1. Introduction
Tomato (
Solanum lycopersicum) counts same as e.g. potato, pepper, and eggplant to the Solanaceae family. Vegetables from this plant family are by far the most consumed once in the world [
1]. After potatoes, tomatoes are the second most produced and consumed vegetable crop. In 2021 almost 189 million tonnes of fresh tomato on a global area of 5.16 million hectares were produced. The main production path is the cultivation in greenhouses. Each ton of produced fresh tomato leads to up to 1.34 t residues consisting of stems, leaves, and green cull tomatoes [
2,
3,
4,
5]. Worldwide hence 253 million tons of tomato plant residues are produced, which are often dumped in landfills or the landscape, which can cause problems such as blocking riverbeds. Uncontrolled burning or decomposition of the residues leads to a high amount of greenhouse gas emissions. Further, the tomato plant residues do have poisoning capacities for cattle and sheep, and they can cause environmental problems by spreading persistent pathogens, which infest the tomato plant at the end of the life cycle [
5,
6].
It seems obvious to develop an added value processing from tomato plant residues. Composting and vermicomposting are two used methods to downgrade plant residues and generate fertile soil. Due to the low C:N ratio and comparably high lignin content in the fibers of tomato residues, microbial composting is not an easy task [
5]. Furthermore, compost is mostly not needed in greenhouse cultivation as the plants are growing on substrates with a controlled nutrient supply. To make use of the tomato plant residues, anaerobic co-digestion can be used for the production of biogas. In the case of tomato biomass, it is hindered by the C:N ratio, which can be fixed by the addition of dairy manure and corn stover, which generates high logistic efforts and reduces thereby the environmental benefits to a certain point [
7].
High amounts of terpenes and terpenoids, polyphenolic compounds, and fatty acids in fruits and vegetables, as well as their pharmaceutical and health-promoting effects, are well-documented [
8,
9]. The antioxidative and antibacterial effects of polyphenol compounds, terpenes, and fatty acids were investigated in literature from a high variety of fruits and vegetables [
9,
10,
11,
12,
13].
Especially for tomato seeds and peels, which are generated as by-products during tomato processing, the constituents, as well as the content of polyphenols and their effects, are well-researched [
14,
15]. Not only the fruits but also the tomato plant residues contain bioactive compounds, which are produced as by-products of primary metabolic processes or for environmental benefits, e.g. protection against fungi, bacteria, and viruses or as attractants for insects [
2]. The bioactive compounds can be isolated via solvent extraction, however, most of the literature uses therefore non-green or toxic solvents, such as methanol, acetone, hexane, or dimethylformamide [
8,
16,
17]. Further, there is no literature available summarizing which molecules are in general present in the vegetative plant matter of tomatoes. The present study investigates the extraction of contained molecules in tomato plant residues by using conventional and green solvents. The extracts are then analyzed using gas chromatography coupled with mass spectrometry (GC-MS) and spectrophotometry.
2. Results & Discussion
2.1. GC-MS Results of Extracts
The main aim of the study is to determine the volatile species (extractables) and the content therefrom in the vegetative plant matter of tomato plants using green (vacuum soxhlet extraction (VSOX) and solid-liquid extraction(SLE) as extraction techniques. The extractables are classified into five groups, namely hydrocarbons, fatty acids/esters, terpenes/terpenoids, phenolic compounds, and uncategorized compounds. After integration and comparison of the chromatograms of all solvents, 383 peaks, including 46 solvent-related impurities are detected, the results are summarized in
Table S1 in the supplementary materials. Linking these peaks to three NIST libraries, and taking the solvent impurities and double detected species into account, 285 different compounds are identified (42 hydrocarbons, including alkanes and waxes, 6 phenolic compounds, 63 fatty acids/ester, and 34 terpenes/terpenoids and 140 uncategorized). In
Table S1 the compounds extracted with a respective solvent are grouped according to type and retention time. The impurities of the used solvents are excluded from tables and concentration determination. In
Table 1 the extraction method, the used solvents, the extracted number of compounds, and the total and individual group concentrations are presented. With hexane 25 to 40 compounds can be extracted with VSOX and SLE, respectively. In hexane overall 938.45 ± 103.64 NEQ kg
dw-1 in the VSOX and 1171.41 ± 58.36 mg NEQ kg
dw-1 in the SLE are determined. Comparing hexane, the most non-polar solvent to the most polar solvent, ethanol, the differences in the number of compounds as well as the concentrations in VSOX and SLE are significant. With ethanol, 240% and 159% more compounds by number in VSOX and SLE are extracted, and the overall concentration increases to 1814.62 ± 92.87 mgNEQ kg
dw-1 and 2068.48 ± 47.98 mgNEQ kg
dw-1, which represent an increase of 93.36% and 76.47%, respectively.
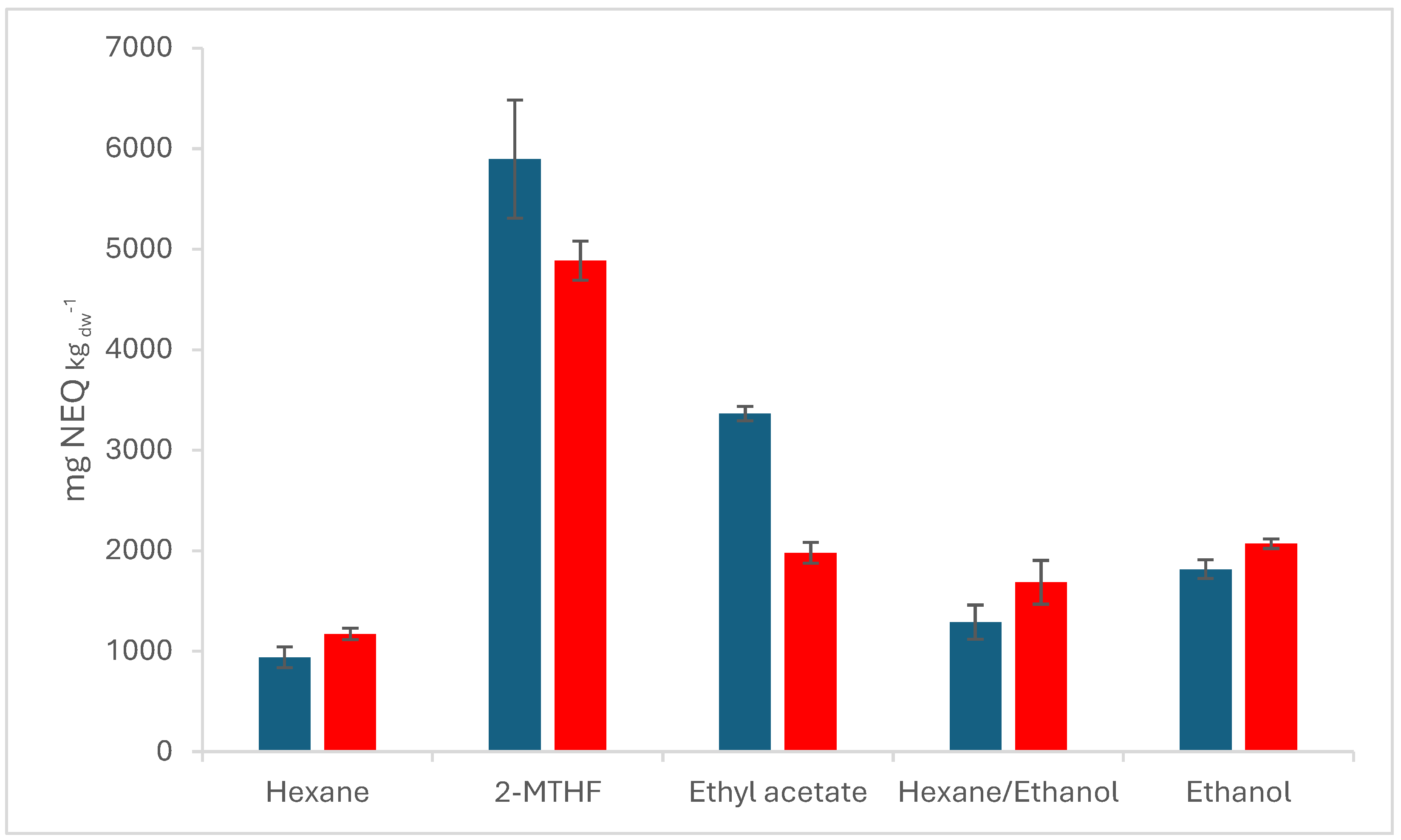
Figure 1 shows the total concentration of all substances as a sum parameter in mgNEQ kg
dw-1 of all used solvents at 50 °C, the 1:1 (v:v) mixture of hexane and ethanol fits with 1288.23 mgNEQ kg
dw-1 and 1684.7 mgNEQ kg
dw-1 into the trend line between the pure solvents for VSOX and SLE, respectively. 2-MTHF 2-methyl-tetrahydrofuran
Compared to pure hexane, the mixture of EtOH and hexane shows a concentration increase of 37% in VSOX and 44% in SLE, while compared to pure ethanol as solvent, the concentration decreases by 29% and 81% for the mixture in VSOX and SLE, respectively. This results indicate a much higher affinity of the compounds to polar and semi-polar solvents. The extracts using 2-methyl-tetrahydrofuran (2-MTHF) and ethyl acetate (EA) as solvents confirm with up to 102 compounds and 5894.82 mgNEQ kgdw-1 that semi-polar solvents, due to their better ability to dissolve polar and non-polar compounds, are preferable to pure hexane or ethanol.
The results of ethanol and its mixture further show, that vacuum has a positive effect on the number of extracted compounds. Under reduced pressure, 3% and 45% more with ethanol and the mixture with hexane are extracted based on the number of substances. At the same time, the application of light vacuum in a range of 510 to 260 mbar, according to the boiling point of the solvent at 50 °C (see
Table 3), in the extraction leads to a high loss of compounds, which can be noted by the decreasing total concentration. Hence, two different effects are working against each other. On the one hand, the vacuum enhances the extraction by disrupting the cell walls for better penetration of the solvent [
18], on the other hand, the reduced pressure compared with the solubility of the compounds in the respective solvent leads to a loss of components due to vapor pressure and flow regime. It can be noted that only for hexane, the worst working solvent in our experiments, the effect of the vacuum on the number of extracted compounds is negative. The correlation between the solubility and the vacuum loss can also be seen in the total concentration in
Figure 1. Although it was expected that VSOX extracts the highest concentration, for hexane, ethanol, and the mixture therefrom, which were the three solvents with the lowest extract concentration, a decrease of the total concentration for VSOX compared to SLE can be seen. Only VSOX for 2-MTHF and EA extracts overall show a higher total extract concentration compared to SLE. When hexane, ethanol, and the mixture with increasing polarity are compared, the differences between VSOX and SLE decrease. Especially for terpenes and terpenoids, and fatty acids and esters this is prominent. While for hexane the total concentration decreases by 78% and 64%, it decreases by 45% and 39% for the mixture of hexane and ethanol and by 15% and 12% for ethanol, respectively. Only hydrocarbons, including alkanes and waxes, show a higher concentration in hexane samples using VSOX. This also shows that the solubility of compounds in a respective solvent influences the total concentration. In our experiments, SLE leads to a 14% higher total concentration than VSOX for ethanol as solvent, 20% higher for hexane as the solvent, and 31% for the mixture of ethanol and hexane. Taticchi et al. also report losses of up to 71.3% for aldehydes, 51.9% for alcohols, and 64.4% for esters [
19]. With a low solubility of compounds in the solvent, the applied vacuum has a higher influence on the total concentration, and the loss of compounds, which are not condensed in cooling traps anymore. The higher solubility of compounds in 2-MTHF and EA results in a higher yield in terms of the number and concentration of compounds in the VSOX than in the SLE experiments, and hinders a relative decrease of the total and individual group concentration.
Both solvents, 2-MTHF and EA, have been tested as green solvents, as both can be produced sustainably and offer a semi-polar behavior, ranging between pure hexane, and pure ethanol with 0.179 and 0.228, respectively. This might also be the reason for the good solvent properties, as polar and non-polar compounds can be extracted. As can be seen in Table 2 both solvents used in the VSOX extraction had 99 ± 3 (EA) and 99 ± 8.83 (2-MTHF) different compounds and 5894 ± 586.48 and 3363.02 ± 71.14 mg NEQ kg
dw-1 for 2-MTHF and EA respectively. As can be seen in
Table 1 the slight pressure drop from 1 atm down to 350 mbar led to a significant increase of compounds with 48% and concentration with 70% that can be extracted from the tomato plant residues with EA.
As shown in
Table 1 the major groups of the extracts are fatty acids and esters, terpenes and terpenoids, and uncategorized compounds. Especially terpenes and terpenoids, and polyphenolic compounds are interesting for their bioactive properties.
2.1.1. Fatty Acids and Esters
Fatty acids are the primary compounds of cell membranes, regulating and signaling the activity of various proteins or acting as an energy source. They can be present in saturated or unsaturated form. Following the principle of solvent extraction, polar compounds are better soluble in polar solvents and vice versa, polar lipids, such as glycolipids, are more soluble in solvents such as ethanol, non-polar lipids, such as triacylglycerols are more soluble in non-polar solvents like hexane. The coexistence of polar and non-polar compounds is supported by the gathered results. During solvent extraction, the solvents 2-MTHF, ethyl acetate, and the mixture therefrom, offering a semi-polarity, lead to high numbers of fatty acids extracted. The highest fatty acid concentration was measured for 2-ethyl-2-methylbutanoic acid in 2-MTHF with 1985.9 ± 417.08 mg NEQ kg
dw-1, which accounts for 76% in VSOX and 89% in SLE of the total fatty acid and ester concentration in the extracts. While ethyl propionate is present in EA as the major compound with 519.57 ± 59.5 mg NEQ kg
dw-1, the content in the other solvents is with 0.92 ± 0.16 up to 8.88 ± 2.24 mg NEQ kg
dw-1 comparably low. Studies for propionate indicate, that it is an effective inhibitor of cholesterol synthesis [
20]. Ethyl butyrate is primarily extracted by hexane with a concentration of up to 683.17 ± 39.29 mg NEQ kg
dw-1 in SLE (58.83% of fatty acids and esters), with 140.52 ± 16.24 mg NEQ kg
dw-1 it is extracted in a smaller concentration by 2-MTHF and with 93 ± 12.77 mg NEQ kg
dw-1 by using EA as solvent. In hexane extracts from VSOX extraction dimethylamino ethyl palmitate is present in a concentration of 112.91 ± 5.36 mg NEQ kg
dw-1, this is related to 50% of the fatty acids and esters in the hexane VSOX extract. Ethanoic extracts contained 6.14 ± 0.13 mg NEQ kg
dw-1 and 37.51 ± 4.18 mg NEQ kg
dw-1 comparably small quantities of ethyl butyrate and dimethylamino ethyl palmitate. The main compound found in ethanol extracts is with 115.05 ± 1.39 mg NEQ kg
dw-1 2-mono palmitin. Minor compounds in ethanol, but richer than in other solvents, are linolenic acid, palmitic acid, and ethyl arachidate. With 8.04 ± 0 mg NEQ kg
dw-1 also 2-hexenoic acid is detected, which has been studied for its anticancer properties [
21]. Some further examples of minor detected fatty acids and esters, such as mandenol [
22], ethyl palmitate [
23]
, or ethyl linolenate [
24]. are summarized in
Table S1 in the supplementary material.
2.1.2. Hydrocarbons
Hydrocarbons, especially
n-alkanes, are present in living species, and plants. Their functions in plants range from functions in water regulation, and pollen viability to pathogen interaction [
25]. The highest hydrocarbon fraction was determined in VSOX extraction with EA (442.47 ± 146.61 mg NEQ kg
dw-1). The high deviation in the VSOX extraction is linked to two compounds, namely tetratriacontane, and 2-methyl-dotriacontane, which were found just in one EA VSOX sample in a quantity of 110.49 ± 0 and 106.03 ± 0 mg NEQ kg
dw-1, respectively. The high content of these two compounds is a hint to the presence of green cull tomatoes in the batch, as fruits have a higher hydrocarbon content on the surface than leaves, which are mostly present as waxes for fruit protection. Wu et al. reported a leave wax content of 8.26 µg/cm², whereas fruits have been reported by Bauer et al. with a mean surface wax amount of 50 µg/cm² [
26,
27]. The major compounds found in the extracts were tetratriacontane (110.49 ± 0 mg NEQ kg
dw-1) in EA, hexatriacontane (217.94 ± 13.89 mg NEQ kg
dw-1) in ethanol and hexane extracts, and tetratetracontane (152.68 ± 37.05 mg NEQ kg
dw-1) in 2-MTHF. The summarized research indicates antimicrobial and antioxidant activity related to the content of tetratriacontane and/or hexatriacontane as the major compounds [
28,
29,
30,
31]. Hydrocarbons, such as eicosane, pentadecane, heneicosane, and nonadecane have been reported to have bioactive properties, however, the concentration thereof is negligible [
32,
33,
34]. Further, anticancer properties are reported for the substance tetracosane that is present in the extracts in an amount of up to 56.81 mg NEQ kg
dw-1[
35].
2.1.3. Terpenes and Terpenoids
Terpenes and terpenoids are with approximately 40000 structures besides fatty acids and esters the largest occurring class of compounds in nature. A high number of terpenes and terpenoids are known for their bioactivity, playing an important role in plant growth, defense, symbiosis, and reproduction [
36,
37]. Especially the defense properties against pathogens and insects make terpenes and terpenoids interesting for research also related to the farm-to-fork strategy of the European Union to reduce permissible synthetic pesticides by 50% by 2030 [
38,
39]. The share of extracted terpenes and terpenoids to the total number of compounds is higher in non-polar solvents such as hexane (40.0 ± 4.0%) than in polar solvents such as ethanol or 2-MTHF with around 20%. Jiang et al. prove the behavior found in the present study. Primary terpenes and terpenoids are hardly soluble in water, which is linked to the non-polar properties of the terpene structure. The active groups on the terpene structure can shift the polarity. Especially hydroxyl groups are known to shift the polarity toward higher polarity. By that also the solubility of the terpenes shifts [
37]. Although the share of the terpenoids to the total number is decreasing with higher polarity, the share of the concentration of terpenes and terpenoids is increasing in solvents with higher polarity, such as hexane/ethanol with up to 73% of the concentration share. This can be attributed to the naturally occurring modifications of the compounds. The comparison of pure ethanol and its mixture with hexane shows that pure ethanol with up to 1354.7 ± 5.54 mg NEQ kg
dw-1 offers a 42% better solubility for terpenes. In SLE experiments 2-MTHF with 1407.97 ± 22.61 mg NEQ kg
dw-1 has reached higher concentrations than EA with 949.85 ± 167.84 mg NEQ kg
dw-1. In VSOX on the other hand EA reaches a 20% higher terpene concentration. It can be concluded that semi-polar solvents are better suited for the extraction of terpenes and terpenoids as EA and 2-MTHF show higher concentrations than ethanol, hexane, and the mixture therefrom.
The most abundant terpenes and terpenoids in the extracts are neophytadiene and its precursor phytol, which are researched for their antimicrobial, anticancer, anti-inflammatory, antipyretic, analgesic, and diuretic properties [
40]. The highest concentration of neophytadiene with 781.2 ± 112.66 mg NEQ kg
dw-1 is found in EA extracts followed by extracts using the solvent 2-MTHF with 718.25 ± 54.89 mg NEQ kg
dw-1. Nevertheless. ethanol extracts are also rich in neophytadiene with 523.98 ± 43.17 and 472.53 ± 96.88 mg NEQ kg
dw-1 for pure ethanol and the mixture, respectively. The neophytadiene concentration therefore is responsible for about 51% of the total terpene and terpenoids concentration of EA and 2-MTHF and about 38% for ethanol and its mixture as solvent. Only hexane shows with 26.75 ± 12.15 mg NEQ kg
dw-1 a significantly lower solubility for neophytadiene. As expected, the extractability of the neophytadiene precursor phytol in hexane is with 10.9 mg NEQ kg
dw-1 similar. The extractability of phytol increases significantly with increasing polarity of the solvent. Therefore, the phytol richest fractions are samples with ethanol as the solvent with a sum of 732.34 ± 91.44 mg NEQ kg
dw-1, followed by hexane and ethanol as the solvent with 574.83 ± 133.7 mg NEQ kg
dw-1. Extracts with EA and 2-MTHF show a concentration of 50 to 60% compared to pure ethanol. Besides these two major compounds, and minor fractions of different phytol isomers, bioactive terpenes and terpenoids, such as
D-limonene,
α-terpinene, squalene,
p-cymene,
trans-β-ionone, sabinene, and carophyllene have been detected, as shown in
Table S1 [
41,
42,
43,
44,
45].
2.1.4. Uncategorized
The group of uncategorized compounds summed up to 2203.7 mg NEQ kg
dw-1 based on the GC-MS evaluations. This category includes compounds such as alkaloids, aldehydes, metabolites, ketones, alcohols, amines, imines, ethers, pyranones, or furans. It can be seen, that the amount of these compounds is much higher in VSOX than in SLE extracts. The solvent 2-MTHF shows the highest content with 2203.7 ± 140,41, followed by EA with 653.51 ± 210.13, hexane with 623.76 ± 36.47, and ethanol with 460.87 ± 81.28 mg NEQ kg
dw-1. In SLE experiments 2-MTHF leads with 990.07 ± 154.39 mg NEQ kg
dw-1 the highest amount for `uncategorized` compounds, which is 123% less compared to VSOX. Ethanol extracted 531.3 ± 32.39 mg NEQ kg
dw-1, 15% more than in VSOX, but with almost similar value and the significantly higher variance of the VSOX with 460.87 ± 81.28 mg NEQ kg
dw-1 a better performance in the extraction of this group cannot be stated. SLE with EA and hexane lead to a content of 236.19 ± 122.14 and 9.75 ± 1.87 mg NEQ kg
dw-1, respectively. The high deviation in the EA extracts is related to three compounds, cis-9-hexadecenal (103.51 mg NEQ kg
dw-1) and the sugars 1,6-anhydro-
α-
D-galactofuranose (83.09 mg NEQ kg
dw-1) and
β-
D-glucopyranoside-methyl (54.33 mg NEQ kg
dw-1). All three compounds are valuable antifungal [
46] and antiviral [
47] agents. Although it was tried to uniformly mix the feedstock, the high content of these three compounds suggests the presence of a highly infected plant part in one EA extraction batch. While cis-9-hexadecenal was also present in a lower concentration in the other extraction batch using ethyl acetate as solvent (11.56 mg NEQ kg
dw-1), 1,6-anhydro-
α-
D-galactofuranose and
β-
D-glucopyranoside-methyl have been identified in only one batch using ethyl acetate.
β-D-Glucopyranoside-methyl was mostly present in hexane extracts with 438.26 ± 86.31 mg NEQ kg
dw-1 compared to 54.33 mg NEQ kg
dw-1 in one EA extract and 2.9 ± 0.47 mg NEQ kg
dw-1 in 2-MTHF.
Other major compounds in this group are tetrahydro-2
H-pyran-2-carboxylic acid,
(S)-1,3-butanediol, hexylene glycol diacetate, 2-methyl- pentanal, trimethylene acetate, and isocitric lactone. Except for trimethylene acetate, all these compounds appear only in 2-MTHF extracts. It can be concluded that 2-MTHF offers superior solubility for the compounds in this group compared to all other solvents. The data are summarized in
Table S1.
2.1.4. Phenolic Compounds
Phenolic compounds and derivatives are essential for plant growth and reproduction. Like terpenes, these group of compounds occur in all plants often functioning as antibacterial, -viral, and -oxidative agents acting as natural repellents and pesticides [
14]. As can be seen in
Table S1 the phenolic compounds 4,6-di(1,1-dimethylethyl)-2-methyl-phenol, 2,4-di-tert-butylphenol, 4-vinylguaiacol, 2-bromo-4-fluorophenol, 2,4-dimethyl-6-(1-phenylethyl)phenol and
DL-
α-tocopherol, have been identified using GC-MS. While the solvents EA, ethanol, and the mixture of hexane and ethanol extracted phenolic compounds in a concentration range between 1.23 and 46.15 mg NEQ kg
dw-1, no phenolic compounds were found in the GC-MS analysis using hexane and 2-MTHF as solvents. The identified compounds have been extracted with a maximum total phenolic concentration of 46.15 ± 23.91 mg NEQ kg
dw-1 using EA as solvent and VSOX. 4,6-Di(1,1-dimethylethyl)-2-methyl-phenol, 2,4-di-tert-butylphenol, and 2-bromo-4-fluorophenol are only found in extracts with EA as solvent. The phenols, 4-vinyl guaiacol and 2,4-dimethyl-6-(1-phenylethyl)phenol are only found in ethanol and ethanol hexane mixture, respectively.
DL-
α-tocopherol has been detected in all three extracts with EA, the 1:1 (v:v) mixture of ethanol and hexane, and ethanol, resulting in a maximum concentration of 15.46 ± 2.64 mg NEQ kg
dw-1 in EA.
DL-
α-tocopherol is an important substance in the human diet that has significant health benefits, such as neuroprotective, cardioprotective, and anti-inflammatory activities [
48].
Studies of industrial by-products of tomato skin and seeds reported a total phenolic content (TPC) of up to 44.18 mg GAE 100 g
-1 [
49] and 125.5 and 83.35 mg GAE g
extract-1 from leaves of two tomato cultivars by using the Folin-Ciocalteau method [
50]. These studies implicate a higher concentration of polyphenols in the biomass, which could not be identified and quantified in the present study. Reasons therefore are the non-volatile behaviour and thermoliability of these compounds. A more suitable analysis method for single phenolic compounds, which was not available, is high-performance liquid chromatography coupled with mass spectrometry, as in this method the compounds are not thermally stressed. To get comparable results with the literature, the total phenolic content of the extracts has been further determined.
2.2. Total Phenolic Content
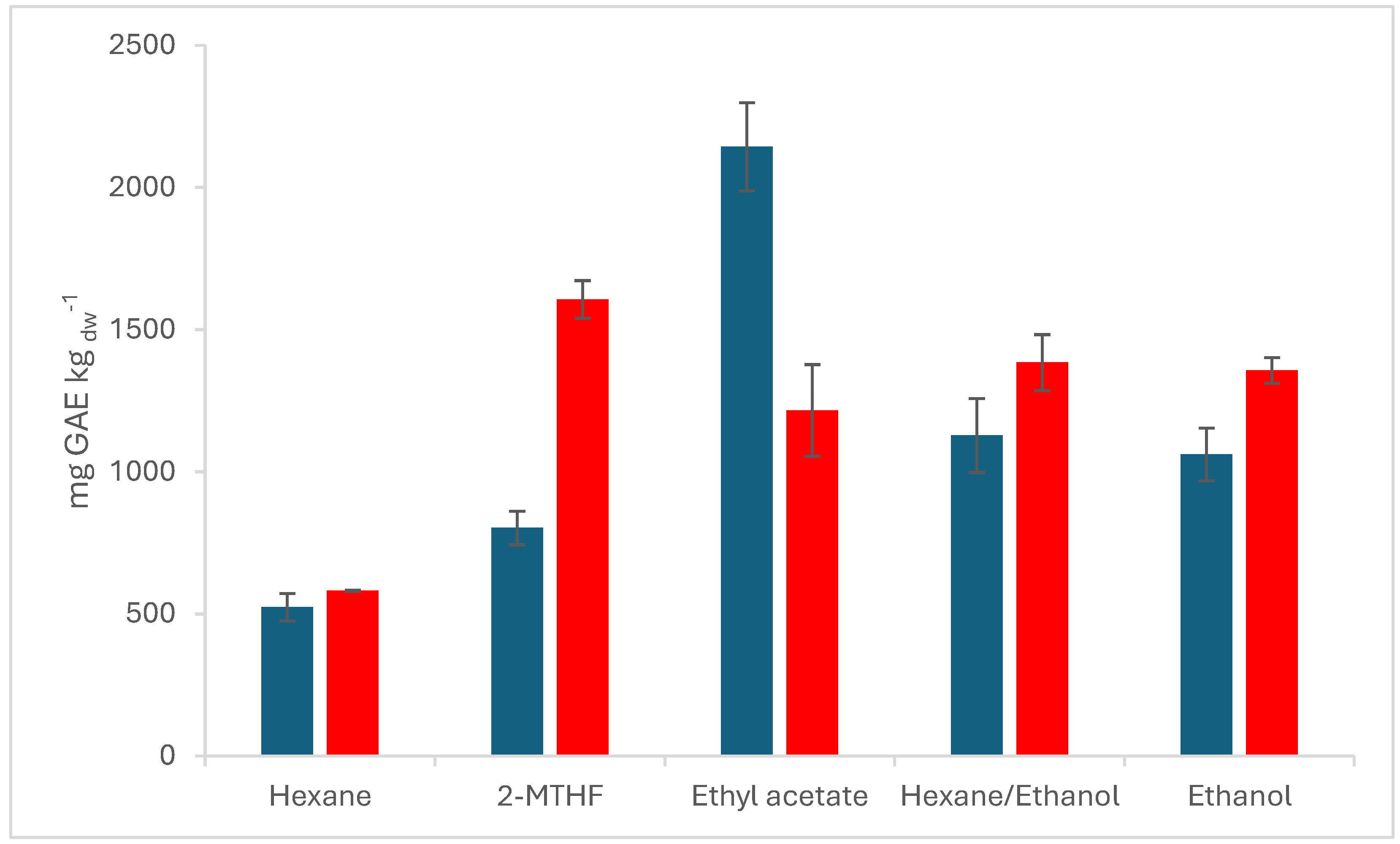
The TPC of the extracts at 50 °C ranged from 523 ± 49 mg kg
dw-1 for hexane to 2143 ± 155 mg gallic acid equivalent (GAE) kg
dw-1 in EA (see
Figure 2).
Silva-Beltrán et al. and Añibarro-Ortega et al. measured a total phenolic content of up to 125.5 mg g
extract-1 for the whole plant and 70.8 mg GAE g
extract-1 for pruning material and end-of-cycle aerial biomass [
50,
51], respectively. In the present study, the total phenolic content was 3 mg GAE g
extract-1 for hexane and 14 mg GAE g
extract-1 for EA, which is comparably low. However, the present study uses air drying as a preparation method, while the others used a vacuum oven- or freeze-drying. These methods are known to be more protective for the polyphenol content [
19,
52,
53]. Research from peach fruits shows that a temperature increase from 25 C to 70 C yielded a TPC decrease of 22%, while red wine freeze-drying preserved 70% of the four major polyphenolic compounds (gallic acid, catechin, epicatechin, and quercetin) [
52,
53].
The present research focuses on the extraction and identification of volatile compounds rather than on single phenolics or the optimization of a high total phenolic content. Further, to ensure a high extraction yield of compounds, the extraction time was set to 24 h, which strongly favors the oxidation of phenolic compounds, and thereof the loss of primary phenolic compounds [
54]. Mokrani et al. showed that the TPC of peach fruits reached a maximum after 3 h, while other compounds, such as flavonoids increased after 7.5 h. To get access to the phenolic compounds, temperatures below 60 °C, extraction times slower than 3 h, and fresh or freeze-dried feedstock shall be applied for the extraction of phenolics.
Taticchi et al. report an increased yield of phenolic compounds of up to 50.1% by applying a high vacuum and 60°C for the extraction of phenolic compounds. These findings could not be confirmed in the present study, as the TPC is higher in samples from SLE than in samples from VSOX extraction. Only in samples, where EA is used as the solvent, the TPC in VSOX is 2143 ± 155 mgGAE kg
dw-1 76% higher. EA was also the second-best working solvent from GC-MS analysis. The significantly high total phenolic content incorporated with the high
DL-
α-tocopherol content and higher hydrocarbon content from GC-MS analysis indicates the presence of green cull tomatoes in the sample. Samples using 2-MTHF as solvent leads with 1605.92 ± 66.46 mg GAE kg
dw-1 to double the concentration of TPC compared to VSOX with 801.98 ± 59.02 mg GAE kg
dw-1, ethanol as solvent performed 28%, the mixture of hexane and ethanol 23%, and hexane 11% better compared to their VSOX counterparts. A significant difference between polar and semi-polar solvents cannot be proclaimed. The data proves that semi-polar and polar solvents are favorable for the extraction of phenolic compounds, which is, due to the hydrophilicity of the phenolic compounds, expected [
55].
3. Materials and Methods
3.1. Reagents and Standards
As solvents hexane (99%, Roth, Karlsruhe, Germany), ethanol (99.9%, Austr Alco, Spillern, Austria), ethyl acetate (EA, 99.5%, Honeywell, Charlotte, North Carolina, US), and 2-methyl-tetrahydrofuran (2-MTHF, 99%, Sigma Aldrich, Darmstadt, Germany) were used. Naphthalene (99%, Roth, Karlsruhe, Germany) was used as an internal standard during GC-MS measurements. The solutions for the total phenolic content were prepared with the Folin-Ciocalteu reagent (2 M, Sigma-Aldrich, Darmstadt, Germany), sodium carbonate (≥99.5, Sigma Aldrich, Darmstadt, Germany), gallic acid (
TraceCERT, Sigma Aldrich, Darmstadt, Germany) and ultra-pure water (<2 µS, Model CB-1703, Adrona SIA, Riga, Latvia).
Table 3 summarizes the polarity value and the vapor pressure of the pure solvent at 50 °C [
56].
Table 3.
Polarity, type of pure solvent, and vapor pressure at 50°C.
Table 3.
Polarity, type of pure solvent, and vapor pressure at 50°C.
| Solvent |
Green solvent |
Polarity |
Extraction pressure soxhlet [mbara] |
| Ethanol |
Yes |
0.654 |
260 ± 10 |
| EA |
Yes |
0.228 |
350 ± 10 |
| 2-MTHF |
Yes |
0.179 |
340 ± 10 |
| Hexane |
No |
0.009 |
510 ± 10 |
3.2. Plant Material
For this study, fresh pruning and older whole tomato plant residues were provided by Frutura GmbH, Bad Blumau, Austria. The tomato plant is classified as a vine tomato. The exact species cannot be named due to business confidentiality.
The tomatoes were planted at the beginning of January on a substrate in glasshouses equipped with nutrition by drip watering. The sample collection was always done between 8 am to 10:30 am in one of the facilities, in April 2021 and consisted of leaves, stems, blossoms, and small green cull tomatoes. The fresh material was immediately transported to the lab, cut with sharp blades, to avoid too high crushing of cell membranes, into pieces of 3-5 mm and dried on paper towels for 72 h in a dark and highly ventilated room at ambient pressure and temperature. Fast processing after harvesting is mandatory, as mold formation of fresh material is a problem. After drying, the tomato plant residues were packed in sample bags and stored at -18°C in a freezer until usage.
3.3. Determination of Moisture and Ash Content
To evaluate the moisture content nine samples of fresh plant matter were investigated with air drying, freeze drying, and drying on a heated scale until no weight change was further measurable. On a heated scale at 80 °C a weight loss of 90.39 ± 0.1 wt% was measured. Drying fresh frozen samples in a freeze-dryer at -40 °C and 0.04 mbara for 96 hours also lead to a mass loss of 91.13 ± 0.39 wt%. Although this drying method is the gentlest and most preservative one for polyphenol compounds, the investigation of the extracts shows a significant loss of volatile compounds, due to the extremely low pressure. By air drying 79.01 ± 1.83 wt% in 48 h and 85.63 ± 2.86 wt% in 72 h at 22 ± 0.8 °C with a relative air humidity of 30 ± 2% were removed.
The ash content was determined by burning a defined amount of plant material at 600 °C in a muffle furnace for 12 hours. The samples were removed from the muffle furnace and cooled down in a desiccator. After cooling down, the samples were analyzed on a laboratory scale. The ash content of all samples was determined with 2 ± 0.1%.
3.4. Vacuum Soxhlet Extraction (VSOX)
During VSOX extraction the solvents hexane, ethanol, 2-MTHF, EA, and a mixture of hexane:ethanol 1:1 (v/v) were used. The VSOX experiments were performed at a constant boiling temperature of 50 °C and adjusted pressure of 260-510 mbara. To guarantee constant operation a solvent amount of 150 ml in the flask was necessary. The temperature of the solvent in the flask was controlled by a heating plate equipped with a flask heating block and a PT1000. The thimble was filled with 4-6 g of dried feedstock and placed in the apparatus. The vapor was condensed into the thimble using a pre-dimroth condenser at 0 °C and a coil condenser at -9 °C to prevent loss of solvent and compounds. After the extraction, the solvent was prepared for analysis and the extraction thimble was dried in an oven at 60 °C for 4 days and stored in a desiccator.
3.5. Solid-Liquid Extraction (SLE)
Samples were prepared with 4-6 g of dried plant material and a solid/liquid weight-to-volume ratio (w/v) of 1:10 in an Erlenmeyer flask. The flask was placed in a shaking water bath for 24 h at 50 °C and ambient pressure with a shaking rate of 120 rpm. After 24 h the samples were vacuum filtered with 150 mbara through a Whatman 589/3 filter and transferred to further sample preparation.
3.6. Sample Preparation
The respective solvent was evaporated at 250-350 mbara at 50 °C in a rotavapor until no further evaporation or solvent was detectable. The extracts were diluted with 1 ml of ethanol or hexan, depending on the solvent polarity, including naphthalene with a concentration of 150-450 ppm as internal standard. This procedure was also performed for pure solvents to determine the quality thereof and to account for failures due to impurities. The liquid sample was transferred into a 1.5 ml centrifuge tube and placed in a BioFuge (9000 rpm, 3 min) to separate the remaining small particles. The liquid phase was carefully raised with a syringe and pressed through a 0.2 µm PTFE filter (polar or non-polar) into a 0.2 ml GC vial and a 1.5 ml glass vial. The air in the vials was displaced with argon to avoid oxidation and stored at -18 °C until analysis.
3.7. GC-MS Analysis
The samples were analyzed using a Shimadzu (Kyoto, Japan) GC-MS-QP2010 SE with a VF-1701 MS column (60m × Ø 0.25mm, 0.25μm film thickness), an injection volume of 1 µl and a split ratio of 1:50. The temperature was increased from 40 °C up to 280 °C within 1 h. The generated chromatograms are integrated and the peaks are aligned with the NIST libraries NIST20R, NIST20M2, and NIST20M1. All compound concentrations were referred to the standard area of naphthalene as mg naphthalene equivalent (NEQ) * kg of dry weight (dw)-1.
3.8. Sample Preparation for the Measurement of the Total Phenolic Content (TPC)
The determination of the TPC was performed by using a modified Folin-Ciocalteu method described by Dahmoune et al. [
57]. For the method first, the sample was diluted with ethanol in a ratio of 1:80, then 100 µl of the sample was mixed with 750 µl of 10 times diluted (v/v) Folin-Ciocalteu reagent. The solution was incubated in the dark for 5 min at ambient temperature. Afterward, 750 µl of a 7.5% sodium carbonate solution was added and the vial was mixed on a shaking plate and stored for 90 min in the dark at room temperature. The reaction was stopped by cooling the vials in a refrigerator at 4 °C. The absorbance was measured with a Shimadzu (Kyoto, Japan) UV-1800 UV spectrophotometer at 765 nm and 22 °C with a reference. The reference was prepared the same as the samples, but instead of the extract pure solvent was used. Gallic acid (0.32 – 5.73 mg/ml) was used to create the calibration curve (y = 14890x - 16.87; R² = 0.9928). The total phenolic content is expressed as mg gallic acid equivalent (GAE) kg
dw-1. The extracts were prepared in duplicates and measured independently in triplicates. The results are expressed in mean value ± standard deviation.
4. Conclusions
The present study investigates the composition of the vegetative mass of tomato plants. The material mainly consisted of pruning material and stems. Methods applied to the plant material are vacuum-assisted and conventional solid-liquid extraction using conventional and green solvents. The extracts were analyzed using gas chromatography coupled with mass spectrometry and evaluated using common NIST libraries. The total phenolic concentration was determined using spectrophotometry. A total of 383 peaks were measured with GC-MS, which could be assigned to 285 different components, consisting of 63 fatty acids and esters, 42 hydrocarbons, 6 phenolic compounds, 34 terpenes and terpenoids, and 140 uncategorized compounds, such as aldehydes, ketones, sugars, alcohols, amine, and amides. With a maximum number of 99 compounds and a maximum total concentration of 5894.82 ± 586.48 and 3363.02 ± 71.14 mg NEQ kgdw-1, 2-MTHF, and EA lead to the highest extraction efficiency, the solvents such as hexane and ethanol were able to extract 36.5 and 58 compounds, and 1171.41 ± 58.38 and 2068.48 ± 47.98 mg NEQ kgdw-1, respectively. Especially for compounds counting to the groups of fatty acids and esters, and terpenes and terpenoids, the alternative green solvents show beneficial extraction results. The results from GC-MS show, that the vacuum has a negative influence on the total extraction efficiency. In the case of the green solvents, 2-methyl-tetrahydrofuran and ethyl acetate, this vacuum-induced component loss was compensated by a better extraction efficiency.
For the extraction of phenolic compounds, semi-polar and polar solvents are favorable. The results from the spectrophotometry, using the Folin-Cioalteau method, compared to the GC-MS results show that further analysis of the samples is necessary to close the gap between the total phenolic content and the identified compounds from GC-MS.
Besides their non-toxicity and sustainability, the present study shows that green solvents, such as 2-methyl-tetrahydrofuran and ethyl acetate, have a better extraction efficiency compared to conventional solvents for the extraction of compounds from vegetative plant biomass, and are therefore a valid alternative.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Mean compound table for solvents. RT retention time, 2-MTHF 2-methyltetrahydrofuran, NEQ naphthalene equivalent, kgdw kilogram dry weight.
Author Contributions
Conceptualization, A.D. and M..K..; methodology, A.D. and L.S.; validation, A.D., formal analysis, A.D. and L.S.; investigation, A.D.; data curation, A.D..; writing—original draft preparation, A.D.; writing— editing, M.K.; visualization, A.D.; supervision, M.K., funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access Funding by the Graz University of Technology Open Access Publishing Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
To get access to data sets from GC-MS or UV-Vis spectrophotometry please contact the author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
No samples are available.
References
- Mueller, L.A.; Solow, T.H.; Taylor, N.; Skwarecki, B.; Buels, R.; Binns, J.; Lin, C.; Wright, M.H.; Ahrens, R.; Wang, Y.; et al. The SOL Genomics Network: a comparative resource for Solanaceae biology and beyond. Plant Physiol. 2005, 138, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Arab, M.; Bahramian, B.; Schindeler, A.; Valtchev, P.; Dehghani, F.; McConchie, R. Extraction of phytochemicals from tomato leaf waste using subcritical carbon dioxide. Innovative Food Science & Emerging Technologies 2019, 57, 102204. [Google Scholar] [CrossRef]
- Cáceres, L.A.; McGarvey, B.D.; Briens, C.; Berruti, F.; Yeung, K.K.-C.; Scott, I.M. Insecticidal properties of pyrolysis bio-oil from greenhouse tomato residue biomass. Journal of Analytical and Applied Pyrolysis 2015, 112, 333–340. [Google Scholar] [CrossRef]
- Fritsch, C.; Staebler, A.; Happel, A.; Cubero Márquez, M.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.; Montanari, A.; López, M.; et al. Processing, Valorization and Application of Bio-Waste Derived Compounds from Potato, Tomato, Olive and Cereals: A Review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Fernández-Gómez, M.J.; Díaz-Raviña, M.; Romero, E.; Nogales, R. Recycling of environmentally problematic plant wastes generated from greenhouse tomato crops through vermicomposting. Int. J. Environ. Sci. Technol. 2013, 10, 697–708. [Google Scholar] [CrossRef]
- Reinoso Moreno, J.V.; Pinna-Hernández, G.; Fernández Fernández, M.D.; Sánchez Molina, J.A.; Rodríguez Díaz, F.; López Hernández, J.C.; Acién Fernández, F.G. Optimal processing of greenhouse crop residues to use as energy and CO2 sources. Industrial Crops and Products 2019, 137, 662–671. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, D.; Li, G.; Lu, J.; Li, S. Solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover for biogas production. Bioresour. Technol. 2016, 217, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Baltacıoğlu, H.; Baltacıoğlu, C.; Okur, I.; Tanrıvermiş, A.; Yalıç, M. Optimization of microwave-assisted extraction of phenolic compounds from tomato: Characterization by FTIR and HPLC and comparison with conventional solvent extraction. Vibrational Spectroscopy 2021, 113, 103204. [Google Scholar] [CrossRef]
- Popescu, M.; Iancu, P.; Plesu, V.; Todasca, M.C.; Isopencu, G.O.; Bildea, C.S. Valuable Natural Antioxidant Products Recovered from Tomatoes by Green Extraction. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Abreu-Naranjo, R.; Paredes-Moreta, J.G.; Granda-Albuja, G.; Iturralde, G.; González-Paramás, A.M.; Alvarez-Suarez, J.M. Bioactive compounds, phenolic profile, antioxidant capacity and effectiveness against lipid peroxidation of cell membranes of Mauritia flexuosa L. fruit extracts from three biomes in the Ecuadorian Amazon. Heliyon 2020, 6, e05211. [Google Scholar] [CrossRef]
- Asif, A.; Farooq, U.; Akram, K.; Hayat, Z.; Shafi, A.; Sarfraz, F.; Sidhu, M.A.I.; Rehman, H.; Aftab, S. Therapeutic potentials of bioactive compounds from mango fruit wastes. Trends in Food Science & Technology 2016, 53, 102–112. [Google Scholar] [CrossRef]
- Dellai, A.; Souissi, H.; Borgi, W.; Bouraoui, A.; Chouchane, N. Antiinflammatory and antiulcerogenic activities of Pistacia lentiscus L. leaves extracts. Industrial Crops and Products 2013, 49, 879–882. [Google Scholar] [CrossRef]
- Drescher, A.; Kienberger, M. A Systematic Review on Waste as Sustainable Feedstock for Bioactive Molecules—Extraction as Isolation Technology. Processes 2022, 10, 1668. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Pyriochou, V.; Peristeraki, A.; Karathanos, V.T. Bioactive phytochemicals in industrial tomatoes and their processing byproducts. LWT 2012, 49, 213–216. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends in Food Science & Technology 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Luengo, E.; Condón-Abanto, S.; Condón, S.; Álvarez, I.; Raso, J. Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Separation and Purification Technology 2014, 136, 130–136. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Light, D.M. Tomato leaf volatile aroma components. J. Agric. Food Chem. 1987, 35, 1039–1042. [Google Scholar] [CrossRef]
- SONG, X.; LI, Y. CELL MEMBRANE DAMAGE BY VACUUM TREATMENT AT DIFFERENT PRESSURE REDUCTION RATES. J Food Process Engineering 2012, 35, 915–922. [Google Scholar] [CrossRef]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Minnocci, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Daidone, L.; Sebastiani, L.; Servili, M. High vacuum-assisted extraction affects virgin olive oil quality: Impact on phenolic and volatile compounds. Food Chem. 2021, 342, 128369. [Google Scholar] [CrossRef]
- Demigné, C.; Morand, C.; Levrat, M.A.; Besson, C.; Moundras, C.; Rémésy, C. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br. J. Nutr. 1995, 74, 209–219. [Google Scholar] [CrossRef]
- Sianipar, N.F.; Assidqi, K.; Hadisaputri, Y.E.; Salam, S.; Purnamaningsih, R.; So, I.G. Mutant Plant Tipobio Variety of Rodent Tuber (Typhonium Flagelliforme): Fatty Acids Compounds and in Vitro Anticancer Activity. E3S Web of Conf. 2023, 388, 1032. [Google Scholar] [CrossRef]
- Park, S.Y.; Seetharaman, R.; Ko, M.J.; Kim, D.Y.; Kim, T.H.; Yoon, M.K.; Kwak, J.H.; Lee, S.J.; Bae, Y.S.; Choi, Y.W. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264. 7 cells. Int. Immunopharmacol. 2014, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Kim, Y.-S.; Jung, E.; Lim, J.; Jung, K.S.; Kim, M.-O.; Lee, J.; Park, D. Melanogenesis inhibitory effect of fatty acid alkyl esters isolated from Oxalis triangularis. Biol. Pharm. Bull. 2010, 33, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Lea-Smith, D.J.; Ortiz-Suarez, M.L.; Lenn, T.; Nürnberg, D.J.; Baers, L.L.; Davey, M.P.; Parolini, L.; Huber, R.G.; Cotton, C.A.R.; Mastroianni, G.; et al. Hydrocarbons Are Essential for Optimal Cell Size, Division, and Growth of Cyanobacteria. Plant Physiol. 2016, 172, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Wu, H. ; Le Liu; Chen, Y. ; Liu, T.; Jiang, Q.; Wei, Z.; Li, C.; Wang, Z. Tomato SlCER1-1 catalyzes the synthesis of wax alkanes which increases the drought tolerance and fruit storability. Hortic. Res. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Schulte, E.; Thier, H.-P. Composition of the surface wax from tomatoes. Eur Food Res Technol 2004, 219, 487–491. [Google Scholar] [CrossRef]
- Swamy, M.K.; Arumugam, G.; Kaur, R.; Ghasemzadeh, A.; Yusoff, M.M.; Sinniah, U.R. GC-MS Based Metabolite Profiling, Antioxidant and Antimicrobial Properties of Different Solvent Extracts of Malaysian Plectranthus amboinicus Leaves. Evid. Based Complement. Alternat. Med. 2017, 2017, 1517683. [Google Scholar] [CrossRef]
- Karabay-Yavasoglu, N.U.; Sukatar, A.; Ozdemir, G.; Horzum, Z. Antimicrobial activity of volatile components and various extracts of the red alga Jania rubens. Phytother. Res. 2007, 21, 153–156. [Google Scholar] [CrossRef]
- Selvan, A.T.; Subramanian, N.S.; Ramadevi, M.; Prasad, B.S.G.; Muthu, S.K. Bioactive compound identification, phytochemical estimation, in-vitro antiinflammatory and antioxidant activity of Pupalia lappacea. Int. J. Pharmacogn. 2014, 596–604. [Google Scholar]
- Yassa, N.; Masoomi, F.; Rohani, R.S.; Hadjiakhoondi, A. Chemical composition and antioxidant activity of the extract and essential oil of Rosa damascena from Iran, population of Guilan. DARU Journal of Pharmaceutical Sciences 2009, 175–180. [Google Scholar]
- OKECHUKWU, P.N. Evaluation of anti-inflammatory, analgesic, antipyretic effect of of eicosane, pentadecane, octacosane, and heneicosane. Asian J Pharm Clin Res 2020, 29–35. [Google Scholar] [CrossRef]
- Wijayanti, D.R.; Dewi, A.P. Extraction and Identification Potent Antibacterial Bioactive Compound of Streptomyces sp. MB 106 from Euphorbia sp. Rhizosphere. BES 2022, 6, 84–88. [Google Scholar] [CrossRef]
- El-Shahir, A.A.; El-Wakil, D.A.; Abdel Latef, A.A.H.; Youssef, N.H. Bioactive Compounds and Antifungal Activity of Leaves and Fruits Methanolic Extracts of Ziziphus spina-christi L. Plants (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Eswaraiah, G.; Peele, K.A.; Krupanidhi, S.; Kumar, R.B.; Venkateswarulu, T.C. Identification of bioactive compounds in leaf extract of Avicennia alba by GC-MS analysis and evaluation of its in-vitro anticancer potential against MCF7 and HeLa cell lines. Journal of King Saud University - Science 2020, 32, 740–744. [Google Scholar] [CrossRef]
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant J. 2008, 54, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Kempinski, C.; Chappell, J. Extraction and Analysis of Terpenes/Terpenoids. Curr. Protoc. Plant Biol. 2016, 1, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Farm to Fork Strategy: For a fair, healthy and environmentally-friendly food system, 2020. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 8 April 2024).
- Vats, S.; Gupta, T. Evaluation of bioactive compounds and antioxidant potential of hydroethanolic extract of Moringa oleifera Lam. from Rajasthan, India. Physiol. Mol. Biol. Plants 2017, 23, 239–248. [Google Scholar] [CrossRef]
- Miller, J.A.; Lang, J.E.; Ley, M.; Nagle, R.; Hsu, C.-H.; Thompson, P.A.; Cordova, C.; Waer, A.; Chow, H.-H.S. Human breast tissue disposition and bioactivity of limonene in women with early-stage breast cancer. Cancer Prev. Res. (Phila) 2013, 6, 577–584. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Oncel, S.S. Recent Progress in Microalgal Squalene Production and Its Cosmetic Application. Biotechnol. Bioprocess Eng. 2022, 27, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials (Basel) 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Hoda, S.; Gupta, L.; Shankar, J.; Gupta, A.K.; Vijayaraghavan, P. cis-9-Hexadecenal, a Natural Compound Targeting Cell Wall Organization, Critical Growth Factor, and Virulence of Aspergillus fumigatus. ACS Omega 2020, 5, 10077–10088. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, L.; Marino, C. Expedient synthesis of 1,6-anhydro-α-D-galactofuranose, a useful intermediate for glycobiological tools. Beilstein J. Org. Chem. 2014, 10, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Trela, A.; Szymańska, R. Less widespread plant oils as a good source of vitamin E. Food Chem. 2019, 296, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Peschel, W.; Sánchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzía, I.; Jiménez, D.; Lamuela-Raventós, R.; Buxaderas, S.; Codina, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.d.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total Phenolic, Flavonoid, Tomatine, and Tomatidine Contents and Antioxidant and Antimicrobial Activities of Extracts of Tomato Plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Ćirić, A.; Martins, V.; Rocha, F.; Soković, M.D.; Barata, A.M.; Carvalho, A.M.; Barros, L.; Ferreira, I.C. Valorisation of table tomato crop by-products: Phenolic profiles and in vitro antioxidant and antimicrobial activities. Food and Bioproducts Processing 2020, 124, 307–319. [Google Scholar] [CrossRef]
- Abascal, K.; Ganora, L.; Yarnell, E. The effect of freeze-drying and its implications for botanical medicine: a review. Phytother. Res. 2005, 19, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L. ) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- González, G.C.; Rugolo, M.; Finimundy, T.C.; Ohaco, E.; Pildain, M.B.; Barroetaveña, C. Impact of Air- and Freeze-Drying Methods on Total Phenolic Content and Antioxidant Activity of Fistulina antarctica and Ramaria patagonica Fructification. Applied Sciences 2023, 13, 8873. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and solvent effects in organic chemistry, Fourth, updated and enlarged edition; Wiley-VCH: Weinheim, 2011; ISBN 3527632220. [Google Scholar]
- Dahmoune, F.; Boulekbache, L.; Moussi, K.; Aoun, O.; Spigno, G.; Madani, K. Valorization of Citrus limon residues for the recovery of antioxidants: Evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind. Crop. Prod. 2013, 50, 77–87. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).