Submitted:
05 June 2024
Posted:
06 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study population
2.2. Inflammatory scores: INFLA-score and SIRI
2.3. Immunological scores: ARIP
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caruso, C.; Passarino, G.; Puca, A.; Scapagnini, G. "Positive biology": the centenarian lesson. Immun Ageing 2012, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: the challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Evert, J.; Lawler, E.; Bogan, H.; Perls, T. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci 2003, 58, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Ligotti, M.E.; Cossarizza, A. Centenarian Offspring as a Model of Successful Ageing. In Centenarians, 1st ed.; Caruso, C., Ed.; Springer Cham: Switzerland, 2019. [Google Scholar]
- Accardi, G.; Aiello, A.; Aprile, S.; Caldarella, R.; Cammarata, G.; Carru, C.; Caruso, C.; Ciaccio, M.; Colomba, P.; Galimberti, D.; Gambino, C.M.; Davinelli, S.; De Vivo, I.; Ligotti, M.E.; Vasto, S.; Zinellu, A.; Candore, G. The Phenotypic Characterization of the Cammalleri Sisters, an Example of Exceptional Longevity. Rejuvenation Res 2020, 23, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Accardi, G.; Aiello, A.; Calabrò, A.; Ligotti, M.E.; Candore, G. Centenarians born before 1919 are resistant to COVID-19. Aging Clin Exp Res 2023, 35, 217–220. [Google Scholar] [CrossRef]
- Caruso, C.; Marcon, G.; Accardi, G.; Aiello, A.; Calabrò, A.; Ligotti, M.E.; Tettamanti, M.; Franceschi, C.; Candore, G. Role of Sex and Age in Fatal Outcomes of COVID-19: Women and Older Centenarians Are More Resilient. Int J Mol Sci 2023, 24, 2638. [Google Scholar] [CrossRef]
- Poulain, M.; Chambre, D.; Pes, G.M. Centenarians exposed to the Spanish flu in their early life better survived to COVID-19. Aging (Albany NY) 2021, 13, 21855–21865. [Google Scholar] [CrossRef]
- Aoki, Y. The number of centenarians continues to increase during the COVID-19 pandemic in Japan. Geriatr Gerontol Int 2023, 23, 395–396. [Google Scholar] [CrossRef]
- de Castro, M.V.; Silva, M.V.R.; Naslavsky, M.S.; Scliar, M.O.; Nunes, K.; Passos-Bueno, M.R.; Castelli, E.C.; Magawa, J.Y.; Adami, F.L.; Moretti, A.I.S.; de Oliveira, V.L.; Boscardin, S.B.; Cunha-Neto, E.; Kalil, J.; Jouanguy, E.; Bastard, P.; Casanova, J.L.; Quiñones-Vega, M.; Sosa-Acosta, P.; Guedes, J.S.; de Almeida, N.P.; Nogueira, F.C.S.; Domont, G.B.; Santos, K.S.; Zatz, M. The oldest unvaccinated Covid-19 survivors in South America. Immun Ageing 2022, 19, 57. [Google Scholar] [CrossRef]
- Ligotti, M.E.; Accardi, G.; Aiello, A.; Aprile, S.; Calabrò, A.; Caldarella, R.; Caruso, C.; Ciaccio, M.; Corsale, A.M.; Dieli, F.; Di Simone, M.; Giammanco, G.M.; Mascarella, C.; Akbar, A.N.; Meraviglia, S.; Candore, G. Sicilian semi- and supercentenarians: Identification of age-related T cell immunophenotype to define longevity trait. Clin Exp Immunol 2023, 214, 61–78. [Google Scholar] [CrossRef]
- Pounis, G.; Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; de Curtis, A.; Persichillo, M.; Sieri, S.; Donati, M.B.; Cerletti, C.; de Gaetano, G.; Iacoviello, L. Polyphenol intake is associated with low-grade inflammation, using a novel data analysis from the Moli-sani study. Thromb Haemost 2016, 115, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, R.; Meier, H.C.S.; Vivek, S.; Klopack, E.; Crimmins, E.M.; Faul, J.; Nikolich-Žugich, J.; Thyagarajan, B. Evaluation of T-cell aging-related immune phenotypes in the context of biological aging and multimorbidity in the Health and Retirement Study. Immun Ageing 2022, 19, 33. [Google Scholar] [CrossRef]
- Aiello, A.; Accardi, G.; Aprile, S.; Caldarella, R.; Carru, C.; Ciaccio, M.; De Vivo, I.; Gambino, C.M.; Ligotti, M.E.; Vasto, S.; Zinellu, A.; Caruso, C.; Bono, F.; Candore, G. Age and Gender-related Variations of Molecular and Phenotypic Parameters in A Cohort of Sicilian Population: from Young to Centenarians. Aging Dis 2021, 12, 1773–1793. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Nazmi, A.; Victora, C.G. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health 2007, 7, 212. [Google Scholar] [CrossRef]
- Incalcaterra, E.; Accardi, G.; Balistreri, C.R.; Caimi, G.; Candore, G.; Caruso, M.; Caruso, C. Pro-inflammatory genetic markers of atherosclerosis. Curr Atheroscler Rep, 2013, 15, 329. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.A.; Hales, N.; Burnett, R.T.; Jerrett, M.; Mix, C.; Dockery, D.W.; Pope, C.A. Particulate Air Pollution, Exceptional Aging, and Rates of Centenarians: A Nationwide Analysis of the United States, 1980-2010. Environ Health Perspect 2016, 124, 1744–1750. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front Immunol 2018, 8, 1960. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Flores, R.R.; Zhu, Y.; Schmiechen, Z.C.; Brooks, R.W.; Trussoni, C.E.; Cui, Y.; Angelini, L.; Lee, K.A.; McGowan, S.J.; Burrack, A.L.; Wang, D.; Dong, Q.; Lu, A.; Sano, T.; O'Kelly, R.D.; McGuckian, C.A.; Kato, J.I.; Bank, M.P.; Wade, E.A.; Pillai, S.P.S.; Klug, J.; Ladiges, W.C.; Burd, C.E.; Lewis, S.E.; La Russo, N.F.; Vo, N.V.; Wang, Y.; Kelley, E.E.; Huard, J.; Stromnes, I.M.; Robbins, P.D.; Niedernhofer, L.J. An aged immune system drives senescence and ageing of solid organs. Nature 2021, 594, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Ligotti, M.E.; Accardi, G.; Aiello, A.; Candore, G. An immunologist’s guide to immunosenescence and its treatment. Expert Rev Clin Immunol 2022, 18, 961–981. [Google Scholar] [CrossRef] [PubMed]
- Zanini, G.; Selleri, V.; Lopez, D.S.; Malerba, M.; Nasi, M.; Mattioli, A.V.; Pinti, M. Mitochondrial DNA as inflammatory DAMP: a warning of an aging immune system? Biochem Soc Trans 2023, 51, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Kouno, T.; Ikawa, T.; Hayatsu, N.; Miyajima, Y.; Yabukami, H.; Terooatea, T.; Sasaki, T.; Suzuki, T.; Valentine, M.; Pascarella, G.; Okazaki, Y.; Suzuki, H.; Shin, J.W.; Minoda, A.; Taniuchi, I.; Okano, H.; Arai, Y.; Hirose, N.; Carninci, P. . Single-cell transcriptomics reveals expansion of cytotoxic CD4 T cells in supercentenarians. Proc Natl Acad Sci 2019, 116, 24242–24251. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, T.T.; Dowrey, T.W.; Villacorta-Martin, C.; Montano, M.; Reed, E.; Belkina, A.C.; Andersen, S.L.; Perls, T.T.; Monti, S.; Murphy, G.J.; Sebastiani, P. Multi-modal profiling of peripheral blood cells across the human lifespan reveals distinct immune cell signatures of aging and longevity. EBioMedicine 2023, 90, 104514. [Google Scholar] [CrossRef] [PubMed]
- Ligotti, M.E.; Accardi, G.; Aiello, A.; Calabrò, A.; Caruso, C.; Corsale, A.M.; Dieli, F.; Di Simone, M.; Meraviglia, S.; Candore, G. Sicilian semi- and supercentenarians: Age-related NK cell immunophenotype and longevity trait definition. Transl Med UniSa 2023, 25, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ligotti, M.E.; Accardi, G.; Aiello, A.; Calabrò, A.; Caruso, C.; Corsale, A.M.; Dieli, F.; Di Simone, M.; Meraviglia, S.; Candore, G. Sicilian semi- and supercentenarians: Age-related Tγδ cell immunophenotype contributes to longevity trait definition. Clin Exp Immunol 2024, 216, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Di Castelnuovo, A.; Pounis, G.; De Curtis, A.; Costanzo, S.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Moli-sani Study Investigators. A score of low-grade inflammation and risk of mortality: prospective findings from the Moli-sani study. Haematologica 2016, 101, 1434–1441. [Google Scholar] [CrossRef]
- Costanzo, S.; Magnacca, S.; Bonaccio, M.; Di Castelnuovo, A.; Piraino, A.; Cerletti, C.; de Gaetano, G.; Donati, M.B.; Iacoviello, L.; Moli-sani Study Investigators. Reduced pulmonary function, low-grade inflammation and increased risk of total and cardiovascular mortality in a general adult population: Prospective results from the Moli-sani study. Respir Med 2021, 184, 106441. [Google Scholar] [CrossRef]
- Mignogna, C.; Costanzo, S.; Di Castelnuovo, A.; Ruggiero, E.; Shivappa, N.; Hebert, J.R.; Esposito, S.; De Curtis, A.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonaccio, M. Moli-sani Study Investigators. The inflammatory potential of the diet as a link between food processing and low-grade inflammation: An analysis on 21,315 participants to the Moli-sani study. Clin Nutr 2022, 41, 2226–2234. [Google Scholar] [CrossRef]
- Andreis, A.; Solano, A.; Balducci, M.; Picollo, C.; Ghigliotti, M.; Giordano, M.; Agosti, A.; Collini, V.; Anselmino, M.; De Ferrari, G.M.; Rinaldi, M.; Alunni, G.; Imazio, M. INFLA-score: A new diagnostic paradigm to identify pericarditis. Hellenic J Cardiol 2024, 21, S1109–9666. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Guo, Z.; Zhu, Y.; Ma, M.; Jia, G. Peripheral blood inflammatory indexes in breast cancer: A review. Medicine (Baltimore) 2023, 102, e36315. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, L.; Lou, Z.; Zhu, Y. Association between Systemic Immune-Inflammation Index and Systemic Inflammation Response Index and Outcomes of Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Ann Indian Acad Neurol 2023, 26, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Jarmuzek, P.; Kozlowska, K.; Defort, P.; Kot, M.; Zembron-Lacny, A. Prognostic Values of Systemic Inflammatory Immunological Markers in Glioblastoma: A Systematic Review and Meta-Analysis. Cancers (Basel) 2023, 15, 3339. [Google Scholar] [CrossRef]
- Sahin, R.; Tanacan, A.; Serbetci, H.; Agaoglu, Z.; Karagoz, B.; Haksever, M.; Kara, O.; Şahin, D. The role of first-trimester NLR (neutrophil to lymphocyte ratio), systemic immune-inflammation index (SII), and, systemic immune-response index (SIRI) in the prediction of composite adverse outcomes in pregnant women with systemic lupus erythematosus. J Reprod Immunol 2023, 158, 103978. [Google Scholar] [CrossRef]
- Olson, N.C.; Doyle, M.F.; Jenny, N.S.; Huber, S.A.; Psaty, B.M.; Kronmal, R.A.; Tracy, R.P. Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. PLoS One 2013, 8, e71498. [Google Scholar] [CrossRef]
- Olson, N.C.; Doyle, M.F.; de Boer, I.H.; Huber, S.A.; Jenny, N.S.; Kronmal, R.A.; Psaty, B.M.; Tracy, R.P. Associations of Circulating Lymphocyte Subpopulations with Type 2 Diabetes: Cross-Sectional Results from the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One 2015, 10, e0139962. [Google Scholar] [CrossRef]
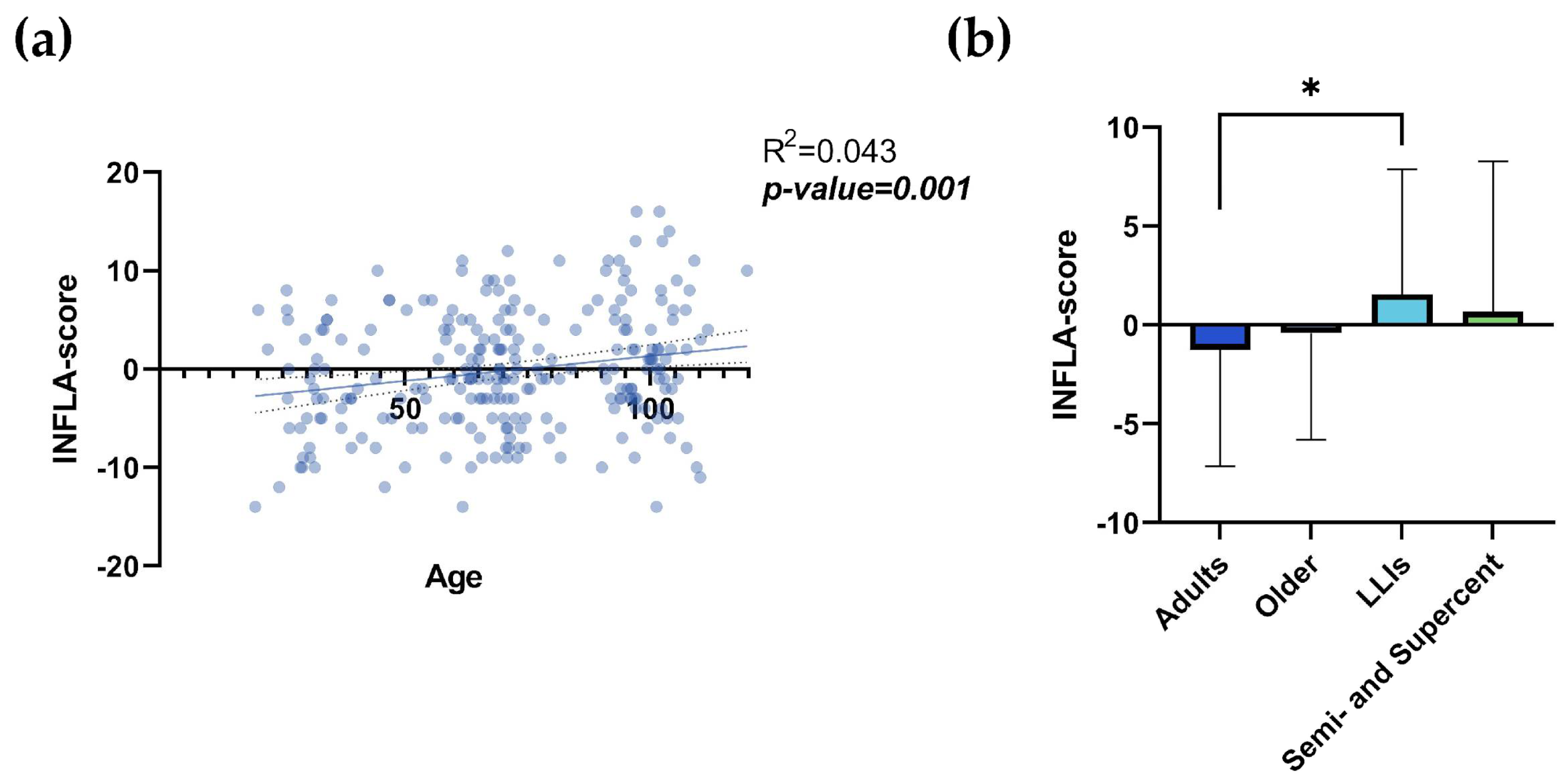
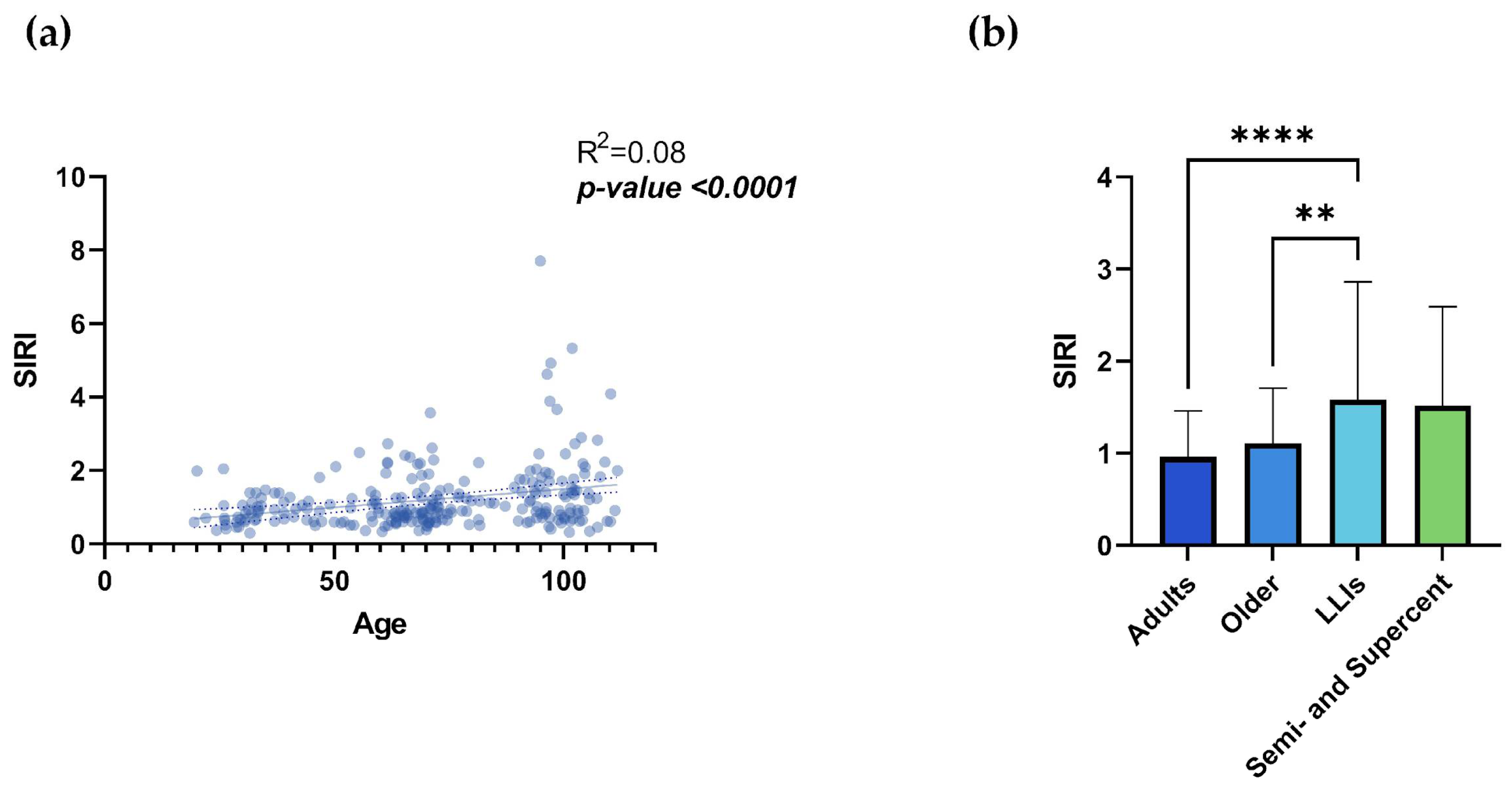
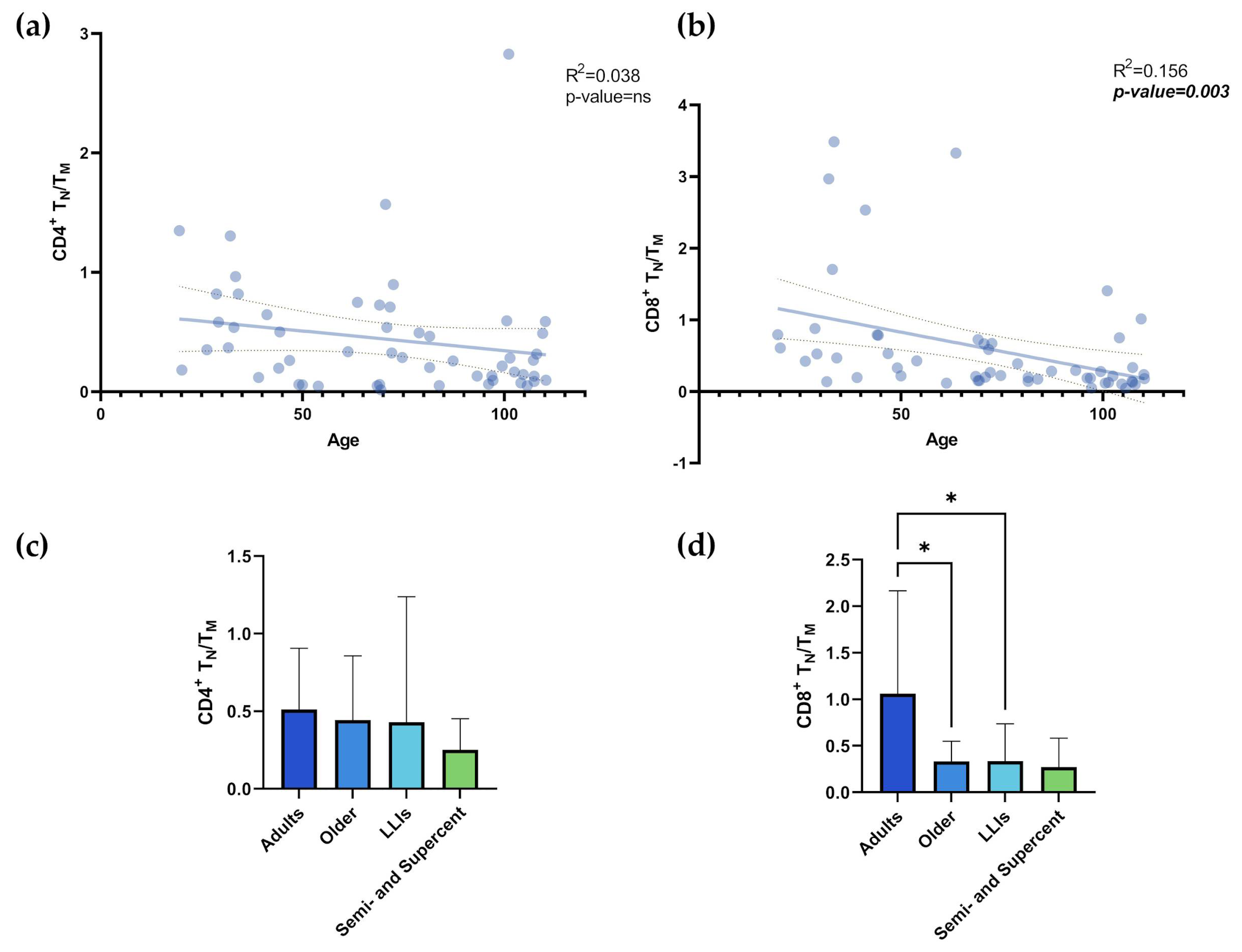
| Variable | Adults (N= 91) |
Older (N= 76) |
LLIs (N= 69) |
Semi- and Supercentenarians (N= 13) |
Significant comparisons | p-value |
|---|---|---|---|---|---|---|
| INFLA-score1 | -1.253±5.891 | -0.408±5.394 | 1.559±6.316 | 0.667±7.608 | Adults vs LLIs | 0.018 |
| SIRI | 0.963±0.5 | 1.107±0.6 | 1.583±1.28 | 1.519±1.073 | Adults vs LLIs | <0.0001 |
| Older vs LLIs | 0.005 | |||||
| Variable |
Adults (N= 20) |
Older (N= 15) |
LLIs (N= 11) |
Semi- and Supercentenarians (N= 8) |
Significant comparisons | p-value |
| CD4+ TN/TM | 0.512±0.393 | 0.443±0.415 | 0.429±0.809 | 0.252±0.2 | None | ns |
| CD8+ TN/TM | 1.061±1.104 | 0.335±0.215 | 0.337±0.401 | 0.272±0.312 | Adults vs Older | 0.024 |
| Adults vs LLIs | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





