1. Introduction
Oral surgery encompasses a wide range of procedures performed within the oral cavity. These procedures can involve tooth extraction, wisdom tooth removal, implant placement, jaw surgery, and other corrective or restorative interventions. While these procedures aim to improve oral health and function, they inevitably cause tissue disruption and initiate an inflammatory response [
1]. Inflammation is a complex biological process that serves a crucial role in wound healing by isolating and eliminating pathogens, promoting tissue repair, and restoring homeostasis. However, in the context of oral surgery, the inflammatory response can also contribute to postoperative discomfort, swelling, and potential complications [
2]. Understanding the mechanisms of inflammation after oral surgery is essential for optimizing patient recovery and developing novel therapeutic strategies.
The surgical procedure itself triggers a cascade of inflammatory events. Tissue injury leads to the release of various inflammatory mediators, including cytokines, chemokines, and vasoactive substances. These mediators promote the recruitment and activation of immune cells, such as neutrophils, macrophages, and lymphocytes, to the surgical site. Neutrophils are the first line of defense, engulfing and destroying bacteria and debris. Macrophages play a pivotal role in phagocytosis, debris clearance, and tissue repair by releasing growth factors and stimulating angiogenesis (new blood vessel formation). Lymphocytes participate in the adaptive immune response, providing long-term immunological memory [
3].
Vasoactive substances cause vasodilation (widening of blood vessels) and increased blood flow, leading to redness and swelling at the surgical site. This increased blood flow delivers essential immune cells, oxygen, and nutrients for healing [
4]. However, excessive inflammation can cause uncontrolled vasodilation and edema, contributing to facial puffiness and discomfort [
5].
Macrophages orchestrate the deposition of collagen, a key structural protein, to rebuild damaged tissues. However, prolonged or dysregulated inflammation can lead to excessive scar tissue formation, which can impair function and aesthetics [
6].
Postoperative inflammation after oral surgery typically manifests as swelling, pain, and tenderness at the surgical site. The degree of swelling can vary depending on the type and complexity of the surgery. Swelling usually peaks within 2-3 days and gradually subsides over the following week. Pain is another well-documented consequence of surgical intervention, and its management is typically achieved through the administration of analgesic medications [7-9].
While inflammation is a natural part of healing, excessive or prolonged inflammation can lead to complications. One such complication is dry socket, a painful condition that occurs when the blood clot protecting the exposed bone socket dissolves prematurely [
10].
Currently, the management of postoperative inflammation primarily relies on pharmacological interventions. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the cornerstone of therapy, acting by inhibiting the enzymes responsible for the production of prostaglandins, key inflammatory mediators. NSAIDs effectively alleviate pain and reduce swelling [
11]. However, NSAIDs can have gastrointestinal side effects and may not be suitable for all patients. Corticosteroids are another class of medications used to suppress inflammation. They are typically used for short periods due to potential side effects like delayed wound healing and increased susceptibility to infection [
12].
Maintaining oral hygiene after oral surgery procedures is crucial for a successful outcome, additionally it prevents the administration of the antibiotic therapy [
13]. Disruption of the oral tissues during surgery creates a vulnerable environment susceptible to bacterial colonization and potential infection. This can delay healing, increase discomfort, and even lead to complications [14-16]. Oral antiseptics have emerged as a valuable tool in the postoperative care regimen. These agents offer a targeted approach to reducing bacterial load and promoting a more favorable healing environment within the oral cavity [
17].
Chlorhexidine has been a mainstay in oral antisepsis for decades. Its broad-spectrum antimicrobial activity effectively targets a wide range of bacteria associated with oral infections [
18]. Studies have demonstrated its effectiveness in reducing plaque formation and promoting gingival healing after periodontal procedures [
19]. However, CHX can have drawbacks, including potential staining of teeth and taste alterations [
20]. Cetylpyridinium chloride presents itself as an alternative antiseptic option. It possesses antimicrobial properties and demonstrates efficacy in reducing plaque and preventing gingivitis [
21]. Additionally, CPC may be less likely to cause taste disturbances compared to CHX [
22]. Despite the established benefits of both antiseptics, the optimal use of CHX and CPC in the context of oral surgery remains a topic of ongoing research.
This study aims to evaluate the clinical outcomes of two different antiseptic protocols after various oral surgical procedures.
2. Materials and Methods
Oral surgery encompasses a wide range of procedures performed within the oral cavity. These procedures can involve tooth extraction, wisdom tooth removal, implant placement, jaw surgery, and other corrective or restorative interventions. While these procedures aim to improve oral health and function, they inevitably cause tissue disruption and initiate an inflammatory response [
1]. Inflammation is a complex biological process that serves a crucial role in wound healing by isolating and eliminating pathogens, promoting tissue repair, and restoring homeostasis. However, in the context of oral surgery, the inflammatory response can also contribute to postoperative discomfort, swelling, and potential complications [
2]. Understanding the mechanisms of inflammation after oral surgery is essential for optimizing patient recovery and developing novel therapeutic strategies.
The surgical procedure itself triggers a cascade of inflammatory events. Tissue injury leads to the release of various inflammatory mediators, including cytokines, chemokines, and vasoactive substances. These mediators promote the recruitment and activation of immune cells, such as neutrophils, macrophages, and lymphocytes, to the surgical site. Neutrophils are the first line of defense, engulfing and destroying bacteria and debris. Macrophages play a pivotal role in phagocytosis, debris clearance, and tissue repair by releasing growth factors and stimulating angiogenesis (new blood vessel formation). Lymphocytes participate in the adaptive immune response, providing long-term immunological memory [
3].
Vasoactive substances cause vasodilation (widening of blood vessels) and increased blood flow, leading to redness and swelling at the surgical site. This increased blood flow delivers essential immune cells, oxygen, and nutrients for healing [
4]. However, excessive inflammation can cause uncontrolled vasodilation and edema, contributing to facial puffiness and discomfort [
5].
Macrophages orchestrate the deposition of collagen, a key structural protein, to rebuild damaged tissues. However, prolonged or dysregulated inflammation can lead to excessive scar tissue formation, which can impair function and aesthetics [
6].
Postoperative inflammation after oral surgery typically manifests as swelling, pain, and tenderness at the surgical site. The degree of swelling can vary depending on the type and complexity of the surgery. Swelling usually peaks within 2-3 days and gradually subsides over the following week. Pain is another well-documented consequence of surgical intervention, and its management is typically achieved through the administration of analgesic medications [7-9].
While inflammation is a natural part of healing, excessive or prolonged inflammation can lead to complications. One such complication is dry socket, a painful condition that occurs when the blood clot protecting the exposed bone socket dissolves prematurely [
10].
Currently, the management of postoperative inflammation primarily relies on pharmacological interventions. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the cornerstone of therapy, acting by inhibiting the enzymes responsible for the production of prostaglandins, key inflammatory mediators. NSAIDs effectively alleviate pain and reduce swelling [
11]. However, NSAIDs can have gastrointestinal side effects and may not be suitable for all patients. Corticosteroids are another class of medications used to suppress inflammation. They are typically used for short periods due to potential side effects like delayed wound healing and increased susceptibility to infection [
12].
Maintaining oral hygiene after oral surgery procedures is crucial for a successful outcome, additionally it prevents the administration of the antibiotic therapy [
13]. Disruption of the oral tissues during surgery creates a vulnerable environment susceptible to bacterial colonization and potential infection. This can delay healing, increase discomfort, and even lead to complications [14-16]. Oral antiseptics have emerged as a valuable tool in the postoperative care regimen. These agents offer a targeted approach to reducing bacterial load and promoting a more favorable healing environment within the oral cavity [
17].
Chlorhexidine has been a mainstay in oral antisepsis for decades. Its broad-spectrum antimicrobial activity effectively targets a wide range of bacteria associated with oral infections [
18]. Studies have demonstrated its effectiveness in reducing plaque formation and promoting gingival healing after periodontal procedures [
19]. However, CHX can have drawbacks, including potential staining of teeth and taste alterations [
20]. Cetylpyridinium chloride presents itself as an alternative antiseptic option. It possesses antimicrobial properties and demonstrates efficacy in reducing plaque and preventing gingivitis [
21]. Additionally, CPC may be less likely to cause taste disturbances compared to CHX [
22]. Despite the established benefits of both antiseptics, the optimal use of CHX and CPC in the context of oral surgery remains a topic of ongoing research.
This study aims to evaluate the clinical outcomes of two different antiseptic protocols after various oral surgical procedures.
3. Results
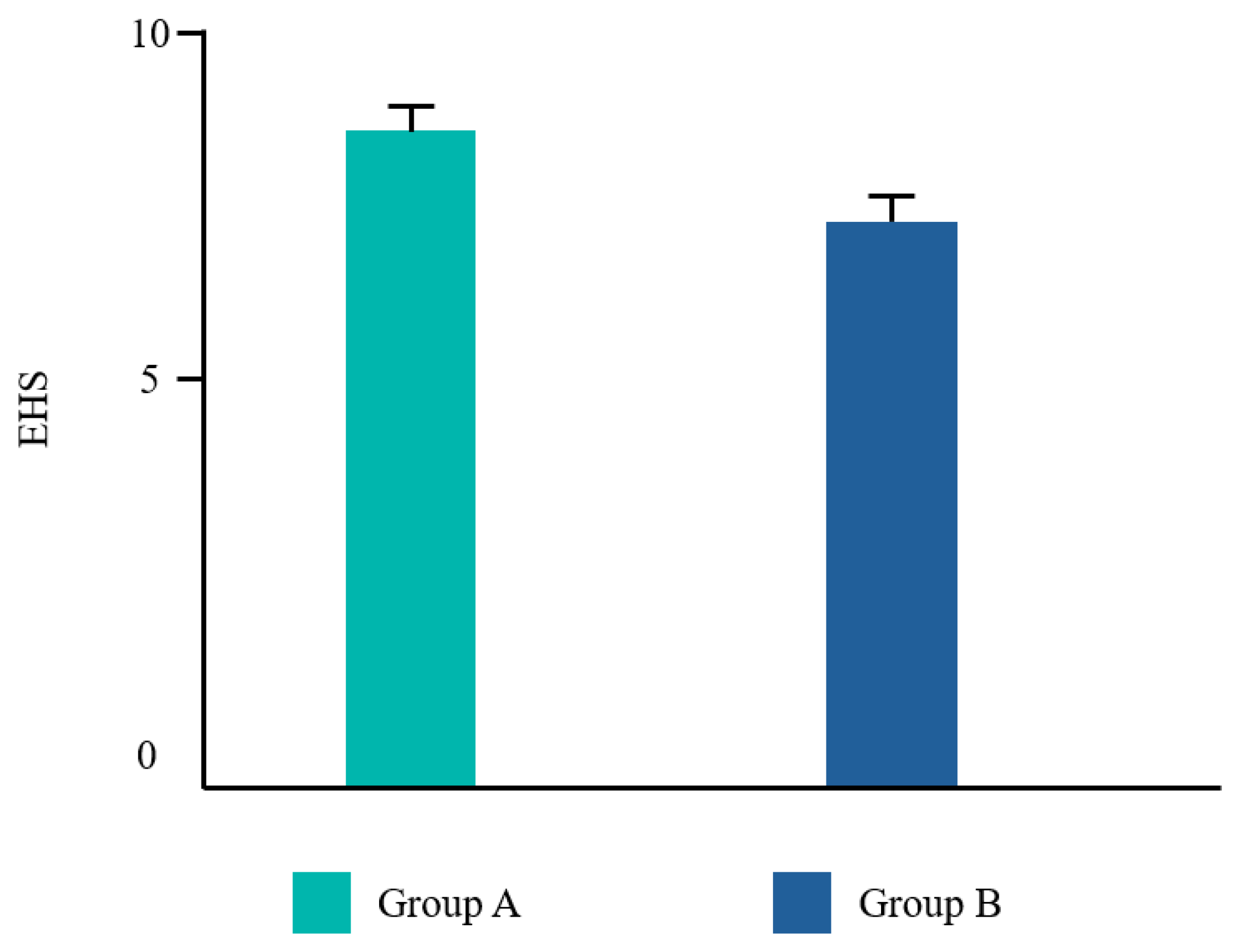
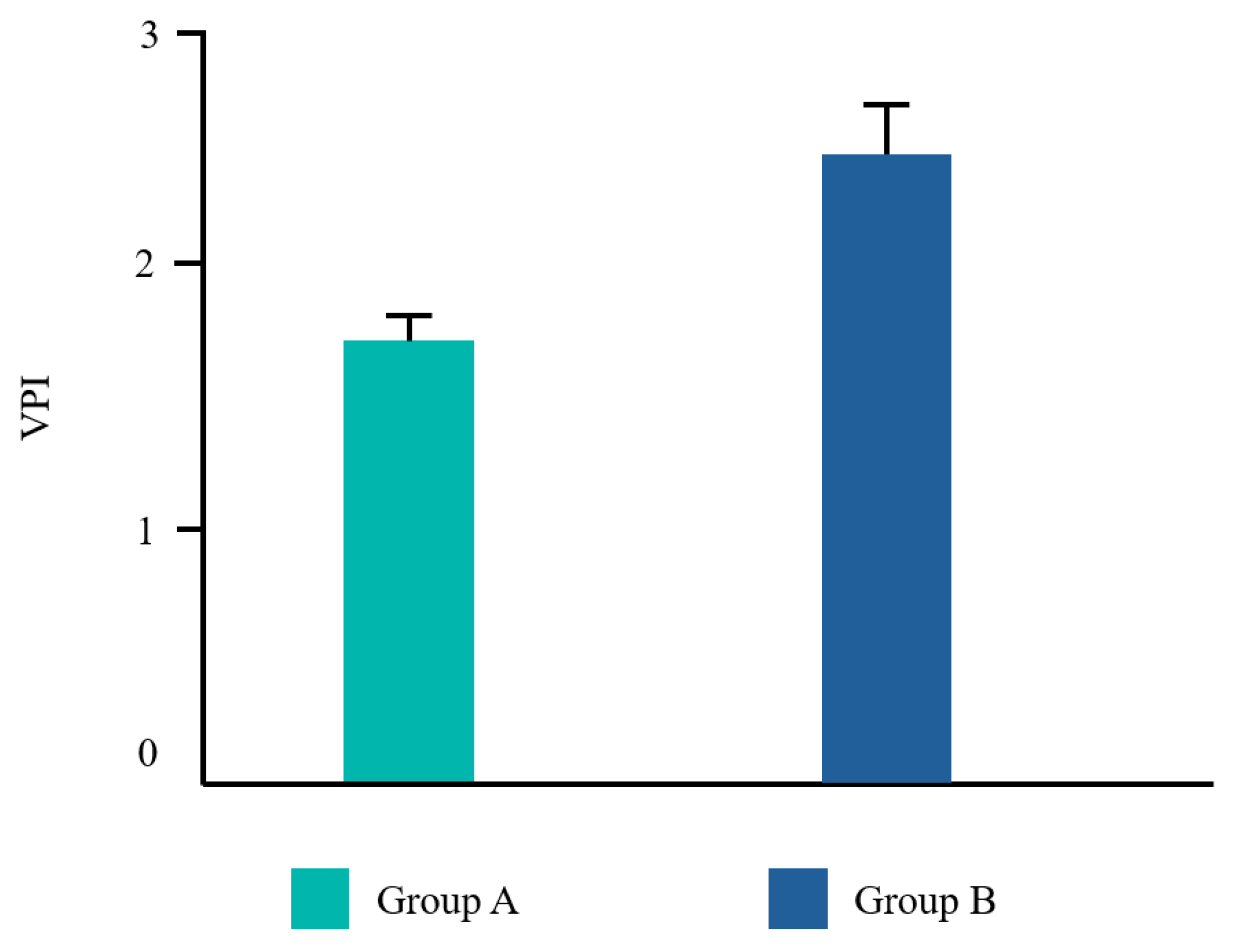
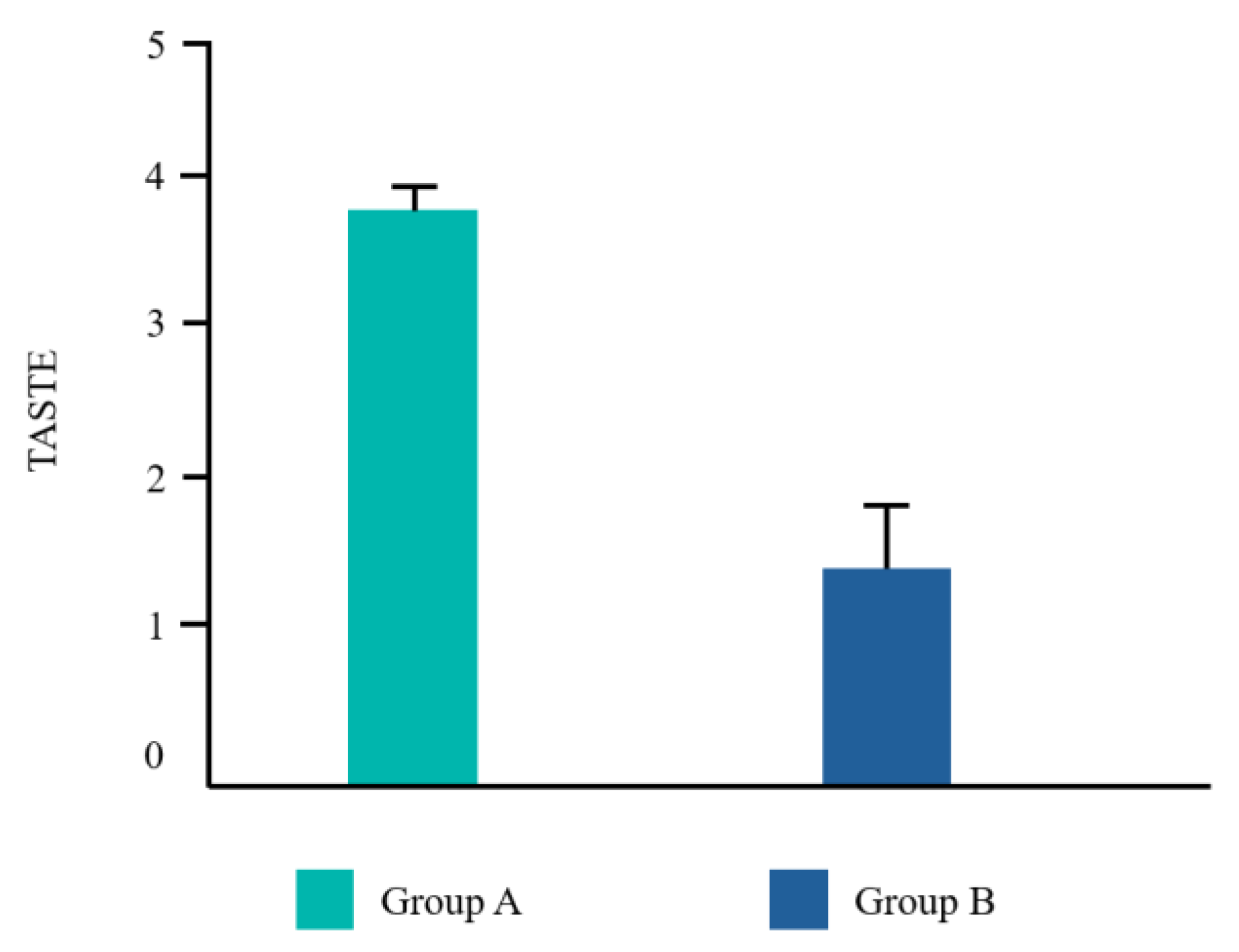
Twenty-two patients from the Dental Clinic of University G. d’Annunzio of Chieti, Italy, 15–68 years old (mean age 39,2±14,19) were included, 45% (10/22) male and 55% (12/22) female. All patients completed the study. No adverse events related to the treatments applied were reported by any of the participants. Frequency distributions of all the analyzed parameters showed a normal profile of the data. Each variable was reported as follows in
Figure 1,
Figure 2,
Figure 3 and
Figure 4.
Main findings were summarized in
Table 1.
The Student T test was significant for EHS and taste. No difference was found for VPI and NRS (
Table 2).
Limitation of the Study
The protocol shown presents some limitations. The first issue was that group B did not receive an antiseptic toothpaste like the group A. The second issue was that the EHS has some intrinsic subjective aspects like every clinical index for wound healing. An objective parameter might has been the histological analysis of a tissue sample of the wound. Obviously, this invasive detection would not have been endorsed by the ethics committee without a clinical/therapeutical reason.
4. Discussion
While CHX is a well-established antiseptic molecule in oral healthcare, limited research exists comparing its single use to combinations with CPC. This study aimed to bridge this gap by evaluating the clinical efficacy of two at-home antiseptic regimens following dental surgery.
CHX is a broad-spectrum antiseptic agent in dentistry, demonstrably effective in reducing plaque and gingivitis [
19]. However, its clinical utility is not without a shadow. While generally well-tolerated, CHX can induce a range of adverse effects that warrant consideration, particularly for long-term use. One of the most commonly reported drawbacks of CHX is taste alteration. Studies have documented a metallic or bitter taste sensation following CHX use, potentially impacting patient compliance [
28,
29]. This can be particularly concerning for patients already experiencing taste disturbances such as dysgeusia. Furthermore, CHX can trigger hypersensitivity reactions, manifesting as localized burning or irritation of the oral mucosa [
30]. While these reactions are typically mild and transient, they can deter patients from continued use. Additionally, prolonged CHX exposure has been linked to staining of teeth and tongue especially with 0,2% concentration, presenting an aesthetic concern for some patients [
29]. To overcome these problems, recent research suggests a potential synergistic effect when used in combination. This synergy translates to enhanced antimicrobial activity compared to the individual agents alone [31, 32]. The mechanism underlying this synergy remains under investigation, but several hypotheses have been proposed. One possibility involves the disruption of microbial cell membranes. Both CHX and CPC possess cationic properties that electrostatically attract to the negatively charged bacterial membranes. CHX is theorized to primarily target the cytoplasmic membrane leading to cell death [
33]. CPC, on the other hand, might disrupt the outer membrane, facilitating CHX penetration and amplifying its effect [
31].
In the present study, subjects in Group A exhibited statistically significant improvements in both the EHS and taste satisfaction. This observation suggests that the comprehensive regimen employed in Group A, which incorporated CHX alongside vitamin B3 in the toothpaste and cetylpyridinium chloride CPC in the mouthwash, yielded superior clinical outcomes and patient satisfaction compared to the regimen in Group B, which solely relied on a higher concentration of CHX mouthwash. Vitamin B3, also known as niacinamide, is a water-soluble essential nutrient that plays a crucial role by strengthening the cellular barrier enhancing the ceramides biosynthesis [
34]. According to Wessels Q. et al. [
35], fibroblast collagen synthesis was increased alongside with cellular migration and proliferation after a topical application of niacinamide. Adding this molecule to a fluoride toothpaste, may be useful to increase the permeability barrier of oral mucosa [
36].
Regarding the mouthwash employed, the combination of CHX and CPC allows to use lower concentrations of CHX which are responsible of side effects. This led to a more pleasant flavor which may enhance the patient concordance to the prescription [
37].
Even though VPI was lower in group A compared to group B, the results were not statistically significant. This may be explained by the fact that group B still used an antiseptic mouthwash which may contrast dental biofilm from spread. The same can be assumed for NRS. Our results were in line with Guerra F. et al. [
36], for the authors CHX with ADS showed a limited ability to reduce bacterial plaque and gingival bleeding. Additionally, the authors concluded that the anti-stain molecule, added to the formulation did not reduce pigmentation in comparison with mouthwashes without it after the spectrophotometric assessment. Finally, Li et al. [
19] found that CHX with ADS did not completely eliminate the side-effect of staining.
With the limitations of the present study, CHX is the cornerstone of the antiseptic management of post-surgical inflammation in dentistry. This study suggests a potential synergistic interaction between CHX and CPC that could offer a promising avenue for optimizing post-surgical care. The observed improvements in patient satisfaction with the CHX-CPC combination highlight the importance of addressing potential adverse effects associated with traditional CHX regimens. Future research with larger sample sizes and longer follow-up periods is necessary to definitively establish the efficacy and safety profile of CHX-CPC combinations in post-surgical dental management.
5. Conclusions
The implementation of a well-designed antiseptic regimen plays a pivotal role in the post-surgical management of inflammatory processes following dental procedures. This regimen likely exerts its effects through a multifaceted approach, including reducing the burden of pathogenic bacteria that can contribute to post-surgical complications. Both the groups tested showed a good plaque reduction. Group A revealed a better EHS maybe due to a more pleasant taste of the mouthwash used. The employment of a protocol based on a manual ultra soft toothbrush, an antiseptic toothpaste with 0,12% chlorhexidine with vitamin B3, and an antiseptic mouthwash with 0,12% chlorhexidine and 0,07% cetylpyridinium chloride proved to be effective in the management of the flogosis that follows oral surgery.
Author Contributions
Conceptualization, S.D.; methodology, S.D.; software, S.D.; validation, S.D., investigation, S.D.; data curation, S.D.; writing—original draft preparation, S.D.; writing—review and editing, S.D.; visualization, S.D.; supervision, S.D.; project administration, S.D. . The author has read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Local Institutional Review Board of Department of Medical, Oral And Biotechnological Sciences – University G. d’Annunzio (protocol code 136/02-04-2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data supporting reported results can be found at University G. d’Annunzio, 31, Via Dei Vestini, 66100 Chieti, Italy.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Lynham, A.; Hsu, E. Postoperative interventions to reduce inflammatory complications after third molar surgery: review of the current evidence. Aust. Dent. J. 2017, 62, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.M.; Niessen, F.B.; Gibbs, S. The Bigger Picture: Why Oral Mucosa Heals Better Than Skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- Andrzej, K.; Jamka-Kasprzyk, M.; Panaś, M.; Grażyna, W.-P. Analysis of complications after the removal of 339 third molars. Dent. Med Probl. 2021, 58, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: what can we learn? Periodontology 2000 2015, 68, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Rossi-Fedele, G.; Doğramacı, E.J. Post-operative instructions following minor oral surgery-the quality and level of evidence: a cross-sectional study. Br. Dent. J. 2020, 228, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, A.P. Minimizing post-operative edema and ecchymoses by the use of an oral enzyme preparation (bromelain). A controlled study of 53 rhinoplasty cases. Eye Ear Nose Throat Mon. 1962, 41, 813–7. [Google Scholar]
- Shibl, M.; Ali, K.; Burns, L. Effectiveness of pre-operative oral corticosteroids in reducing pain, trismus and oedema following lower third molar extractions: a systematic review. Br. Dent. J. 2021, 1–8. [Google Scholar] [CrossRef]
- Cardoso, C.L.; Rodrigues, M.T.V.; Júnior, O.F.; Garlet, G.P.; de Carvalho, P.S.P. Clinical Concepts of Dry Socket. J. Oral Maxillofac. Surg. 2010, 68, 1922–1932. [Google Scholar] [CrossRef]
- Fletcher, M.C.; Spera, J.F. Management of Acute Postoperative Pain after Oral Surgery. Dent. Clin. North Am. 2012, 56, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Brar, P.; Jakubowski, J.; Kaltman, S.; Lopez, E. The use of corticosteroids and nonsteroidal antiinflammatory medication for the management of pain and inflammation after third molar surgery: A review of the literature. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 107, 630–640. [Google Scholar] [CrossRef] [PubMed]
- D'Agostino S, Dolci M. Antibiotic therapy in oral surgery: a cross sectional survey among Italian dentists. J Biol Regul Homeost Agents. 2020 Jul-Aug,;34(4):1549-1552. [CrossRef] [PubMed]
- Bouloux, G.F.; Steed, M.B.; Perciaccante, V.J. Complications of Third Molar Surgery. Oral Maxillofac. Surg. Clin. North Am. 2007, 19, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Camps-Font, O.; Martín-Fatás, P.; Clé-Ovejero, A.; Figueiredo, R.; Gay-Escoda, C.; Valmaseda-Castellón, E. Postoperative infections after dental implant placement: Variables associated with increased risk of failure. J. Periodontol. 2018, 89, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Laraki M, Chbicheb S, El Wady W. Les alvéolites: revue de littérature [Alveolitis: review of the literature]. Odontostomatol Trop. 2012 Sep;35(139):19-25. French. [PubMed]
- Norman, G.; Dumville, J.C.; Mohapatra, D.P.; Owens, G.L.; Crosbie, E.J. Antibiotics and antiseptics for surgical wounds healing by secondary intention. Cochrane Database Syst. Rev. 2016, 2022, CD011712. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, A.; Kaspar, S.; Kathirvelu, R.; Srinivasan, B.; Srinivasan, S.; Sundram, R. Chlorhexidine: An Elixir for Periodontics. J. Pharm. Bioallied Sci. 2020, 12, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, R.E.; Finger, M.; Lang, N.P. Evaluation of the antigingivitis effect of a chlorhexidine mouthwash with or without an antidiscoloration system compared to placebo during experimental gingivitis. J. Investig. Clin. Dent. 2013, 5, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Deus, F.P.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Pitten, F.-A.; Kramer, A. Efficacy of Cetylpyridinium Chloride Used as Oropharyngeal Antiseptic. Arzneimittelforschung 2001, 51, 588–595. [Google Scholar] [CrossRef]
- Navabi, N.; Afshari, Z.; Kamyabi, H.; Mohammadi, M. Side effects and short effects of using three common mouthwashes on oral health and quality of life: A quasi-experimental study. Int. J. Dent. Hyg. 2023. [Google Scholar] [CrossRef]
- Urbaniak GC, Plous, S. Research Randomizer (Version 4.0). 2013. Available online: http://www.randomizer.org/ (accessed on 3 January 2019).
- Wilson, W.R.; Gewitz, M.; Lockhart, P.B.; Bolger, A.F.; DeSimone, D.C.; Kazi, D.S.; Couper, D.J.; Beaton, A.; Kilmartin, C.; Miro, J.M.; et al. Prevention of Viridans Group Streptococcal Infective Endocarditis: A Scientific Statement From the American Heart Association. Circ. 2021, 143, e963–e978. [Google Scholar] [CrossRef] [PubMed]
- Marini, L.; Rojas, M.A.; Sahrmann, P.; Aghazada, R.; Pilloni, A. Early Wound Healing Score: a system to evaluate the early healing of periodontal soft tissue wounds. J. Periodontal Implant. Sci. 2018, 48, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar] [PubMed]
- Downie, W.W.; Leatham, P.A.; Rhind, V.M.; Wright, V.; Branco, J.A.; Anderson, J.A. Studies with pain rating scales. Ann. Rheum. Dis. 1978, 37, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Gent, J.F.; Frank, M.E.; Hettinger, T.P. Taste Confusions Following Chlorhexidine Treatment. Chem. Senses 2002, 27, 73–80. [Google Scholar] [CrossRef] [PubMed]
- McCoy, L.C.; Wehler, C.J.; Rich, S.E.; Garcia, R.I.; Miller, D.R.; Jones, J.A. Adverse events associated with chlorhexidine use: results from the Department of Veterans Affairs Dental Diabetes Study. J. Am. Dent. Assoc. 2008, 139, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.S.; Abdullah, D.; Liew, A.K.C.; Khazin, S.M. Chlorhexidine hypersensitivity. Br. Dent. J. 2021, 230, 273–273. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent Development of Active Ingredients in Mouthwashes and Toothpastes for Periodontal Diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Y.; Zhang, X.; Zhang, P.; Tian, Q.; Ma, C.; Shi, C. Combination of Cetylpyridinium Chloride and Chlorhexidine Acetate: A Promising Candidate for Rapid Killing of Gram-Positive/Gram-Negative Bacteria and Fungi. Curr. Microbiol. 2023, 80, 1–12. [Google Scholar] [CrossRef]
- Kuyyakanond, T.; Quesnel, L.B. The mechanism of action of chlorhexidine. FEMS Microbiol. Lett. 1992, 100, 211–215. [Google Scholar] [CrossRef]
- Tanno, O.; Ota, Y.; Kitamura, N.; Katsube, T.; Inoue, S. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br. J. Dermatol. 2000, 143, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Wessels, Q.; Pretorius, E.; Smith, C.M.; Nel, H. The potential of a niacinamide dominated cosmeceutical formulation on fibroblast activity and wound healing in vitro. Int. Wound J. 2014, 11, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W. Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 5229. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Pasqualotto, D.; Rinaldo, F.; Mazur, M.; Corridore, D.; Nofroni, I.; Ottolenghi, L.; Nardi, G.M. Therapeutic efficacy of chlorhexidine-based mouthwashes and its adverse events: Performance-related evaluation of mouthwashes added with Anti-Discoloration System and cetylpyridinium chloride. Int. J. Dent. Hyg. 2019, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).