2. Materials and Methods
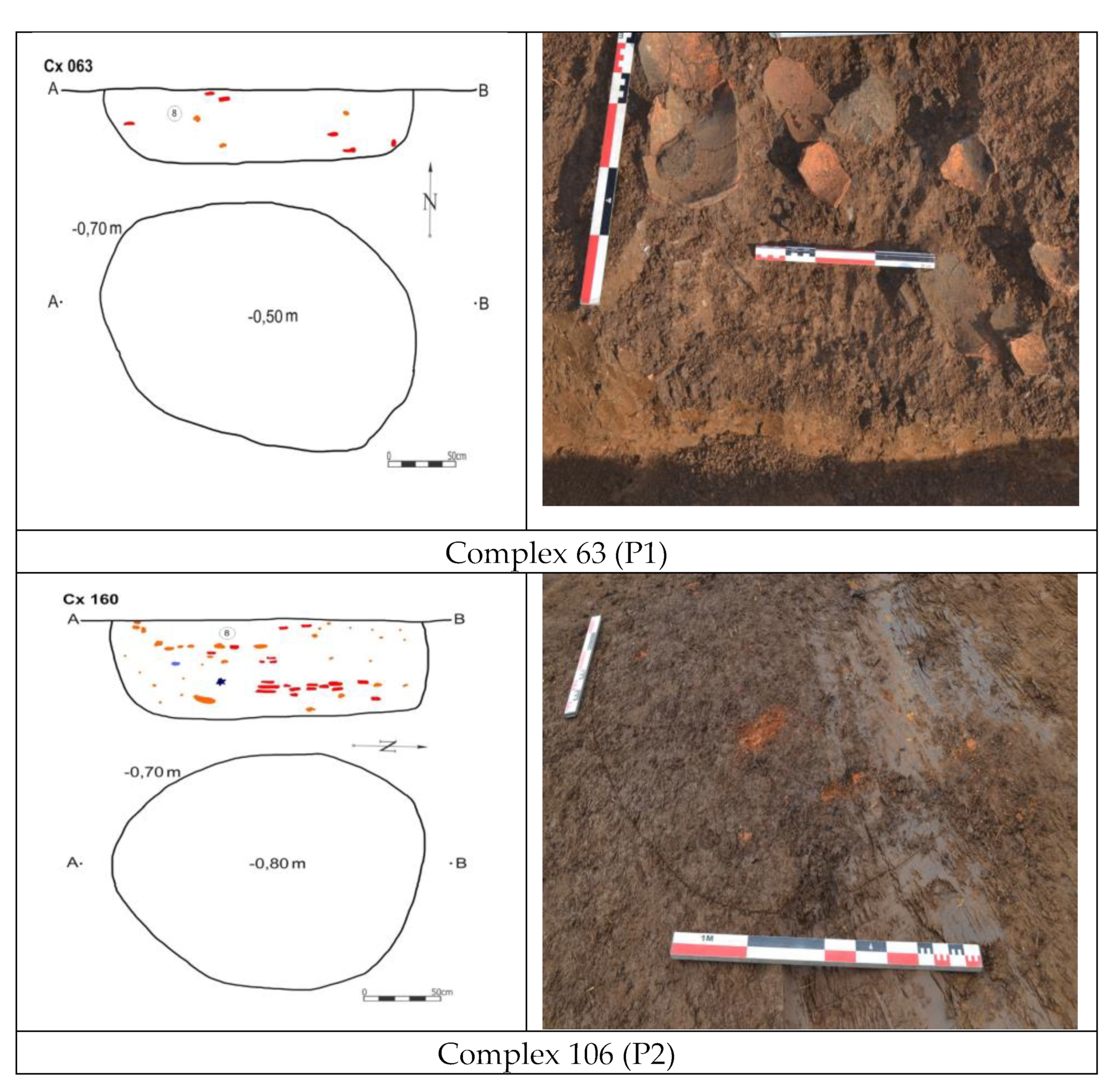
The ceramic fragments discovered at various archaeological sites exhibit a classification into thick-walled and thin-walled ceramics, with prevalent hues of dark red and black, while certain pieces showcase painted designs adorning their surfaces. Two important pieces have been investigated, as follows: Fragment of vessel, Dudeşti Culture Milcov, Olt, D.Ex.12, Sit 3, Cx. 63 (nominated as (P1), and Supply vessel, Dudeşti Culture, Milcov, Olt, D.Ex.12, Sit 3, Cx.106, (nominated as (P2),
Figure 1. All these samples have been collected in agreement with the national regulations [
9].
The samples were discovered during the works for the express road Pitești - Craiova, in Milcov locality, Olt County.
Methods
The analysis involved the utilization of a Rigaku ZSX Primus II spectrometer for wavelength dispersion X-ray fluorescence spectroscopy (WDXRF). The instrument is equipped with an X-ray tube featuring a Rh anode and 4.0 kW power, and a front Be window (30µm thickness). Employing an EZ-scan in conjunction with the Rigaku SQX fundamental parameters software (ZSX version) facilitated spectra recording and data processing, ensuring precise and comprehensive results from the spectroscopic examination.
X-ray diffraction (XRD) analysis was carried out utilizing a Rigaku Ultima IV model diffractometer with the following characteristics: Cu Kα radiation (λ = 1.5406 Å), generator radiation acceleration voltage of 40 kV and the emission current of 200 mA. Diffractograms were captured in parallel beam geometry within a 2θ range of 5° to 100° at a scanning rate of 4°/min.
Additionally, optical microscopy (OM) imaging was conducted using a Novex Microscope BBS trinocular microscope at various magnifications, equipped with a digital video camera for real-time data acquisition through ZenPro software and subsequent analysis with ImageJ 1.50. Stereomicroscopy was performed using a Stereo trinocular stereomicroscope from EUROMEX Microscopen B.V., model 1903, providing magnification options within the range of 7–45×.
Environmental scanning electron microscopy (ESEM-FEI Quanta 200, Eindhoven, The Netherlands) has been used for morphology evaluation of the samples. The working parameters were high vacuum, an LFD detector, and magnification between 50×–100 000×. For preparing the samples for SEM investigations, a small piece of each sample has been taken and covered with a 5 nm layer of Au (through sputter coating), by using a Q150R-ES system (Quorum Technologies Ltd, West Sussex, UK). This coating thin films of Au is used to minimize the charging effects during SEM imaging, and to make them conductive before observation.
FT-IR spectroscopy utilizing a Perkin Elmer GX spectrometer (manufacturer Perkin Elmer, Waltham, USA), (attenuated total reflection mode-ATR), resolution of 4 cm-1 and accumulation of 32 spectra, measurements within the range of 4000 - 400 cm-1 for analysis.
Raman spectroscopy was conducted with a Rigaku portable analyzer (Rigaku, USA) with 785 and 1064 nm stabilized laser to ensure high sensitivity. The measurements were carried out at a resolution of 4 cm-1 using a laser power of 252 mW. For data processing the Opus 7.0 software from Bruker Optics GmbH, has been used.
The textural properties of the investigated samples were determined using the Brunauer-Emmett-Teller (BET) method, where nitrogen adsorption/desorption isotherms recorded at 77 K over a relative pressure range of 0.005-1.0 using a NOVA2200e gas absorption analyzer from Quantachrome Instruments in Florida, USA. For data processing the NovaWin version 11.03 software, has been used. Before the adsorption measurements, the samples underwent a 4-hour degassing process at 180°C under vacuum. The BJH model was applied to evaluate the pore size distribution and pore volume informations.
The chromatic parameters were measured using a CR-410 colorimeter from Konica Minolta, Tokyo, Japan, following the CIELAB standard [
10,
11]. These included L* for brightness (ranging from 0 for black to 100 for white), a* for red/green variation (where +a* denotes red and -a* denotes green), and b* for yellow/blue variation (+b* for yellow and -b* for blue).
The thermogravimetric analyses were conducted with a Pyris 1 TGA analyzer from Perkin Elmer (TGA-7) in Waltham, MA, USA, with a temperature range of 50-800 °C, a heating rate of 10 °C/min, and nitrogen flow at a rate of 50 mL/min.
3. Results and Discussion
Pottery is indeed a crucial artifact in archaeological studies, offering valuable information about past civilizations. Analyzing pottery can reveal details about cultural practices, trade routes, and social interactions among ancient societies. By studying pottery, archaeologists can offer a better understanding of the lifestyles and historical contexts of different cultural groups, enabling them to piece together a more comprehensive picture of human history. [
7]. The composition of pottery paste is recognized as a valuable tool in archaeological investigations, providing insights into the sourcing of raw materials, production techniques, and chronological sequences of artifacts. By analyzing the chemical composition of pottery paste, archaeologists can determine patterns of trade, cultural exchange, and technological advancements in ancient societies. This information enhances the interpretation of archaeological finds and helps reconstruct past human activities with greater accuracy and depth. [
12].
Various chemical composition and visual characteristics of the examined samples provide important insights into the production techniques and firing processes of the pottery. The two-layer texture observed in the ceramic walls suggests different firing conditions, such as uneven firing or firing in a reducing atmosphere followed by rapid oxidation. These findings can help archaeologists understand the technological choices and skills employed by ancient artisans, shedding light on ancient manufacturing practices and the cultural context in which the pottery was produced [
13,
14].
The detailed description of the first sample collected from the household waste pit in Complex 63 provides crucial contextual information about the archaeological site. The composition of the filling material, including sedimentary, loamy, compact soil pigmented with adobe, ceramic fragments, and stones, offers insights into the daily activities and waste disposal practices of the ancient inhabitants. This data aids in understanding the living conditions, material culture, and socio-economic aspects of the people who occupied the site, enriching the archaeological interpretation and reconstruction of past human behaviors [
4].
Figure 2.
The locations where the samples have been collected from.
Figure 2.
The locations where the samples have been collected from.
The detailed description of the second sample, a fragment from a supply vessel found in the Dudești Culture belonging to the Cernica phase, provides valuable information about the archaeological context and material culture of the Middle Neolithic period. The vessel, discovered in Complex 106 within household waste, is significant as one of the few complete pieces from the Cernica phase, highlighting the craftsmanship and decorative patterns characteristic of this cultural period. The presence of linear incised decoration on the vessel adds to our understanding of artistic expressions and technological practices of the Dudești culture, contributing to a comprehensive narrative of ancient societies in the region.
The cultural name, Dudești Culture, derives from the site found in the Dudești area of Bucharest and extends across southeastern Oltenia and southern Wallachia. Dating back to the beginning of the 5th millennium BCE, this culture showcases ceramics with strong geo-Mediterranean influences, incorporating elements from the Starčevo-Criş culture and the linear pottery culture. The assimilation of these diverse cultural elements points to the interconnectedness and exchange of ideas among ancient societies, shedding light on the complex dynamics of cultural interactions in the prehistoric period of the region.[
8]
.
It is highly probable that this vessel was utilized for storing liquids, given its polished surface and intricate decorations. Supply vessels like these exhibit a high frequency in Milcov pottery, indicating their functional importance in ancient societies for storing and transporting liquids. The craftsmanship and attention to detail seen in the polished and decorated surface of the container highlight the significance of such vessels in daily activities and cultural practices of the time, providing insights into the material culture and societal interactions of the ancient Milcov pottery tradition [
4].
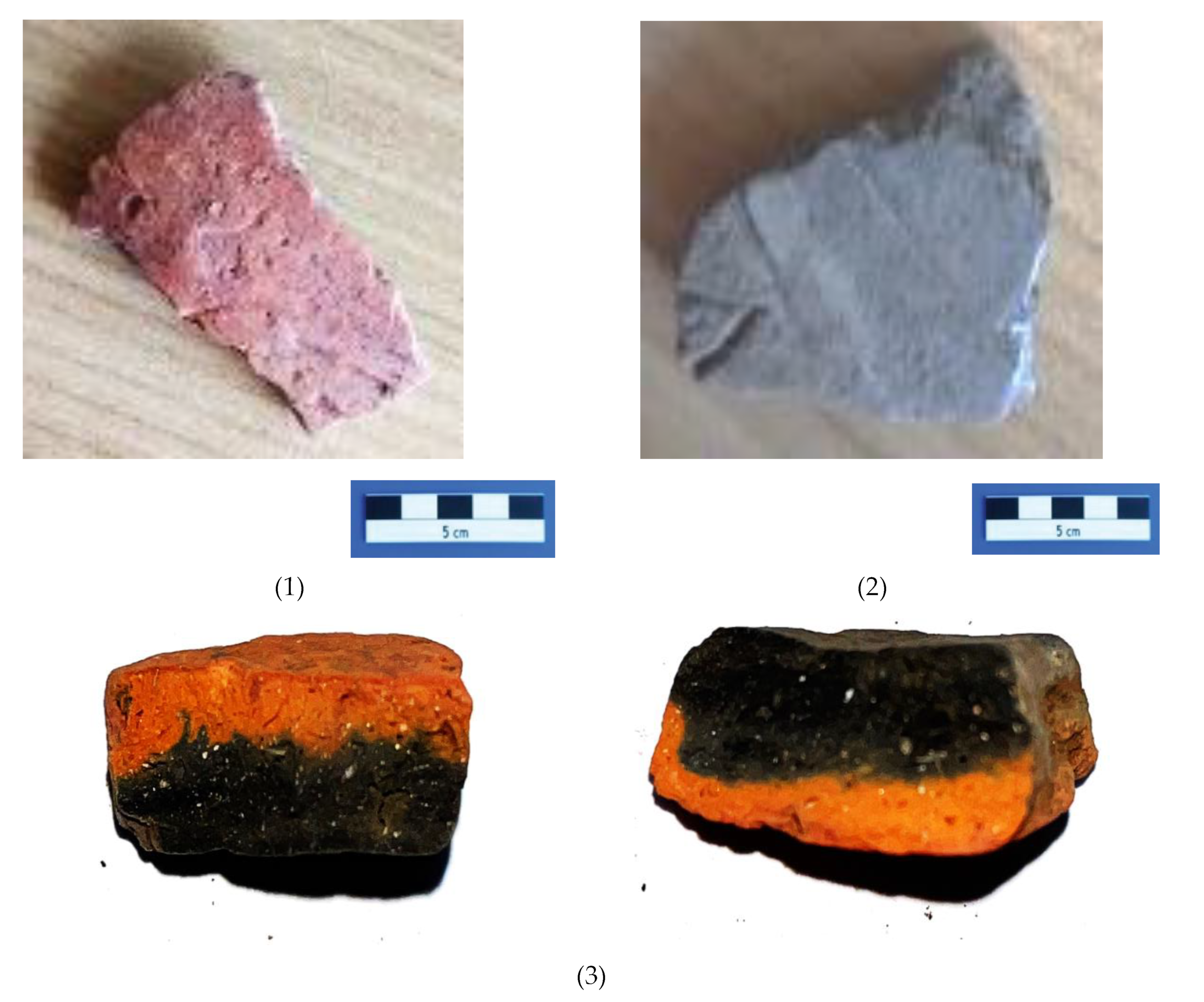
The clay piece was shaped by hand, the firing being oxidizing. The paste is compact with fine slip in composition, with scarlet angoba. The surface has been finely leveled and shows signs of secondary burning.
WDXRF
The investigated samples are rich in alumina, silica with traces of iron oxide, but with minor content of calcium oxide, potassium oxide, magnesium, titanium, according to
Table 1. This method allows for a detailed examination of the elemental composition of the pottery fragments, providing valuable insights into the raw materials used in their production and potentially indicating regional sourcing or technological preferences in pottery-making practices. The precise quantification of mineral components through WDXRF analysis offers a nuanced understanding of the chemical composition of the pottery fragments, aiding in archaeological interpretations and material studies.
The intensity distribution maps reveal varying compositions in the pottery fragments, with a notable presence of calcium and trace amounts of Al, Mg, K, Si, Ti, Fe elements. This indicates the potential presence of minerals such as calcite, silicates, K-feldspar, and hematite, among others. To further analyze these results, the composition of soils in Oltenia, including Sandy chernozem, Meadow chernozem, reddish-brown forest soil, brown forest soils, brown mountain forest soils, and Gleice or Lacovistile soils, must be considered. These soils encompass different types, each with varying compositions and characteristics, which can inform the understanding of the mineral composition found in the pottery fragments. By correlating the elemental compositions in both the pottery and soil samples, a comprehensive analysis can be conducted to unravel potential relationships between the materials used in pottery making and the local geological environment.[
1]. The WDXRF data analysis enables the tentative identification of mineral inclusions in the investigated clays, with intensity distribution maps highlighting Si, K, and Fe-rich particles suggestive of minerals like silicates, K-feldspar, and hematite. The pottery samples exhibit predominant concentrations of SiO2, Al2O3, and various fluxes (K2O, Fe2O3, CaO, MgO, and TiO2), indicative of non-calcareous ceramics with low refractoriness and a lack of a smooth surface, indicating firing temperatures below 750 ℃. Additionally, partially smooth surfaces, non-calcareous compositions, and low refractoriness suggest firing temperatures around 750-800 ℃. These findings offer valuable insights into the materials used and firing processes employed in the creation of the archaeological pottery, shedding light on production techniques and material characteristics [
2].
The analysis conducted using X-ray diffraction revealed that the shards mainly consist of quartz and feldspar as the primary mineral phases, with muscovite and aluminosilicates present in some shards. The mineralogical compositions indicated similarity in raw materials used for the shards across all sides. Furthermore, the presence of hematite in addition to calcite suggests variations in the geological formations in this site area, influencing the composition of the pottery shards. This insight connects the material composition of the artifacts to the geological characteristics of the regions, providing valuable information about the resources available and the potential trade networks that may have existed.
The examination of pottery sherds from Neolithic sites in Dudești through WDXRF analysis uncovered notable concentrations of Fe-Ca and Zr-Sr in the clay bodies of all samples, offering initial insights into the raw materials utilized for their production based on their chemical compositions. The presence of low concentrations of Ca in the samples suggests the use of low-lime raw materials, indicative of low-Ca clay in the ceramics manufacturing process. Typically, clays with CaO levels below 5% (Ca < 3.6%) are deemed non-calcareous, emphasizing the significance of understanding the elemental composition of the clay in determining the production techniques and material selection in ancient ceramic production within the archaeological context of Dudești [
15]. Moreover, the analysis revealed consistent concentrations of Fe ranging from 3% to 8% across nearly all samples, indicating a prevalent presence of iron in the ceramics. Additionally, the similar concentration range of Zr in all samples suggests a potential correlation with the specific clay deposits utilized in the production process. This insight into the elemental composition of the pottery shards from Dudești facilitates a deeper understanding of the raw materials selected and the manufacturing techniques employed in crafting these Neolithic artifacts [
16].
The findings from the WDXRF investigations revealed that calcium is not a major component in the investigated samples as a raw material, with only trace amounts found in a few samples in forms such as calcite, phosphate, or sulfate. These small concentrations of calcium could be provided by the clay and gypsum present in the geological formations of the areas where the sites are located. The research provided insight into the raw materials used for product production and the chemical composition of the samples. Based on the results, it can be concluded that the samples are non-calcareous with low limestone content, indicating the use of poor calcium clays in the ceramics manufacturing process. Additionally, the clay paste in the samples showed a consistent iron concentration of around 7%, highlighting the presence of iron in the composition of the ceramics [
17], [
18]. Additionally, the clay pastes in the samples showed a consistent iron concentration of around 7%, highlighting the presence of iron in the composition of the ceramics.
XRD
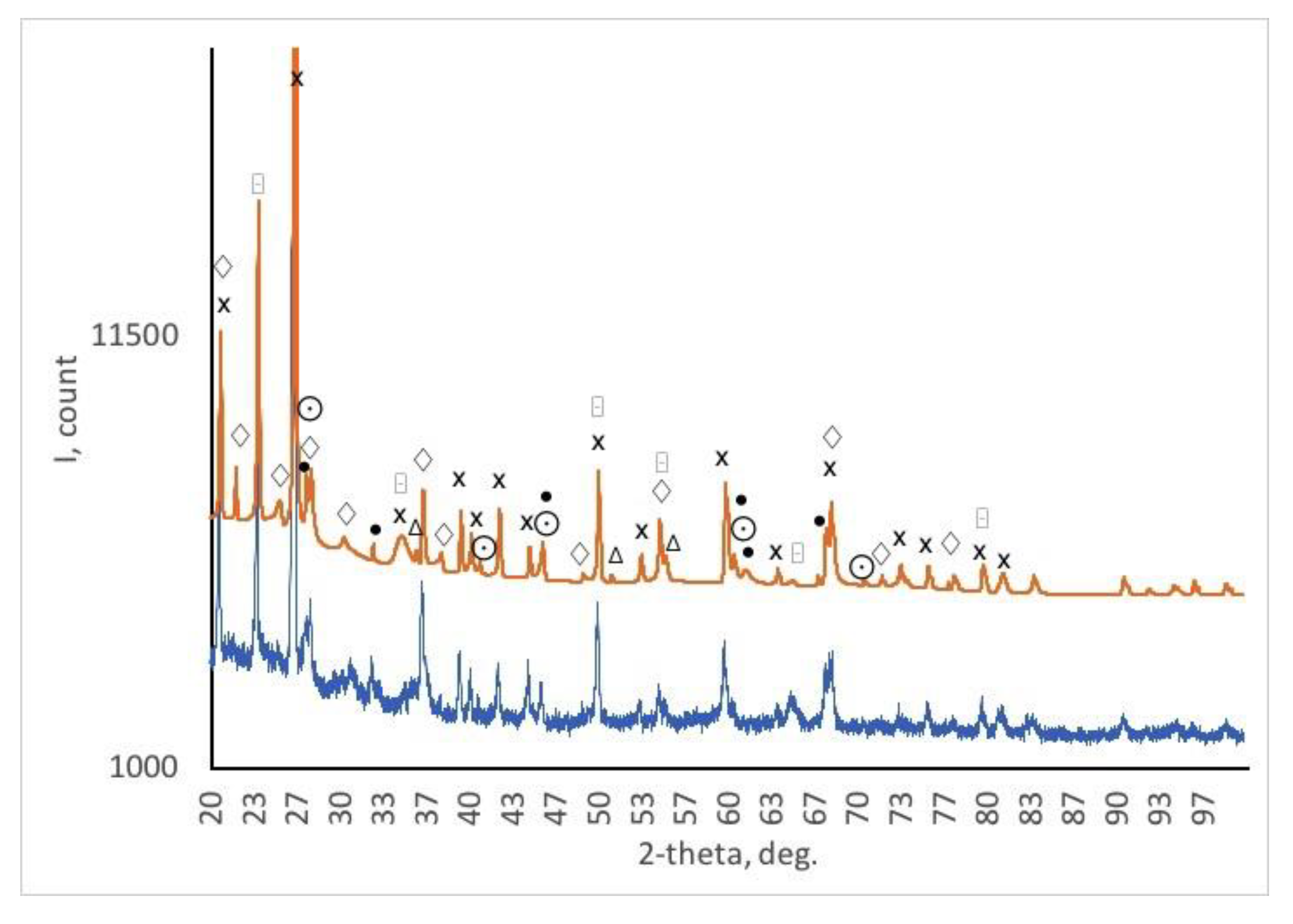
The concentration of the main components of these ceramics are determined by XRD measurements,
Figure 3 and
Table 2.
Composition of samples, as determined by XRD are marked directly on XRD diagram and reflected in
Table 2.
Microscopy
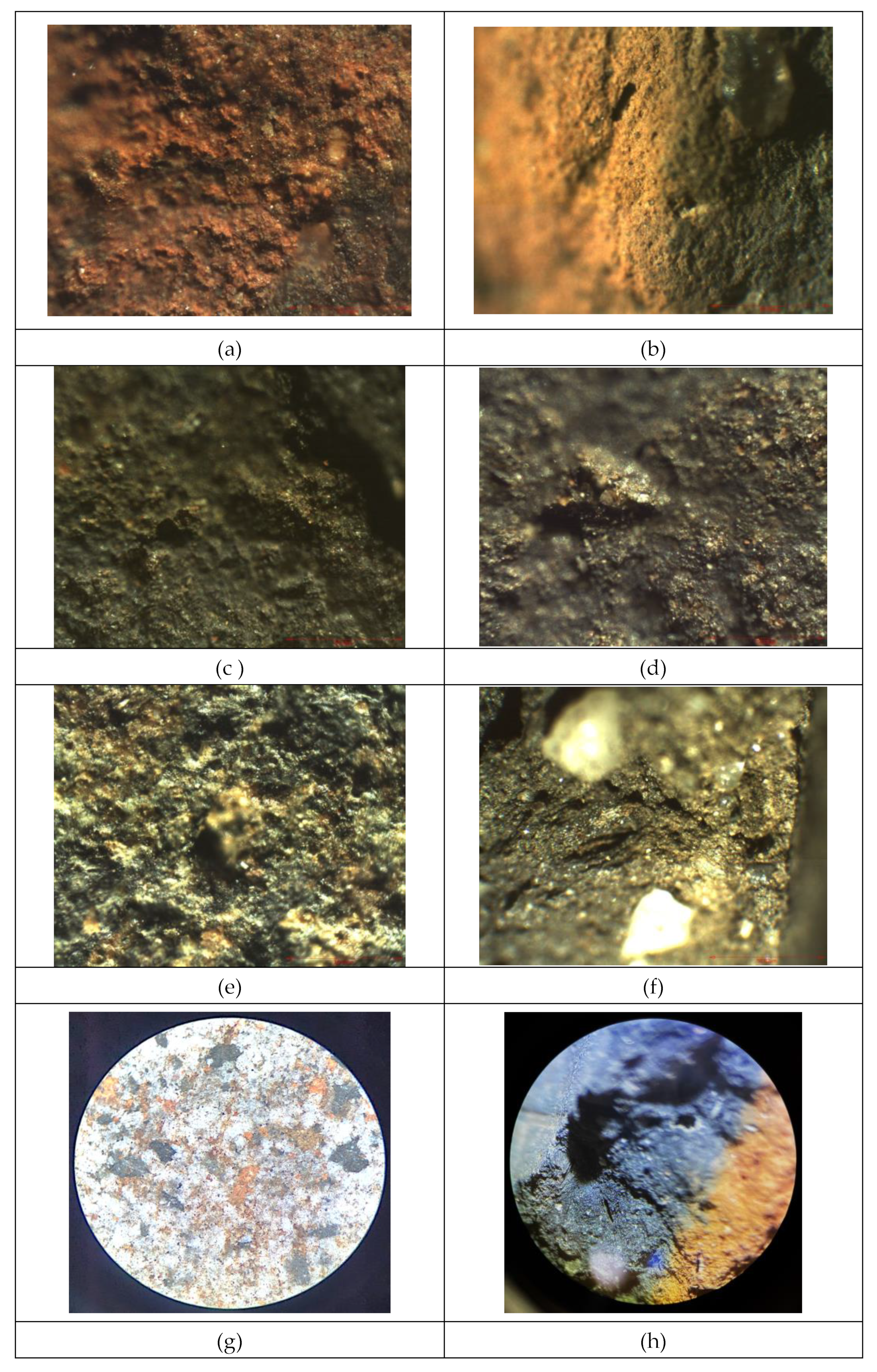
Optical microscopy analysis of the samples revealed fine crystal-like traces, distinct groove-type patterns, as well as a cracked texture, indicating specific characteristics and formations present in these ceramics. The detailed observations in the optical and stereomicroscopic images provide insight into the composition and structure of the ceramic bodies, with previous studies suggesting that the ceramics are made from semi-fine clays with an amorphous-microcrystalline structure containing non-plastic natural inclusions, either from raw materials or added during processing. These findings contribute to a better understanding of the manufacturing processes and material composition of the studied ceramics [
12,
14,
17,
18]. In this case, the non-plastic inclusions found in the ceramics primarily consist of materials such as quartz, muscovite, melanterite, and feldspar group clays, with sizes ranging from 1 to 3 mm and accounting for 5% to 10% of the shard volumes. These inclusions also include components like calcite, hematite, and even small plant remains within the sherds. Notably, red ocher, soot, hematite, and possibly maghemite were identified in the red and black decorations of the Neolithic pottery from southern Romania, serving as indicators of firing conditions and providing valuable insights into the cultural and technological aspects of the ceramics studied [
19].
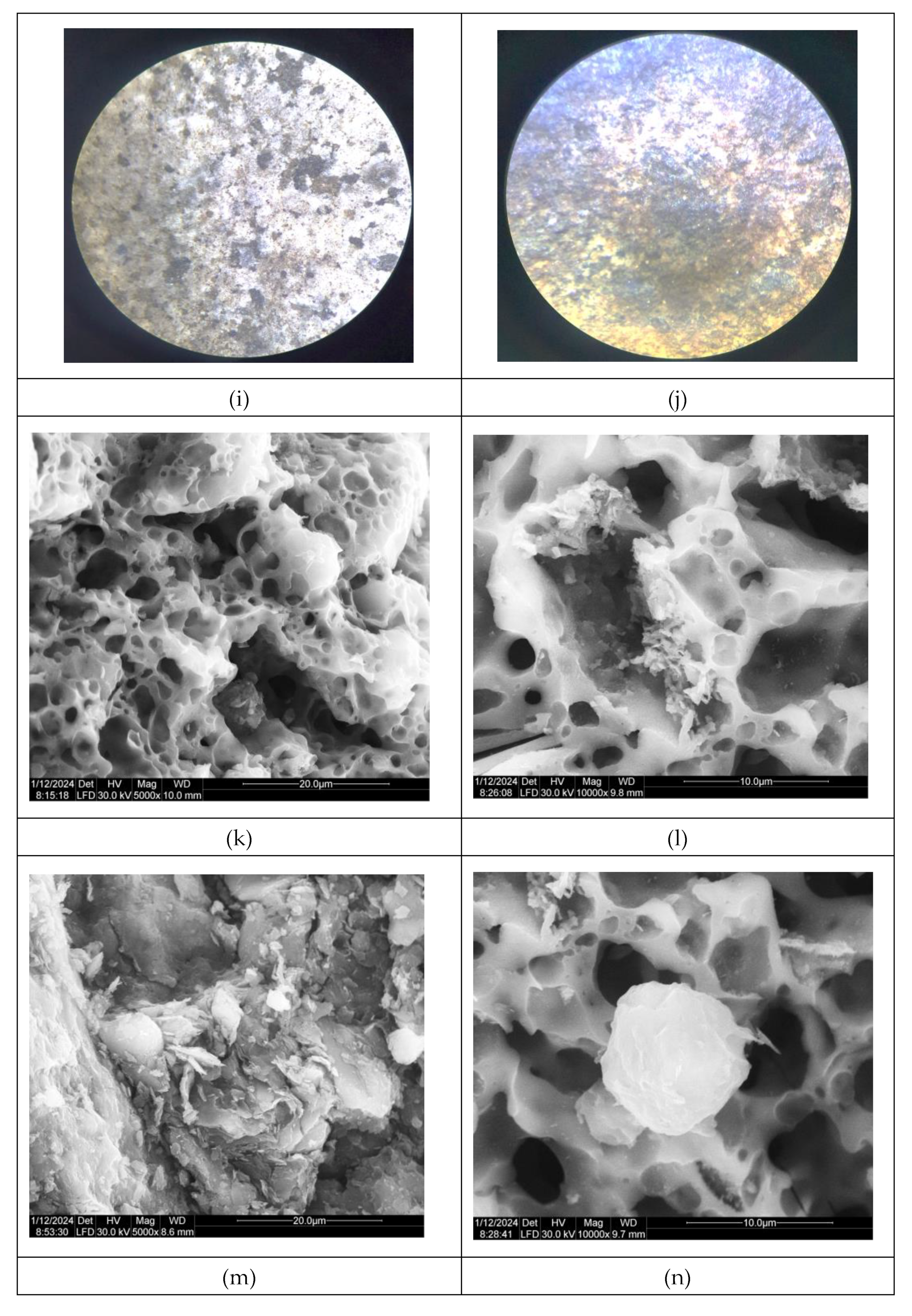
All these inclusion, more visible by stereomicroscopy have been identified as small fragment reddish, black or intermediate colors, visible for sample P1-red part, while for black part, this color seems to be deposited, most probably due to an anaerobic firing atmosphere, or to the obtained soot,
Figure 4 g,h. The same observations could be not so visible for sample P2,
Figure 4 i,j.
The analysis revealed that various Fe-rich minerals were present in the red colors of the ceramics, while dark brown hues were predominantly achieved using Mn-rich minerals, except for one case where Fe-rich minerals were utilized, likely influenced by a reducing atmosphere. The black decorations on the sherds are believed to have been created post-firing with bitumen, as the sulfurous content in the black areas indicates. Interestingly, similar pigment types have been identified by other researchers in Neolithic pottery from different locations across the Balkans and Europe, highlighting potential similarities and shared practices in pigment preparation and decoration techniques during that period [
20,
21].
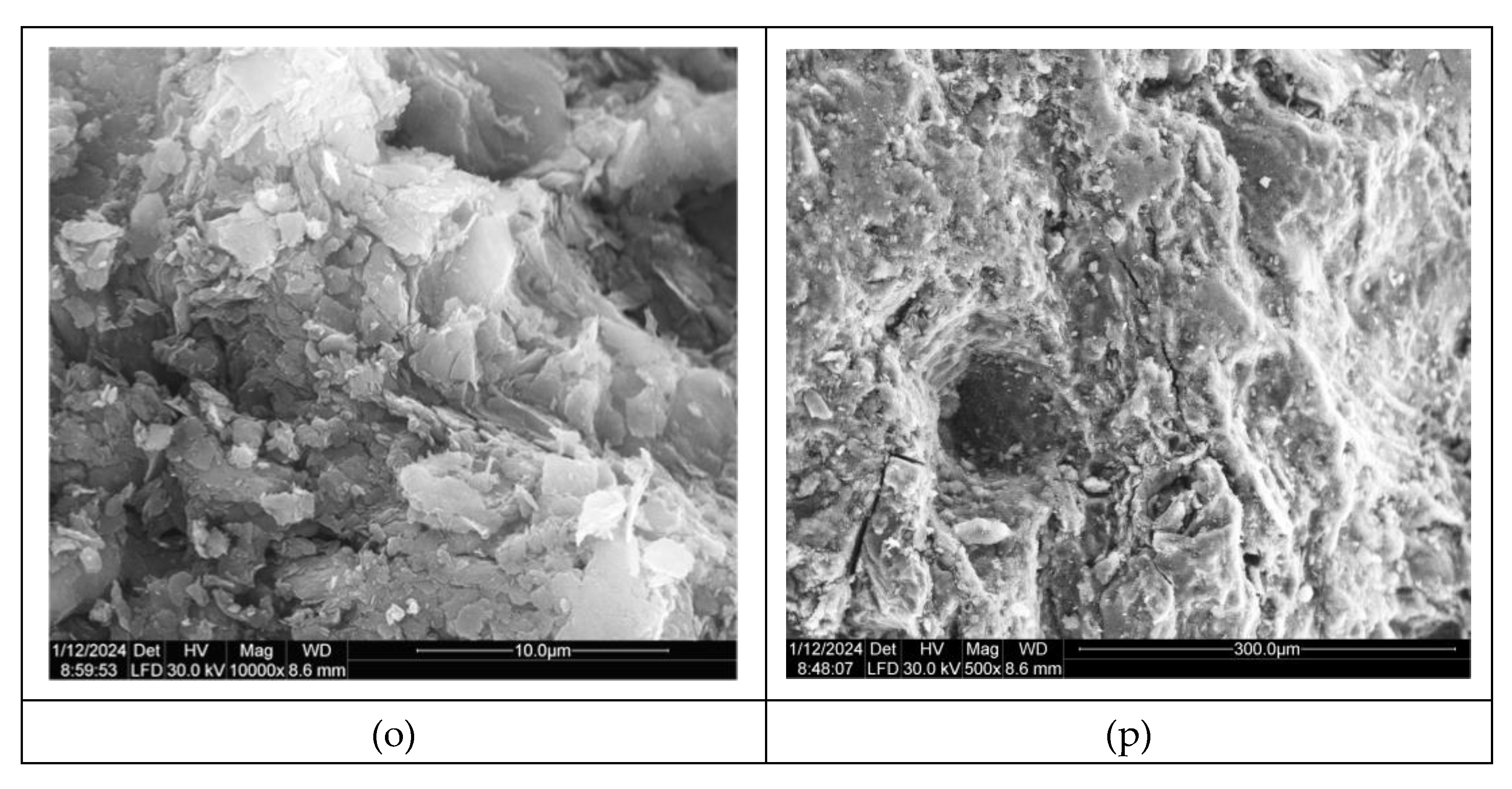
The SEM micrographs displaying representative samples of vessel sherds can be viewed in
Figure 4 (k-m), providing a visual insight into the characteristics and composition of the analyzed samples. These micrographs likely offer detailed information on the surface morphology, elemental composition, and structural features of the vessel sherds, aiding in further understanding and interpretation of the materials under investigation. For sample P1, only the red part presented significant structures, not the black part. Being a non-calcareous ceramic, with a low concentration of CaO, and with a heterogeneous distribution of it in the ceramic mass, in sample P1 a zone dominated by nanometric pores (
Figure 4 k,l) was observed inside the calcite crystals, which suggests an early stage of decomposition and release of CO
2, as demonstrated by Rodriguez Navarro et al. [
22]. The entire surface of calcite crystals exhibits nanoporosity, which is primarily concentrated along the weaker regions of the crystals called cleavage surfaces. Combustion of the coal mass dispersed in the sample may be a source of these pores. The amorphous glassy mass together with cryptocrystalline minerals is organized in the pore walls of the boundary layer (
Figure 4, m), where some spherical aggregates can be formed, and their size makes the pore walls even more visible (
Figure 4 n). Obviously, such an organization contributes to the increase in porosity, decreasing the water absorption capacity of the ceramic vessel [
23]. If these structures are very clear at P1 sample, at P2 samples, are not. At this sample, only small layered crystalized and non-homogeneous dispersed could be observed,
Figure 4 o, which surround large pores created by burning,
Figure 4 p.
The scanning electron microscope (SEM) has been used for investigating and analyzing the morphology and degree of vitrification in archaeological ceramics. This method allows for a detailed examination of the surface features, composition, and structural characteristics of the ceramics, providing insights into the vitrification process and the resulting structures present in the samples. SEM analysis allows researchers to gain a deeper understanding of the firing conditions, mineral composition, and potential cultural practices associated with the production of these archaeological ceramics [
24]. The study suggests that clay-calcareous ceramics with insufficient calcite content and fine grain exhibit vitrification behaviors similar to non-calcareous clay ceramics when fired in a reducing atmosphere. Vitrification does not occur at temperatures below 800°C in a reducing atmosphere, but higher temperatures lead to extensive vitrification characterized by fine pores and swellings. The ceramics studied are primarily medium and fine in texture, and their firing in a reducing atmosphere resulted in vitrification structures at temperatures around 50°C lower than in an oxidizing atmosphere. This highlights the importance of firing conditions in influencing the vitrification process and resulting structures in archaeological ceramics [
25,
26].
Firing the ceramics in a reducing atmosphere not only lowered the vitrification temperatures but also led to the formation of a significant concentration of fine bubble pores, approximately 4 nm in diameter. This indicates that the reduction atmosphere plays a crucial role in creating these unique microstructural features in the ceramics, further demonstrating the impact of firing conditions on the final characteristics of the archaeological artifacts.[
27,
28].
As firing temperature increases in a reducing atmosphere, there is a transition in the ceramic microstructure characterized by larger pore sizes and reduced pore numbers until a network of unconnected pores formed during the final vitrification stage. This leads to full collapse or significant bulging, resulting in a brittle and highly porous body, as observed in sample P2. The samples (P1 and P2) were likely fired below and slightly above 800 °C, respectively, in a reducing atmosphere [
29,
30,
31], these pore networks could indicate the use of plants or their remnants, further microscopic analysis did not support this claim
Though some references suggest that these pore networks may indicate the use of plants or their remnants, further microscopic analysis did not support this claim. Analysis through FTIR and Raman techniques revealed traces of organic oils, potentially from coatings applied to the ceramics rather than originating from plant materials [
32].
FTIR
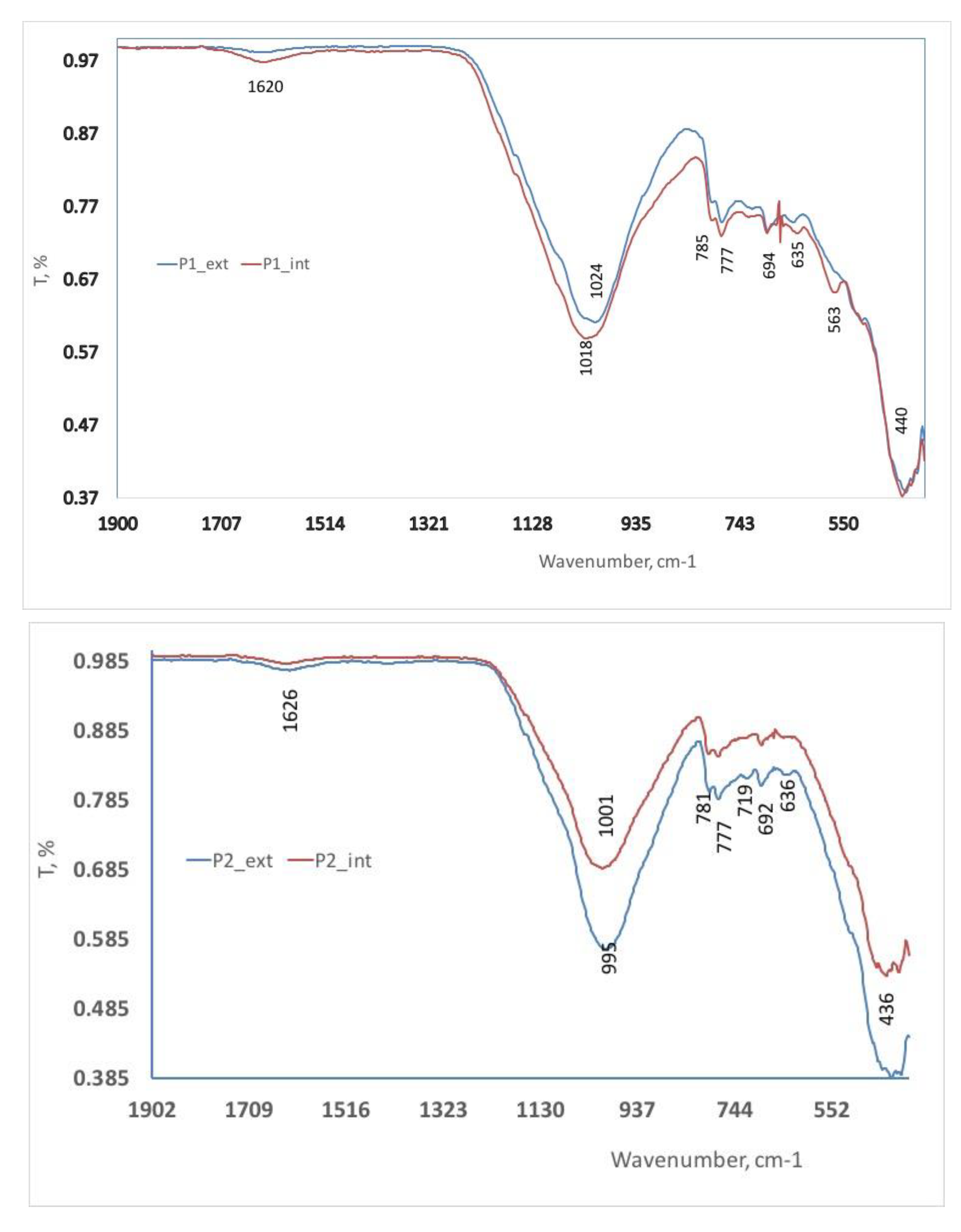
The FTIR spectra obtained from the samples display prominent peaks corresponding to aluminosilicates and quartz, indicating the presence of these compounds (as shown in
Figure 5). The distinctive peak at 635 cm
-1 is attributed to hydroxyl groups, while vibrations at 694 cm
-1 along with doublets at 777 cm
-1 and 785 cm
-1 are characteristic of quartz, emphasizing its presence. The Si-O absorptions and bands at 436-440 cm
-1 further confirm the existence of quartz, with the broadband signal at around 1000 cm
-1 highlighting the crystalline nature of quartz within the samples. These spectral features provide valuable insights into the structure and composition for the analyzed ceramics. [
23]. The presence of bands at 797, 777, 510, and 440 cm
–1 in quartz indicates specific vibrational modes within the material, while the band at 1630 cm
–1 is assigned to the H-O-H bending of water molecules. Additionally, the FTIR spectra showed prominent bands related to silicates and aluminate hydrates, such as strong silicate bands at 1011–1022 cm
−1 and various internal bonds involving Si, O, and Al atoms. These observations provide valuable insights into the composition and structural characteristics of the sample [
22].
The band at 694 cm
–1, attributed to the Si-O-Al group, Si-O-Si group, and Al-OH modes, suggests the presence of muscovite within the sample. Additionally, specific peaks at 563 and 476 cm
–1 indicate the presence of hematite in the studied region. These findings further support the characterization of the material and provide important information about its mineral composition [
14].
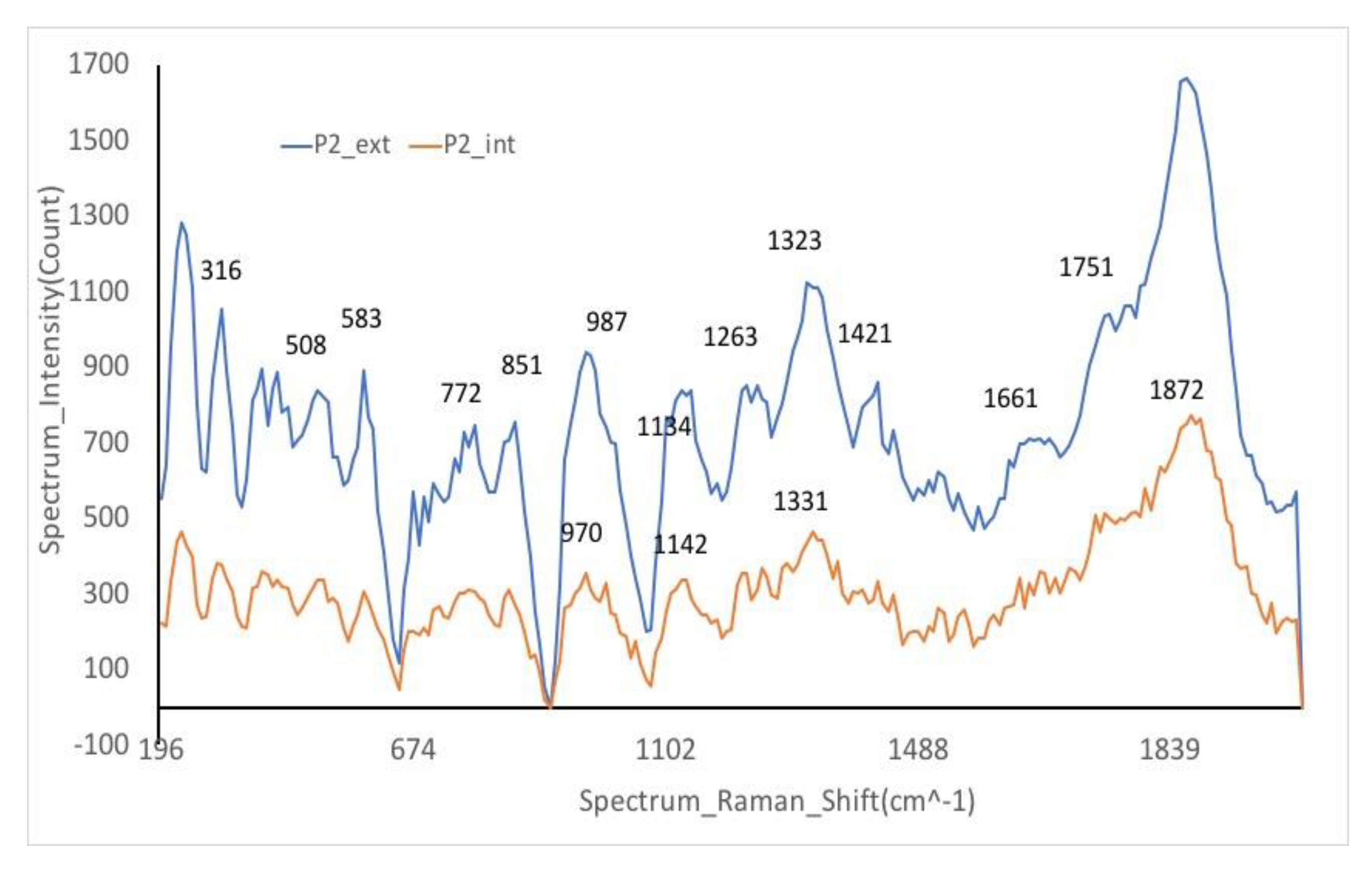
Raman spectra
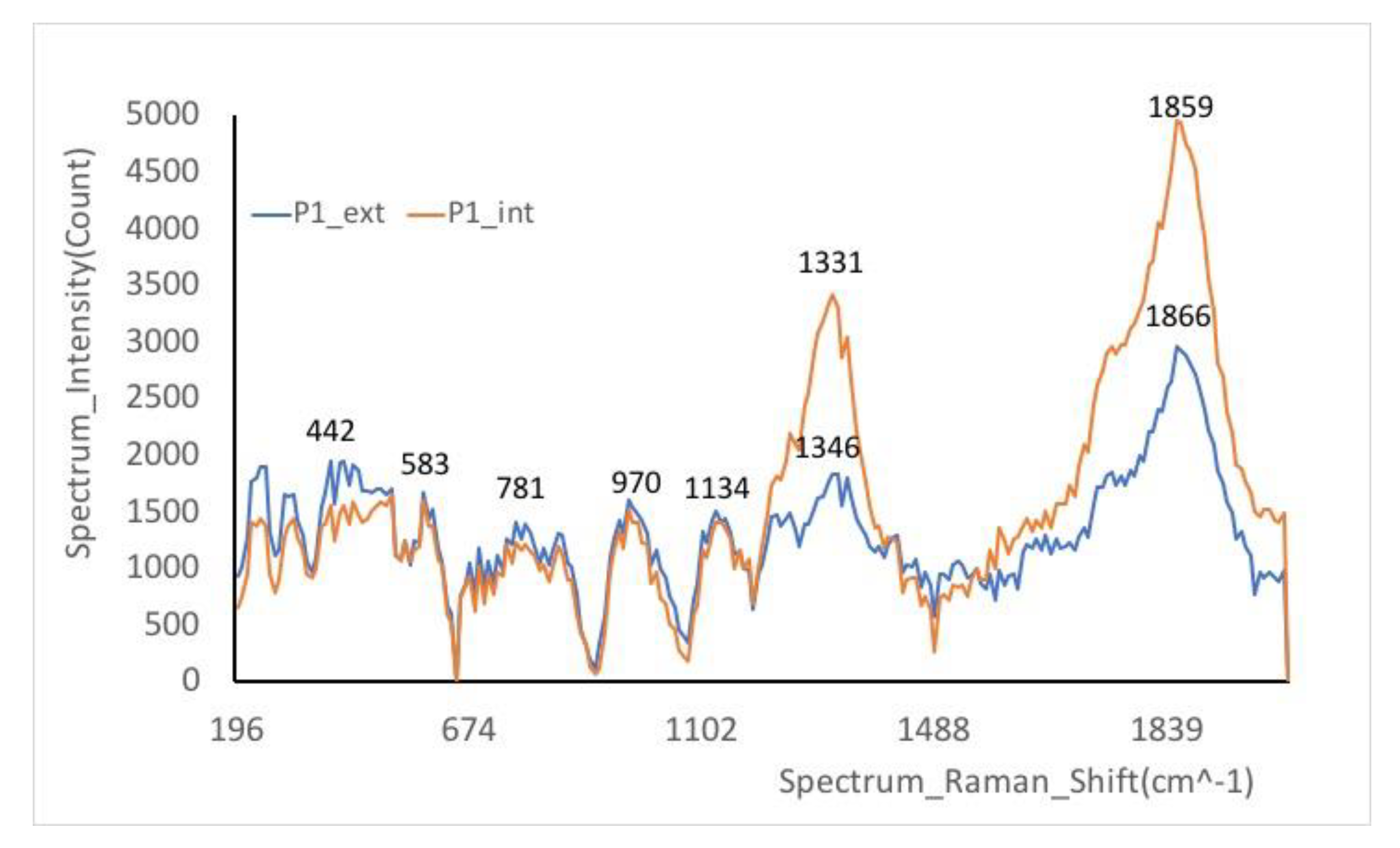
Besides FTIR spectra, the study of ceramics has been completed with Raman spectroscopy,
Figure 6, useful to identify and classify the artifacts according to socio-cultural, compositional criteria, and raw material sources [
33].
Of a real interest is the Raman signature of the silicate matrix consisting of two pairs of bands: the first between ~850 and 1200 cm−1 and the second between 400 and 600 cm−1, being attributes of the silicate vibrational unit, SiO4 tetrahedron. As can be seen, the spectrum is a fingerprint of the nanostructure of the amorphous silicate network.
The peaks at 475 cm
−1 in P1 and 476 cm
−1 in P2 are noteworthy as they closely align with the diagnostic peak of 480 cm−1 associated with iron-bearing mineral oxides. This indicates a potential presence of such minerals in the sample, further corroborating the mineral composition findings. The proximity of these peaks to the known diagnostic peak strengthens the identification of iron-bearing mineral oxides within the sample [
34].
The white color observed in the sample is likely attributed to quartz, as indicated by the presence of peaks at 443 and 465 cm
−1 at P1. Additionally, the band at 1331 cm
−1 is indicative of graphite, which contributes to the composition and characteristics of the material under study. These findings help in identifying the mineral components within the sample and provide insights into its physical properties [
28,
35,
36]. The band at 972 cm
-1 is a stretching vibration of the SiO
4 tetrahedra in the structural chain.
The average intensity band at 507 cm–1, characteristic of feldspars, serves as the representative Raman band in the analysis. In addition, the presence of hematite is suggested by the band at 506 cm–1 along with the shoulder around 583 cm–1, with these features being more pronounced in the red sample. These findings provide crucial insights into the mineral composition of the samples, pointing towards the presence of feldspars and hematite, with variations observed between the red and other samples.
The broad band around 1350–1500 cm
–1 is due to amorphous carbon, the band at 461 cm
–1 is attributed to β-quartz. The band at 1315 cm
-1 was recorded as belonging to hematite (α-Fe
2O
3), as a crystalline phase of iron, for red ocher [
37].
The bands from 1328–1370 cm
−1 and 1594–1597 cm
−1 are indicative of carbon black presence within the sample. Additionally, the band detected at 955 cm
−1 may be associated with a broad P-O stretching band, as illustrated in
Figure 6. These identified bands provide valuable information regarding the composition and molecular structure of the material under investigation, contributing to a more comprehensive understanding of its properties [
38,
39].
The Raman spectroscopy analysis revealed distinct carbon bands at 1591 cm
-1 for Sample P1 and 1599 cm
-1 for Sample P2, suggesting the presence of carbon black in these samples. Moreover, besides the hematite and gypsum, used as pigments, specific oil compounds were identified on the ceramic surface, indicating their application as a primer before painting using the dry oil technique. The detection of residual oil traces was linked to a characteristic vibration at 1870 cm
-1, attributed to the C-C stretching of cyclopropenoid compounds found in certain fatty acids. This finding has enabled the identification of oils derived from plants, aiding in estimating the initial oil content and its degradation over time, providing valuable insights into the materials and techniques used in the creation of the artwork [
40].
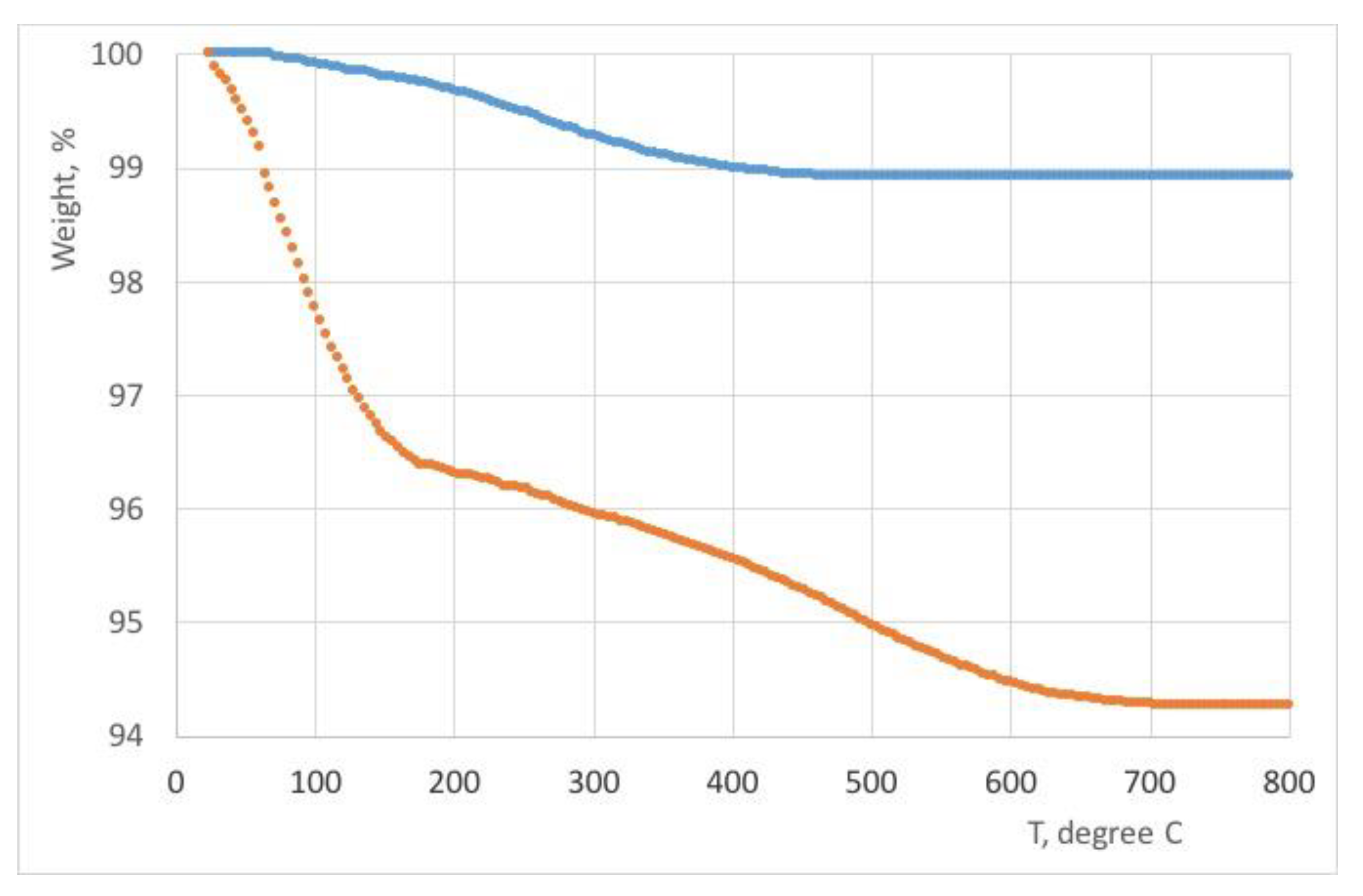
Thermal analysis
The thermal behavior of the samples was examined as depicted in
Figure 7. The firing temperature determination was conducted by analyzing the light-colored regions (ranging from yellow to red) and comparing them with controlled fired clay samples of a similar nature, demonstrating a firing temperature range spanning from 500 °C to 700 °C. By leveraging this comparative approach, insight into the firing conditions and temperature ranges employed during the production of the samples was successfully obtained.
The thermal analysis conducted within the temperature range of 100-250 °C was an indicator for removing of the absorption water specific to each clay deposit, while the elimination of chemically bound water took place around 400 °C. It is crucial to control the temperature escalation beyond these critical points, as rapid heating towards values near the maximum tolerance level of each clay type could potentially result in the vitrification of the clay mass and deformation of the pieces. The attribution of firing temperatures, such as the suggestion of 600 °C for achieving a light brown color, highlights the importance of precise temperature control in the production process to attain the desired ceramic properties. The study’s findings, revealing low calcium concentrations and the absence of calcite, are indicative of lower firing temperatures, providing valuable insights into the firing conditions necessary for optimal ceramic outcomes. Iron oxides play a significant role in the coloration of ceramics during firing. The colors produced, ranging from rust-red to dark brown or black, depend on the concentration of iron oxide and the firing conditions. The atmospheric conditions, such as the presence of oxygen in the kiln, can impact the chemical reactions involving iron oxides, influencing the final color of the ceramics. In oxidation firing, where there is sufficient oxygen, iron oxide tends to be refractory and does not melt well, resulting in lighter colors. In reduction firing, with limited oxygen, iron oxide acts as a strong flux, creating a more fluid glaze with darker colors [
41,
42].
Sample P1 exhibits red and trifle exteriors with partially black insides, showcasing variations in color transformation under different combustion environments. The red angoba experienced similar alterations to the shard’s core while showcasing color changes under oxidizing conditions. Within the same angoba type, diverse shades of red were observed. The presence of carbon deposition in vessel pores can affect the external color of white pigment in ceramic samples by potentially altering the purity and brightness of the white color due to the darkening effect of carbon. Different combustion conditions leading to varying levels of carbon residue can impact the overall appearance and properties of the ceramic material, potentially affecting its optical characteristics and surface quality. [
43]. The presence of calcite in ceramic samples can provide informations about the firing temperature and combustion conditions used in their production. Calcite presence indicates low firing temperatures (650-800°C) characteristic of bonfire firing technology, in a reducing atmosphere. The chemical composition and mineralogical differences in the samples can further support these temperature and combustion condition assessments. The presence of iron oxides, such as hematite (α-Fe
2O
3) or magnetite (Fe
3O
4), in ceramic samples can provide valuable information about the firing atmosphere in which they were produced. The conversion of magnetite to hematite under oxidizing conditions and vice versa under reducing conditions can indicate whether the ceramics were fired in an oxidizing or reducing atmosphere. Furthermore, the use of magnetite as a stable phase for producing a black pigment can influence the final color of the ceramic samples, highlighting the significant impact of firing conditions on the overall appearance and aesthetics of the finished ceramics [
33]. These findings suggest that the physical accidents observed in some pieces, such as circular exfoliation due to the sudden evaporation of water, may be attributed to the dehydration processes occurring at different temperature ranges during the firing of the ceramics. This process is similar to exfoliation in rocks, where tension from water entering cracks leads to the formation of new low-density minerals, enhancing cracks and causing slabs to detach.
The thermal analysis results indicate that the studied ceramic samples underwent three distinct temperature ranges of thermal decomposition. The total mass loss (ML) of the samples ranged from 1.17 mg to 5.63 mg. The decomposition process occurred within the temperature ranges of room temperature (RT) to 220 °C, 220 to 600 °C, and 600 to 700 °C. During the initial temperature range of RT to 100 °C, dehydration of hygroscopic water (humidity) resulted in a mass loss change from 0.1 to 1.3%. Between 100 and 220 °C, the dehydration of phyllosilicate minerals took place.
The thermal analysis results shown in
Figure 7 indicate that the total mass loss (ML) of the studied samples varied from 1.17 mg (sample P1) to 5.63 mg (sample P2). The thermal decomposition of the samples was observed to occur in three temperature ranges: room temperature (RT) to 220 °C, 220 to 600 °C, and 600 to 700 °C. Within the RT to 220 °C range, the mass loss (ML) can be further divided into two ranges: RT to 100 °C and 100 to 200 °C. These findings suggest the presence of different components or phases within the samples that exhibit distinct thermal decomposition behaviors at different temperature ranges. The dehydration of hygroscopic water (humidity) occurs between RT and 100 °C, [
7,
11] accompanied by ML change from 0.2 to 1.6%. After that, in the range 100 - 220 °C, the dehydration of phyllosilicate takes place [
44,
45,
46,
47].
According to Moropoulou et al. [
16], the ML in the temperature range RT—220 °C reflects the hygroscopic properties of mortar by affecting the heat of desorption and vaporization, as well as the amount and form of water availability at different relative humidity levels. The high sensitivity of muscovite to milling results in the rearrangement and activation of Al-OH bonds, as well as the diffusion of hydroxyls from the inner part of the mineral to its surface due to the mechanochemical activation treatment. This process leads to surface aluminum enrichment and reduction in binding energies of both Si and Al elements, enhancing the muscovite reactivity [
35]. The hygroscopic properties of muscovite in mortar are determined by the amount of hygroscopic water evolved between room temperature (RT) and 100°C. A measured moisture loss of around 6% in this temperature range indicates that the samples do not exhibit significant hygroscopic properties. This lack of hygroscopic behavior can have implications for the performance and durability of the mortar, as materials with high hygroscopicity may be more susceptible to moisture-related degradation [
48].
The thermal reactions and effects that take place between 220-600 °C, with a total ML range of 0.14-4.16%, are related to the decomposition and combustion of organic matter. At these temperatures, organic matter undergoes thermal decomposition, breaking down into simpler compounds such as carbon dioxide, water, and other by-products. Combustion reactions can also occur, especially if there is sufficient oxygen present, leading to the oxidation of the organic matter and the production of carbon dioxide and water as the main products [
49].
Taking into account that ML derived from organic components and hydraulic phases decomposition was observed in 220—600 °C temperature range [
3,
11,
12,
36,
37], it can be deduced the behavior to the decomposition of organic materials under different temperature conditions. Also, should be mentioned that the hydraulic components formation could be provided from chemically active phases such as clays, ceramic fragments, or volcanic ash [
37].
The dehydroxylation of phyllosilicates in the temperature range of 600-720 °C can lead to structural changes and the breakdown of the layer structure of the minerals. This process may result in the formation of chemically active phases as the dehydroxylated phases no longer resemble a phyllosilicate. The presence of chemically active phases can impact the specific heat, crystal phase transition, surface functional groups, and coordination of silicon and aluminum in the minerals [
44,
45].
Porosity
Porosity is a crucial property in ceramic materials as it can provide valuable insights into the behavior and characteristics of the samples. For the ancient pottery, the porosity of ceramic samples can offer significant information about the firing temperature, type of clay and tempering materials used, as well as the manufacturing and forming techniques employed by the ancient pottery. Different textured samples with varying levels of porosity can reflect the processes involved in the creation of the pottery, the type of raw materials utilized, and the level of craftsmanship exhibited. By analyzing the porosity of ancient pottery, a better understanding of the cultural and technological aspects of the civilizations that produced these artifacts, could be obtained.
All these vessels have been used for storing or boiling various liquids. A high degree of porosity is observed, due both to the relatively low temperature and to the characteristics of ceramic vessels to have their own porosity. By repeated use, when storing certain products, a decrease in this porosity was observed by clogging the external pores with particles of these products, which penetrated deeply through the capillary absorption process.
Textural measurements are essential for studying the composition-dependent changes in properties of materials. In the case of ceramic samples, specific surface area plays a significant role. A larger specific surface area, such as 42 m2/g, can suggest a lower firing temperature of the material, as an indicator about the conditions which the ceramic was formed.
In addition, the presence of calcite in the ceramic composition can impact the shrinkage behavior during firing. The amount of calcite can influence the porosity and texture of the final ceramic product. By analyzing the mesopore size distribution, it is observed that the pore diameters in the samples vary between 4 and 10 nm. This variation in pore sizes can affect the overall properties of the ceramic, such as its strength, density, and thermal properties.
Overall, textural measurements, specific surface area, and pore size distribution are crucial parameters in understanding the composition and firing conditions of ceramic materials. They provide valuable information about the processing steps involved in the production of ceramics and can help researchers interpret the technological aspects of ancient pottery,
Table 3.
Surface and pore structure analysis for the two ceramic samples were performed using N2 physisorption.
Table 3 shows that all the sample P1 show large pores, while sample P2 reveals partially blocked mesoporous structure, with very small pores. The value of pore diameter (Dp) is relevant for the above-mentioned assumption.
Chromatic parameters
The color of ceramic bodies can provide valuable insights into the firing and production techniques employed by ancient potters. In the case of the ceramic samples described, the color variation ranging from reddish-yellow to brown or reddish-brown indicates different firing conditions and atmospheres during the production process.
The bi-layered texture observed in the cross-section of the ceramic wall, lighter color for the outer layer and a darker color for the inner layer, suggests specific firing conditions. This distinct layering can be an indication of uneven firing throughout the ceramic body. It may signify incomplete firing time for the organic clay material to burn completely, leading to variations in color and texture.
Alternatively, the bi-layered texture can also suggest a firing process of ceramics in a reducing atmosphere followed by a rapid oxidation process. The outer layer with a lighter color may result from reduction firing, where oxygen is restricted, leading to color changes in the clay body. The subsequent rapid oxidation can cause the inner layer to have a darker color, creating this distinct bi-layered appearance.
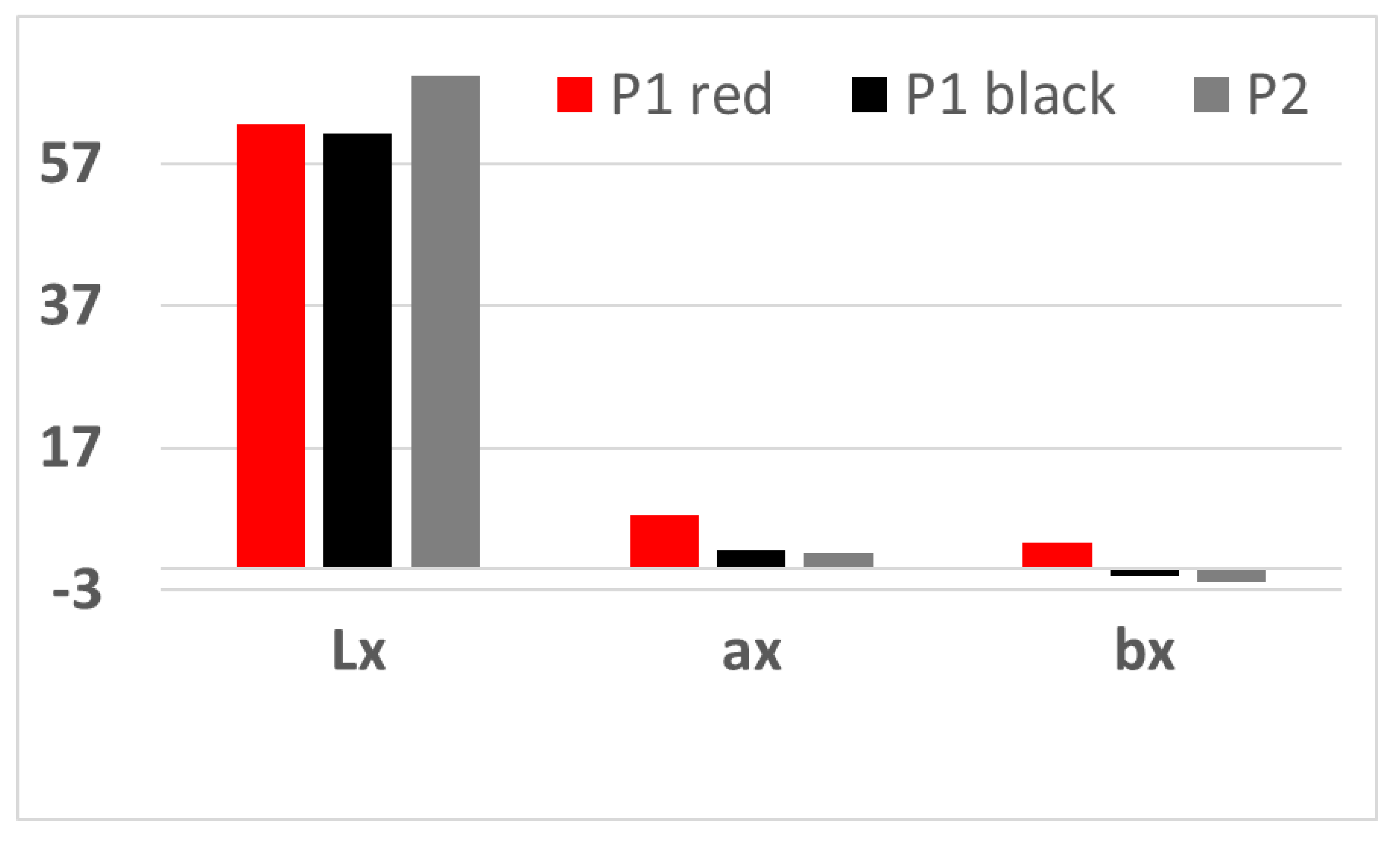
The color analysis of the ceramic samples using the L*a*b* color space parameters provides further quantitative information about the brightness (L*), red/green variation (a*), and yellow/blue variation (b*) of the material. These color coordinates can help in understanding the color characteristics and variations in the ceramic samples and aid in deciphering the firing techniques and atmospheres used in their production.
Analyzing the graphical representation (
Figure 8), it could be observed that P1 has for both faces Lx, ax values with positive values, which means red and yellow for the red face of the sample, and the black face has Lx and ax lower in value, but still positive, while bx has negative values, for both girls which means the chromatic tendency towards blue color. In sample P2, there is the same tendency, but bx has an even lower value, considering the relatively homogeneous gray-black color. These observations complete the optical microscopy observations presented previously.