it is considered to be associated with extrahepatic portosystemic shunts. The total serum bile acid level at the time of SVR was significantly correlated with LGV diameters (
Figure 4). We then analyzed predictors for post-SVR aggravated EGV, including serum total bile acid. Univariate analysis showed that platelet count, LSM, total bile acid level, autotaxin level, history of HCC, and the diameter of the LGV at the time of SVR were significantly associated with aggravated EGV (
Table 2). Multivariate analysis identified platelet count <11.0 × 10
4/μL (hazard ratio [HR] 3.769 for ≥11.0 × 10
4/μL, P = 0.008), LSM ≥18.0 kPa (HR 4.834 for <18.0 kPa; P = 0.006), total bile acid ≥33.0 μmol/L (HR 3.341 for <33.0 μmol/L, P = 0.009), and the diameter of LGV ≥5.0 mm when HCV was eradicated (HR 5.891 for <5.0 mm, P < 0.001) as independent risk factors for aggravated EGV after achieving SVR. Receiver operating characteristic curves were generated for both values, and the optimal cutoff values were identified as 11.0 × 10
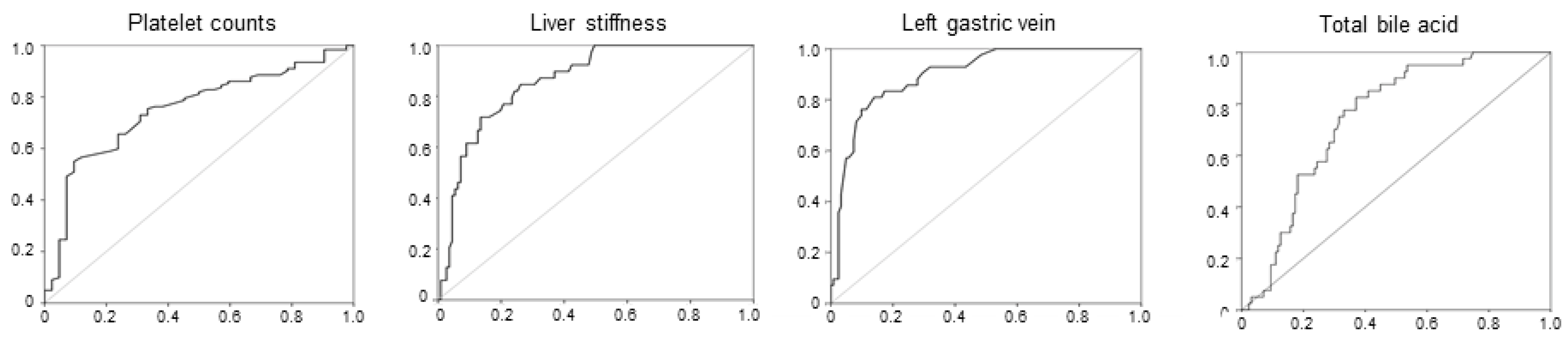
4/μL for platelet count at SVR, with an area under the curve (AUC) of 0.786 (P < 0.001), 18.0 kPa for liver stiffness at SVR with an AUC of 0.883 (P < 0.001), 5.0 mm for the maximal diameters of the LGV with an AUC of 0.901 (P < 0.001), and 33 μmol/L for total bile acid at SVR with an AUC of 0.767 (P < 0.001) (
Figure 5).
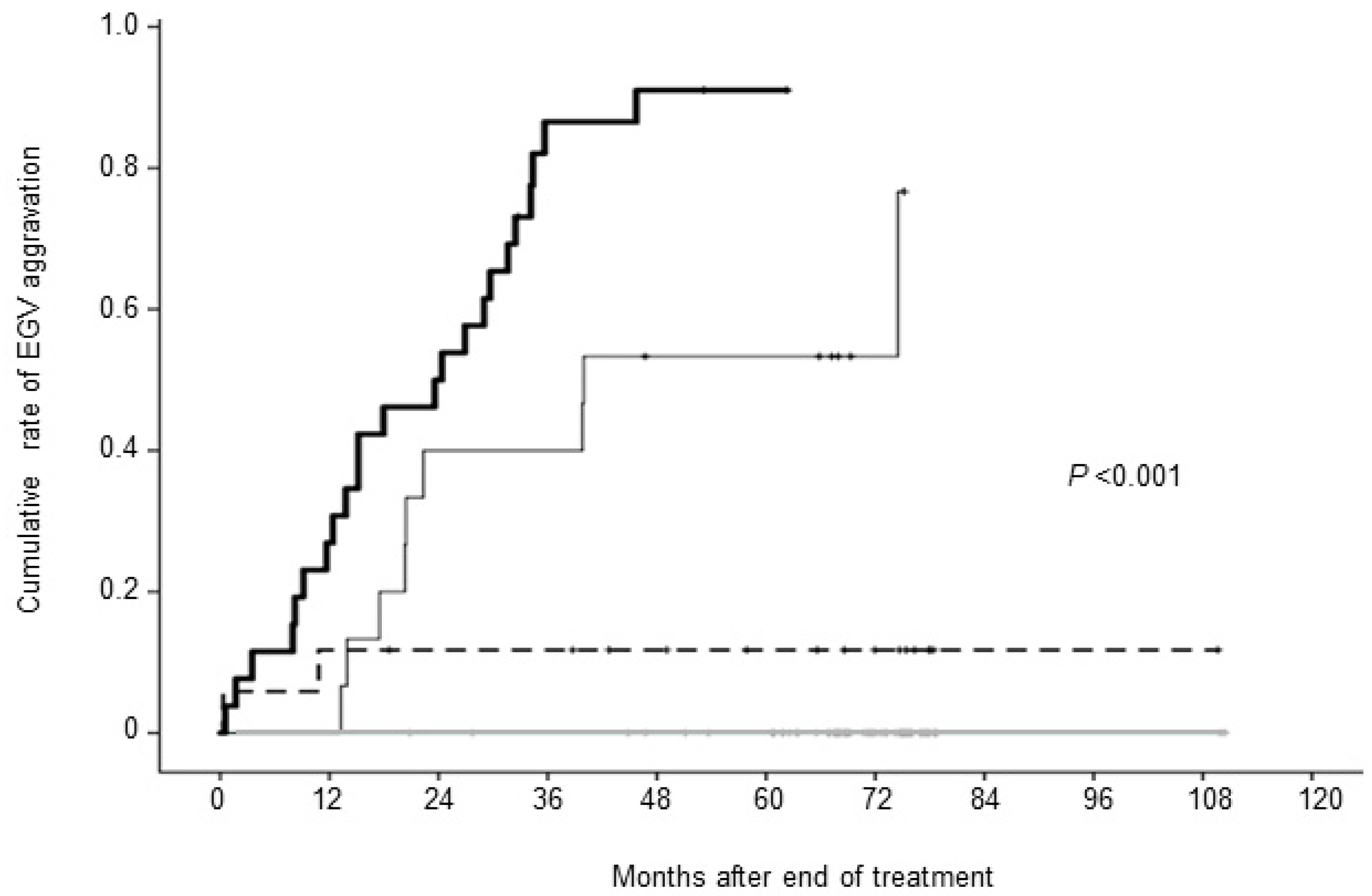
We analyzed the cumulative rate of aggravated EGV according to the number of risk factors. In patients who had all four risk factors, LGV diameter ≥5.0 mm, LSM ≥18.0 kPa, platelet count <11.0 × 10
4/µL, and total bile acid ≥33.0 µmol/L, the cumulative EGV aggravation rates at 1, 3, and 5 years were 27%, 87%, and 91%, respectively. In patients with three risk factors, the cumulative aggravation rates at 1, 3, and 5 years were 7%, 40%, and 53%, respectively. In patients with two risk factors, the cumulative aggravation rates at 1, 3, and 5 years were 0%, 12%, and 12%, respectively. By contrast, none of the patients who had zero or one risk factors experienced aggravated EGV during the observation period (P < 0.001) (
Figure 6).
4. Discussion
During the median observation period of 69 months, 42 (25%) patients with HCV-related liver cirrhosis experienced aggravation of their EGV despite achieving SVR. This finding is consistent with previous reports that EGV was aggravated in 13%–58% of patients with HCV-related cirrhosis who achieved SVR [
13,
14].
Liver function and liver fibrosis estimates based on the ALBI score and FIB-4 index improved after SVR regardless of whether EGV was aggravated. However, the median LSM failed to improve for the first two years when LGV worsened. Moreover, in patients who had LSM ≥30 kPa before treatment, the median LSM did not decrease until one year after EOT, and subsequently improved by two years after EOT. Furthermore, the cumulative aggravated EGV rates at 1, 3, and 5 years were 14%, 63%, and 74% for the group with a pretreatment LSM ≥30 kPa, aggravation rates were significantly higher in the LSM ≥30 kPa group.
LSM is widely used as a non-invasive test for liver cirrhosis and portal hypertension [
15]. Recently, spleen stiffness has been measured in the same way as liver stiffness, and there are reports that spleen stiffness is also related to EGV thickness and HVPG [
16]. Furthermore, liver stiffness is positively correlated with EGV in patients with HCV-related cirrhosis [
17]. Ogasawara et al. reported that liver stiffness at 24 weeks after EOT predicts aggravated EGV in post-SVR patients with HCV-related cirrhosis [
18]. Based on these facts, we believe that it is important to understand the status of EGV before administering DAA therapy to patients with HCV-related cirrhosis.
This study newly found that total bile acids are associated with the diameter of LGV and are an independent aggravation factor for post-SVR EGV. Bile acids are produced in the liver and stored in the gallbladder. After meals, the stored bile acids are released into the small intestine. Approximately 95% of the bile acids in the intestinal tract are reabsorbed and return to the liver through the portal vein as part of the bile acid enterohepatic circulation [
19]. Serum total bile acids are elevated in patients with liver cirrhosis [
20,
21], and portosystemic shunts have been reported to be associated with elevated serum bile acids in peripheral blood [
22,
23]. Hayashi et al. measured portal pressure in patients who underwent percutaneous transhepatic portal vein puncture and showed that bile acid levels were positively associated with portal pressure [
24]. Certainly, in portal hypertension, it is necessary to understand the status of portal venous pressure, and HVPG is used as a substitute for portal venous pressure [
11]. However, HVPG measurement is currently not covered by insurance in Japan, and although there are reports involving invasive tests, there are almost no reports on non-invasive tests. Therefore, we believe that total bile acids should be measured and may be an important indicator for monitoring changes in portal hypertension and its progress.
Here, we identified the following independent risk factors for post-SVR aggravated EGV: platelet count <11.0 × 10
4/μL, LSM ≥18.0 kPa, total bile acid ≥33.0 μmol/L, and LGV diameter ≥5.0 mm. These findings are consistent with the Baveno VII guidelines [
25], which recommend surveillance of EGV based on platelet count and LSM. The existence of EGV or portosystemic collateral vessels increases the risk of aggravated EGV and the incidence of portosystemic encephalopathy in patients with HCV-related cirrhosis, even after successfully eradicating HCV through DAA therapy [7-10]. By adding serum bile acid levels to these previously reported factors, the risk of aggravated EGV can be stratified with higher accuracy. Patients with all four risk factors had a significantly higher risk of aggravated EGV; thus, strict surveillance by dynamic CT and endoscopic examination is warranted for such patients. By contrast, no patients with one or none of these risk factors developed aggravated EGV. These patients seem to have an extremely low risk of aggravated EGV after SVR, suggesting that surveillance of EGV is not needed for such patients.
5. Conclusions
In conclusion, we found that even among patients who successfully achieved SVR following DAA therapy, portal hypertension did not immediately improve in patients with compensated liver cirrhosis, particularly those with at least two of the following risk factors: LGV diameter ≥5.0 mm, LSM >18.0 kPa, platelet count <11.0×104/μL, and total bile acid ≥33.0 μmol/L. These patients may require monitoring for aggravated EGV after SVR is achieved.
6. Patents
This section is not mandatory but may be added if there are patents resulting from the work reported in this manuscript.
Supplementary Materials
The following supporting information can be downloaded at: Figure S1: title; Correlation between the diameter of left gastric vein and autotaxin at the end of treatment.
Author Contributions
Conception and design: YN, MT, HA, SO. Acquisition of data: YN, KY, YF, SU, HF, AO, TN, EM, TK, DM, HC, MT, HA, SO. Analysis and interpretation of data: YN. Drafting of the manuscript: YN. Statistical analysis: YN. Study supervision: NC, TM, HA, SO. Critical revision of the manuscript: MT, HA, NC, SO. All authors read the final version of this article and approved its content and submission for publication. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The funders had no role in the design of the study, collection and analysis of data, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Institutional Review Board Statement
All patients provided written informed consent to participate in the study in accordance with the ethical guidelines of the Declaration of Helsinki and in accordance with a program approved by the ethics committee of Hiroshima University Hospital.
Informed Consent Statement
All patients provided written informed consent to participate in the study in accordance with the ethical guidelines of the Declaration of Helsinki.
Data Availability Statement
All data generated or analyzed during this study are included in the published article.
Acknowledgments
The authors thank Emi Nishio for clerical assistance.
Conflicts of Interest
All authors declare no competing interests.
References
- Carrat, F.; Fontaine, H.; Dorival, C.; Simony, M.; Diallo, A.; Hezode, C.; De Ledinghen ,V.; Larrey, D.; Haour, G,; Bronowicki, J.; et al. French ANRS CO22 Hepather cohort. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019, 393, 1453–64.
- Mauro, E.; Crespo, G.; Montironi, C.; Londoño, MC.; Hernández-Gea, V.; Ruiz, P.; Lombardo, J.; Mariño, Z.; Díaz, A.; Colmenero, J.; et al. Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology. 2018, 67, 1683–94.
- Mandorfer, M.; Kozbial. K,; Schwabl, P.; Freissmuth, C.; Schwarzer, R.; Stern, R.; Chromy, D.; Stättermayer, AF.; Reiberger, T.; Beinhardt, S.; et al. Sustained virologic response to interferon- free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016, 65,692–99.
- Afdhal, N.; Everson, GT.; Calleja, JL.; McCaughan, GW.; Bosch, J.; Brainard, DM.; McHutchison, JG.; De-Oertel, S.; An, D.; Charlton, M.; et al. Effect of viral suppression on hepatic venous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J Viral Hepatol. 2017, 24, 823–31.
- Lens, S.; Alvarado-Tapias.; E, Mariño, Z.; Londoño, MC.; LLop, E.; Martinez, J.; Fortea, JI.; Ibañez, L.; Ariza, X.; Baiges, A.; et al. Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis. Gastroenterology. 2017, 153, 1273–83.
- Mandorfer, M.; Kozbial, K.; Schwabl, P.; Chromy, D.; Semmler, G.; Stättermayer, AF.; Pinter, M.; Hernández-Gea, V.; Fritzer-Szekeres, M.; Steindl-Munda, P.; et al. Changes in hepatic venous pressure gradient predict hepatic decompensation in patients who achieved sustained virologic response to interferon-free therapy. Hepatology. 2020, 71, 1023–36.
- Nagaoki, Y.; Aikata, H.; Kobayashi, T.; Fukuhara, T.; Masaki, K.; Tanaka, M.; Naeshiro, N.; Nakahara, T.; Honda, Y.; Miyaki, D.; at al. Risk factors for the exacerbation of esophageal varices or portosystemic encephalopathy after sustained virological response with IFN therapy for HCV-related compensated cirrhosis. J Gastroenterol. 2013, 48, 847–55.
- Ogasawara, N.; Saitoh, S.; Akuta, N.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; Hosaka, T.; Kobayashi, M.; Suzuki, F.; Suzuki, Y.; et al. Long-term outcome of hepatocellular carcinoma occurrence, esophageal varices exacerbation, and mortality in hepatitis C virus-related liver cirrhosis after interferon-based therapy. Hepatol Res. 2019, 49, 1441–50.
- Nagaoki, Y.; Imamura, M.; Teraoka, Y.; Morio, K.; Fujino, H.; Ono, A.; Nakahara, T.; Murakami, E.; Yamauchi, M.; Kawaoka, T.; et al. Impact of viral eradication by direct-acting antivirals on the risk of hepatocellular carcinoma development, prognosis, and portal hypertension in hepatitis C virus-related compensated cirrhosis patients. Hepatol Res. 2020, 50, 1222–33.
- Tsuji, S.; Uchida, Y.; Uemura, H.; Kouyama, JI.; Naiki, K.; Nakao, M.; Motoya D.; Sugawara K.; Nakayama N.; Imai Y.; et al. Involvement of portosystemic shunts in impaired improvement of liver function after direct-acting antiviral therapies in cirrhotic patients with hepatitis C virus. Hepatol Res. 2020, 50, 512–23.
- de Franchis, R. Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015, 63, 743–52.
- The Japan Society for Portal Hypertension. The General Rules for Study of Portal Hypertension, 3rd ed. Tokyo: Kanehara, 2013, 37–8.
- Puigvehí, M.; Londoño, MC.; Torras, X.; Lorente, S.; Vergara, M.; Morillas, RM.; Masnou, H.; Serrano, T.; Miquel, M.; Gallego, A.; et al. Impact of sustained virological response with DAAs on gastroesophageal varices and Baveno criteria in HCV- cirrhotic patients. J Gastroenterol. 2020, 55, 205–16.
- Di Marco, V.; Calvaruso, V.; Ferraro, D.; Bavetta, MG.; Cabibbo, G.; Conte, E.; Cammà, C.; Grimaudo, S.; Pipitone, RM.; Simone, F.; et al. Effects of eradicating hepatitis C virus infection in patients with cirrhosis differ with stage of portal hypertension. Gastroenterology. 2016, 151, 130–9.
- Berzigotti, A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017, 67, 399–411.
- Tseng, Y.; Li, F.; Wang, J.; Chen, S.; Jiang, W.; Shen, X.; Wu, S. Spleen and liver stiffness for noninvasive assessment of portal hypertension in cirrhotic patients with large esophageal varices. J Clin Ultrasound. 2018, 46, 442–9.
- Vizzutti, F.; Arena, U.; Romanelli, RG.; Rega, L.; Foschi, M.; Colagrande, S.; Petrarca, A.; Moscarella, S.; Belli, G.; Zignego, AL.; et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007, 45, 1290–7.
- Ogasawara, N.; Saitoh, S.; Akuta, N.; Sezaki, H.; Suzuki, F.; Fujiyama, S.; Kawamura, Y.; Hosaka, T.; Kobayashi, M.; Suzuki, Y.; et al. Advantage of liver stiffness measurement before and after direct-acting antiviral therapy to predict hepatocellular carcinoma and exacerbation of esophageal varices in chronic hepatitis C. Hepatol Res .2020, 50, 426–38.
- Liu, X.; Wang, Y. An overview of bile acid synthesis and its physiological and pathological functions. Yi Chuan. 2019, 41,365–74.
- Tarantino, G.; Cambri, S.; Ferrara, A.; Marzano, M.; Liberti, A.; Vellone, G.; Ciccarelli, AF. Serum concentration of bile acids and portal hypertension in cirrhotic patients. Possible correlations. Riv Eur Sci Med Farmacol.1989, 11,195–205.
- Siciliano, M.; Milani, A.; Marra, L.; Rossi, L. Serum bile acids in cirrhosis: correlation with liver function parameters and with the severity of the disease. Quad Sclavo Diagn. 1986, 22,355–61.
- Poupon, RE.; Poupon, RY.; Grosdemouge, ML.; Erlinger, S. Effect of portacaval shunt on serum bile acid concentration in patients with cirrhosis. Digestion. 1977, 16, 138–45.
- Ohkubo, H.; Okuda, K.; Lida, S.; Ohnishi, K.; Ikawa, S.; Makino, I. Role of portal and splenic vein shunts and impaired hepatic extraction in the elevated serum bile acids in liver cirrhosis. Gastroenterology.1984, 86, 514–20.
- Hayashi, H.; Beppu, T.; Okabe, H.; Nitta, H.; Imai, K.; Doi, K.; Chikamoto, A.; Baba, H. Combined measurements of serum bile acid level and splenic volume may be useful to noninvasively assess portal venous pressure. J Gastroenterol. 2012, 47, 1336–41.
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C. Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022, 76, 959–974.
Figure 1.
Cumulative rate of esophagogastric varices (EGV) aggravation after achievement of sustained virological response.
Figure 1.
Cumulative rate of esophagogastric varices (EGV) aggravation after achievement of sustained virological response.
Figure 2.
Changes in liver function and liver fibrosis following achievement of sustained virological response. Changes in ALBI score, FIB-4 index, and liver stiffness at baseline (Pre), SVR, and 6 (PT6M), 12 (PT12M) and 36 (PT36M) months post treatment in patients with and without aggravation of esophagogastric varices (EGV). In these box-and-whisker plots, lines within the boxes represent median values. The upper and lower lines of the boxes represent the 75th and 25th percentiles, respectively, and the upper and lower bars outside the boxes represent the 90th and 10th percentiles, respectively.
Figure 2.
Changes in liver function and liver fibrosis following achievement of sustained virological response. Changes in ALBI score, FIB-4 index, and liver stiffness at baseline (Pre), SVR, and 6 (PT6M), 12 (PT12M) and 36 (PT36M) months post treatment in patients with and without aggravation of esophagogastric varices (EGV). In these box-and-whisker plots, lines within the boxes represent median values. The upper and lower lines of the boxes represent the 75th and 25th percentiles, respectively, and the upper and lower bars outside the boxes represent the 90th and 10th percentiles, respectively.
Figure 3.
(a) Changes in liver stiffness measurement (LSM). Patients were classified into three groups with respect to LSM at the start of antiviral therapy 15–20 kPa, 20–30 kPa, and ≥30 kPa. LSM was measured at baseline (Pre), SVR and 6 (PT6M) and 12 (PT12M) months post-treatment. (b) Cumulative rate of esophagogastric varices (EGV) aggravation after end of treatment according to liver stiffness measurement (LSM) at the start of antiviral therapy.
Figure 3.
(a) Changes in liver stiffness measurement (LSM). Patients were classified into three groups with respect to LSM at the start of antiviral therapy 15–20 kPa, 20–30 kPa, and ≥30 kPa. LSM was measured at baseline (Pre), SVR and 6 (PT6M) and 12 (PT12M) months post-treatment. (b) Cumulative rate of esophagogastric varices (EGV) aggravation after end of treatment according to liver stiffness measurement (LSM) at the start of antiviral therapy.
Figure 4.
Correlation between the diameter of the left gastric vein and total bile acid at the end of treatment.
Figure 4.
Correlation between the diameter of the left gastric vein and total bile acid at the end of treatment.
Figure 5.
Receiver operating characteristic curves for platelet count, liver stiffness measurement (LSM), and diameter of the left gastric vein (LGV) and total bile acid at SVR.
Figure 5.
Receiver operating characteristic curves for platelet count, liver stiffness measurement (LSM), and diameter of the left gastric vein (LGV) and total bile acid at SVR.
Figure 6.
Cumulative rate of esophagogastric varices (EGV) aggravation after the end of treatment. Patients were divided into four groups based on risk factors (the diameter of left gastric vein ≥5.0 mm, liver stiffness measurement >18.0 kPa, platelet count <11.0×104/µL, and total bile acid ≥33.0 µmol/L). Patients having all four risk factors (thick solid line), three risk factors (thin solid line), two risk factors (long dotted line), ad with one or no risk factors (gray solid line).
Figure 6.
Cumulative rate of esophagogastric varices (EGV) aggravation after the end of treatment. Patients were divided into four groups based on risk factors (the diameter of left gastric vein ≥5.0 mm, liver stiffness measurement >18.0 kPa, platelet count <11.0×104/µL, and total bile acid ≥33.0 µmol/L). Patients having all four risk factors (thick solid line), three risk factors (thin solid line), two risk factors (long dotted line), ad with one or no risk factors (gray solid line).
Table 1.
Clinical characteristics of 167 patients.
Table 1.
Clinical characteristics of 167 patients.
| Category |
|
|
| Age, years |
|
74 (48–90) |
| Sex, male/female |
|
82/85 |
| Body mass index, kg/m2
|
|
22.3(14.7–39.1) |
| Total bilirubin, mg/dL |
|
0.8(0.3–3.6) |
| Aspartate aminotransferase, IU/L |
|
49(12–351) |
| Alanine aminotransferase, IU/L |
|
37(82–54) |
| Albumin, g/dL |
|
3.9(2.3–5.1) |
| Total cholesterol, mg/dL |
|
140(75–256) |
| Ammonia, μg/dL |
|
40(10–128) |
| Platelet count, ×104/μL |
|
9.8(3.0–29.5) |
| Prothrombin activity, % |
|
83(31–112) |
| Alfa-fetoprotein, ng/mL |
|
7.3(1.1–482.9) |
| FIB-4 index |
|
5.98(3.27–26.09) |
| ALBI score |
|
–2.56(–3.43– –1.28) |
| Liver stiffness measurement, kPa |
|
18.9(5.6–44.2) |
| Total bile acid, µmol/L |
|
32.3(1.73–17.7) |
| Autotaxin, mg/L |
|
1.89(0.76–43.29) |
| Past history of HCC, yes/no |
|
89/78 |
| Diameter of left gastric vein, mm |
|
4.9(2.8–13.9) |
| Diameter of splenorenal shunt, mm |
|
8.1(6.7–23.1) |
| Esophagogastric varices, F1/ F2 |
|
41/7 |
| Gastric varices, F1/F2 |
|
1/2 |
Table 2.
Univariate and multivariate analyses of risk factors associated with aggravation of esophagogastric varices after eradicating HCV.
Table 2.
Univariate and multivariate analyses of risk factors associated with aggravation of esophagogastric varices after eradicating HCV.
| Category |
Univariate analysis
P-value |
Multivariate analysis
HR (95% CI) P-value |
| Age, <70/70≤ years |
0.559 |
– |
– |
| Sex, male/female |
0.649 |
– |
– |
| Body mass index, <23/23≤kg/m2
|
0.562 |
– |
– |
| Total bilirubin, <1.0/1.0≤mg/dL |
0.656 |
– |
– |
| Aspartate aminotransferase, <30/30≤IU/L |
0.317 |
– |
– |
| Alanine aminotransferase, <20/20≤IU/L |
0.258 |
– |
– |
| Albumin, <4.2/4.2≤g/dL |
0.086 |
– |
– |
| Total cholesterol, <170/170≤mg/dL |
0.382 |
– |
– |
| Ammonia, <40/40≤μg/dL |
0.426 |
– |
– |
| Platelet count, <11.0/11.0≤×104/μL |
<0.001 |
3.769 (1.424–9.977) |
0.008 |
| Prothrombin activity, <80/80≤% |
0.351 |
– |
– |
| Alfa-fetoprotein, <5.0/5.0≤ng/mL |
0.763 |
– |
– |
| FIB-4 index, <4.39/4.39≤ |
0.482 |
– |
– |
| ALBI score, <–2.82/–2.82≤ |
0.095 |
– |
– |
| Liver stiffness measurement, <18.0/18.0≤kPa |
<0.001 |
4.834 (1.706–10.794) |
0.006 |
| Total bile acid, <33.0/33.0≤µmol/L |
<0.001 |
3.341 (1.350–8.173) |
0.009 |
| Autotaxin, <1.9/1.9≤mg/L |
0.008 |
1.921 (0.685–5.832) |
0.286 |
| Past history of HCC, yes/no |
0.022 |
1.532 (0.523–4.386) |
0.485 |
| Diameter of left gastric vein, <5.0/5.0≤mm |
<0.001 |
5.891(2.596–14.228) |
<0.001 |
| Diameter of splenorenal shunt, <8.0/8.0≤mm |
0.051 |
– |
– |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).