Introduction
Insulin resistance is a central feature of Metabolic Syndrome (MetS) , a cluster of cardio-metabolic features, and has been advanced as a pivotal pathophysiological mechanism for this disorder [
1,
2,
3]. Furthermore insulin resistance predisposes to both T2DM and ASCVD [
3,
4]. Although the hyperinsulinemic-euglycemic clamp (HIEC) is the gold standard for quantifying insulin resistance, it is expensive, laborious, and not routinely available. Therefore, the use of other measures to detect insulin resistance, such as the homeostasis model assessment insulin resistance (HOMA-IR) and triglyceride-glucose index (TyG), have been studied and validated against the HIEC as reliable surrogates of insulin resistance.
The TyG index has been shown to be a reliable cost effective and valid measure of insulin resistance and has been validated against the HIEC technique [
5,
6,
7,
8,
9,
10]. The majority of these studies, validating the TyG index emanate from Asian and Latin American populations. Surprisingly, the TyG index has not been embraced by investigators in the US.
In a recent report we showed that the TyG index was superior to HOMA-IR in predicting MetS in a US adult population [
11]. In addition to predicting MetS and diabetes it also appears to predict cardiovascular diseases [
12,
13,
14,
15] . Also a recent report showed it predicted severity of albuminuria independent of insulin resistance and MetS [
16]. In all of these disorders both inflammation and oxidative stress have been suggested to be pathogenic mechanisms [
4,
17]. However there is scantly data investigating a detailed repertoire of circulating and cellular biomarkers of inflammation and oxidative stress in persons with increasing TyG indices [
6,
18].
Accordingly, in the present report we investigate the relationship between the TyG index and biomarkers of inflammation, free fatty acids( FFA) levels, oxidative stress and adipokine dysregulation in a select cohort of nascent MetS without the confounding of T2DM, ASCVD, smoking, chronic inflammation and hypolipidemic drug therapy using both tertiles of TyG index and correlations with relevant variables .
Patients and Methods
In a series of papers, significant findings in this cohort focusing on adipokine dysregulation, inflammation and oxidative stress have been reported [
19,
20,
21,
22,
23]. MetS participants (n=47) and controls (n=41) aged 21-69 years were recruited from Sacramento County, CA using the criteria of the Adult Treatment Panel III (ATP III) as described previously[
1,
2]. The 5 MetS risk factors include higher waist circumference (≥ 102 cm or 40 inches for men and ≥ 88 cm or 35 inches for women), elevated triglycerides (≥ 150 mg/dL), low HDL-cholesterol levels (<40 mg/dL for men and <50 mg/dL for women), high blood pressure (systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥85 mm Hg) and high glucose level (≥100 mg/dL). Participants were defined as having MetS, if they had at least three cardio-metabolic features of MetS. Exclusion criteria for healthy control subjects included current use of any blood pressure medications, elevated triglyceride levels (>200 mg/dl) and having 3 or more of the ATP III criteria. Other important exclusion criteria for all subjects which were determined by a screening questionnaire, clinical examination and baseline chemistries included diabetes defined by fasting blood glucose level >125 mg/dl and HbA1C>6.4%, clinical ASCVD, acute or chronic inflammatory disorders, and history of smoking. Major medication exclusion criteria for subjects with MetS included anti-diabetic medications, anti-coagulants, steroids, oral contraceptive therapy, estrogen replacement therapy ,anti-inflammatory drugs, statins as well as other lipid lowering agents and angiotensin 2 receptor blockers. Additionally, all participants in the study had a high-sensitive C-reactive protein (hsCRP) level <10.0 mg/L and a normal white cell count. The study was approved by the institutional review board at the University of California, Davis and informed consent was obtained from all participants.
Fasting blood samples were taken from participants after histories and physical examinations. The details of the different assays have been reported previously [
19,
20,
21,
22,
23].Plasma lipids, lipoprotein profiles, and glucose were assayed by standard laboratory techniques in the Clinical Pathology Laboratory as described previously. Insulin levels were assayed by ELISA (Linco Biosystems, St. Charles, MO) and homeostasis model assessment insulin resistance index (HOMA-IR) was calculated from glucose and insulin levels as described previously. Endotoxin levels were quantitated using reagents from Lonza (Limulus Amebocyte Lysate, QCL 1000; Walkersville, MD). Levels of oxidized low density lipoprotein (ox-LDL) and nitrotyrosine were measured in the plasma by a sandwich ELISA using reagents from Mercodia (U.S. branch, Winston-Salem, NC) and Bioxytech (Oxis Research International, Inc., Foster City, CA) respectively. Surface expression of Toll like receptors (TLR2 and TLR4) on monocytes were analyzed by flow cytometry using BD FACS Array as reported previously[
20]. Retinol binding protein 4(RBP-4), Chemerin , adiponectin and leptin levels were measured using reagents from Linco. Interleukin-1 (IL-1) , IL-6, and IL-8 were measured using a multiplex cytokine/chemokine array (Bioplex, San Jose, CA). Nuclear factor -Kappa-beta (NFkB) activity and cytosolic phospho-P38- mitogen activated protein (MAP) Kinase activity (pP38MAPKinase) were assayed as reported[
20,
23].
The triglyceride-glucose (TyG) index was calculated as reported previously:
Ln [fasting triglycerides (mg/dl) x fasting plasma glucose (mg/dl)/2] [
5,
7].
Adipose tissue insulin resistance was calculated as the product of FFA and fasting insulin levels as reported previously[
24].
Statistical Analysis
SAS version 9.4 (SAS Institute, Cary, NC) was used for statistical analysis and significance was defined as a two-sided p-value <0.05. Results are expressed as median and interquartile range. Trend analysis of TyG index tertiles in our MetS and control participants was evaluated using the Jonckheere-Terpstra (J-T) test for trend. Combining the control and MetS groups, Spearman rank correlation coefficients were also determined to assess the association between TyG index and relevant variables. Logistic regression models were used to compute Receiver Operating Characteristic (ROC) Area under the curve (AUC) for assessing the efficacy of TyG in the prediction of MetS.
Results
As depicted in
Table 1, all cardio-metabolic features increased significantly with increasing tertiles of the TyG index except high density lipoprotein-cholesterol (HDL-C) which decreased significantly .In addition fasting insulin , non-HDL-cholesterol , FFA levels, hsCRP and HOMA-IR also increased significantly with increasing tertiles of TyG index .Importantly a valid measure of adipose tissue insulin resistance (Adipo-IR) also increased significantly with increasing tertiles of TyG index .The TyG index correlated significantly with both HOMA-IR (r=0.53, p<0.0001) and Adipo-IR (0.77,p<0.0001).
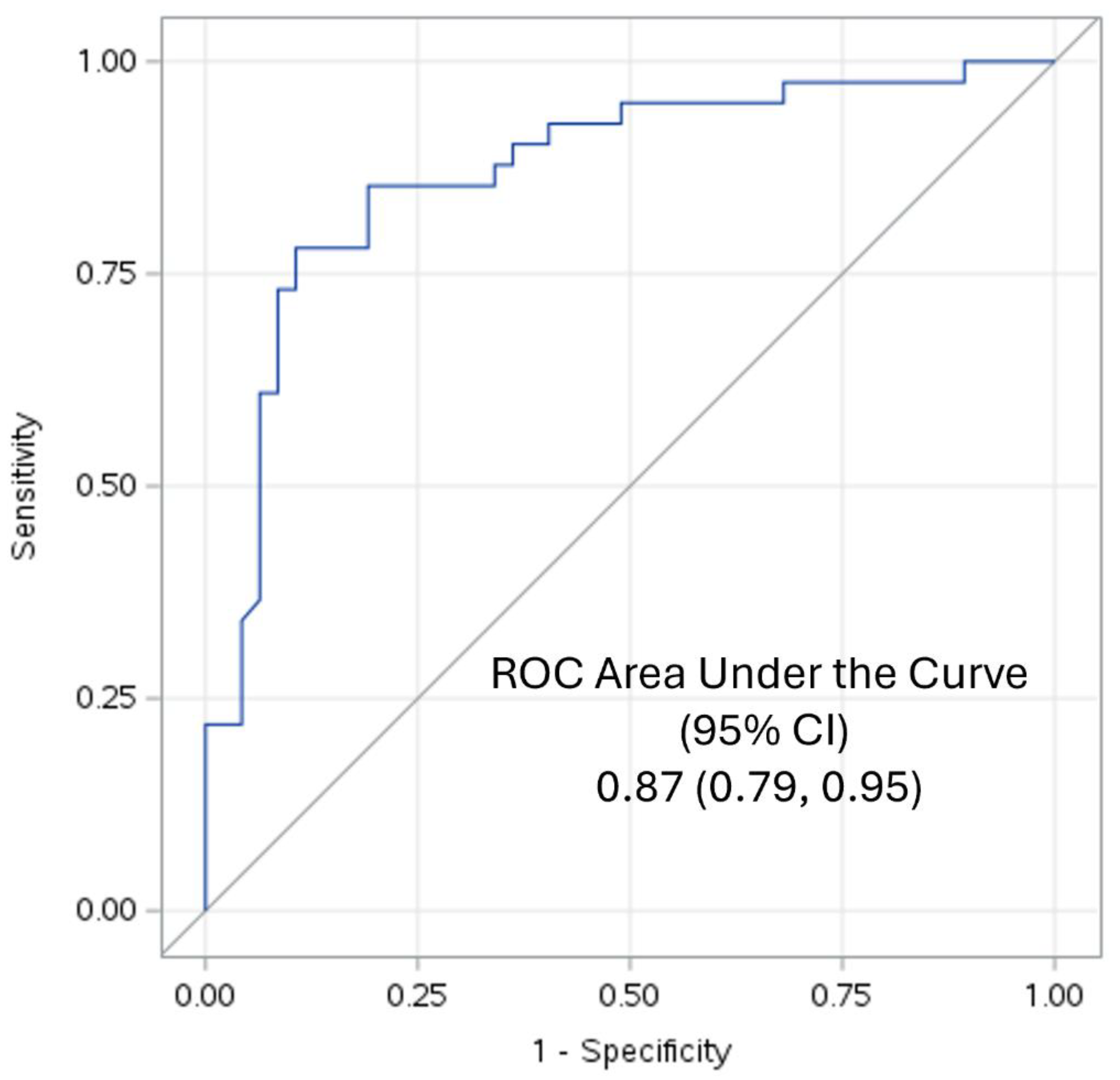
Since 47 patients had MetS and 41 were controls ROC-AUC analyses were undertaken to determine the validity of the TyG index in predicting MetS. It revealed that TyG was an excellent predictor of MetS according to the criteria of Hosmer and Lemeshow[
25]; ROC-AUC of 0.87 with 95% confidence interval of 0.79 to 0.95 as shown in
Figure 1.
In previous reports biomarkers of inflammation, oxidative stress and dysregulation of adipokine biology have been detailed in these patients[
19,
20,
21,
22,
23,
24]. In the present communication the focus was on those biomarkers that were significantly abnormal in those published studies focusing on their relationships with increasing tertiles of TyG index.
In
Table 2 are shown various biomarkers of oxidative stress, inflammation and adipokines across tertiles of TyG index. Whilst oxidized LDL levels were significantly increased over TyG index tertiles, plasma nitrotyrosine levels were not significant. With respect to circulating biomarkers of inflammation, the prototypic downstream marker of inflammation , hsCRP and endotoxin levels were increased significantly. However none of the cytokines including IL-1, IL8 and IL-6 were statistically increased over TyG index tertiles.
The two important toll-like receptors (TLR) relevant to diabetes and cardiovascular diseases are TLR2 and TLR4 [
26] .Whilst TLR2 abundance on monocytes were not increased( p=0.10) , there was a significant increase in TLR4 abundance on monocytes with increasing tertiles of TyG index. Furthermore two important signal transduction pathways, nuclear factor -Kappa-beta (NFkB) activity and cytosolic phospho-P38- mitogen activated protein (MAP) Kinase activity (pP38MAPKinase), were also increased in monocytes across tertiles.
We examined four adipokines in this report .Whilst serum chemerin levels were significantly increased across TyG index tertiles, there was a trend with retinol binding protein 4 (p=0.08). Both leptin and adiponectin were non-significant over tertiles.
We also undertook correlations to understand relationships with TyG index given the paucity of data with respect to these biomarkers in the published literature. For all cardio-metabolic features presented in
Table 1 the Spearman correlation coefficients paralleled the tertile analyses and were significant. To avoid redundancy the data is not shown.
In
Table 3 is shown the correlations with biomarkers reported in
Table 2. Interestingly the trends across tertiles for IL-6, retinol binding protein 4 (RBP-4) and monocyte TLR2 abundance revealed significant correlations: IL-6, r=0.27, p=0.01, RBP-4, r=0.32, p=0.008 and TLR2, r=0.23, p=0.04.
Since a previous study has shown that the TyG index increased significantly over quartiles of the SII index [
18] we also determined this relationship in our cohort. The median SII index over tertiles were 424, 514 and 505, respectively, p for trend =0.28 with a non-significant correlation; r=0.11, p=0.33.
For simplicity and due to the paucity of data on TyG and these biomarkers, either significance defined by tertiles or correlations are interpreted as significant for the discussion.
Discussion
The present report was prompted by the paucity of data on the relationship between the TyG index and biomarkers of inflammation and oxidative stress [
6,
18] to explain the increased risk for T2DM, ASCVD and severity of albuminuria.
Our volunteers included controls and patients with nascent MetS without the confounding of T2DM, ASCVD, macro-inflammation, smoking and hypolipidemic drug therapy . Whilst the TyG index is a validated measure of insulin resistance it also predicts MetS, diabetes, severity of albuminuria and cardiovascular diseases[
12,
13,
14,
15,
16]. Our ROC-AUC of 0.87 confirms it is an excellent discriminant of MetS. However mechanistic insights to explain these associations are sparse.
Firstly we make the novel observation that with increasing tertiles of TyG index there is a significant increase in a measure of adipose tissue insulin resistance, Adipo-IR. The strong correlation between TyG index and Adipo-IR ( r=0.77) supports our previous hypothesis that it captures both hepatic and adipose tissue insulin resistance[
11] . As expected all cardio-metabolic features reported in
Table 1 increased significantly except HDL-C which decreased. Levels of non-HDL-C increased significantly with increasing tertiles of TyG index with a correlation of 0.64, p<0.0001. This could also be advanced as a mediating mechanism for increased risk for cardiovascular diseases [
27,
28].
With respect to oxidative stress as a mediating mechanism our data on 2 biomarkers does not allow for a firm conclusion since there was a significant increase over tertiles for oxidized-LDL and significant correlation with TyG index. However this was not evident with plasma nitrotyrosine levels.
The most interesting associations were with our panel of biomarkers of inflammation. In addition to hsCRP, endotoxin levels were significantly increased and IL-6 correlated significantly with TyG index .Endotoxin is the classical ligand for the pattern recognition receptor, TLR-4[
29]. FFA levels can contribute to activation of TLR-4 and thus contribute to both insulin resistance and inflammation[
30,
31] Furthermore both cell surface receptors, TLR-2 and TLR-4 displayed significant correlations with the TyG index. In addition there were significant correlations with the downstream signal transduction pathways especially for TLR-4 including the master switch of inflammation, NFKB activity and pP38 MAPKinase activity. Thus the activation of the TLR pathway could be a crucial mechanism linking TyG index with inflammation and its sequelae.
In summary, the above data on circulating biomarkers (increased hsCRP, endotoxin, IL-6 and chemerin levels) and cellular biomediators (increase in abundance of TLR2 and TLR4 and their signal transduction pathways) support a pro-inflammatory state associated with increasing TyG index.
The Systemic Immune Inflammation (SII) index has been shown to correlate with the TyG index .We failed to confirm this in our well curated cohort controlling for confounders. The authors acknowledge that since this index is the product of neutrophils x platelets over lymphocytes it is subject to confounding by comorbidities and drug therapy etc [
18]
The data with respect to adipokine dysregulation revealed mixed results. There was a significant correlation between TyG index and RBP-4, the latter promotes insulin resistance [
19] . Chemerin, a chemoattractant for macrophages and dendritic cells is also an adipokine and appears to contribute to insulin resistance [
32]. In a prospective study, chemerin predicted the onset of T2DM over a period of 5.3 years [
33]. The increase in chemerin levels could also explain the relationship between TyG index and risk for diabetes. Unlike the report from Brazil [
6], we found no significant correlation between plasma adiponectin levels with the TyG index. This could be explained by the different populations and assays used. There was a trend to an increase in leptin levels with increasing tertiles of TyG index (p=0.08) with a correlation of 0.24, p=0.06. However much further work is needed to establish links between TyG index and adipokine dysregulation.
In conclusion, in participants without the confounding of T2DM, ASCVD, smoking, macro-inflammation and lipid therapy, the TyG index is an excellent predictor of MetS and captures both hepatic and adipose tissue insulin resistance. With respect to mechanistic insights, it appears based on the above findings that elevated FFA levels, increased non-HDL-C and a pro-inflammatory state evidenced collectively by increases in hsCRP, endotoxin, TLR-2 and TLR-4 abundance and activity and elevated chemerin levels could be advanced as mediating mechanisms explaining the increased risk for T2DMand ASCVD. However, given the cross-sectional nature of this report it cannot imply cause and effect. This can only be settled with prospective studies.
Author Contributions
IJ generated the idea for this publication. BAH undertook the statistical analyses. Both generated the original version and edited multiple iterations. Both approved the final version for submission.
Funding
There was no funding for this present report.
Institutional Review Board Statement
This study was approved by UC Davis IRB:200715074.
Informed Consent Statement
All volunteers provided written informed consent.
Acknowledgments
We thank the volunteers for participating in our study.
Conflicts of Interest
No conflicts of interest, financial or otherwise, are declared by any of the authors.
Availability of data and materials
The data is available from the senior author for review on reasonable request.
References
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith Jr SC, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112(17):2735-2752.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, and Smith SC Jr. International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute: American Heart Association: World Heart Federation; International Atherosclerosis Society; and International Association for the study of Obesity. Circulation. 2009;120(16):1640-1645.
- Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121-31.
- DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010 Jul;53(7):1270-87. [CrossRef] [PubMed] [PubMed Central]
- Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010 Jul;95(7):3347-51. [CrossRef] [PubMed]
- Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011 Sep;93(3):e98-e100. [CrossRef] [PubMed]
- Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008 Dec;6(4):299-304. [CrossRef] [PubMed]
- Guerrero-Romero F, Villalobos-Molina R, Jiménez-Flores JR, Simental-Mendia LE, Méndez-Cruz R, Murguía-Romero M, Rodríguez-Morán M. Fasting Triglycerides and Glucose Index as a Diagnostic Test for Insulin Resistance in Young Adults. Arch Med Res. 2016 Jul;47(5):382-387. [CrossRef] [PubMed]
- Nabipoorashrafi SA, Seyedi SA, Rabizadeh S, Ebrahimi M, Ranjbar SA, Reyhan SK, Meysamie A, Nakhjavani M, Esteghamati A. The accuracy of triglyceride-glucose (TyG) index for the screening of metabolic syndrome in adults: A systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2022 Dec;32(12):2677-2688. [CrossRef] [PubMed]
- Park HM, Lee HS, Lee YJ, Lee JH. The triglyceride-glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res Clin Pract. 2021 Oct;180:109042. [CrossRef] [PubMed]
- Adams-Huet B, Zubirán R, Remaley A, Jialal I. The triglyceride-glucose index is superior to homeostasis model assessment of insulin resistance in predicting metabolic syndrome in an adult population in the United States. Journal of Clinical Lipidology. [CrossRef]
- Moon JH, Kim Y, Oh TJ, Moon JH, Kwak SH, Park KS, Jang HC, Choi SH, Cho NH. Triglyceride-Glucose Index Predicts Future Atherosclerotic Cardiovascular Diseases: A 16-Year Follow-up in a Prospective, Community-Dwelling Cohort Study. Endocrinol Metab (Seoul). 2023 Aug;38(4):406-417. [CrossRef] [PubMed] [PubMed Central]
- Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022 May 6;21(1):68. [CrossRef] [PubMed] [PubMed Central]
- Lopez-Jaramillo P, Gomez-Arbelaez D, Martinez-Bello D, Abat MEM, Alhabib KF, Avezum Á, Barbarash O, Chifamba J, Diaz ML, Gulec S, Ismail N, Iqbal R, Kelishadi R, Khatib R, Lanas F, Levitt NS, Li Y, Mohan V, Mony PK, Poirier P, Rosengren A, Soman B, Wang C, Wang Y, Yeates K, Yusuf R, Yusufali A, Zatonska K, Rangarajan S, Yusuf S. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. 2023 Jan;4(1):e23-e33. [CrossRef] [PubMed]
- D'Elia L, Masulli M, Virdis A, Casiglia E, Tikhonoff V, Angeli F, Barbagallo CM, Bombelli M, Cappelli F, Cianci R, Ciccarelli M, Cicero AFG, Cirillo M, Cirillo P, Dell'Oro R, Desideri G, Ferri C, Gesualdo L, Giannattasio C, Grassi G, Iaccarino G, Lippa L, Mallamaci F, Maloberti A, Masi S, Mazza A, Mengozzi A, Muiesan ML, Nazzaro P, Palatini P, Parati G, Pontremoli R, Quarti-Trevano F, Rattazzi M, Reboldi G, Rivasi G, Russo E, Salvetti M, Tocci G, Ungar A, Verdecchia P, Viazzi F, Volpe M, Borghi C, Galletti F. Triglyceride-glucose Index and Mortality in a Large Regional-based Italian Database (Urrah Project). J Clin Endocrinol Metab. 2024 Mar 14:dgae170. [CrossRef] [PubMed]
- Wang Z, Qian H, Zhong S, Gu T, Xu M, Yang Q. The relationship between triglyceride-glucose index and albuminuria in United States adults. Front Endocrinol (Lausanne). 2023 Aug 23;14:1215055. [CrossRef] [PubMed] [PubMed Central]
- Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. 2021 Nov 22;117(13):2525-2536. [CrossRef] [PubMed] [PubMed Central]
- Xiao S, Wang X, Zhang G, Tong M, Chen J, Zhou Y, Ji Q, Liu N. Association of Systemic Immune Inflammation Index with Estimated Pulse Wave Velocity, Atherogenic Index of Plasma, Triglyceride-Glucose Index, and Cardiovascular Disease: A Large Cross-Sectional Study. Mediators Inflamm. 2023 Feb 15;2023:1966680. [CrossRef] [PubMed] [PubMed Central]
- Bremer AA, Devaraj S, Afify A, and Jialal I. Adipose tissue dysregulation in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011; 96:E1782-E1788.
- Jialal I, Huet BA, Kaur H, Chien A, and Devaraj S. Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care. 2012; 35:900-904.
- Jialal I, Devaraj S, Adams-Huet B, Chen X, and Kaur H. Increased cellular and circulating biomarkers of oxidative stress in nascent metabolic syndrome. J Clin Endocrinol Metab. 2012; 97:E1844-E1850.
- Jialal I, Devaraj S, Kaur H, Adams-Huet B, and Bremer AA. Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndrome. J Clin Endocrinol Metab. 2013; 98:E514-E517.
- Jialal I, Adams-Huet B, and Pahwa R. Selective increase in monocyte p38 mitogen-activated protein kinase activity in metabolic syndrome. Diab Vasc Dis Res. 2016 Jan; 13(1):93-96.
- Adams-Huet B, Devaraj S, Siegel D, and Jialal I. Increased adipose tissue insulin resistance in metabolic syndrome: Relationship to circulating adipokines. Metab Syndr Relat Disord. 2014. Dec;12(10):503-508.
- Hosmer DW and Lemeshow S. Applied Logistic Regression.2nd edition. Hoboken, NJ :Wiley and Sons ,Inc 2000:156-164.
- Jialal I, Kaur H. The Role of Toll-Like Receptors in Diabetes-Induced Inflammation: Implications for Vascular Complications. Curr Diab Rep. 2012 Feb 8. [CrossRef] [PubMed]
- Welsh C, Celis-Morales CA, Brown R, Mackay DF, Lewsey J, Mark PB, Gray SR, Ferguson LD, Anderson JJ, Lyall DM, Cleland JG, Jhund PS, Gill JMR, Pell JP, Sattar N, Welsh P. Comparison of Conventional Lipoprotein Tests and Apolipoproteins in the Prediction of Cardiovascular Disease. Circulation. 2019 Aug 13;140(7):542-552. [CrossRef]
- Raja V, Aguiar C, Alsayed N, Chibber YS, ElBadawi H, Ezhov M, Hermans MP, Pandey RC, Ray KK, Tokgözoglu L, Zambon A, Berrou JP, Farnier M. Non-HDL-cholesterol in dyslipidemia: Review of the state-of-the-art literature and outlook. Atherosclerosis. 2023 Oct;383:117312. [CrossRef] [PubMed]
- Jialal I, Kaur H, and Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J Clin Endocrinol Metab. 2014; 99:39-48.
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006 Nov;116(11):3015-25. [CrossRef] [PubMed] [PubMed Central]
- Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab. 2011 Jan;300(1):E145-54. [CrossRef] [PubMed] [PubMed Central]
- Jialal I. Chemerin levels in metabolic syndrome: a promising biomarker. Arch Physiol Biochem. 2021 Apr 12:1-3. [CrossRef] [PubMed]
- Bobbert T, Schwarz F, Fischer-Rosinsky A, Maurer L, Möhlig M, Pfeiffer AF, Mai K, Spranger J. Chemerin and prediction of Diabetes mellitus type 2. Clin Endocrinol (Oxf). 2015 Jun;82(6):838-43.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).