1. Introduction
Reconstruction of individual patient data from Kaplan-Meier (KM) plots is a novel methodological approach that is increasingly being used to investigate therapeutic questions based on time-to-event endpoints [
1,
2,
3,
4], especially when the focus is on the long term. The Individual Patient Data from published Kaplan-Meier survival curves (IPDfromKM), also known as “Shiny” method, represents a groundbreaking approach to the study of anti-cancer agents that was implemented using artificial intelligence [
5]. Recently, the IPDfromKM methodology has been applied to several other areas, including cardiology and surgery [
6,
7,
8]. For these reasons, we chose to use this methodology to conduct an updated analysis of the evidence for first-line treatment of locally advanced or metastatic urothelial cancer, focusing on clinical trials that included long-term follow-up.
In this setting, first-line chemotherapy with platinum plus gemcitabine has been the standard of care for decades. Recently, maintenance treatment with avelumab, an immune checkpoint inhibitor, has been added in patients who respond to platinum, with encouraging results. In the Javelin-100 trial, avelumab reduced the risk of death by a quarter regardless of PD-L1 expression [
9]. It should however be noted that approximately 50% of patients are ineligible to cisplatin [
10]; these patients who usually receive carboplatin plus gemcitabine have generally a lower survival rate. Recently, several regimens have attempted to address this clinical need by offering new alternatives [
11].
Although some standard meta-analyses have been performed to compare the efficacy of these alternatives in this disease condition (especially immunotherapies [
12,
13]), long-term survival can be examined more accurately by presenting results using a KM curve rather than a hazard ratio. The IPDfromKM approach, based on reconstructing patient-level data, has been shown to perform well in these situations [
3,
6] and replaces the conventional forest plot used in standard binary meta-analyses with a survival plot containing as many KM curves as treatments considered. In this study we used the IPDfromKM method to provide a comparative overview of the main treatment options for locally advanced or metastatic urothelial cancer, determining their respective positions in the armamentarium and their comparative effectiveness.
2. Materials and Methods
2.1. Literature Search
A systematic search of the PubMed database was conducted to identify randomized controlled trials (RCTs) that met the eligibility criteria for the analysis. The final search was conducted on 22 April 2024. The search term was constructed as follows: [("urothelial carcinoma" OR "urothelial cancer" OR "bladder carcinoma" OR "bladder cancer") AND ("immune checkpoint inhibitor" OR ipilimumab OR nivolumab OR pembrolizumab OR atezolizumab OR durvalumab OR cemiplimab OR avelumab)]. The selection of articles was conducted in accordance with the PRISMA algorithm [
14].
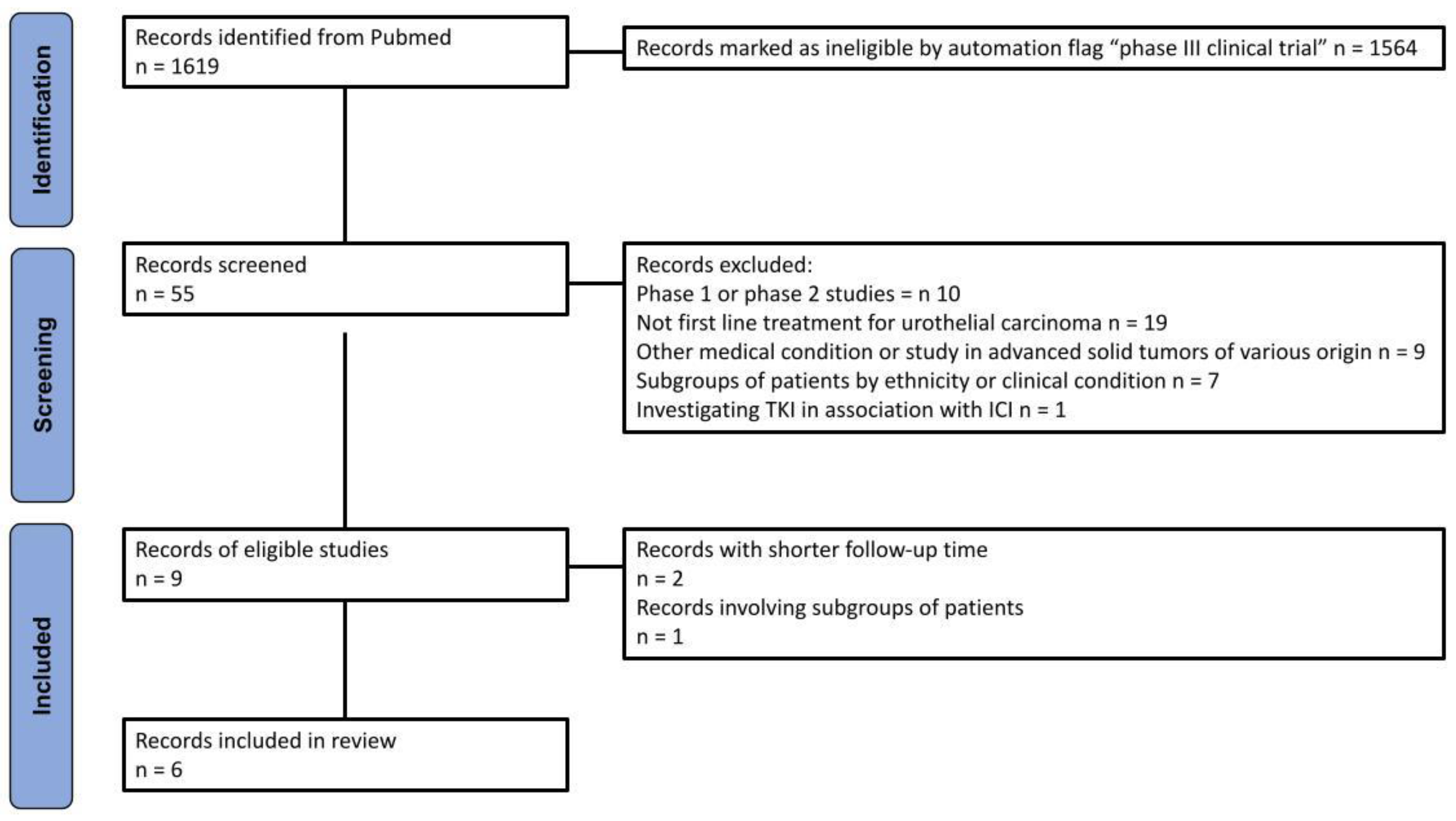
Our query identified 1,619 records. A total of 55 randomized interventional clinical trials were selected using the automated flag filter option, representing the only trials selected for this phase of the process. The main inclusion criteria were: (a) phase III trial; (b) first line treatment for urothelial carcinoma; (c) treatment with ICI in combination or not with other therapies (except tyrosine-kinase inhibitors); (d) OS endpoint; (e) results reported as a KM curve. In order to prevent the inclusion of the same patients in different trials, we prioritized the most recent publication.
2.2. Reconstruction of Individual Patient Data
Individual patient data were reconstructed from the KM curves of the treatment and control arms of each randomized clinical trial (RCT) using the IPDfromKM approach [
3,
5]. To begin, the KM curves were digitized utilizing Webplotdigitizer (version 4.7 online;
https://apps.automeris.io/wpd/, accessed 22 April 2024). Following this process, the x,y coordinates derived from the digitized curves, along with the total number of patients and events, were input into the 'Reconstruct Individual Patient Data' function of the IPDfromKM software (version: 1.2.3.0 online; last update: 22 March 2022). The software generated the reconstructed individual patient data for each RCT arms reporting survival time, defined as the difference between the date of enrolment and the last follow-up date. In addition, patient outcomes were reported, categorized as alive, dead or censored.
Platinum-based chemotherapy was considered the common comparator against which all other treatments were compared. Control groups were established by aggregating patients who received chemotherapy within the trials included in each step of analysis.
2.3. Inclusion and Exclusion of Individual Therapeutic Regimens in Our Main Analysis
To avoid an excessive number of treatments in the final analysis, it was necessary to consider three categories of experimental treatments separately:
- a)
ICI given as monotherapy;
- b)
Combinations of ICI with chemotherapy;
- c)
Combinations of ICI with other drugs.
A preliminary analysis of comparative efficacy based on indirect comparisons was conducted for each of the three categories above. The objective of this analysis was to identify the regimen exhibiting the most favorable outcome in terms of overall survival (OS) compared to chemotherapy. Then, in our main analysis, we included only the regimens that showed a survival benefit in categories (a) and (b) and finally we compared them with each regimen of category (c ).
2.4. Statistical Analysis
The efficacy of the different therapeutic options was evaluated in comparison with that of the pooled control arms by applying the Cox statistic to overall survival. The results were presented as hazard ratios (HR) with 95% confidence intervals (95% CI). To ascertain whether the control groups were homogenous, both the likelihood ratio test and the concordance test were employed.
A Cox model and restricted mean survival calculation were employed to perform indirect comparisons between active treatments (across all head-to-head combinations). The statistical analyses were conducted using the survival package in the R platform (version 4.3.2).
3. Results
3.1. Trial Selection and Design of Analysis
Figure 1 outlines the methodology employed for the literature search and identification of included trials, which was based on the PRISMA algorithm. A total of five trials, corresponding to six publications, were included in our analysis, which is presented in
Table 1. The efficacy of ICI as monotherapy or in combination with chemotherapy or other drugs was investigated, with gemcitabine plus platinum-based regimen serving as the common comparator.
In the initial phase of our OS analyses, we reconstructed individual patient data from the original KM curves of the six cohorts of patients listed in
Table 1. Subsequently, as previously described in
Section 2.3, we proceeded to assess OS in the following groups:
- a)
OS of ICIs used as monotherapy vs standard chemotherapy;
- b)
OS of ICIs combined with chemotherapy vs standard chemotherapy;
- c)
OS of ICI combined with other drugs vs standard chemotherapy.
All reconstructed patients deriving from the control arms of the studies included in each group were pooled to create a common comparator. A preliminary heterogeneity analysis was conducted to study these control arms composed of patients receiving platinum-based chemotherapy plus gemcitabine. Therefore, if no significant heterogeneity was present, patients deriving from the control arms of each trial were merged into a single control group, which was used as a common comparator.
3.2. Regimens’ Selection Based on Overall Survival Analyses
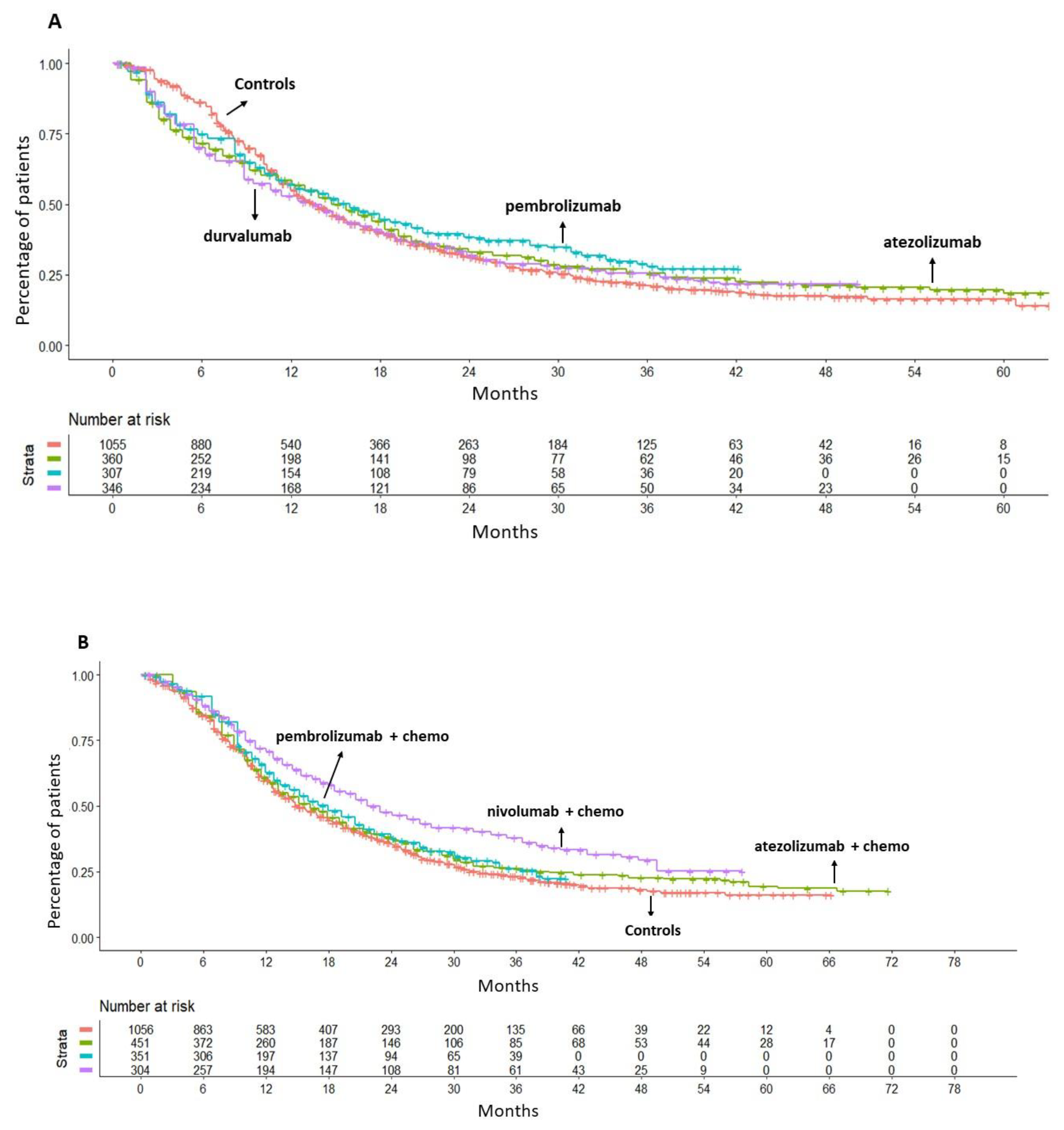
The efficacy of monotherapy with an ICI (atezolizumab, pembrolizumab or durvalumab) was compared to gemcitabine plus platinum-based chemotherapy. According to the results of this analysis, giving an ICI as monotherapy did not produce any significant OS benefit compared to chemotherapy; the values of HR were as follows: atezolizumab, HR= 0.98 (95%CI 0.85-1.13); pembrolizumab, HR= 0.89 (95%CI 0.76-1.05); durvalumab, HR=1.03 (95%CI 0.89-1.19) (
Figure 2A). Owing to the lack of a survival benefit, none of these three regimens was eventually included in our final analysis.
The preliminary analysis revealed no significant heterogeneity between the groups treated with chemotherapy in Imvigor-130, Keynote-361 and CheckMate-901 studies. The observed OS was found to be similar for patients treated with chemotherapy in the three selected trials. This finding provided the rationale for our decision to merge the three control groups into a single control group, which was used as a common comparator (
Figure S1A in the Supplementary material). Then, when ICI plus chemotherapy regimens were compared with this common comparator, neither atezolizumab plus chemotherapy (HR= 0.91, 95%CI 0.80-1.04) nor pembrolizumab plus chemotherapy (HR=0.89, 95%CI 0.76-1.03) were superior to chemotherapy alone in terms of OS. Conversely, nivolumab plus chemotherapy demonstrated a significant survival advantage in comparison to chemotherapy (HR=0.70, 95%CI 0.59-0.82) with a reduction of the death risk of 30% at a median follow-up of 33.6 months (
Figure 2B).
3.3. Main Analysis: Overall Survival of Chemotherapy-Free Regimens Compared with ICI Plus Chemotherapy
In consideration of the results presented in previous sections, our main analysis was undertaken to assess the efficacy of ICI combined with chemotherapy-free treatments compared to the only regimen of ICI plus chemotherapy that proved to be superior to chemotherapy alone in terms of OS (i.e. nivolumab plus chemotherapy). Therefore, the following three treatments were evaluated: i) nivolumab plus chemotherapy; ii) durvalumab plus tremelimumab; and iii) enfortumab vedotin plus pembrolizumab.
A preliminary heterogeneity analysis (
Figure S1B) involved the control arms of three trials: Danube, EV-302, and CheckMate-901. As shown in
Figure S1B, the KM curve of the CheckMate-901 control arm (study by van der Heiden et al., 2023) suggests that the controls in this study survived longer than those in the control arms of the Danube (study by Powles et al., 2020) and EV-302 (study by Powles et al., 2024) studies.
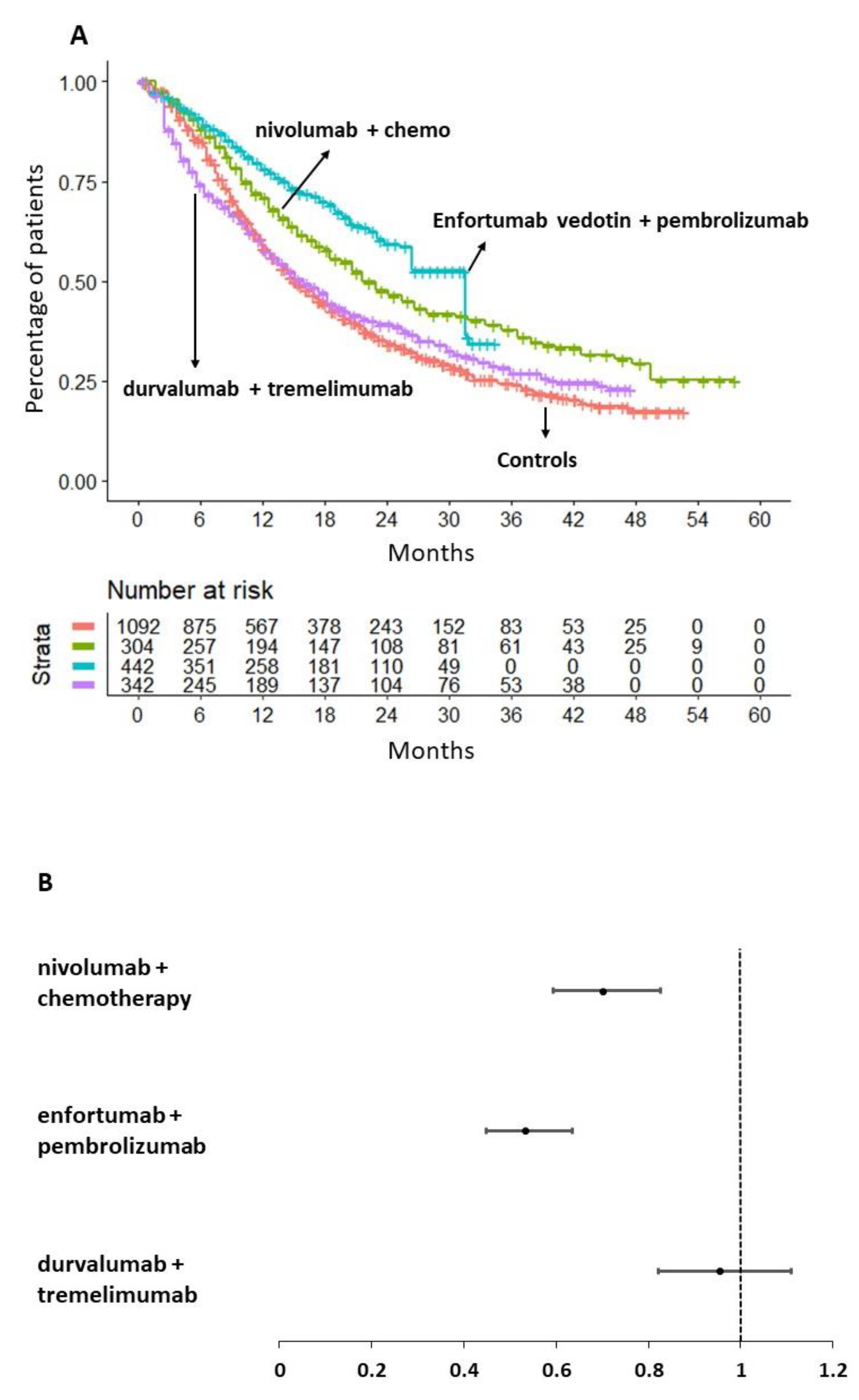
Our main analysis is shown in
Figure 3 (panels A and B). Its results indicate that nivolumab in combination with chemotherapy demonstrated superior efficacy in terms of OS compared to chemotherapy alone (HR= 0.70, 95%CI: 0.59-0.82). In contrast, durvalumab in combination with tremelimumab did not demonstrate any OS benefit compared to the aforementioned controls (HR=0.95, 95%CI 0.82-1.11).
It is noteworthy that the combination of enfortumab vedotin and pembrolizumab demonstrated a significantly superior OS compared to chemotherapy alone (HR=0.53, 95%CI 0.45-0.63) but also compared to nivolumab plus chemotherapy (HR= 0.76, 95%CI 0.60-0.97). The magnitude of the survival benefit was considerable in both cases. In
Figure 3A, the multi-treatment Kaplan-Meier curves based on reconstructed patients, which are typical of the IPDfromKM method, provide an effective graphical synthesis of all results generated by our main analysis.
Figure 3B shows the forest plot of HRs with 95% CI for the three therapeutic approaches selected for this analysis in comparison with the pooled control groups given chemotherapy.
To obtain a more comprehensive understanding of the survival benefit produced by enfortumab vedotin plus pembrolizumab over nivolumab plus chemotherapy, we calculated the RMST at 34 months. It was found that the combination of nivolumab plus chemotherapy provided a survival advantage of 3.5 months in comparison to chemotherapy alone (RMST, 21.78 months, 95%CI 20.40-23.15 vs RMST= 18.88, 95%CI 17.6-19.06, respectively). Conversely, the combination of enfortumab vedotin plus pembrolizumab was found to provide a survival advantage of two additional months (RMST=24.12, 95%CI 22.92-25.31) in comparison to nivolumab plus chemotherapy. Therefore, the combination of enfortumab vedotin and pembrolizumab was identified to be the optimal therapeutic option for the treatment of locally advanced or metastatic urothelial cancer.
4. Discussion
The present study describes the use of the IPDfromKM method to provide an updated overview of the current evidence for first-line treatment of locally advanced and metastatic urothelial cancer. Our systematic approach and simple layout allows us to highlight, at a glance, not only the superiority of enfortumab vedotin plus pembrolizumab over standard chemotherapy, as described in the pivotal trial [
18], but also over all other treatment options. The heterogeneity of survival in the control arms may have overestimated the effect of nivolumab plus chemotherapy, limiting the power of the results in the final analysis. Nevertheless, the significance of the additional benefit seen with enfortumab vedotin plus pembrolizumab is clear, and indeed this regimen has quickly emerged as the new standard of care for this disease. The benefit of this combination is independent of PD-L1 expression (consistent with previous reports for pembrolizumab in the first-line setting) but also independent of clinical factors such as Eastern Cooperative Oncology Group Performance Status (ECOG-PS) or the presence of liver metastases [
17,
18]. Further analyses are welcome to assess whether the efficacy of enfortumab vedotin plus pembrolizumab can be related to other patient selection biases.
The editorial by Niegisch et al. accompanying the publication of this study in the New England Journal of Medicine [
21] noted that while the increased efficacy seen with this new combination is undeniable, the question of cost arises. In the US, Ike et al. calculated that the estimated annual cost of treatment with enfortumab vedotin and pembrolizumab was 3.8 times higher than the cost of platinum-based chemotherapy followed by maintenance avelumab (
$455,630 vs
$120,253), which is the reference treatment for pricing in this context [
22]. As the Javelin Bladder 100 results would introduce a selection bias by including only platinum-eligible responders, we excluded them from the comparison in our study.
The preliminary heterogeneity of the final analysis (
Figure S1B), which included the control arms of three trials, indicates that patients in the CheckMate-901 control arm lived longer than patients in the Danube and EV-302 control arms. The probable selection bias in CheckMate-901 is related to the fact that patients had to be eligible for cisplatin therapy, without the treatment alternative of carboplatin, yielding a population of more fit patients with higher survival probabilities. However, this would further strengthen the superiority of enfortumab vedotin plus pembrolizumab and the results of this head-to-head comparison. It is important to emphasize that the platinum eligibility criteria will be less and less used in clinical practice after the widespread adoption of enfortumab vedotin + pembrolizumab in the first-line setting, and this categorisation will be considered for the choice of the subsequent lines of therapy. Another aspect that needs to be considered is the long-term safety profile of this combination; despite a lower percentage of grade 3-4 adverse events compared to platinum-based chemotherapy, peripheral sensory neuropathy associated with enfortumab vedotin could have a negative impact on quality of life [
23], given the expected high number of cycles of this drug administered in the first-line setting. Long-term safety data and patient-reported outcomes will help to clarify this issue [
24,
25].
5. Conclusions
This study used an innovative evidence-based technique that reconstructs individual patient data from published clinical trials focusing on first-line treatments for locally advanced or metastatic urothelial cancer. The aim of our analysis was twofold: firstly, to provide an updated overview of the main first-line treatments available for advanced urothelial cancer, highlighting the significant increase in efficacy seen with the latest regimen based on enfortumab vedotin, published just a few months ago in 2024. On the other hand, this study provides another important confirmation of the excellent performance of the IPDfromKM method in generating an original analysis based on indirect comparisons. Taking into account the inherent limitations of this technique, our results confirm that enfortumab vedotin plus pembrolizumab is the most effective combination in treatment-naive patients in terms of survival benefit, but other aspects - namely cost and safety - should be further investigated to draw the conclusions and assess the overall benefit of this new first-line therapy.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1A, Fig, S1B
Author Contributions
Conceptualization, Lorenzo Gasperoni, Luna Del Bono and Andrea Messori; Data curation, Luna Del Bono, Andrea Ossato and Vera Damuzzo; Formal analysis, Lorenzo Gasperoni, Andrea Ossato, Andrea Messori and Vera Damuzzo; Investigation, Lorenzo Gasperoni, Andrea Ossato and Emilio Giunta; Methodology, Andrea Ossato and Andrea Messori; Software, Andrea Messori; Supervision, Lorenzo Gasperoni and Luna Del Bono; Validation, Lorenzo Gasperoni, Luna Del Bono and Andrea Messori; Writing – original draft, Emilio Giunta, Andrea Messori and Vera Damuzzo; Writing – review & editing, Emilio Giunta, Andrea Messori and Vera Damuzzo.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and in the supplementary material.
Acknowledgments
Italian Society for Clinical Pharmacy and Therapeutics (SIFaCT), Turin, Italy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leung, J.H.; Wang, S.Y.; Leung, H.W.C.; Chan, A.L.F. Comparative efficacy and safety of multimodality treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: patient-level network meta-analysis. Front Oncol. 2024, 14, 1344798. [Google Scholar] [CrossRef] [PubMed]
- Nichetti, F.; Rota, S.; Ambrosini, P.; Pircher, C.; Gusmaroli, E.; Droz Dit Busset, M.; Pusceddu, S.; Sposito, C.; Coppa, J.; Morano, F. NALIRIFOX, FOLFIRINOX, and Gemcitabine With Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. JAMA Netw Open. 2024, 7, e2350756. [Google Scholar] [CrossRef] [PubMed]
- Messori, A.; Damuzzo, V.; Rivano, M.; Cancanelli, L.; Di Spazio, L.; Ossato, A. Application of the IPDfromKM-Shiny method to compare the efficacy of novel treatments aimed at the same disease condition: a report of 14 analyses. Cancers. 2023, 15, 1633. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C. , Rothschild, S., Villacampa, G., Heinrich, M.C., George, S., Blay, J.Y., Sicklick, J.K., Schwartz, G.K., Rastogi, S.; Jones, R.L.; et al. Rethinking placebos: embracing synthetic control arms in clinical trials for rare tumors. Nat Med. 2023, 29, 2689–2692. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021, 21, 111. [Google Scholar] [CrossRef] [PubMed]
- Messori, A. Synthetizing Published Evidence on Survival by Reconstruction of Patient-Level Data and Generation of a Multi-Trial Kaplan-Meier Curve. Cureus. 2021, 13, e19422. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Nie, Z.; Shi, R.; Yu, D.; Wang, Q.; Shao, F.; Wu, G.; Wu, Z.; Chen, T.; Li, C. Time to Benefit of Sodium-Glucose Cotransporter-2 Inhibitors Among Patients With Heart Failure. JAMA Netw Open. 2023, 6, e2330754. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Li, A.; Qu, M.; Nilius, H.; Shargall, Y. Prognostic significance of lymph nodes assessment during pulmonary metastasectomy: a systematic review and meta-analysis. J Thorac Dis. 2023, 15, 6447–6458. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Ullén, A.; Loriot, Y.; Sridhar, S.S.; Sternberg, C.N.; Bellmunt, J. , et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up. J Clin Oncol, 2023; 41, 3486–3492. [Google Scholar] [CrossRef]
- Moussa, M.J.; Campbell, M.T.; Alhalabi, O. Revisiting Treatment of Metastatic Urothelial Cancer: Where Do Cisplatin and Platinum Ineligibility Criteria Stand? Biomedicines. 2024, 12, 519. [Google Scholar] [CrossRef]
- Roviello, G.; Santoni, M.; Sonpavde, G.P.; Catalano, M. The evolving treatment landscape of metastatic urothelial cancer. Nat Rev Urol. 2024. [Google Scholar] [CrossRef]
- Maisch, P.; Hwang, E.C.; Kim, K.; Narayan, V.M.; Bakker, C.; Kunath, F. ; Dahm. P. Immunotherapy for advanced or metastatic urothelial carcinoma: an abridged Cochrane review. BJU Int, 2024. [Google Scholar] [CrossRef]
- Monteiro, F.S.M.; Soares, A.; Mollica, V.; Leite, C.A.; Carneiro, A.P.C.D.; Rizzo, A.; Bourlon, M.T.; Sasse, A.D.; Santoni, M.; Gupta, S.; Massari, F. Efficacy of immune checkpoint inhibitors combinations as first-line systemic treatment in patients with advanced urothelial carcinoma: A systematic review and network meta-analysis. Crit Rev Oncol Hematol. 2024, 196, 104321. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015, 350, g7647. Erratum in: BMJ 2016, 354, i4086. [CrossRef]
- Grande, E.; Arranz, J.Á.; De Santis, M.; Bamias, A.; Kikuchi, E.; Del Muro, X.G.; Park, S.H.; De Giorgi, U.; Alekseev, B.; Mencinger, M.; et al. Atezolizumab plus chemotherapy versus placebo plus chemotherapy in untreated locally advanced or metastatic urothelial carcinoma (IMvigor130): final overall survival analysis results from a randomised, controlled, phase 3 study. Lancet Oncol. 2024, 25, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Bamias, A.; Davis, I.D.; Galsky, M.D.; Arranz, J.Á.; Kikuchi, E.; Grande, E.; Del Muro, X.G.; Park, S.Hh; De Giorgi, U.; Alekseev, B.; et al. Atezolizumab monotherapy versus chemotherapy in untreated locally advanced or metastatic urothelial carcinoma (IMvigor130): final overall survival analysis from a randomised, controlled, phase 3 study. Lancet Oncol. 2024, 25, 46–61. [Google Scholar] [CrossRef]
- Powles, T.; Csoszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Morales Barrera, R; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. In Lancet Oncol.; 2021; Volume 22, pp. 931–945. [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J. , et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024, 390, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.L.; De Giorgi, U.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574-1588. Erratum in: Lancet Oncol. 2021, 22, e5. [CrossRef]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Niegisch, G. Enfortumab Vedotin and Pembrolizumab - A New Perspective on Urothelial Cancer. N Engl J Med. 2024, 390, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Ike, C.; Kongnakorn, T.; Tichy, E.; Benedict, A.; Sanchez Alvarez, J.; Kearney, M. Healthcare costs associated with first-line (1L) treatment of patients with locally advanced or metastatic urothelial carcinoma (la/mUC) in the United States (US). JCO Oncol Pract. 2023, 19, 11–11. [Google Scholar] [CrossRef]
- Fu, Z.; Gao, C.; Wu, T.; Wang, L.; Li, S.; Zhang, Y.; Shi, C. Peripheral neuropathy associated with monomethyl auristatin E-based antibody-drug conjugates. iScience. 2023, 26, 107778. [Google Scholar] [CrossRef] [PubMed]
- Milowsky, M.I.; O'Donnell, P.H.; Hoimes, C.J.; Petrylak, D.P.; Flaig, T.W.; Moon, H.H.; Friedlander, T.W.; Mar, N.; McKay, R.R.; Srinivas, S.; et al. Patient-Reported Outcomes in Patients With Advanced Urothelial Cancer Who Are Ineligible for Cisplatin and Treated With First-Line Enfortumab Vedotin Alone or With Pembrolizumab. J Clin Oncol. 2024, 42, 1403–1414. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Mamtani, R.; Sonpavde, G.P.; Loriot, Y.; Duran, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Sridhar, S.S.; Pappot, H.; et al. Health-related Quality of Life in Patients with Previously Treated Advanced Urothelial Carcinoma from EV-301: A Phase 3 Trial of Enfortumab Vedotin Versus Chemotherapy. Eur Urol. 2024, 85, 574–585. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).