Introduction
Stroke, a significant cause of morbidity and mortality globally, is a primary precipitant of seizures and epilepsy in adults. The occurrence of seizures following a stroke, commonly referred to as post-stroke seizures (PSS), can complicate recovery and significantly impact the quality of life. When seizures recur over time, this condition may evolve into post-stroke epilepsy (PSE). Understanding the distinction between these two conditions and their management is crucial for improving patient outcomes. [
1]
Post-Stroke Seizures (PSS)
Post-stroke seizures are relatively common, occurring in a notable subset of stroke survivors[
2,
3]. These seizures can be classified based on their timing:
Early Seizures: Occurring within the first week after a stroke, these are typically provoked by the acute effects of the stroke, such as brain injury, hemorrhage, or metabolic disturbances.
Late Seizures: These occur after the first week post-stroke and are considered more likely to indicate the development of epilepsy.
The pathophysiology of post-stroke seizures involves acute neuronal injury, excitotoxicity, and metabolic changes that lead to hyperexcitability in the affected brain regions. Hemorrhagic strokes, large cortical infarcts, and specific stroke subtypes are more likely to result in early seizures. [
4,
5]
Post-Stroke Epilepsy (PSE)
When a patient experiences recurrent seizures beyond the acute phase of stroke recovery, the condition is termed post-stroke epilepsy. PSE is a form of secondary epilepsy where the stroke acts as the initial insult that triggers chronic epileptogenic processes. [
6,
7]
Factors Contributing to PSE Development:
Stroke Severity: Larger and more severe strokes have a higher risk of resulting in PSE.
Stroke Type: Hemorrhagic strokes carry a greater risk compared to ischemic strokes.
Location: Cortical involvement significantly increases the risk of developing PSE.
Early Seizures: The presence of early post-stroke seizures is a strong predictor of subsequent epilepsy. (
Table 1)
Clinical Implications and Management
Diagnosis
The diagnosis of PSS and PSE requires a detailed medical history, neurological examination, and appropriate imaging studies to identify the stroke and its aftermath. Electroencephalography (EEG) is essential for detecting abnormal electrical activity indicative of seizures or epilepsy [
8,
9]
Treatment
Antiseizure Medications (ASMs): The choice of ASM is crucial and should be tailored to individual patient profiles, considering factors such as stroke type, patient age, and comorbidities. Lacosamide, levetiracetam, and lamotrigine are commonly used due to their favorable side effect profiles and efficacy [
10,
11].
Levetiracetam, commonly used in post-stroke epilepsy, may induce P-glycoprotein (P-gp), potentially interacting with direct oral anticoagulants (DOACs). Many recent studies analysis confirmed interactions between each DOAC and levetiracetam, but no interaction was found for hemorrhagic stroke. A strong signal was also observed with carbamazepine. However, the role of levetiracetam as a P-glycoprotein inducer in humans remains debated. Study results are conflicting; some data support the combination of NOACs and levetiracetam, suggesting that levetiracetam does not alter the plasma levels of NOACs. [
12,
13,
14].
Primary and Secondary Prophylaxis: There is no consensus on the use of prophylactic ASMs for preventing PSS or PSE, particularly in the absence of early seizures. The decision to initiate ASMs prophylactically should be based on the clinical context and individual risk factors.[
15,
16]
NOACs and ASMs: For patients with atrial fibrillation (AF) and a history of stroke, NOACs are often prescribed to prevent further thromboembolic events. The combination of NOACs and ASMs, such as lacosamide, has been shown to be safe and effective, without increasing the risk of recurrent ischemic or hemorrhagic events. A recent study has demonstrated that Levetiracetam is preferred over enzyme-inducing drugs when used in combination with apixaban and/or rivaroxaban, as it does not decrease their plasma concentrations, thereby preserving their anticoagulant efficacy [
17,
18].
Aim of the Study
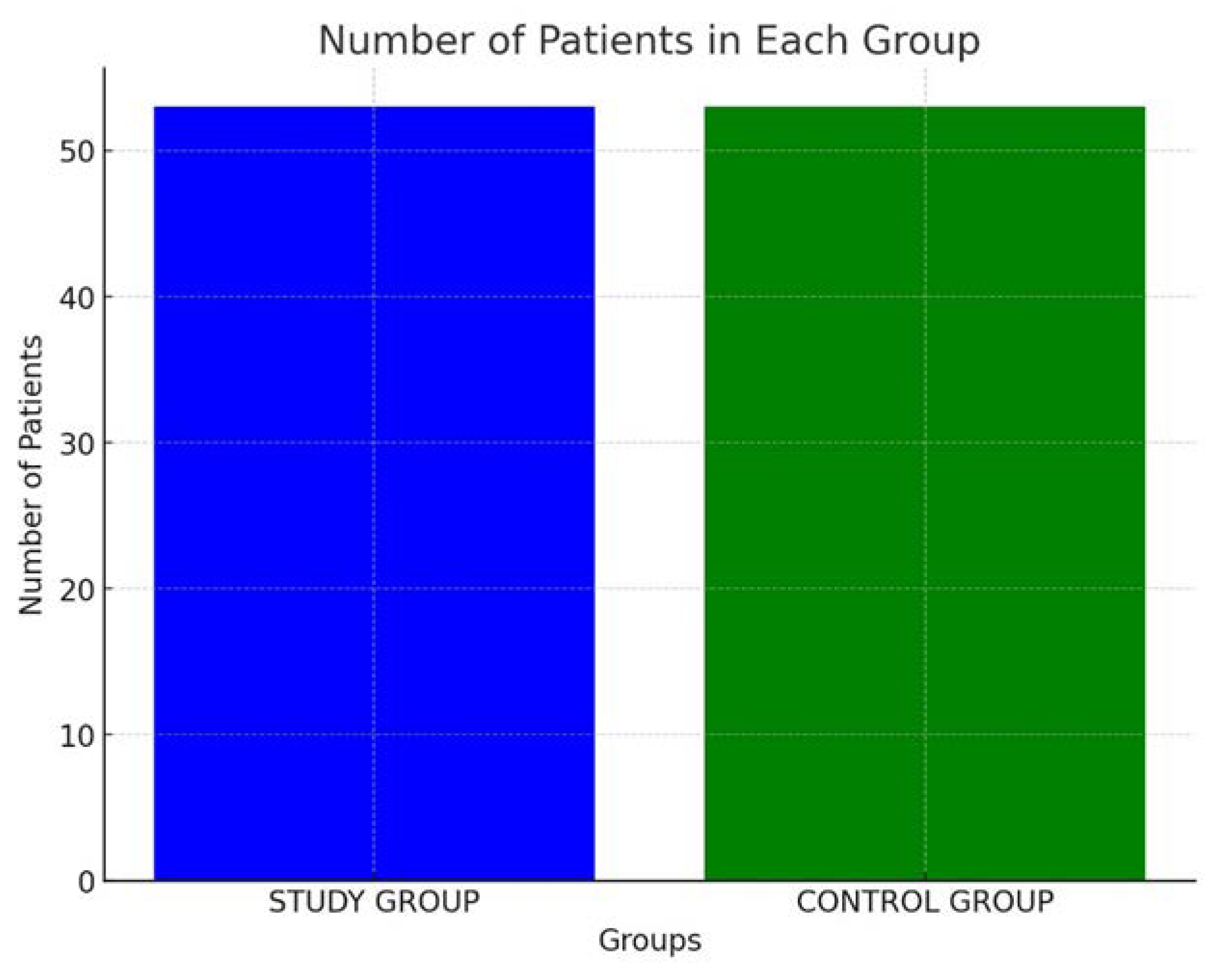
This prospective longitudinal study was conducted to evaluate the safety and efficacy of lacosamide in combination with non-vitamin K antagonist oral anticoagulants (NOACs) in patients with post-stroke epilepsy (PSE) and atrial fibrillation (AF). The study was carried out from September 2022 to May 2023, involving a cohort of 53 patients admitted to the stroke unit department. After discharge from the ward, the patients were followed in the outpatient clinic for 12 months, undergoing four follow-up neurological visits.
Study Design and Population
Study Group: The study group consisted of 53 patients diagnosed with PSE and AF. Each patient in this group was administered NOACs to manage AF and lacosamide to control seizures. The choice of NOACs was based on individual patient profiles, considering factors such as renal function, bleeding risk, and patient preference. Lacosamide, a newer ASM known for its efficacy and safety profile, was selected to address the seizure activity [
19,
20].
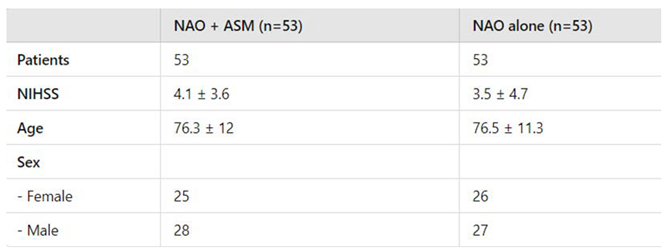
Control Group: A control group comprising 53 patients with cardioembolic stroke was established. These patients were receiving NOACs as part of their standard treatment protocol. The control group was meticulously matched to the study group based on several parameters, including age, sex, and mean National Institutes of Health Stroke Scale (NIHSS) scores, to ensure comparability and minimize confounding variables. (
Figure 1)
The characteristics of the study population are summarized in
Table 2
Objectives
Primary Outcome: The primary outcome of the study was twofold:
- 3.
Efficacy of Seizure Control: The effectiveness of lacosamide in managing and controlling seizures in patients with PSE.
- 4.
Safety Profile: The safety of the combination therapy, particularly focusing on the recurrence rate of minor or major embolic events over a 24-month follow-up period. This included monitoring for ischemic strokes, hemorrhagic strokes, and other embolic complications.
Methodology
Patient Monitoring and Follow-Up
Patients were monitored regularly through clinical visits, telemedicine consultations, and routine diagnostic tests, including EEG and MRI, to track seizure activity and detect any new embolic events. Blood tests were conducted periodically to monitor the efficacy of NOACs and to ensure no adverse interactions with lacosamide. Patient adherence to the medication regimen was reinforced through regular counseling sessions, and potential side effects were meticulously recorded and managed.
Data Collection and Analysis
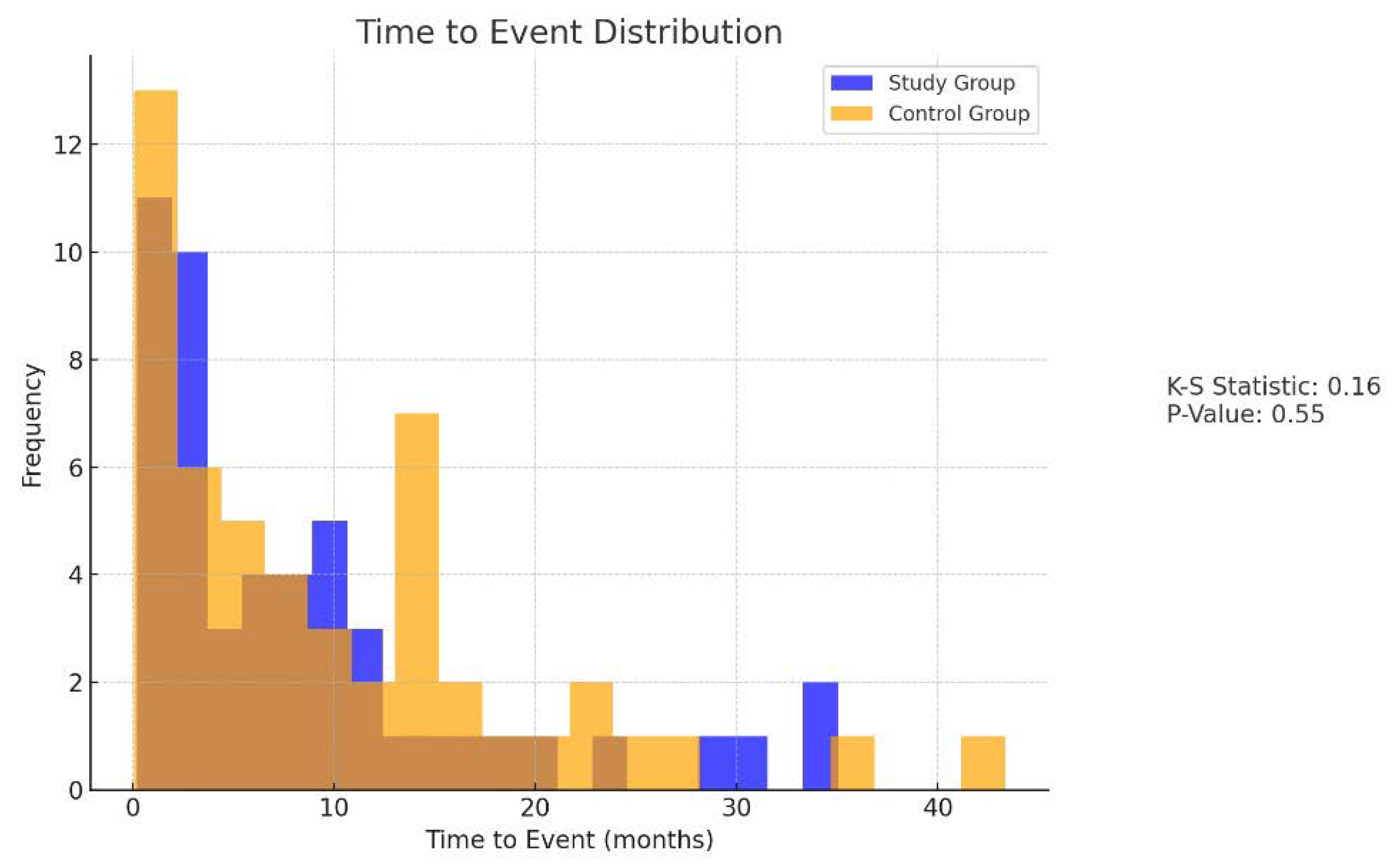
Detailed patient records were maintained, documenting demographic data, stroke characteristics, treatment regimens, and outcomes. Statistical analysis was performed to compare the incidence of embolic events and seizure control between the study and control groups. Kaplan-Meier survival analysis and Cox proportional hazards models were used to evaluate time-to-event data and identify predictors of recurrent embolic events. Mean, standard deviation, and proportions were calculated to summarize the baseline characteristics of the study population. Chi-square tests and Fisher’s exact tests were employed to compare categorical variables (e.g., sex, incidence of embolic events) between the groups. Independent t-tests were used to compare continuous variables (e.g., age, NIHSS scores). Multivariable Cox regression models were constructed to identify predictors of recurrent embolic events while adjusting for potential confounders. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated to quantify the association between variables (e.g., treatment group, age, stroke severity) and the risk of recurrent embolic events. Repeated measures ANOVA was employed to compare seizure control metrics between the study and control groups across multiple time points. (
Figure 2)
Results
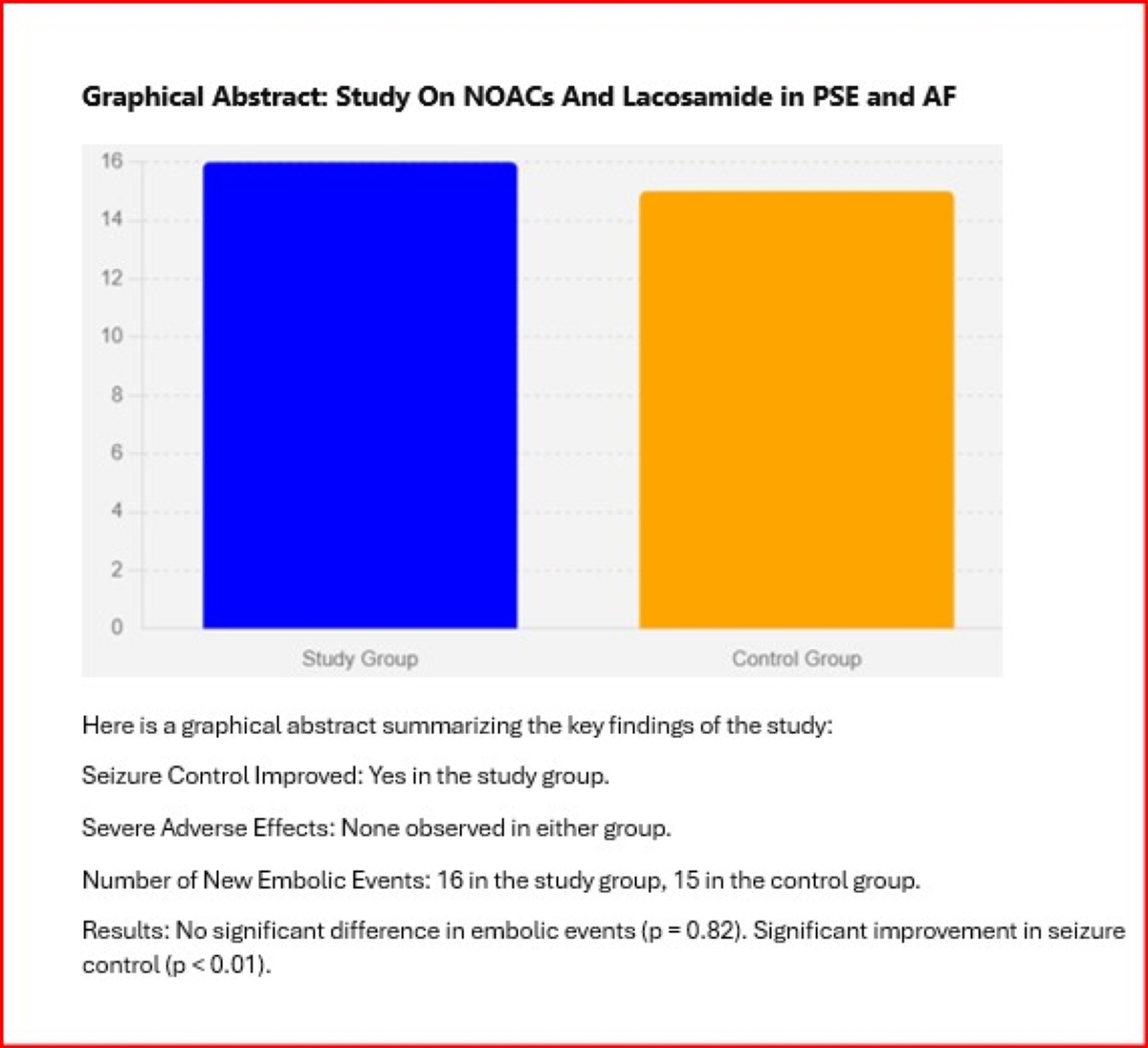
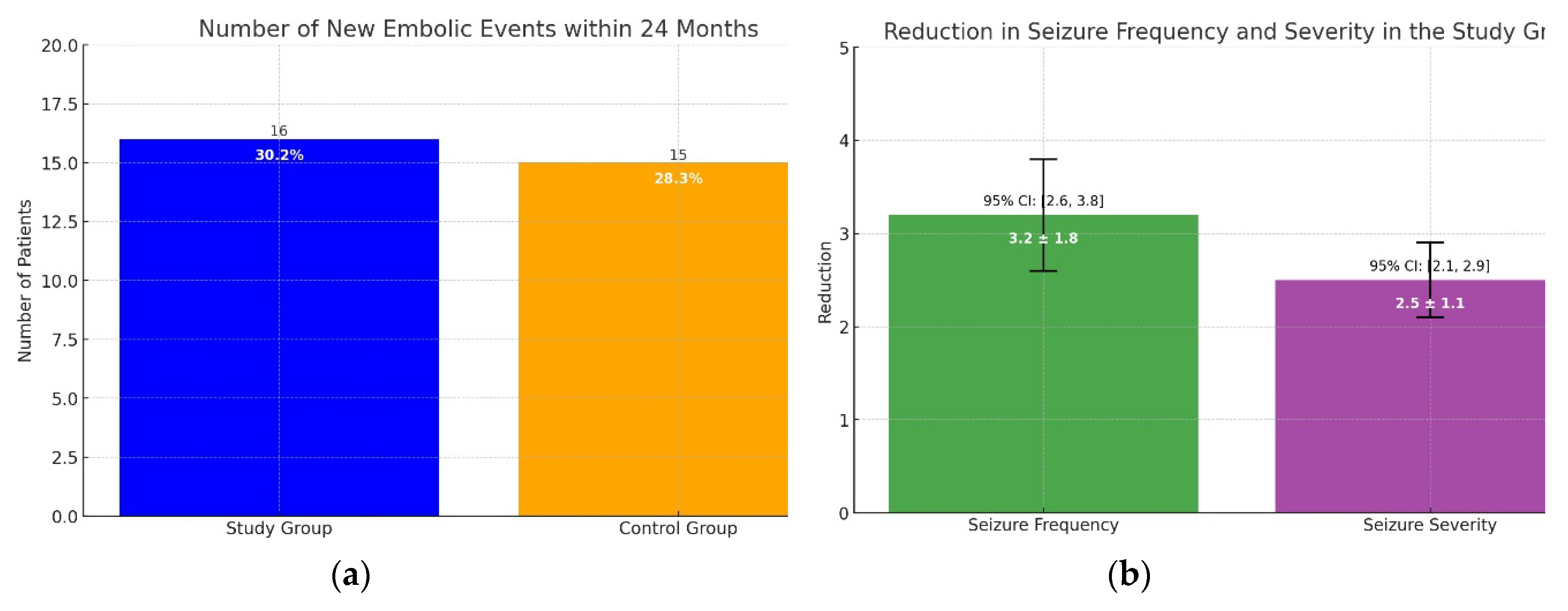
In the study group, 16 patients (30.2%) experienced a new embolic event within 24 months, compared to 15 patients (28.3%) in the control group. The difference between the two groups was not statistically significant (p = 0.82). The Kaplan-Meier survival analysis showed similar survival curves for both groups (Log-rank test, p = 0.78), indicating that the combination of NOACs and lacosamide did not increase the risk of recurrent ischemic or hemorrhagic events in patients with AF and PSE. Seizure control was effectively achieved in the majority of patients in the study group, with a significant reduction in seizure frequency and severity reported during the follow-up period. The mean reduction in seizure frequency was 3.2 ± 1.8 episodes per month (p < 0.01, 95% CI: 2.6 to 3.8), and the severity of seizures, measured using a standardized seizure severity scale, decreased by 2.5 ± 1.1 points (p < 0.01, 95% CI: 2.1 to 2.9). No severe adverse effects attributable to lacosamide were observed, reinforcing its safety profile when used alongside NOACs. Only one patient (1.9%) developed a second-degree atrioventricular block after taking lacosamide. Consequently, lacosamide was discontinued, and the patient was withdrawn from the study. These findings suggest that the combination of NOACs and lacosamide is both safe and effective for managing AF and PSE without increasing the risk of recurrent embolic events. (
Figure 3A,B).
Only one patient developed a second-degree atrioventricular block after taking lacosamide. Consequently, lacosamide was discontinued, and the patient was withdrawn from the study.
Discussion
These findings support the hypothesis that lacosamide, in conjunction with NOACs, is a safe and effective treatment strategy for patients with post-stroke epilepsy (PSE) and atrial fibrillation (AF). The absence of an increased risk of embolic events highlights the potential of this therapeutic combination to provide comprehensive management of both seizure activity and stroke prevention in this high-risk population. The results suggest that combining lacosamide with NOACs can offer dual benefits: reducing the frequency and severity of seizures while also preventing recurrent ischemic events. This dual approach is particularly advantageous for patients with AF, who are already at an elevated risk of stroke, and for those with PSE, where seizure control is paramount to improving quality of life. The study’s findings underscore the importance of safety when managing patients with comorbid conditions. The fact that no significant increase in embolic events was observed is crucial, as it reassures healthcare providers about the safety of adding lacosamide to the treatment regimen of patients already taking NOACs. This is particularly significant given the delicate balance required to manage anticoagulation in patients at risk of both seizures and thromboembolic events. While the results are promising, several limitations must be acknowledged:
Sample Size: The relatively small sample size of 53 patients in each group limits the generalizability of the findings. Larger, multicenter trials are needed to validate these results and ensure they are applicable to a broader population.
Follow-Up Duration: The follow-up period of 24 months may not be sufficient to capture all long-term outcomes and potential delayed adverse effects. Extended follow-up is necessary to assess the sustained efficacy and safety of the treatment combination.
Single-Center Design: As the study was conducted at a single center, there may be site-specific factors that influence the results. Multi-center studies would help mitigate this limitation and provide more robust data.
Real-World Applicability: The controlled clinical environment of the study may not fully reflect real-world clinical practice. Patient adherence, comorbid conditions, and other variables in everyday clinical settings could impact the outcomes.
Future studies with larger sample sizes and longer follow-up periods are warranted to confirm these data and further refine treatment protocols.
These future studies should aim to:
- Validate Findings: larger, multicenter trials are needed to validate these initial findings and ensure they are generalizable across different populations.
- Long-Term Outcomes: longer follow-up periods will help assess the long-term safety and efficacy of the lacosamide and NOAC combination, including any delayed adverse effects or changes in seizure control and stroke prevention over time.
- Comparative Studies: research comparing different NOACs and antiseizures medicaments (ASMs) will be valuable in identifying the most effective and safest combinations. This could lead to more personalized treatment strategies, optimizing outcomes based on individual patient profiles.
Exploring the impact of different NOACs and ASMs on patient outcomes could indeed provide more personalized treatment strategies for individuals with PSE and AF. Personalized medicine, which tailors treatment to the individual characteristics of each patient, has the potential to significantly improve clinical outcomes. Future studies should focus on:
-
Genetic and Biomarker Analysis: Identifying genetic markers or other biomarkers that predict response to specific ASMs and NOACs can help tailor treatments to individual patients.
Recent research has investigated the role of the glymphatic system and perivascular spaces as potential biomarkers for post-stroke epilepsy (PSE) [
21]
. Disruptions in these systems may contribute significantly to the development of epilepsy following a stroke. The findings suggest that abnormalities in the glymphatic system and perivascular spaces could be crucial indicators for the onset and progression of PSE. This discovery offers promising new avenues for early diagnosis and targeted therapy. The research underscores the potential of these biomarkers to enhance the understanding and management of epilepsy in stroke patients, potentially leading to more personalized and effective treatment strategies. Protein expression profiles in patients with PSE significantly differ from those in non-epileptic stroke patients, suggesting the involvement of multiple proteins in the development of post-stroke epileptogenesis. Notably, TNFSF-14 has been identified as a key predictive biomarker for PSE, with its downregulated levels showing strong potential in forecasting the risk of PSE. [
22,
23]
-
Drug-Drug Interactions: Knowledge about the pharmacokinetic and pharmacodynamic drug-drug interactions (DDIs) between antiseizure drugs and direct oral anticoagulants is limited, primarily based on in vitro and in vivo studies. Currently, there is no available data on DDIs with NOACs for approximately 67% of ASMs. As the number of patients requiring combined therapy with both, there is a critical need for comprehensive research in this area to inform clinical practice and ensure patient safety. Understanding the pharmacokinetics and pharmacodynamics of different ASMs and NOACs in combination is essential to avoid adverse interactions and optimize therapeutic efficacy [
24,
25]
- Patient Subgroups: Analyzing outcomes in various subgroups (e.g., age, sex, comorbidities) will help determine which patients are most likely to benefit from specific treatment combinations.
While the current study presents a positive outlook, it is essential to approach these findings with a degree of caution. The limitations of the study, including sample size and duration, must be acknowledged. Additionally, real-world applicability may vary, and thus, ongoing vigilance in monitoring and reporting adverse events in clinical practice is crucial. Moreover, as healthcare moves towards more integrative and holistic approaches, it is vital to consider the broader context of patient care. This includes the role of lifestyle modifications, patient education, and support systems in managing chronic conditions like PSE and AF. Future research should also explore these aspects to provide a more comprehensive understanding of how best to support patients in achieving optimal health outcomes.
Conclusions
Post-stroke seizures and epilepsy represent significant challenges in the rehabilitation of stroke survivors. Early recognition and appropriate management are critical to improving outcomes and enhancing the quality of life for affected individuals. While current guidelines provide a framework for treatment, ongoing research is essential to refine therapeutic strategies and develop more effective interventions for PSS and PSE. This study provides valuable insights into the management of PSE in patients with AF, demonstrating that the combination of lacosamide and NOACs is both safe and effective. These preliminary findings pave the way for further research and potential updates to clinical guidelines, aiming to improve the quality of life and clinical outcomes for stroke survivors with PSE.
References
- Feyissa, A.M.; Hasan, T.F.; Meschia, J.F. Stroke-related epilepsy. Eur. J. Neurol. 2018, 26, 18. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ihara, M. Post-stroke epilepsy. Neurochem. Int. 2017, 107, 219–228. [Google Scholar] [CrossRef]
- Zelano, J.; Holtkamp, M.; Agarwal, N.; Lattanzi, S.; Trinka, E.; Brigo, F. How to diagnose and treat post-stroke seizures and epilepsy. Epileptic Disord. 2020, 22, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Tröscher, A.R.; Gruber, J.; Wagner, J.N.; Böhm, V.; Wahl, A.-S.; von Oertzen, T.J. Inflammation Mediated Epileptogenesis as Possible Mechanism Underlying Ischemic Post-stroke Epilepsy. Front. Aging Neurosci. 2021, 13, 781174. [Google Scholar] [CrossRef] [PubMed]
- Altman, K.; Shavit-Stein, E.; Maggio, N. Post Stroke Seizures and Epilepsy: From Proteases to Maladaptive Plasticity. Front. Cell. Neurosci. 2019, 13, 397. [Google Scholar] [CrossRef] [PubMed]
- Freiman, S.; Hauser, W.A.; Rider, F.; Gulyaeva, N.; Guekht, A. Post-stroke epilepsy: From clinical predictors to possible mechanisms. Epilepsy Res. 2024, 199, 107282. [Google Scholar] [CrossRef]
- Mafla-Mendoza, A.P.; Paredes-Urbano, E.D.; Gea-Izquierdo, E. Risk Factors Associated with Epilepsy Related to Cerebrovascular Disease: A Systematic Review and Meta-Analysis. Neuropsychiatr. Dis. Treat. 2023, ume 19, 2841–2856. [Google Scholar] [CrossRef]
- Lee, D.A.; Jang, T.; Kang, J.; Park, S.; Park, K.M. Functional Connectivity Alterations in Patients with Post-stroke Epilepsy Based on Source-level EEG and Graph Theory. Brain Topogr. 2024, 1–10. [Google Scholar] [CrossRef]
- Šmigelskytė, A.; Rimkuvienė, G.; Žukaitė, D.; Repečkaitė, G.; Jurkevičienė, G. The Association of Epileptic Seizures after Acute Ischemic Stroke with Cerebral Cortical Involvement and Electroencephalographic Changes. Medicina 2024, 60, 768. [Google Scholar] [CrossRef]
- Wen, L.-M.; Li, R.; Wang, Y.-L.; Kong, Q.-X.; Xia, M. Electroencephalogram findings in 10 patients with post-stroke epilepsy: A retrospective study. World J. Clin. Cases 2024, 12, 249–255. [Google Scholar] [CrossRef]
- Trinka, E. , & Höfler, J. (2019). Antiepileptic drug treatment in post-stroke seizures: An update. Journal of Neurology, Neurosurgery & Psychiatry, 90(2), 142-149. [CrossRef]
- Kaoud, M.A.; Nissan, R.; Segev, A.; Sabbag, A.; Orion, D.; Maor, E. Levetiracetam Interaction with Direct Oral Anticoagulants: A Pharmacovigilance Study. CNS Drugs 2023, 37, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Mavri, A.; Ilc, S. The efficacy of direct oral anticoagulants in patients on concomitant treatment with levetiracetam. Sci. Rep. 2023, 13, 9257. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.; Rabkin, N.; Buchman, N.; Jacobs, A.R.; Sandouka, K.; Raccah, B.; Negev, T.F.; Matok, I.; Bialer, M.; Muszkat, M. The Effect of Levetiracetam Compared with Enzyme-Inducing Antiseizure Medications on Apixaban and Rivaroxaban Peak Plasma Concentrations. CNS Drugs 2024, 38, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A. A., Straus, S. E., & Rücker, G. (2018). Comparative safety and effectiveness of anti-seizure medications for post-stroke seizures: A systematic review and network meta-analysis. PLoS ONE, 13(7), e0200702. [CrossRef] [PubMed]
- Holtkamp, M.; Beghi, E.; Benninger, F.; Kälviäinen, R.; Rocamora, R.; Christensen, H.; European Stroke Organisat. European Stroke Organisation guidelines for the management of post-stroke seizures and epilepsy. Eur. Stroke J. 2017, 2, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, F.; Brandt, C.; Bozorg, A.; Dimova, S.; Steiniger-Brach, B.; Zhang, Y.; Ferrò, B.; Holmes, G.L.; Kälviäinen, R. Lacosamide in patients with epilepsy of cerebrovascular etiology. Acta Neurol. Scand. 2020, 141, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T., Battino, D., & Perucca, E. (2020). The management of epilepsy in the elderly: A critical review. Epileptic Disorders, 22(5), 441-461. [CrossRef] [PubMed]
- Hirsch, L. J., & LaRoche, S. M. (2021). Optimizing antiseizure medication therapy: Evidence-based recommendations and clinical guidance. Neurology Clinical Practice, 11(1), 49-61. [CrossRef] [PubMed]
- Chen, Y.; Li, W.; Lu, C.; Gao, X.; Song, H.; Zhang, Y.; Zhao, S.; Cai, G.; Guo, Q.; Zhou, D.; et al. Efficacy, tolerability and safety of add-on third-generation antiseizure medications in treating focal seizures worldwide: a network meta-analysis of randomised, placebo-controlled trials. EClinicalMedicine 2024, 70, 102513. [Google Scholar] [CrossRef]
- Rosenow, F.; Brandt, C.; Bozorg, A.; Dimova, S.; Steiniger-Brach, B.; Zhang, Y.; Ferrò, B.; Holmes, G.L.; Kälviäinen, R. Lacosamide in patients with epilepsy of cerebrovascular etiology. Acta Neurol. Scand. 2020, 141, 473–482. [Google Scholar] [CrossRef]
- Hlauschek, G.; Nicolo, J.; Sinclair, B.; Law, M.; Yasuda, C.L.; Cendes, F.; Lossius, M.I.; Kwan, P.; Vivash, L. Role of the glymphatic system and perivascular spaces as a potential biomarker for post-stroke epilepsy. Epilepsia Open 2023, 9, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Abraira, L.; López-Maza, S.; Quintana, M.; Fonseca, E.; Toledo, M.; Campos-Fernández, D.; Lallana, S.; Grau-López, L.; Ciurans, J.; Jiménez, M.; et al. Exploratory study of blood biomarkers in patients with post-stroke epilepsy. Eur. Stroke J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Abraira, L.; Santamarina, E.; Cazorla, S.; Bustamante, A.; Quintana, M.; Toledo, M.; Fonseca, E.; Grau-López, L.; Jiménez, M.; Ciurans, J.; et al. Blood biomarkers predictive of epilepsy after an acute stroke event. Epilepsia 2020, 61, 2244–2253. [Google Scholar] [CrossRef]
- Stöllberger, C.; Finsterer, J.; Schneider, B. Interactions between antiepileptic drugs and direct oral anticoagulants for primary and secondary stroke prevention. Expert Opin. Drug Metab. Toxicol. 2024, 359–376. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).