1. Introduction
Glycogen is a branched polysaccharide with a molecular weight of nine to ten million Da. The average glycogen molecule contains about 55,000 glucose residues linked by α-1,4 (92%) and α-1,6 (8%) glycosidic bonds [
1]. The synthesis of glycogen is primed by glycogenin [
2] (even though aberrant forms of glycogen are also formed when glycogenin is suppressed [
3]), and catalyzed by glycogen synthase (GYS) and glycogen branching enzyme (GBE): GYS links glucose residues to each other by α-1,4 glycosidic bonds to form linear chains. GBE, in turn, attaches a short linear stretch of α-1,4-linked glucose units from the outer non-reducing end of a growing glycogen chain into an α-1,6 position of a pre-existing chain thus generating a branch. Repetitive cycles of elongation and branching generate a large (110–290 nm in liver) [
4] spherical molecule with a hydrophilic surface and increased number of reactive termini, facilitating glycogen synthesis and degradation [
5,
6]. Glycogen degradation, on the other hand, takes place in the cytosol and in lysosomes. In the cytosol, glycogen is degraded by glycogen phosphorylase (GP) and glycogen debranching enzyme (GDE). GP phosphorylates outer glucose residues and releases each one as glucose-1-hosphate. GP can degrade an outer chain only until 4 residues from the branch point. Then, GDE is required to complete glycogenolysis. GDE catalyzes two reactions: transferring a three-glucosyl glucan to the non-reducing end of another linearstrand (α-1,4-glucanotransferase reaction) and hydrolyzing the α-1-6-glycosidic bond remaining on the branch point, releasing glucose (amylo-α-1,6-glucosidase reaction,
n.b., glucose, rather than glucose-1-phosphate, is released). In the lysosomes, glycogen degradation is catalyzed by α-glucosidase.
Glycogen is stored primarily in liver and muscle, where it respectively serves as a reservoir for regulating blood glucose levels and as a readily mobilizable energy reserve. Therefore, aberrations in glycogen metabolism, or glycogen storage disorders (GSDs) are primarily hepatic or muscular disorders, with two notable exceptions, APBD and LD, involving specifically the central and peripheral nervous systems. The most common disorder of glycogen metabolism is observed in diabetes, in which abnormal amount of insulin or abnormal insulin response result in accumulation or depletion of liver glycogen. However, GSDs are normally associated with hereditary deficiencies in enzymes of glycogen and glucose metabolism causing glycogen accumulation (except for GSD type 0, which is a GYS deficiency and is therefore associated with glycogen insufficiency).
In this work we focused on GSD type III (GSDIII, Cori disease), the third most prevalent muscle GSD (incidence of 1:100,000) after GSD types V and II [
7,
8,
9], which is caused by GDE deficiency leading to accumulation of glycogen due to its reduced degradation. Phosphorylase-limit dextrin (PLD), the type of glycogen which accumulates in GSDIII, is structurally abnormal, containing shorter outer branches [
10]. While phosphorylase-limit dextrin is sensitive to diastase digestion and does not form polyglucosan bodies [
10], it is still associated with large vacuoles and can cause histological damage to myofibrils [
11,
12]. GSDIII usually starts as a liver disorder characterized by hepatomegaly, hypoglycemia, hyperlipidemia and hyperketonemia. These result from the limited ability to breakdown glycogen to glucose, leading to excessive use of lipid oxidation as an alternative energy source with ensuing attenuation of lipid uptake by adipocytes and increase in ketone bodies as byproducts of fatty acid oxidation (FAO). Later on, myopathy pursues [
13], with occasional cardiomyopathy [
14], liver cirrhosis and hepatocellular adenoma [
15].
GSDIII implicates vacuolar myopathy and myofibrillar damage [
12,
16], also observed in GSDII [
17], which shares with GSDIII muscle glycogen over accumulation (albeit lysosomal in GSDII and mostly cytoplasmic in GSDIII). We therefore tested here the GSDIII curative capacity of GHF-201, a novel safe compound capable of reversing glycogen accumulation and enhancing autophagy [
18] (doi.org/10.1101/2023.02.20.529109). We show that GHF-201 was capable of improving motility and grip strength in a
Agl-/- mouse model of GSDIII, where GDE, encoded by the murine
Agl gene, was knocked out. At the same time, GHF-201 did not modify the deficient motor learning capacities of
Agl-/- mice associated with a neurological deficiency not implicated in GSDIII. We further demonstrate that GHF-201 corrected hypoglycemia, hyperlipidemia and liver and muscle damage, as well as carbohydrate over fat fuel preference. Combined with reduction of glycogen levels and muscle atrophy at the tissue level, and improvement of lysosomal and mitochondrial phenotypes at the cell level (in GSDIII patients’ fibroblasts), our results strongly support that GHF-201 has a potential therapeutic capacity for treating GSDIII, either as a standalone, or as part of a combination therapy.
4. Discussion
The long-term aim of this study was to test the capacity of GHF-201 to alleviate disease symptoms in the
Agl-/- mouse model of GSDIII. GHF-201 specifically binds the lysosomal membrane protein LAMP1 [
18]. GHF-201 was further able to re-acidify the aberrant alkaline lysosomal pH. In adult GSDIV (APBD) models, GHF-201 ameliorated the diseased state by enhancing lysosomal glycogen catabolism and autophagy.
GSDIII starts with liver involvement followed by storage of glycogen in skeletal muscle provoking a myopathy. The disease is caused by cytoplasmic glycogen debranching enzyme (GDE) deficiency leading to accumulation of glycogen due to its reduced degradation. Glycogen accumulation in muscle is more extensive in GSDIII, as compared to the other myopathy implicating GSDs GSDII, GSDV, GSDIX, GSDX, and GSDXV [
9]. In particular, human muscle biopsies from GSDIII patients show a typical and constant vacuolar myopathy, characterized by multiple and variably sized vacuoles filled with PAS-positive material [
7]. Phosphorylase-limit dextrin (PLD), the type of glycogen which accumulates in GSDIII, is structurally abnormal, containing shorter outer branches [
10]. While PLD is sensitive to diastase digestion and does not form polyglucosan bodies [
10], it is still associated with large vacuoles and can cause histological damage to myofibrils [
11,
16]. In fact, as also shown by our results (
Figure 5), disruption of myofibrillar structure by glycogen vacuoles is more pronounced in GSDIII than in other GSDs with prominent muscular involvement [
9].
GSDIII usually starts as a liver disorder and later on myopathy pursues [
13], with occasional cardiomyopathy [
14], liver cirrhosis and hepatocellular adenoma [
15]. Interestingly, possibly due to higher overall glycogen accumulation, GSDIII manifests with excess glycogen in both cytoplasm and lysosomes, as opposed to GSDII, where excess glycogen is primarily found in lysosomes due to the aberrant function of GAA [
29]. The main pathogenic factor in GSDIII, as revealed by ultrastructural analysis of muscle specimens from patients, is autophagosome accumulation. Concordantly, Laforet
et al., using electron microscopy, showed the presence of large non-membrane bound sarcoplasmic deposits of normally structured glycogen as well as smaller rounded sac structures lined by a continuous double membrane containing only glycogen, corresponding to autophagosomes. A consistent SQSTM1/p62 decrease and beclin-1 increase in human muscle biopsies suggested autophagic dysregulation [
7]. The latter can impede the degradation of dysfunctional organelles and proteins, and, notably, also of glycogen, in a specific pathway mediated by the starch binding domain-containing protein 1 (Stbd1), which tags glycogen for its autophagy, interacting with autophagy machinery and glycogen related proteins, including GDE, in other domains [
30]. Interestingly, among these glycogen related proteins, GDE has the highest number of Atg8 binding motifs [
30], also suggesting an important role of autophagy in GSDIII, which might take place if GDE deficiency impairs the interaction with the autophagy machinery. Moreover, dysfunctional autophagy plays a pivotal role in disrupting muscle homeostasis and causing structural damage to sarcomeres [
31]. The tight link between autophagic impairment and GSDIII pathology can thus explain the beneficial effects of GHF-201, a general autophagic activator [
18], in improving motor function (
Figure 2) and skeletal muscle health (
Figure 5) in a GSDIII mouse model.
Our blood biochemistry panel demonstrated that GHF-201 treatment led to improved liver and muscle function and partially corrected blood hypoglycemia and hypertriglyceridemia (
Figure 3), also observed in GSDIII patients [
32]. Both hypoglycemia and hyperlipidemia are expected outcomes of glycogen surcharge since more glucose is shunted from blood to intracellular glycogen and since high glycogen levels can inhibit the energy sensor AMPK [
33,
34,
35], leading to activation of acetyl CoA carboxylase, the rate limiting enzyme of fatty acid synthesis, which is phospho-inhibited by AMPK. Reduction of glycogen levels by GHF-201 (
Figure 5) expectedly mitigated this hyperlipidemia. The blood lipid profile can also reflect fuel utilization. As glycogen mobilization is compromised in GSDIII and enhanced by GHF-201, we expected that GSDIII mice use lipid instead of carbohydrate fuel as a compensatory reaction. However, as suggested by the hypertriglyceridemia observed in untreated GSDIII mice (
Figure 3), the mice are not capable of this metabolic compensation. Notably, as revealed by both indirect calorimetry (
Figure 4) and the blood biochemistry panel (
Figure 3), GHF-201 treatment enabled this metabolic compensation by boosting fat burn (
Figure 4), which is possibly reflected in the reduction in blood triglyceride level (
Figure 3). A similar effect of GHF-201 was observed in the GSDIV modeling
Gbeys/ys mice [
18]. Interestingly, GHF-201-mediated improvement of fat oxidation (
Figure 4A), and other in vivo metabolic effects (TEE, (
Figure 4A), ambulatory activity (
Figure 4B), wheel running (
Figure 2B)) were more pronounced in the light than in the dark. Since mice are nocturnal animals, in which food is mostly consumed in the dark (see also
Figure 4C) and digested in the light, it can be conjectured that GHF-201, affecting fuel digestion, or autophagic substrate catabolism, exerts a larger effect in the light.
In conclusion, we show here that GHF-201, a LAMP-1 targeting small molecule acting as a general activator of autophagic flux, can partially correct aberrant metabolic, motor and myopathologic phenotypes in the Agl-/- mouse model of GDE deficiency, or GSDII, a disease implicating a particularly high glycogen burden, causing liver and myofibrillar injury.
Author Contributions
Conceptualization, OK, MW, and EM; Methodology, KM, SS, SB, US, MM, SB, and HG.; Writing—original draft preparation, OK.; Writing—review and editing, MW, EM; Supervision, JT, HR, MW, EM, OK; funding acquisition, MW, EM, OK. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
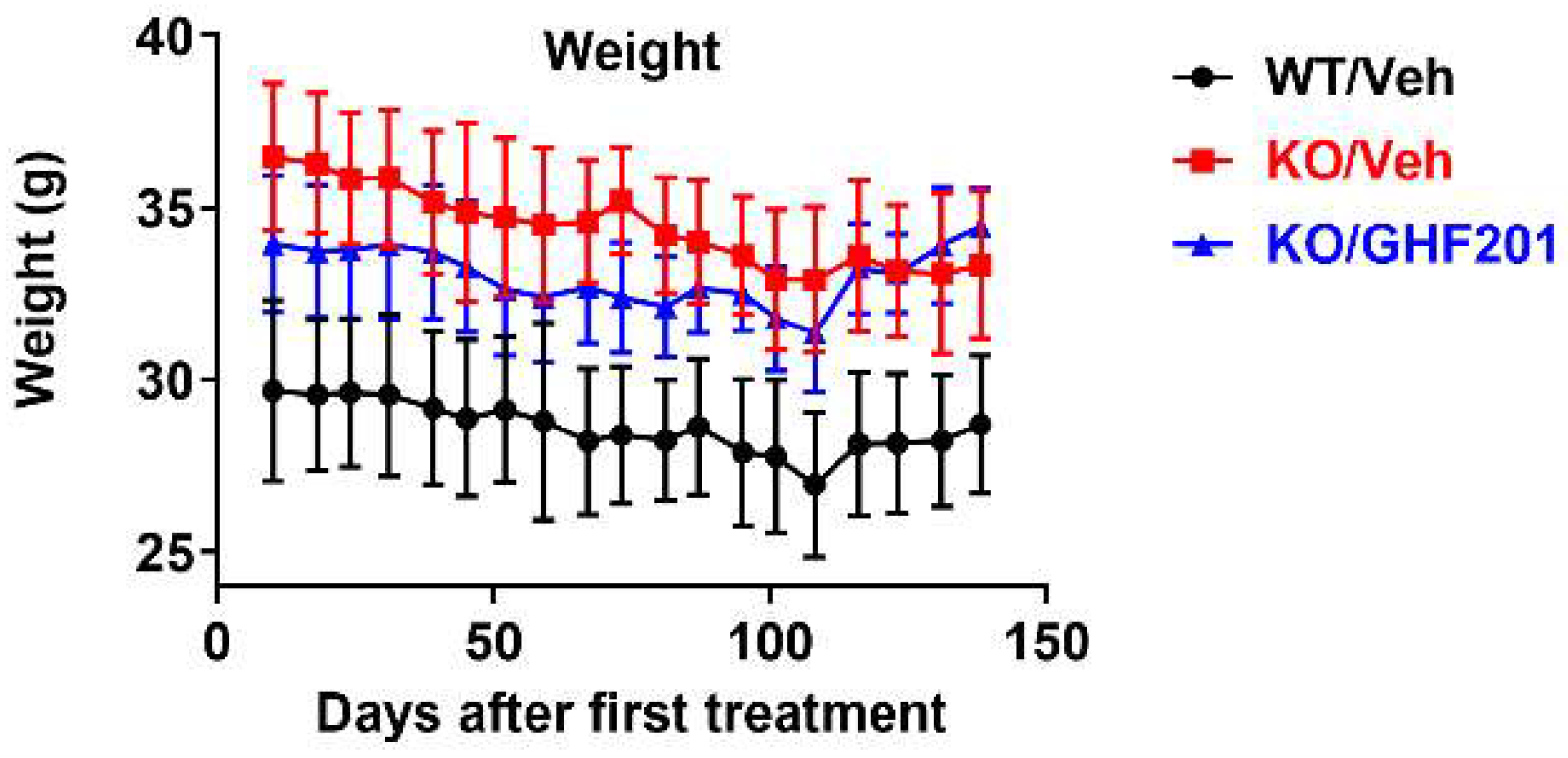
Figure 1.
Weight over time in WT mice, Agl-/- (KO) mice injected intramuscularly biweekly with 10% DMSO solvent control, and KO mice injected in the same way with 250 mg/kg GHF-201.
Figure 1.
Weight over time in WT mice, Agl-/- (KO) mice injected intramuscularly biweekly with 10% DMSO solvent control, and KO mice injected in the same way with 250 mg/kg GHF-201.
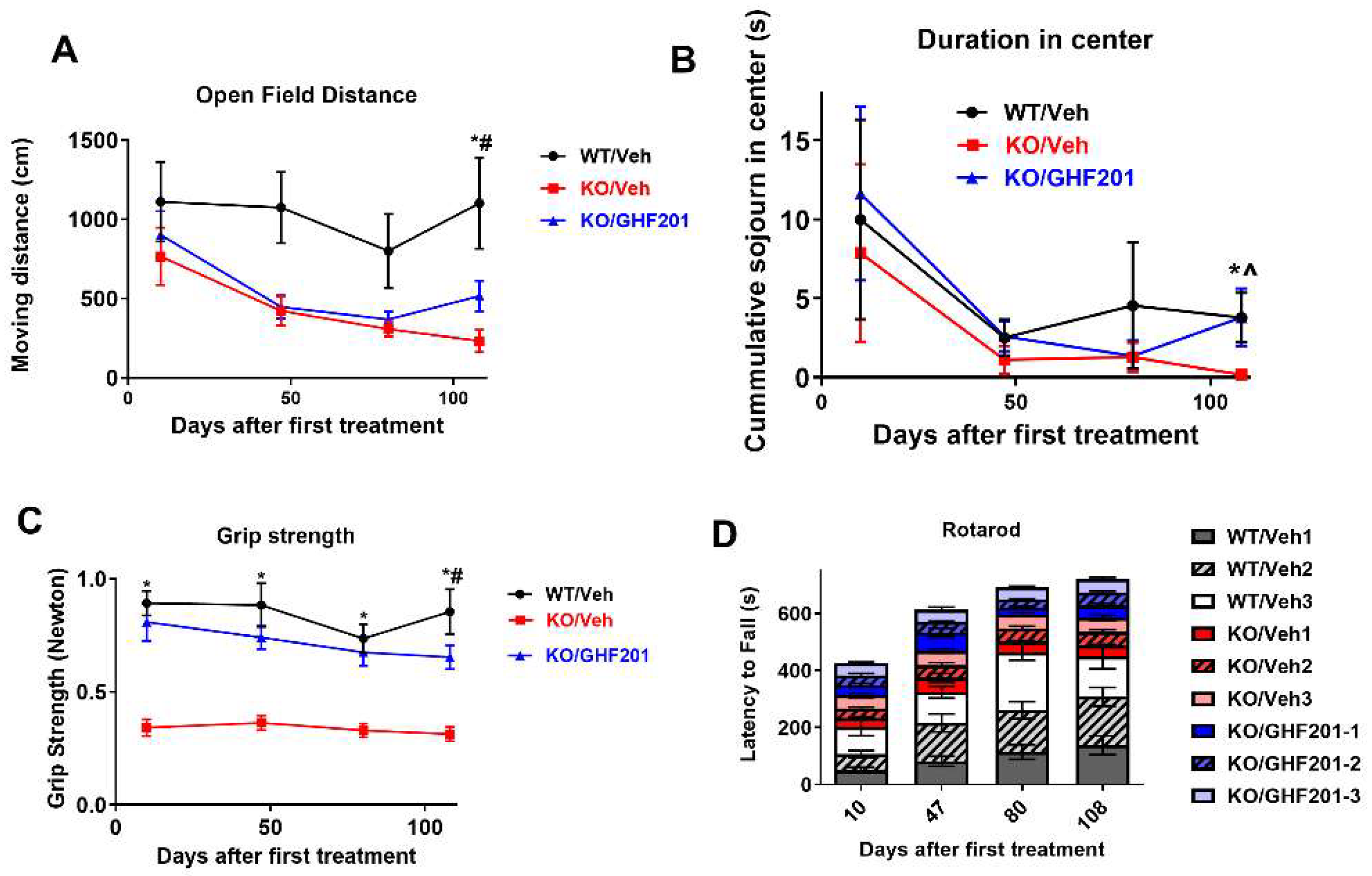
Figure 2.
Movement distance (A), sojourn in the center of open field arena (inset, representative open field sojourn heatmaps) (B), grip strength (C) and latency to fall from a rotating rod (D) were measured in n=8 Agl-/- mice treated with 10% DMSO vehicle (KO/Veh), n=6 wild type mice treated with vehicle (WT/Veh), and n=10 Agl-/- mice treated with GHF-201 (KO/GHF-201). In (D), each rotarod session included three consecutive runs (1-3), separated by 10 min pauses. Two-way ANOVA with repeated measures shows that only in (C) GHF-201 treatment values were higher than vehicle-treated values in KO mice throughout the period (p<0.05). *, KO/GH201 is significantly different from KO/Veh; *#, KO/GHF-201 is significantly different from both KO/Veh and WT/Veh; *^, KO/GHF-201 is significantly different only from KO/Veh and not from WT/Veh (correction effect). Difference significance determined by multiple t-tests with Sidak post-hoc correction. All error bars represent s.e.m.
Figure 2.
Movement distance (A), sojourn in the center of open field arena (inset, representative open field sojourn heatmaps) (B), grip strength (C) and latency to fall from a rotating rod (D) were measured in n=8 Agl-/- mice treated with 10% DMSO vehicle (KO/Veh), n=6 wild type mice treated with vehicle (WT/Veh), and n=10 Agl-/- mice treated with GHF-201 (KO/GHF-201). In (D), each rotarod session included three consecutive runs (1-3), separated by 10 min pauses. Two-way ANOVA with repeated measures shows that only in (C) GHF-201 treatment values were higher than vehicle-treated values in KO mice throughout the period (p<0.05). *, KO/GH201 is significantly different from KO/Veh; *#, KO/GHF-201 is significantly different from both KO/Veh and WT/Veh; *^, KO/GHF-201 is significantly different only from KO/Veh and not from WT/Veh (correction effect). Difference significance determined by multiple t-tests with Sidak post-hoc correction. All error bars represent s.e.m.
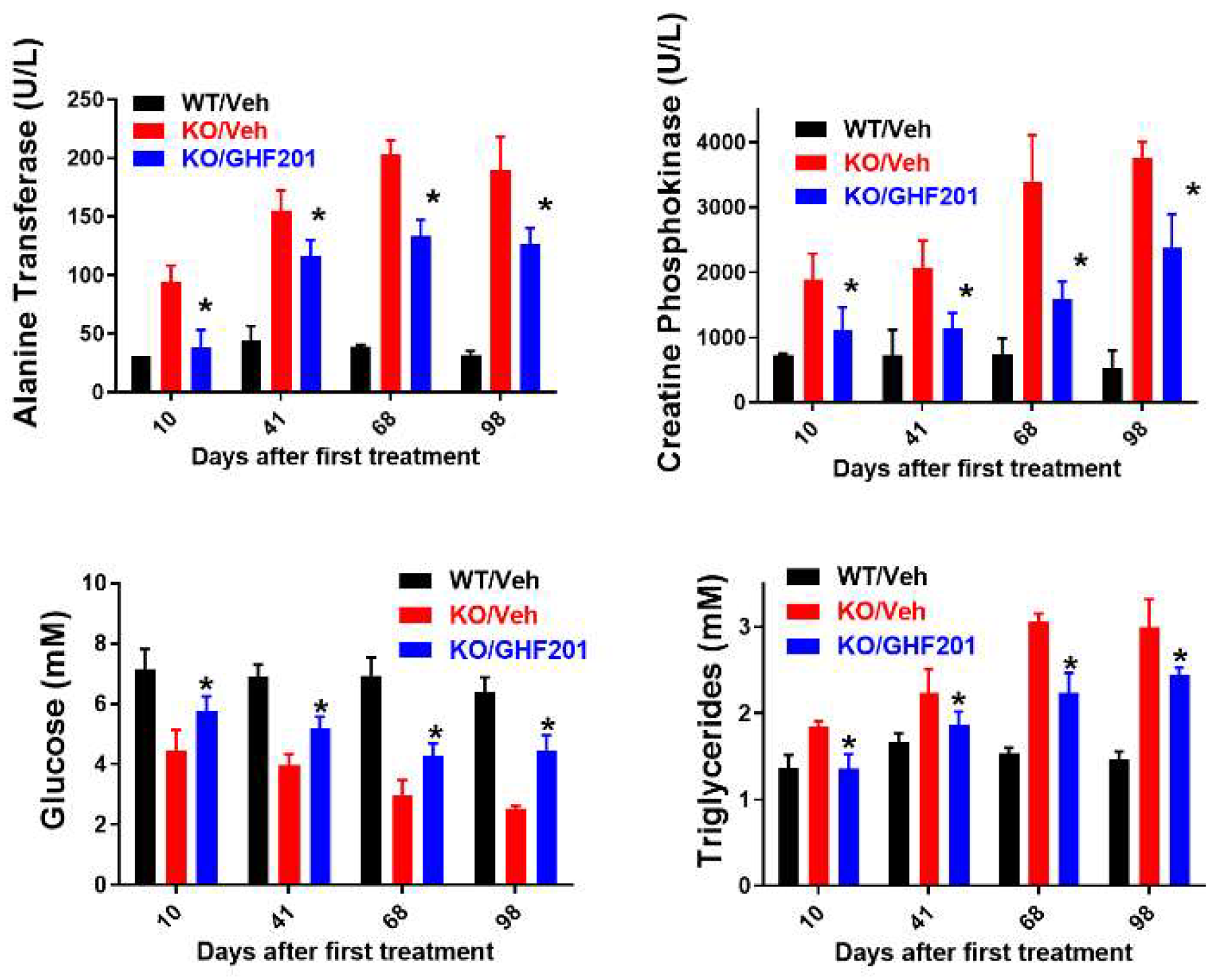
Figure 3.
Blood metabolic panel based on n=8 Agl-/- mice treated with 10% DMSO vehicle (KO/Veh), n=6 wild type mice treated with vehicle (WT/Veh), and n=10 Agl-/- mice treated with GHF-201, as indicated. At all time points, GHF-201 significantly reduced alanine transferase and creatine phosphokinase, demonstrating partial restoration of liver and muscle damages respectively. Additionally, blood glucose was increased and blood triglycerides were decreased by GHF-201 in treated Agl-/- mice. *, significant difference v KO/Veh determined by two-tailed t-tests.
Figure 3.
Blood metabolic panel based on n=8 Agl-/- mice treated with 10% DMSO vehicle (KO/Veh), n=6 wild type mice treated with vehicle (WT/Veh), and n=10 Agl-/- mice treated with GHF-201, as indicated. At all time points, GHF-201 significantly reduced alanine transferase and creatine phosphokinase, demonstrating partial restoration of liver and muscle damages respectively. Additionally, blood glucose was increased and blood triglycerides were decreased by GHF-201 in treated Agl-/- mice. *, significant difference v KO/Veh determined by two-tailed t-tests.
Figure 4.
(next page). Indirect calorimetry and metabolic cage analysis. Following a 48 h habituation period, mice (n=4 from each study arm indicated) were monitored over a 24-hr period. Data are mean±SEM from eight-month-old mice. (A) Vehicle-treated Agl-/- mice demonstrate lower respiratory exchange ratio (RER), total energy expenditure (TEE), and carbohydrate and fat oxidation. These parameters were increased or corrected by GHF-201 where indicated. ANCOVA results suggest that weight-dependent TEE is reduced in Agl-/- compared to WT mice in both light conditions and overall, and that GHF-201 corrects this TEE reduction in Agl-/- mice only in the light. (B) Ambulatory activity and wheel running were also increased or corrected by GHF-201 as indicated. (C) Food and water intake (total cumulative values are shown in the bar graphs) were increased, but not significantly, mainly in the dark period by GHF-201. *#, Significant difference between KO/GHF-201 and both KO/Veh and WT/Veh. *^, Significant difference between KO/GHF-201 and KO/Veh, but not between KO/GHF-201 and WT/Veh.
Figure 4.
(next page). Indirect calorimetry and metabolic cage analysis. Following a 48 h habituation period, mice (n=4 from each study arm indicated) were monitored over a 24-hr period. Data are mean±SEM from eight-month-old mice. (A) Vehicle-treated Agl-/- mice demonstrate lower respiratory exchange ratio (RER), total energy expenditure (TEE), and carbohydrate and fat oxidation. These parameters were increased or corrected by GHF-201 where indicated. ANCOVA results suggest that weight-dependent TEE is reduced in Agl-/- compared to WT mice in both light conditions and overall, and that GHF-201 corrects this TEE reduction in Agl-/- mice only in the light. (B) Ambulatory activity and wheel running were also increased or corrected by GHF-201 as indicated. (C) Food and water intake (total cumulative values are shown in the bar graphs) were increased, but not significantly, mainly in the dark period by GHF-201. *#, Significant difference between KO/GHF-201 and both KO/Veh and WT/Veh. *^, Significant difference between KO/GHF-201 and KO/Veh, but not between KO/GHF-201 and WT/Veh.
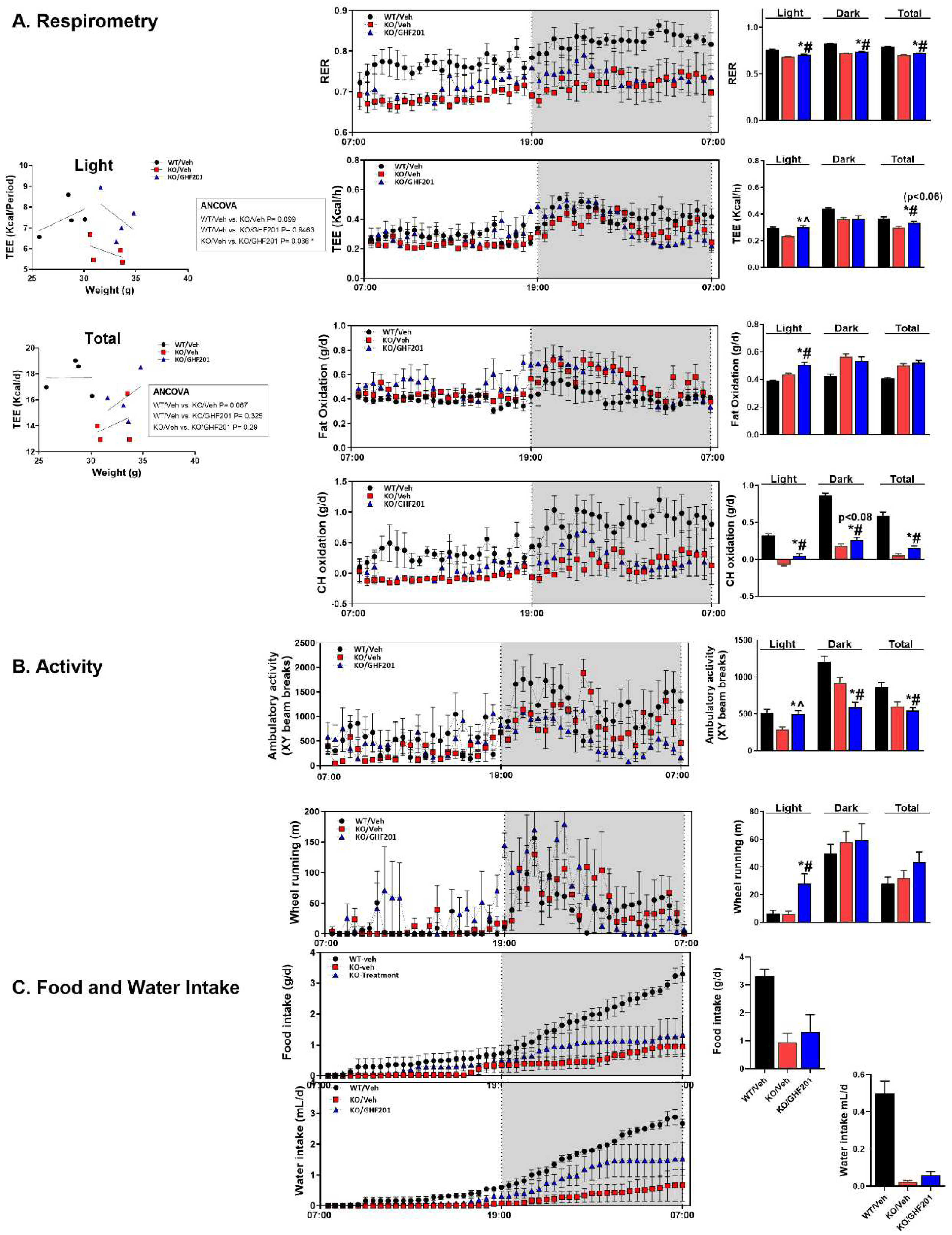
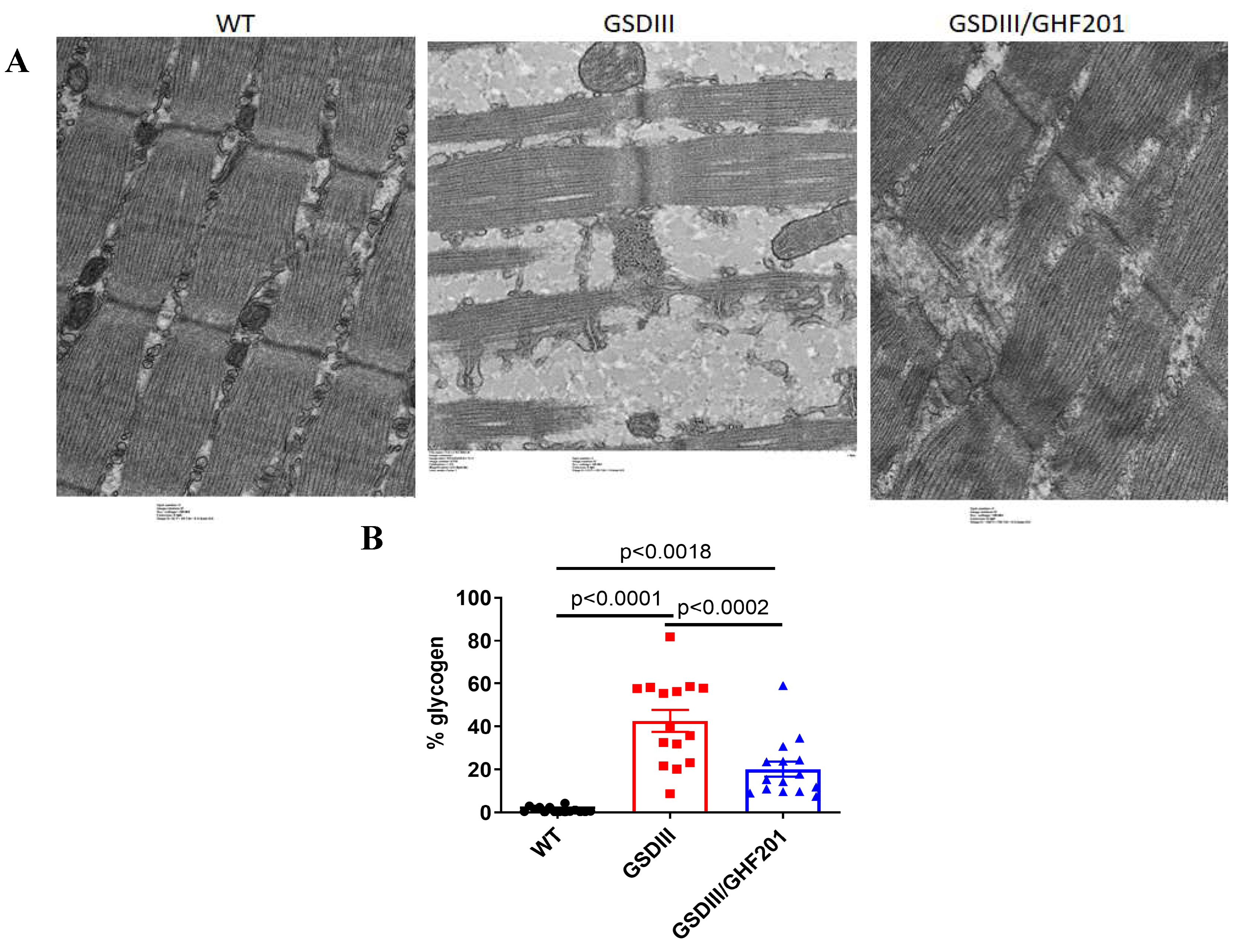
Figure 5.
(A) Transmission electron microscopy images of longitudinal sections of gastrocnemius muscle collected from 8-month-old animals treated as indicated. Note granular glycogen material (arrow) in sections from untreated Agl-/- mouse. Also note variable width of sarcomeres in the Agl-/- mouse sample. These ultrastructural phenotypes were partially corrected in the GHF-201-treated Agl-/- mouse. (B) Quantification of area % of glycogen based on analysis of TEM images.
Figure 5.
(A) Transmission electron microscopy images of longitudinal sections of gastrocnemius muscle collected from 8-month-old animals treated as indicated. Note granular glycogen material (arrow) in sections from untreated Agl-/- mouse. Also note variable width of sarcomeres in the Agl-/- mouse sample. These ultrastructural phenotypes were partially corrected in the GHF-201-treated Agl-/- mouse. (B) Quantification of area % of glycogen based on analysis of TEM images.
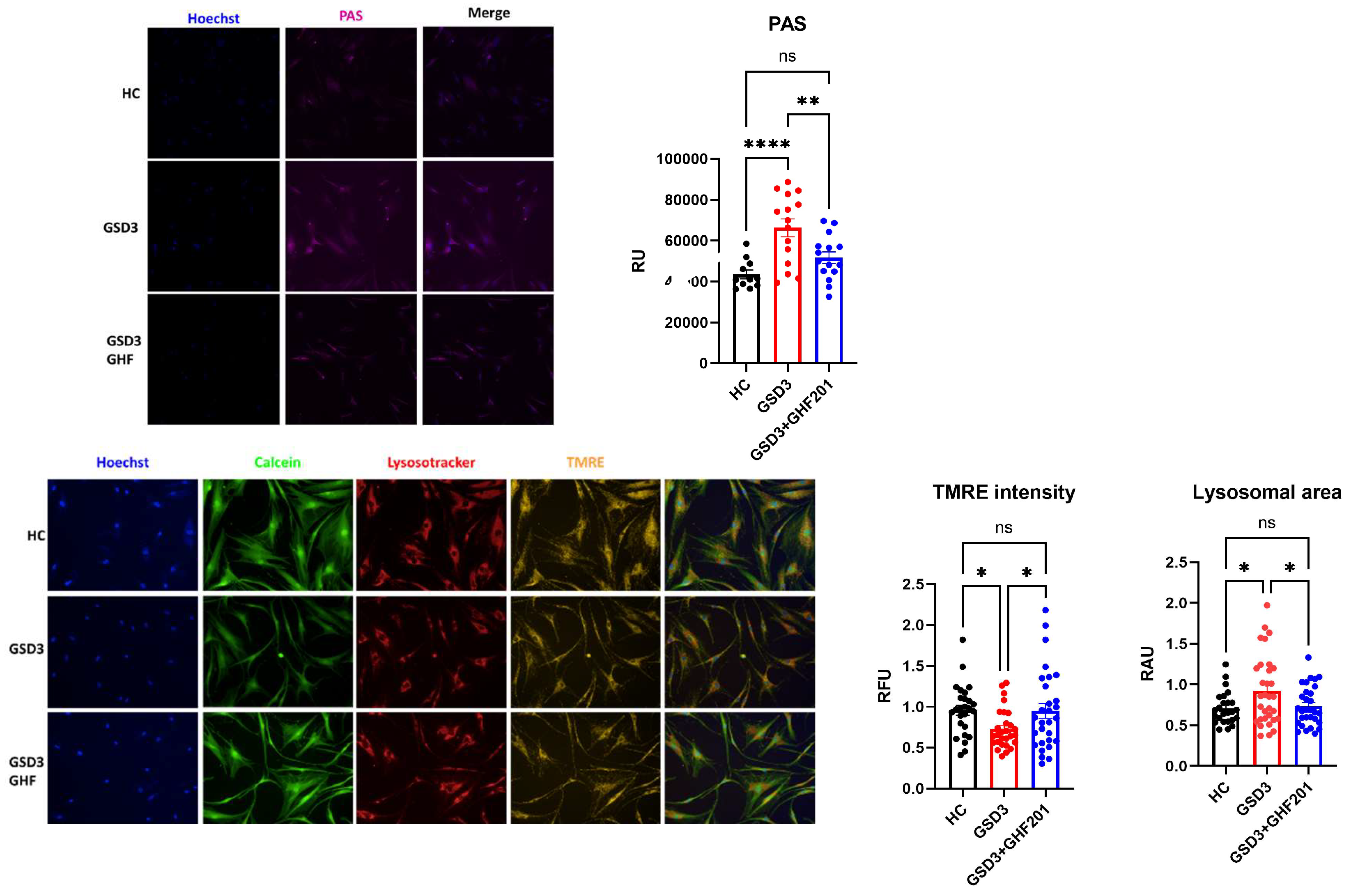
Figure 6.
Skin fibroblasts from 3 GSD3 and 3 HC individuals were cultured in 96 well plates for 48 h in starvation medium without FBS and glucose. Subsequently cells were live-stained with (A) PAS reagent to quantify intracellular glycogen, or (B) a mix of fluorescent dyes which included Hoechst, Calcein-AM, TMRE, and Lysortracker to respectively stain nuclei (blue), cytoplasm (green), respiring mitochondria (yellow) and lysosomes (red). Multiple images from the live-stained cells were automatically obtained by an Operetta G1 image analyzer under environmental controlled conditions. Shown are representative images and their quantification in relative units.
Figure 6.
Skin fibroblasts from 3 GSD3 and 3 HC individuals were cultured in 96 well plates for 48 h in starvation medium without FBS and glucose. Subsequently cells were live-stained with (A) PAS reagent to quantify intracellular glycogen, or (B) a mix of fluorescent dyes which included Hoechst, Calcein-AM, TMRE, and Lysortracker to respectively stain nuclei (blue), cytoplasm (green), respiring mitochondria (yellow) and lysosomes (red). Multiple images from the live-stained cells were automatically obtained by an Operetta G1 image analyzer under environmental controlled conditions. Shown are representative images and their quantification in relative units.