Submitted:
05 June 2024
Posted:
06 June 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
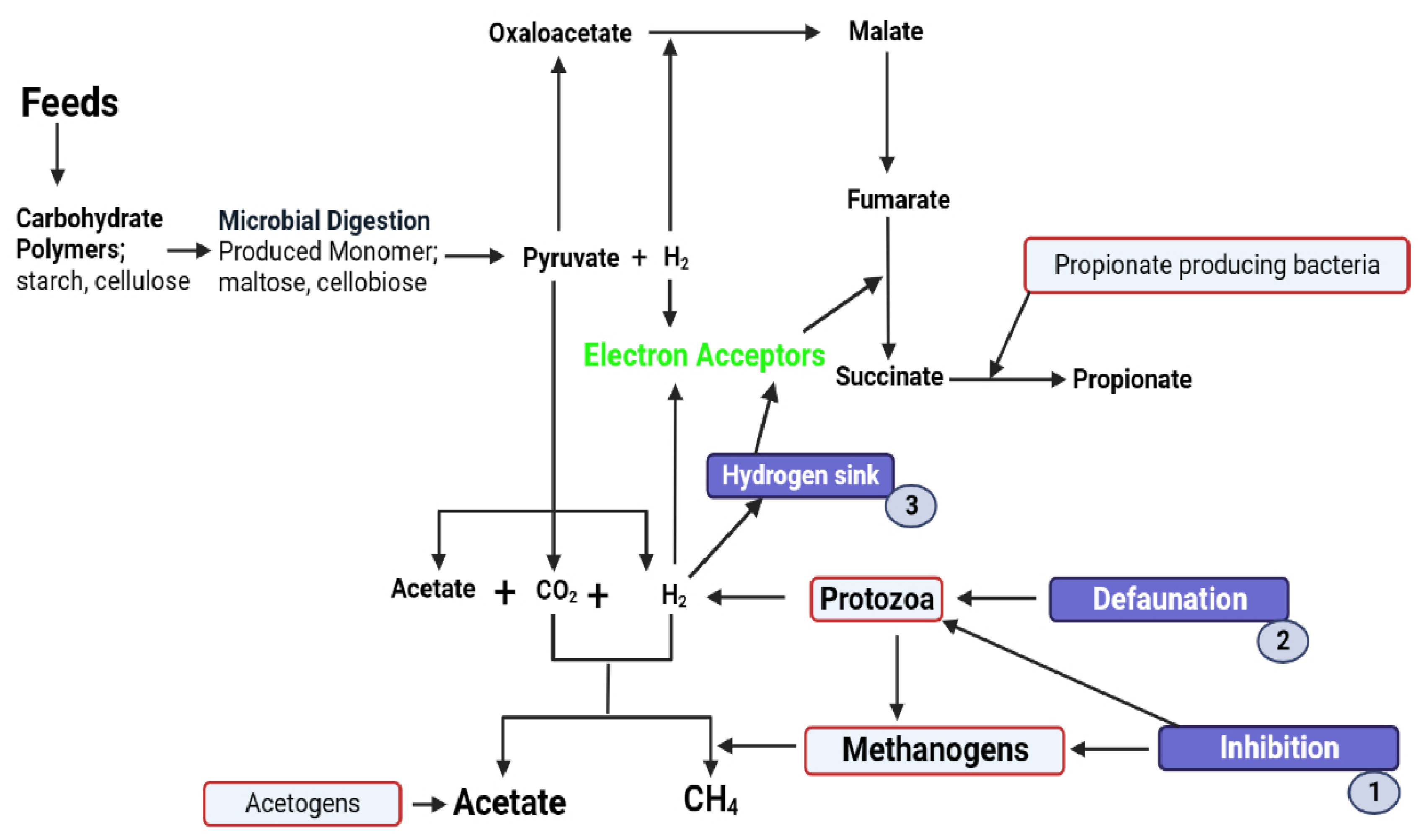
2. Insight into the Role of Rumen Microbial Ecology on Methanogenesis
3. Phytogenic Tools for Reducing Methane Emissions and Their Effects on Ruminal Microbial Ecology
3.1. Mechanism of Action of Phytogenic Feed Additives on Rumen Microbial Cells
3.2. Phytogenic Feed Additives: Sources and Effects
3.2.1. Trees, Shrubs, and Forages
3.2.2. Trees and Shrubs
Italian Plumeless Thistle (Carduus pycnocephalus)
Chinese Peony (Paeonia lactiflora)
Leucaena (Leucaena leucocephala)
Gliricidia (Gliricidia sepium)
Calliandra (Calliandra calothyrsus)
Mulberry (Morus spp.)
3.2.3. Forages
Brassica Forages
Alfalfa (Medicago sativa L)
Clover (Trifolium species)
Chinese Lespedeza (Sericea lespedeza)
3.2.4. Plant Oil Extracts
Rapeseed Oil
Camelina Sativa Oil
Garlic Oil
Palm Oil
3.3. Mechanism of Actions of Plant Bioactive Compounds (PBC) and Their Role in Reducing Methane Emissions
| Sources of PBC | Type of Experiment | Dosage | Diet | Methane Emission | Reference |
|---|---|---|---|---|---|
| Tannins | |||||
| Acacia mimosa Extracts – CT | In vivo (6 Cannulated Nellore cattle) | 1.25% and 2.25% | Grazing | 28% | Fagundes et al. (2020) |
| Extracts of Lipid encapsulated- Acacia Tannin |
In vivo (4 rumen-cannulated Merino withers) | 50 g/kg feed | Eragrotis Lucerne hay |
19% | Adejoro et al. (2019) |
| Extracts of Crude-Acacia Tannin | In vivo (4 rumen-cannulated Merino withers) | 40 g/kg feed | Eragrotis Lucerne hay |
30% | Adejoro et al. (2019) |
| Extracts of Acacia nilotica Leaves and Pods |
In Vitro (Sheep rumen fluid) |
Leaves (187g/kg DM HT) Pods (350g/kg/DM HT) |
Acacia nilotica Leaves and Pods |
64% | Rira et al. (2019) |
| Tannin-containing – Birdsfoot trefoil, Sainfoin, and Small burnet | In Vitro (Heifer) | 2.5% CT 4.5% HT |
Hay | 21 – 34% | Stewart et al. (2019) |
| Tannic acid | in vivo (Beef Cattle) | 6.5, 13.0, or 26.0 g/kg DM | Corn silage and Concentrate mixture | 11.1%, 14.7% and 33.6% | Yang et al. (2017) |
| Purified hydrolyzable (chestnut and sumach) and Condensed tannins (mimosa and quebracho) | In vitro (Cattle) | 0.5, 0.75 and 1.0 mg/ml | 70% Hay 30% Concentrate |
22 – 37% | Jayanegara et al. (2015) |
| Saponins | |||||
| Tea saponin | In vivo (Sheep) | 2.0g/Day | Basal diet | 8.8% | Y. Liu et al. (2019) |
| Tea saponin | In Vitro (Bovine) | 0.50g/L | 54% Corn silage 6% Hay 40% Concentrate |
29% | Guyader et al. (2017) |
| Extracts of Yucca schidigera | In vivo (Sheep) | 170 mg per day | 75% Hay 35% Concentrate |
16% | Wang et al. (2009) |
| Extracts of Knautia arvensis leaves | In vitro (Holstein Cow) | 10.2 & 20.4 g/kg | 50% Hay 50% Concentrate |
5.5 & 6.4% | G Goel et al. (2008) |
| Leaves of Sesbania sesban | In vitro (Holstein Cow) | 174 g/kg |
32% Hay: 68% Concentrate |
12% | Gunjan Goel et al. (2008) |
| Seeds of Trigonella foenum-graecum | In vitro (Holstein Cow) | 30.4 g/kg |
50% Hay 50% Concentrate |
2% | Gunjan Goel et al. (2008) |
| Essential Oil | |||||
| Essential Oil Blend | In vivo (Dairy cow) | 1g/d/cow | Total Mixed Ration | 8.8% | Belanche et al. (2020) |
| Essential Oil Blend (Coriander, geranyl acetate, and eugenol) | In vivo (Dairy cow) | 1 g/d/cow | Total Mixed Ration | 6% | Hart et al. (2019) |
| Anise oil | In vivo (Sheep) | 0, 50, 100, 200, 400 mg/L | 40% Hay 60% corn-based concentrate |
47% | Wang et al. (2018) |
| Garlic oil Eucalyptus oil Origanum oil Clove oil Peppermint oil |
In vitro (Lactating Jersey Cow) | 0.25, 0.50 and 1.0 g/l Fermentation medium | Ground alfalfa hay and concentrate 50% each | 22 – 42% 17 – 26% 12 – 86% 11 – 34%8 – 16% |
Patra and Yu (2015) |
| Plant Bioactive Compound | Effect on Rumen Ecology | Potential Mechanism | References |
|---|---|---|---|
| Tannin | Bacteriostatic in rumen | Inhibit the activities of rumen microbes | McSweeney et. al. (2001); (Jayanegara et al., 2015) |
| Reduce fiber digestion in the rumen. | Reduce methanogenesis by decreasing the level of available H2 needed for the production of methane | Amlan K Patra, (2012); Bodas et al. (2012) | |
| Increase in the abundance of butyrate-producing bacteria and other probiotic bacteria, such as Bifidobacterium and Lactobacillusamino | Decreased the production of short-chain fatty acids like acetate and reduced methane production | Buccioni et al. (2015); (Correa et al., 2020). | |
| Suppressing the archaea communities and increasing total rumen bacteria populations | Lower methane production | Fagundes et al. (2020) | |
| Suppressing the growth of methanogens directly | Reduce CH4 production | (Aboagye & Beauchemin, 2019) | |
| Decreased organic matter digestion in the rumen | Reduce methanogenesis | (Grainger et al., 2009) | |
| Decreased the relative abundance of protozoa, methanogens, and Ruminococcus albus | Reduce methanogenesis by inhibiting methanogen and protozoal growth | Yang et. al. (2017); (Volpe et al., 2018); (Witzig et al., 2018) | |
| Saponins | Inhibition of protozoal ecology in rumen and other methanogens associated with protozoa | Reduce protozoal population by interaction with sterol moiety present in the protozoa membrane thereby reducing methanogenesis | (Patra & Saxena, 2009); (Bodas & Prieto, 2012); (Jayanegara et al., 2014); (Ramírez-Restrepo et al., 2016); (Guyader et al., 2017); (Y. Liu et al., 2019); (Tan et al., 2020) |
| Essential oil | Alteration of rumen microbial ecology. Inhibit the growth of methanogenic Archaea in the rumen | Inhibit the HMG-CoA reductase, which will lead to membrane instability and ultimately, death of methanogenic archaea cells. Reduce methanogenesis | (Patra & Yu, 2015).; (Ye et al., 2018); (Lei et al., 2019); (Belanche et al., 2020) |
| Inhibition activity of gram-positive (+ve) and gram-negative (-ve) bacteria | Antimicrobial capabilities are mainly due to their interface with the cell membrane of rumen microbes by disrupting membrane stability of lips bilayers of bacterial cells. This inhibition in the rumen may lead to an increase in propionate levels in the rumen, thereby reducing the rate of methane production | (Zengin & Baysal, 2014); (Cobellis et al., 2016); (Schären et al., 2017); (Poudel et al., 2019) | |
| Increased the abundance of Succinivibrio species, Bacteroides species, and Succinivibrio species in rumen. | Shift in rumen fermentation pattern, favoring propionate production over acetate. This may reduce methane production | (Evans & Martin, 2000); (Lei et al., 2019). | |
| Flavonoids | Antimicrobial properties | Their interaction with rumen microbes can decrease the population of methanogenic archaea | (Purba et al., 2020) |
| Increase the abundance of Fibrobacter succinogenes diversity and decrease Ruminoccocus albus and Ruminoccocus flavefaciens population | Create a competition for hydrogen between rumen microbes and other methanogens for VFA production and methanogenesis. | (Kim et al., 2015) | |
| Reduce ciliate protozoa and hydrogenotrophic methanogens population | Inhibit methanogenesis | (Oskoueian et al., 2013); (Seradj et al., 2014) | |
| Propolis | Reduce the population of methanogenic Archaea | Inhibit methanogenesis | (Morsy et al., 2021) |
4. Conclusions
Acknowledgments
References
- Aboagye, I. A., & Beauchemin, K. A. (2019). Potential of molecular weight and structure of tannins to reduce methane emissions from ruminants: A review. Animals, 9(11), 856. [CrossRef]
- Adejoro, F. A., Hassen, A., & Akanmu, A. M. (2019). Effect of lipid-encapsulated acacia tannin extract on feed intake, nutrient digestibility and methane emission in sheep. Animals, 9(11), 863. [CrossRef]
- Adelusi, O., Idowu, O., Adebayo, K., Balogun, M., & Oni, A. (2022). In vitro degradability of diets containing varying levels of Gliricidia sepium and cassava leaf meals.
- Ahsan, U., Adabi, S. G., Sayın Özdemir, Ö., Sevim, Ö., Tatlı, O., Kuter, E., & Cengiz, Ö. (2022). Growth performance, carcass yield and characteristics, meat quality, serum biochemistry, jejunal histomorphometry, oxidative stability of liver and breast muscle, and immune response of broiler chickens fed natural antioxidant alone or in combination with Bacillus licheniformis. Archives Animal Breeding, 65(2), 183-197. [CrossRef]
- Alvarez-Hess, P., Williams, S., Jacobs, J., Hannah, M., Beauchemin, K., Eckard, R., Wales, W., Morris, G., & Moate, P. (2019). Effect of dietary fat supplementation on methane emissions from dairy cows fed wheat or corn. Journal of Dairy Science, 102(3), 2714-2723. [CrossRef]
- Animut, G., Puchala, R., Goetsch, A., Patra, A., Sahlu, T., Varel, V., & Wells, J. (2008). Methane emission by goats consuming diets with different levels of condensed tannins from lespedeza. Animal Feed Science and Technology, 144(3-4), 212-227. [CrossRef]
- Armsby, H. P. (1916). The use of energy values in the computation of rations for farm animals. US Department of Agriculture.
- ARRIAGA JORDAN, C., & González Ronquillo, M. (2021). Feeding Forage Mixtures of Ryegrass (Lolium spp.) with Clover (Trifolium spp.) Supplemented with Local Feed Diets to Reduce Enteric Methane Emission E.
- Barry, T. (2013). The feeding value of forage brassica plants for grazing ruminant livestock. Animal Feed Science and Technology, 181(1-4), 15-25. [CrossRef]
- Bayat, A., Kairenius, P., Stefański, T., Leskinen, H., Comtet-Marre, S., Forano, E., Chaucheyras-Durand, F., & Shingfield, K. (2015). Effect of camelina oil or live yeasts (Saccharomyces cerevisiae) on ruminal methane production, rumen fermentation, and milk fatty acid composition in lactating cows fed grass silage diets. Journal of Dairy Science, 98(5), 3166-3181. [CrossRef]
- Bayat, A. R., Tapio, I., Vilkki, J., Shingfield, K., & Leskinen, H. (2018). Plant oil supplements reduce methane emissions and improve milk fatty acid composition in dairy cows fed grass silage-based diets without affecting milk yield. Journal of Dairy Science, 101(2), 1136-1151. [CrossRef]
- Beauchemin, K., & McGinn, S. (2006). Methane emissions from beef cattle: Effects of fumaric acid, essential oil, and canola oil. Journal of animal science, 84(6), 1489-1496. [CrossRef]
- Belanche, A., Newbold, C. J., Morgavi, D. P., Bach, A., Zweifel, B., & Yáñez-Ruiz, D. R. (2020). A meta-analysis describing the effects of the essential oils blend agolin ruminant on performance, rumen fermentation and methane emissions in dairy cows. Animals, 10(4), 620. [CrossRef]
- Bica, R., Palarea-Albaladejo, J., Lima, J., Uhrin, D., Miller, G. A., Bowen, J., Pacheco, D., Macrae, A., & Dewhurst, R. (2022). Methane emissions and rumen metabolite concentrations in cattle fed two different silages. Scientific Reports, 12(1), 5441. [CrossRef]
- Black, J. L., Davison, T. M., & Box, I. (2021). Methane emissions from ruminants in Australia: mitigation potential and applicability of mitigation strategies. Animals, 11(4), 951. [CrossRef]
- Bodas, R., López, S., Fernandez, M., García-González, R., Rodríguez, A., Wallace, R., & González, J. (2008). In vitro screening of the potential of numerous plant species as antimethanogenic feed additives for ruminants. Animal Feed Science and Technology, 145(1-4), 245-258. [CrossRef]
- Bodas, R., & Prieto, N. (2012). Garcia-Gonzales; Andres, S.; Giraldez, F. J.; Lopez, S. Anim. Feed Sci. Technol, 176, 78-93.
- Bodas, R., Prieto, N., García-González, R., Andrés, S., Giráldez, F. J., & López, S. (2012). Manipulation of rumen fermentation and methane production with plant secondary metabolites. Animal Feed Science and Technology, 176(1-4), 78-93. [CrossRef]
- Busquet, M., Calsamiglia, S., Ferret, A., Cardozo, P., & Kamel, C. (2005). Effects of cinnamaldehyde and garlic oil on rumen microbial fermentation in a dual flow continuous culture. Journal of Dairy Science, 88(7), 2508-2516. [CrossRef]
- Cardoso-Gutierrez, E., Aranda-Aguirre, E., Robles-Jimenez, L., Castelán-Ortega, O., Chay-Canul, A., Foggi, G., Angeles-Hernandez, J., Vargas-Bello-Pérez, E., & González-Ronquillo, M. (2021). Effect of tannins from tropical plants on methane production from ruminants: A systematic review. Veterinary and Animal Science, 14, 100214. [CrossRef]
- Casamiglia, S., Busquet, M., Cardoza, P., Castillejos, L., & Ferrett, A. (2017). Essential oils as modifiers of rumen microbial fermentation: A review. J. Dairy Sci, 90, 2580-2595.
- CASTILLO-GONZÁLEZ, A. R., BURROLA-BARRAZA, M. E., RIVAS-MARTÍNEZ, M. I., Dominguez-Viveros, J., ORTEGA-GUTIÉRREZ, J. A., & Dominguez-Diaz, D. (2016). Relationships between ruminal Gram-positive bacteria and methane from lactating dairy cows supplemented with monensin and tallow, alone or in combination. Med. Weter, 72(8), 498-504. [CrossRef]
- Chagas, J. C., Ramin, M., Exposito, R. G., Smidt, H., & Krizsan, S. J. (2021). Effect of a Low-Methane Diet on Performance and Microbiome in Lactating Dairy Cows Accounting for Individual Pre-Trial Methane Emissions. Animals, 11(9), 2597. [CrossRef]
- Chaudhary, S. (2000). Flora of the Kingdom of Saudi Arabia, vol II, parts 1–3. Ministry of Agriculture and Water, Riyadh.
- Cieslak, A., Szumacher-Strabel, M., Stochmal, A., & Oleszek, W. (2013). Plant components with specific activities against rumen methanogens. Animal, 7 Suppl 2, 253-265. [CrossRef]
- Cobellis, G., Trabalza-Marinucci, M., & Yu, Z. (2016). Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Science of the Total Environment, 545, 556-568. [CrossRef]
- Correa, P. S., Mendes, L. W., Lemos, L. N., Crouzoulon, P., Niderkorn, V., Hoste, H., Costa-Júnior, L. M., Tsai, S. M., Faciola, A. P., & Abdalla, A. L. (2020). Tannin supplementation modulates the composition and function of ruminal microbiome in lambs infected with gastrointestinal nematodes. FEMS Microbiology Ecology, 96(3), fiaa024. [CrossRef]
- Dhanasekaran, D. K., Dias-Silva, T. P., Filho, A. L. A., Sakita, G. Z., Abdalla, A. L., Louvandini, H., & Elghandour, M. M. (2020). Plants extract and bioactive compounds on rumen methanogenesis. Agroforestry Systems, 94, 1541-1553. [CrossRef]
- Du Toit, C. J. L., Van Niekerk, W. A., Meissner, H., Erasmus, L. J., & Coertze, R. J. (2020). Methane emissions from sheep fed Eragrostis curvula hay substituted with Lespedeza cuneata. Animal Production Science, 60(15), 1777-1784. [CrossRef]
- Du, W., Hou, F., Tsunekawa, A., Kobayashi, N., Ichinohe, T., & Peng, F. (2019). Effects of the diet inclusion of common vetch hay versus alfalfa hay on the body weight gain, nitrogen utilization efficiency, energy balance, and enteric methane emissions of crossbred Simmental cattle. Animals, 9(11), 983. [CrossRef]
- Durmic, Z., & Blache, D. (2012). Bioactive plants and plant products: Effects on animal function, health and welfare. Animal Feed Science and Technology, 176(1-4), 150-162. [CrossRef]
- Ebeid, H. M., Hassan, F.-u., Li, M., Peng, L., Peng, K., Liang, X., & Yang, C. (2020). Camelina sativa L. Oil Mitigates Enteric in vitro Methane Production, Modulates Ruminal Fermentation, and Ruminal Bacterial Diversity in Buffaloes [Original Research]. Frontiers in Veterinary Science, 7. [CrossRef]
- Ebeid, H. M., Hassan, F.-u., Li, M., Peng, L., Peng, K., Liang, X., & Yang, C. (2020). Camelina sativa L. oil mitigates enteric in vitro methane production, modulates ruminal fermentation, and ruminal bacterial diversity in buffaloes. Frontiers in Veterinary Science, 7, 550. [CrossRef]
- El-Lakany, A., Abdel-Kader, M., Hammoda, H., Ghazy, N., & Mahmoud, Z. (1997). A new flavone glycoside with antimicrobial activity for Carduus pycnocephalus L. Pharmazie, 52(1), 78-79.
- Elevitch, C. R., & Francis, J. K. (2006). Gliricidia sepium (gliricidia). Species Profiles for Pacific Island Agroforestry, 2(1), 1-18.
- Esmaeili, A., Rustaiyan, A., Nadimi, M., Masoudi, S., Tadayon, F., Sedaghat, S., Ebrahimpur, N., & Hajyzadeh, E. (2005). Volatile constituents of Centaurea depressa MB and Carduus pycnocephalus L. two compositae herbs growing wild in Iran. Journal of essential oil research, 17(5), 539-541. [CrossRef]
- Evans, J. D., & Martin, S. A. (2000). Effects of thymol on ruminal microorganisms. Current microbiology, 41(5), 336-340. [CrossRef] [PubMed]
- Fagundes, G. M., Benetel, G., Welter, K. C., Melo, F. A., Muir, J. P., Carriero, M. M., Souza, R. L., Meo-Filho, P., Frighetto, R. T., & Berndt, A. (2020). Tannin as a natural rumen modifier to control methanogenesis in beef cattle in tropical systems: friend or foe to biogas energy production? Research in Veterinary Science, 132, 88-96. [CrossRef]
- Flachowsky, G., Langbein, T., Böhme, H., Schneider, A., & Aulrich, K. (1997). Effect of false flax expeller combined with short-term vitamin E supplementation in pig feeding on the fatty acid pattern, vitamin E concentration and oxidative stability of various tissues. Journal of Animal Physiology and Animal Nutrition, 78(1-5), 187-195. [CrossRef]
- Flachowsky, G., & Lebzien, P. (2012). Effects of phytogenic substances on rumen fermentation and methane emissions: A proposal for a research process. Animal Feed Science and Technology, 176(1-4), 70-77. [CrossRef]
- Flores-Santiago, E. d. J., González-Garduño, R., Vaquera-Huerta, H., Calzada-Marín, J. M., Cadena-Villegas, S., Arceo-Castillo, J. I., Vázquez-Mendoza, P., & Ku-Vera, J. C. (2022). Reduction of Enteric Methane Emissions in Heifers Fed Tropical Grass-Based Rations Supplemented with Palm Oil. Fermentation, 8(8), 349. [CrossRef]
- FrAnKIČ, T., Voljč, M., Salobir, J., & Rezar, V. (2009). Use of herbs and spices and their extracts in animal nutrition. Acta Agric Slov, 94(2), 95-102. [CrossRef]
- Fu, C., Hernandez, T., Zhou, C., & Wang, Z.-Y. (2015). Alfalfa (Medicago sativa L.). Agrobacterium Protocols: Volume 1, 213-221. [CrossRef]
- Goel, G., Makkar, H., & Becker, K. (2008). Changes in microbial community structure, methanogenesis and rumen fermentation in response to saponin-rich fractions from different plant materials. Journal of Applied Microbiology, 105(3), 770-777. [CrossRef]
- Goel, G., Makkar, H. P., & Becker, K. (2008). Effects of Sesbania sesban and Carduus pycnocephalus leaves and Fenugreek (Trigonella foenum-graecum L.) seeds and their extracts on partitioning of nutrients from roughage-and concentrate-based feeds to methane. Animal Feed Science and Technology, 147(1-3), 72-89. [CrossRef]
- Grainger, C., Clarke, T., Auldist, M., Beauchemin, K., McGinn, S., Waghorn, G., & Eckard, R. J. (2009). Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Canadian Journal of Animal Science, 89(2), 241-251. [CrossRef]
- Guyader, J., Eugène, M., Doreau, M., Morgavi, D., Gérard, C., & Martin, C. (2017). Tea saponin reduced methanogenesis in vitro but increased methane yield in lactating dairy cows. Journal of Dairy Science, 100(3), 1845-1855. [CrossRef] [PubMed]
- Haque, M. N. (2018). Dietary manipulation: a sustainable way to mitigate methane emissions from ruminants. Journal of animal science and technology, 60(1), 1-10. [CrossRef]
- Harrison, M. T., McSweeney, C., Tomkins, N. W., & Eckard, R. J. (2015). Improving greenhouse gas emissions intensities of subtropical and tropical beef farming systems using Leucaena leucocephala. Agricultural Systems, 136, 138-146. [CrossRef]
- Hart, K. J., Jones, H. G., Waddams, K. E., Worgan, H. J., Zweifel, B., & Newbold, C. J. (2019). An essential oil blend decreases methane emissions and increases milk yield in dairy cows. Open Journal of Animal Sciences, 9(03), 259. [CrossRef]
- Hassan, F.-u., Arshad, M. A., Ebeid, H. M., Rehman, M. S.-u., Khan, M. S., Shahid, S., & Yang, C. (2020). Phytogenic additives can modulate rumen microbiome to mediate fermentation kinetics and methanogenesis through exploiting diet–microbe interaction. Frontiers in Veterinary Science, 7, 575801. [CrossRef]
- Hassan, F.-u., Arshad, M. A., Li, M., Rehman, M. S.-u., Loor, J. J., & Huang, J. (2020). Potential of mulberry leaf biomass and its flavonoids to improve production and health in ruminants: Mechanistic insights and prospects. Animals, 10(11), 2076. [CrossRef]
- He, D.-Y., & Dai, S.-M. (2011). Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora Pall., a traditional Chinese herbal medicine. Frontiers in pharmacology, 2, 10. [CrossRef]
- Hironaka, R., Mathison, G. W., Kerrigan, B. K., & Vlach, I. (1996). The effect of pelleting of alfalfa hay on methane production and digestibility by steers. Science of The Total Environment, 180(3), 221-227. [CrossRef]
- Houghton, J. T., Ding, Y., Griggs, D. J., Noguer, M., van der Linden, P. J., Dai, X., Maskell, K., & Johnson, C. (2001). Climate change 2001: the scientific basis: contribution of Working Group I to the third assessment report of the Intergovernmental Panel on Climate Change. Cambridge university press.
- Hurtaud, C., & Peyraud, J.-L. (2007). Effects of feeding camelina (seeds or meal) on milk fatty acid composition and butter spreadability. Journal of Dairy Science, 90(11), 5134-5145. [CrossRef] [PubMed]
- Islam, M., & Lee, S.-S. (2019). Advanced estimation and mitigation strategies: a cumulative approach to enteric methane abatement from ruminants. Journal of animal science and technology, 61(3), 122. [CrossRef]
- Jayanegara, A., Goel, G., Makkar, H. P., & Becker, K. (2015). Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Animal Feed Science and Technology, 209, 60-68. [CrossRef]
- Jayanegara, A., Wina, E., & Takahashi, J. (2014). Meta-analysis on methane mitigating properties of saponin-rich sources in the rumen: influence of addition levels and plant sources. Asian-Australasian Journal of Animal Sciences, 27(10), 1426. [CrossRef]
- Johnson, K. A., & Johnson, D. E. (1995). Methane emissions from cattle. Journal of animal science, 73(8), 2483-2492. [CrossRef]
- Kholif, A., Hassan, A., El Ashry, G. M., Bakr, M., El-Zaiat, H., Olafadehan, O., Matloup, O., & Sallam, S. (2021). Phytogenic feed additives mixture enhances the lactational performance, feed utilization and ruminal fermentation of Friesian cows. Animal Biotechnology, 32(6), 708-718. [CrossRef]
- Kholif, A. E., & Olafadehan, O. A. (2021). Essential oils and phytogenic feed additives in ruminant diet: chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochemistry Reviews, 20(6), 1087-1108. [CrossRef]
- Kim, E. T., Lee, S. J., Lee, S. M., Lee, S. S., Lee, I. D., Lee, S. K., & Lee, S. S. (2015). Effects of flavonoid-rich plant extracts on in vitro ruminal methanogenesis, microbial populations and fermentation characteristics. Asian-Australasian Journal of Animal Sciences, 28(4), 530. [CrossRef]
- Kobayashi, Y. (2010). Abatement of methane production from ruminants: trends in the manipulation of rumen fermentation. Asian-Australasian Journal of Animal Sciences, 23(3), 410-416. [CrossRef]
- Kolodziejczyk-Czepas, J. (2012). Trifolium species-derived substances and extracts—Biological activity and prospects for medicinal applications. Journal of ethnopharmacology, 143(1), 14-23. [CrossRef]
- Kongmun, P., Wanapat, M., Pakdee, P., & Navanukraw, C. (2010). Effect of coconut oil and garlic powder on in vitro fermentation using gas production technique. Livestock Science, 127(1), 38-44. [CrossRef]
- Kozłowska, M., Cieślak, A., Jóźwik, A., El-Sherbiny, M., Stochmal, A., Oleszek, W., Kowalczyk, M., Filipiak, W., & Szumacher-Strabel, M. (2020). The effect of total and individual alfalfa saponins on rumen methane production. Journal of the Science of Food and Agriculture, 100(5), 1922-1930. [CrossRef] [PubMed]
- Lamont, E.-J. , Zoghlami, A., Hamilton, R. S., & Bennett, S. J. (2001). Clovers (Trifolium L.). In Plant genetic resources of legumes in the Mediterranean (pp. 79-98). Springer.
- Lawson, L. (1996). The composition and chemistry of garlic cloves and processed garlic. Garlic: The science and therapeutic applications of Allium sativum L. and related species, 37-109.
- Lee, J., Woodward, S., Waghorn, G., & Clark, D. (2004). Methane emissions by dairy cows fed increasing proportions of white clover (Trifolium repens) in pasture. Proceedings of the New Zealand Grassland Association. [CrossRef]
- Lei, Z., Zhang, K., Li, C., Jiao, T., Wu, J., Wei, Y., Tian, K., Li, C., Tang, D., & Davis, D. I. (2019). Ruminal metagenomic analyses of goat data reveals potential functional microbiota by supplementation with essential oil-cobalt complexes. BMC microbiology, 19(1), 1-10. [CrossRef]
- Lillehoj, H., Liu, Y., Calsamiglia, S., Fernandez-Miyakawa, M. E., Chi, F., Cravens, R. L., Oh, S., & Gay, C. G. (2018). Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Veterinary research, 49(1), 1-18. [CrossRef]
- Lim, T. (2014). Paeonia lactiflora. In Edible Medicinal and Non Medicinal Plants (pp. 559-596). Springer.
- Liu, H., Puchala, R., LeShure, S., Gipson, T. A., Flythe, M. D., & Goetsch, A. L. (2019). Effects of lespedeza condensed tannins alone or with monensin, soybean oil, and coconut oil on feed intake, growth, digestion, ruminal methane emission, and heat energy by yearling Alpine doelings. Journal of Animal Science, 97(2), 885-899. [CrossRef]
- Liu, Y., Ma, T., Chen, D., Zhang, N., Si, B., Deng, K., Tu, Y., & Diao, Q. (2019). Effects of tea saponin supplementation on nutrient digestibility, methanogenesis, and ruminal microbial flora in dorper crossbred ewe. Animals, 9(1), 29. [CrossRef]
- McGuffey, R. (2017). A 100-Year Review: Metabolic modifiers in dairy cattle nutrition. Journal of Dairy Science, 100(12), 10113-10142. [CrossRef]
- Mizrahi, I. (2013). Rumen symbioses. In The prokaryotes: prokaryotic biology and symbiotic associations (pp. 533-544). Springer-Verlag Berlin Heidelberg.
- Mohammad, A.-B., & Hassan, A. Effects of in vitro supplementation of mulberry leaf flavonoids on microbial flora, methanogenesis and fermentative products in rumen fluid of sheep.
- Molina-Botero, I. C., Arroyave-Jaramillo, J., Valencia-Salazar, S., Barahona-Rosales, R., Aguilar-Pérez, C. F., Burgos, A. A., Arango, J., & Ku-Vera, J. C. (2019). Effects of tannins and saponins contained in foliage of Gliricidia sepium and pods of Enterolobium cyclocarpum on fermentation, methane emissions and rumen microbial population in crossbred heifers. Animal Feed Science and Technology, 251, 1-11. [CrossRef]
- Molina-Botero, I. C., Montoya-Flores, M. D., Zavala-Escalante, L. M., Barahona-Rosales, R., Arango, J., & Ku-Vera, J. C. (2019). Effects of long-term diet supplementation with Gliricidia sepium foliage mixed with Enterolobium cyclocarpum pods on enteric methane, apparent digestibility, and rumen microbial population in crossbred heifers. Journal of Animal Science, 97(4), 1619-1633. [CrossRef]
- Molina, I., Angarita, E., Mayorga, O., Chará, J., & Barahona-Rosales, R. (2016). Effect of Leucaena leucocephala on methane production of Lucerna heifers fed a diet based on Cynodon plectostachyus. Livestock Science, 185, 24-29. [CrossRef]
- Montoya-Flores, M. D., Molina-Botero, I. C., Arango, J., Romano-Muñoz, J. L., Solorio-Sánchez, F. J., Aguilar-Pérez, C. F., & Ku-Vera, J. C. (2020). Effect of dried leaves of Leucaena leucocephala on rumen fermentation, rumen microbial population, and enteric methane production in crossbred heifers. Animals, 10(2), 300. [CrossRef]
- Morgavi, D. P., Kelly, W., Janssen, P., & Attwood, G. (2013). Rumen microbial (meta) genomics and its application to ruminant production. Animal, 7(s1), 184-201. [CrossRef]
- Morsy, A., Soltan, Y., El-Zaiat, H., Alencar, S., & Abdalla, A. (2021). Role of bee propolis extract on diet digestibility, purine derivatives, mitigating methane formation, and blood metabolites in late pregnant ewes. Animal Feed Science and Technology, 273, 114834.
- Muhlisin, M. (2017). Calliandra calothyrsus as tannins source for in vitro methane production inhibitor agents. International Seminar on Tropical Animal Production (ISTAP).
- Muir, S., Kennedy, A., Kearney, G., Hutton, P., Thompson, A., Vercoe, P., & Hill, J. (2020). Offering subterranean clover can reduce methane emissions compared with perennial ryegrass pastures during late spring and summer in sheep. Animal Production Science, 60(11), 1449-1458. [CrossRef]
- Navarro-Villa, A., O’Brien, M., López, S., Boland, T., & O’kiely, P. (2011). In vitro rumen methane output of red clover and perennial ryegrass assayed using the gas production technique (GPT). Animal Feed Science and Technology, 168(3-4), 152-164. [CrossRef]
- Omojate Godstime, C., Enwa Felix, O., Jewo Augustina, O., & Eze Christopher, O. (2014). Mechanisms of antimicrobial actions of phytochemicals against enteric pathogens–a review. J Pharm Chem Biol Sci, 2(2), 77-85.
- Orhan, I., Deliorman-Orhan, D., & Özçelik, B. (2009). Antiviral activity and cytotoxicity of the lipophilic extracts of various edible plants and their fatty acids. Food chemistry, 115(2), 701-705. [CrossRef]
- Oskoueian, E., Abdullah, N., & Oskoueian, A. (2013). Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. BioMed research international, 2013. [CrossRef]
- Parker, S., May, B., Zhang, C., Zhang, A. L., Lu, C., & Xue, C. C. (2016). A pharmacological review of bioactive constituents of Paeonia lactiflora Pallas and Paeonia veitchii Lynch. Phytotherapy Research, 30(9), 1445-1473. [CrossRef]
- Parnian-Khajehdizaj, F., Noel, S., Johansen, M., Weisbjerg, M., Hellwing, A., Højberg, O., Hall, M., & Lund, P. (2023). Methane emission, nutrient digestibility, and rumen microbiota in Holstein heifers fed 14 different grass or clover silages as the sole feed. Journal of Dairy Science, 106(6), 4072-4091. [CrossRef]
- Patra, A., Park, T., Kim, M., & Yu, Z. (2017). Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. Journal of animal science and biotechnology, 8(1), 1-18. [CrossRef]
- Patra, A., & Yu, Z. (2015). Effects of garlic oil, nitrate, saponin and their combinations supplemented to different substrates on in vitro fermentation, ruminal methanogenesis, and abundance and diversity of microbial populations. Journal of Applied Microbiology, 119(1), 127-138. [CrossRef]
- Patra, A. K. (2013). The effect of dietary fats on methane emissions, and its other effects on digestibility, rumen fermentation and lactation performance in cattle: A meta-analysis. Livestock Science, 155(2-3), 244-254. [CrossRef]
- Patra, A. K., & Saxena, J. (2009). Dietary phytochemicals as rumen modifiers: a review of the effects on microbial populations. Antonie van Leeuwenhoek, 96(4), 363-375. [CrossRef]
- Pech-Cervantes, A. A., Terrill, T. H., Ogunade, I. M., & Estrada-Reyes, Z. M. (2021). Meta-analysis of the effects of dietary inclusion of sericea lespedeza (Lespedeza cuneata) forage on performance, digestibility, and rumen fermentation of small ruminants. Livestock Science, 253, 104707. [CrossRef]
- Piazza, G. J., & Foglia, T. A. (2001). Rapeseed oil for oleochemical usage. European journal of lipid science and technology, 103(7), 450-454.
- Poudel, P., Froehlich, K., Casper, D. P., & St-Pierre, B. (2019). Feeding essential oils to neonatal Holstein dairy calves results in increased ruminal Prevotellaceae abundance and propionate concentrations. Microorganisms, 7(5), 120. [CrossRef] [PubMed]
- Poulsen, M., Schwab, C., Borg Jensen, B., Engberg, R. M., Spang, A., Canibe, N., Højberg, O., Milinovich, G., Fragner, L., & Schleper, C. (2013). Methylotrophic methanogenic Thermoplasmata implicated in reduced methane emissions from bovine rumen. Nature communications, 4(1), 1-9. [CrossRef] [PubMed]
- Purba, R. A. P., Paengkoum, S., Yuangklang, C., & Paengkoum, P. (2020). Flavonoids and their aromatic derivatives in Piper betle powder promote in vitro methane mitigation in a variety of diets. Ciência e Agrotecnologia, 44.
- Ramin, M., Chagas, J. C., Smidt, H., Exposito, R. G., & Krizsan, S. J. (2021). Enteric and fecal methane emissions from dairy cows fed grass or corn silage diets supplemented with rapeseed oil. Animals, 11(5), 1322. [CrossRef]
- Ramírez-Restrepo, C. A., Tan, C., López-Villalobos, N., Padmanabha, J., Wang, J., & McSweeney, C. S. (2016). Methane production, fermentation characteristics, and microbial profiles in the rumen of tropical cattle fed tea seed saponin supplementation. Animal Feed Science and Technology, 216, 58-67. [CrossRef]
- Reddy, P. R. K., Elghandour, M., Salem, A., Yasaswini, D., Reddy, P. P. R., Reddy, A. N., & Hyder, I. (2020). Plant secondary metabolites as feed additives in calves for antimicrobial stewardship. Animal Feed Science and Technology, 264, 114469. [CrossRef]
- Reuter, H. D. (1996). Therapeutic effects and applications of garlic and its preparations. Garlic.
- Ridwan, R., Rusmana, I., Widyastuti, Y., Wiryawan, K., Prasetya, B., Sakamoto, M., & Ohkuma, M. (2014). Methane mitigation and microbial diversity of silage diets containing Calliandra calothyrsus in a rumen in vitro fermentation system. Media Peternakan, 37(2), 121-121. [CrossRef]
- Rira, M., Morgavi, D. P., Genestoux, L., Djibiri, S., Sekhri, I., & Doreau, M. (2019). Methanogenic potential of tropical feeds rich in hydrolyzable tannins. Journal of animal science, 97(7), 2700-2710. [CrossRef]
- Roca-Fernández, A. I., Dillard, S. L., & Soder, K. J. (2020). Ruminal fermentation and enteric methane production of legumes containing condensed tannins fed in continuous culture. Journal of Dairy Science, 103(8), 7028-7038. [CrossRef] [PubMed]
- Rochfort, S., Parker, A. J., & Dunshea, F. R. (2008). Plant bioactives for ruminant health and productivity. Phytochemistry, 69(2), 299-322. [CrossRef] [PubMed]
- Rohela, G. K., Jogam, P., Mir, M. Y., Shabnam, A. A., Shukla, P., Abbagani, S., & Kamili, A. N. (2020). Indirect regeneration and genetic fidelity analysis of acclimated plantlets through SCoT and ISSR markers in Morus alba L. cv. Chinese white. Biotechnology reports, 25, e00417. [CrossRef] [PubMed]
- Rojas-Downing, M. M., Nejadhashemi, A. P., Harrigan, T., & Woznicki, S. A. (2017). Climate change and livestock: Impacts, adaptation, and mitigation. Climate risk management, 16, 145-163. [CrossRef]
- Roldan, M. B., Cousins, G., Muetzel, S., Zeller, W. E., Fraser, K., Salminen, J.-P., Blanc, A., Kaur, R., Richardson, K., & Maher, D. (2022). Condensed tannins in white clover (Trifolium repens) foliar tissues expressing the transcription factor TaMYB14-1 bind to forage protein and reduce ammonia and methane emissions in vitro. Frontiers in Plant Science, 12, 777354. [CrossRef]
- Rustaiyan, A., & Faridchehr, A. (2021). Constituents and biological activities of selected genera of the Iranian Asteraceae family. Journal of Herbal Medicine, 25, 100405. [CrossRef]
- Schären, M., Drong, C., Kiri, K., Riede, S., Gardener, M., Meyer, U., Hummel, J., Urich, T., Breves, G., & Dänicke, S. (2017). Differential effects of monensin and a blend of essential oils on rumen microbiota composition of transition dairy cows. Journal of Dairy Science, 100(4), 2765-2783. [CrossRef] [PubMed]
- Seradj, A. R., Abecia, L., Crespo, J., Villalba, D., Fondevila, M., & Balcells, J. (2014). The effect of Bioflavex® and its pure flavonoid components on in vitro fermentation parameters and methane production in rumen fluid from steers given high concentrate diets. Animal Feed Science and Technology, 197, 85-91. [CrossRef]
- Shelton, H. M. , & Brewbaker, J. L. (1994). Leucaena leucocephala-the most widely used forage tree legume. Forage tree legumes in tropical agriculture.(Eds. RC Gutteridge and HM Shelton). CAB International. Wallingford, UK, 15.
- Soltan, Y. A. Soltan, Y. A., Natel, A. S., Araujo, R., Morsy, A. S., & Abdalla, A. L. (2018). Progressive adaptation of sheep to a microencapsulated blend of essential oils: Ruminal fermentation, methane emission, nutrient digestibility, and microbial protein synthesis. Animal Feed Science and Technology, 237, 8-18. [CrossRef]
- Soltan, Y. A. , & Patra, A. K. (2021). Ruminal Microbiome Manipulation to Improve Fermentation Efficiency in Ruminants. In Animal Feed Science and Nutrition-Production, Health and Environment. IntechOpen London.
- Stewart, E. K., Beauchemin, K. A., Dai, X., MacAdam, J. W., Christensen, R. G., & Villalba, J. J. (2019). Effect of tannin-containing hays on enteric methane emissions and nitrogen partitioning in beef cattle. Journal of animal science, 97(8), 3286-3299. [CrossRef]
- Sukmawati, M. , Permana, I., Thalib, A., & Kompiang, S. (2011). Effect of complete rumen modifier (CRM) and Calliandra calothyrus on productivity and enteric methane productions of PE dairy goat.
- Sun, X. (2020). Invited review: Glucosinolates might result in low methane emissions from ruminants fed Brassica forages. Frontiers in Veterinary Science, 7, 588051. [CrossRef]
- Sun, X., Pacheco, D., & Luo, D. (2016). Forage brassica: a feed to mitigate enteric methane emissions? Animal Production Science, 56(3), 451-456. [CrossRef]
- Sutapa, M., & Analava, M. (2009). Health effects of palm oil. Journal of Human Ecology, 26(3), 197-203. [CrossRef]
- Tan, C., Ramírez-Restrepo, C. A., Shah, A. M., Hu, R., Bell, M., Wang, Z., & McSweeney, C. (2020). The community structure and microbial linkage of rumen protozoa and methanogens in response to the addition of tea seed saponins in the diet of beef cattle. Journal of animal science and biotechnology, 11(1), 1-10. [CrossRef]
- Tan, H., Sieo, C., Abdullah, N., Liang, J., Huang, X., & Ho, Y. (2011). Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Animal Feed Science and Technology, 169(3-4), 185-193. [CrossRef]
- Tapio, I., Snelling, T. J., Strozzi, F., & Wallace, R. J. (2017). The ruminal microbiome associated with methane emissions from ruminant livestock. Journal of animal science and biotechnology, 8(1), 1-11. [CrossRef]
- Tava, A., Biazzi, E., Ronga, D., Pecetti, L., & Avato, P. (2022). Biologically active compounds from forage plants. Phytochemistry Reviews, 1-31. [CrossRef]
- Teoh, E. S., & Teoh, E. S. (2016). Secondary metabolites of plants. Medicinal orchids of Asia, 59-73.
- Terrill, T. H., Smith, V., Morning, B., Courson, E., Muir, J. P., Cherry, N., & Morris, J. Concentration and Bioactivity of Condensed Tannins and Total Phenolics of Lespedeza Species From a Germplasm Collection.
- Tiemann, T. T., Lascano, C. E., Wettstein, H.-R., Mayer, A., Kreuzer, M., & Hess, H. (2008). Effect of the tropical tannin-rich shrub legumes Calliandra calothyrsus and Flemingia macrophylla on methane emission and nitrogen and energy balance in growing lambs. Animal, 2(5), 790-799. [CrossRef]
- Tyagi, N., Mishra, D. B., Vinay, V., & Kumar, S. (2022). Feasible Strategies for Enteric Methane Mitigation from Dairy Animals. Animal Manure: Agricultural and Biotechnological Applications, 335-354.
- Van Lingen, H. J., Niu, M., Kebreab, E., Valadares Filho, S. C., Rooke, J. A., Duthie, C.-A., Schwarm, A., Kreuzer, M., Hynd, P. I., & Caetano, M. (2019). Prediction of enteric methane production, yield and intensity of beef cattle using an intercontinental database. Agriculture, Ecosystems & Environment, 283, 106575. [CrossRef]
- Villar, M. L., Hegarty, R. S., Nolan, J. V., Godwin, I. R., & McPhee, M. (2020). The effect of dietary nitrate and canola oil alone or in combination on fermentation, digesta kinetics and methane emissions from cattle. Animal Feed Science and Technology, 259, 114294. [CrossRef]
- Volpe, M., Goldfarb, J. L., & Fiori, L. (2018). Hydrothermal carbonization of Opuntia ficus-indica cladodes: Role of process parameters on hydrochar properties. Bioresource Technology, 247, 310-318. [CrossRef]
- Wallace, R. J., McEwan, N. R., McIntosh, F. M., Teferedegne, B., & Newbold, C. J. (2002). Natural products as manipulators of rumen fermentation. Asian-Australasian Journal of Animal Sciences, 15(10), 1458-1468. [CrossRef]
- Wang, B., Jia, M., Fang, L., Jiang, L., & Li, Y. (2018). Effects of eucalyptus oil and anise oil supplementation on rumen fermentation characteristics, methane emission, and digestibility in sheep. Journal of animal science, 96(8), 3460-3470. [CrossRef] [PubMed]
- Wang, C., Wang, S., & Zhou, H. (2009). Influences of flavomycin, ropadiar, and saponin on nutrient digestibility, rumen fermentation, and methane emission from sheep. Animal Feed Science and Technology, 148(2-4), 157-166. [CrossRef]
- Wang, C., Zhang, C., Yan, T., Chang, S., Zhu, W., Wanapat, M., & Hou, F. (2020). Increasing roughage quality by using alfalfa hay as a substitute for concentrate mitigates CH4 emissions and urinary N and ammonia excretion from dry ewes. Journal of animal physiology and animal nutrition, 104(1), 22-31. [CrossRef] [PubMed]
- Weimer, P. J. (2015). Redundancy, resilience, and host specificity of the ruminal microbiota: implications for engineering improved ruminal fermentations. Frontiers in microbiology, 6, 296. [CrossRef]
- Williams, S., Moate, P., Deighton, M., Hannah, M., Wales, W., & Jacobs, J. (2016). Milk production and composition, and methane emissions from dairy cows fed lucerne hay with forage brassica or chicory. Animal Production Science, 56(3), 304-311. [CrossRef]
- Wina, E. (2012). The use of plant bioactive compounds to mitigate enteric methane in ruminants and its application in Indonesia. WARTAZOA. Indonesian Bulletin of Animal and Veterinary Sciences, 22(1), 24-34.
- Witzig, M., Zeder, M., & Rodehutscord, M. (2018). Effect of the ionophore monensin and tannin extracts supplemented to grass silage on populations of ruminal cellulolytics and methanogens in vitro. Anaerobe, 50, 44-54. [CrossRef] [PubMed]
- Yang, K., Wei, C., Zhao, G., Xu, Z., & Lin, S. (2017). Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility. Journal of Animal Physiology and Animal Nutrition, 101(2), 302-310. [CrossRef] [PubMed]
- Ye, D., Karnati, S., Wagner, B., Firkins, J., Eastridge, M., & Aldrich, J. (2018). Essential oil and monensin affect ruminal fermentation and the protozoal population in continuous culture. Journal of Dairy Science, 101(6), 5069-5081. [CrossRef] [PubMed]
- Yilmaz, K., & Kara, K. (2022). The effect of vegetable and animal oils added to different forages and concentrates on the in vitro fermentation parameters in ruminants. Journal of Applied Animal Research, 50(1), 548-559. [CrossRef]
- Yusuf, R. O., Noor, Z. Z., Abba, A. H., Hassan, M. A. A., & Din, M. F. M. (2012). Methane emission by sectors: a comprehensive review of emission sources and mitigation methods. Renewable and Sustainable Energy Reviews, 16(7), 5059-5070. [CrossRef]
- Zaina, M. , Putria, E. M., Rusmana, W., & Erpomena, M. M. Effects of Supplementing Gliricidia Sepium on Ration based Ammoniated Rice Straw in Ruminant Feed to Decrease Methane Gas Production and to Improve Nutrient Digestibility (In-Vitro).
- Zayed, M. Z., & Samling, B. (2016). Phytochemical constituents of the leaves of Leucaena leucocephala from Malaysia. Int J Pharm Pharm Sci, 8(12), 174-179. [CrossRef]
- Zengin, H., & Baysal, A. H. (2014). Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules, 19(11), 17773-17798. [CrossRef]


| Sources of PFAs | Plant bioactive compounds (PBC) | Mechanism of Mitigation | Reference |
|---|---|---|---|
| Carduus pycnocephalus | Essential oil, Flavonoids, Terpenes | Inhibit methanogens | Bodas et al. (2008) |
| Paeonia lactiflora | Total glucosides of peony | Inhibition of Gram-positive bacteria | CASTILLO-GONZÁLEZ et al. (2016). |
| Leucaena leucocephala | Tannins | Reduction in the total number of methanogens and protozoa | Tan et al. (2011). |
| Brassica | Glucosinolates | Alter the mean retention time of digestion in the rumen | Sun (2020) |
| Rapeseed oil | Sterols and tocopherols | Decrease protozoal population in the rumen | Villar et al. (2020). |
| Camelina sativa oil | Unsaturated fatty acids and antioxidant | Inhibiting rumen protozoa and methanogens. Hydrogen sink |
Hassan, Arshad, Ebeid, et al. (2020). |
| Garlic oil | Organosulfur | Reducing the abundance of protozoa | Kongmun et al. (2010). |
| Palm oil | Fatty acids | Decrease the number of ciliate protozoa | Yilmaz and Kara (2022) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





