1. Introduction
Proper fit is crucial for the safety, comfort, and functionality of face masks [
1,
2]. Recent advancements have greatly improved filter media, enhancing aerosol filtration efficiency while maintaining an acceptable pressure drop [
3,
4,
5,
6,
7]. However, the effectiveness of even the most advanced filter media is compromised if the mask does not fit the wearer properly. Essentially, a mask only functions as personal protective equipment if air flows through the filter media and not around it through gaps between the mask and the skin. Consequently, the protective level of the mask directly correlates with the amount of air leakage. A poor fit can dramatically decrease a mask’s overall protection efficacy. Notably, unlike tight-fitting respirators, disposable 3-layer surgical masks often have a loose fit that can worsen during physical activities, incorrect usage, or extended wear [
8,
9,
10,
11,
12].
Gaps between a mask and skin can significantly affect airflow and aerosol movement, depending on their size and location. However, current standardized tests cannot measure these gaps or link them to mask fit. While mask-fit testers exist for tight-fitting respirators, similar tools for loose-fitting masks, like disposable surgical masks, are lacking [
13,
14,
15,
16,
17,
18]. For instance, directly applying the TSI Portacount mask-fit tester to surgical masks yields unrealistically low scores (0–40) with large variability. Also, while we can measure air speed through gaps, measuring gap size is difficult. These gaps change with activity, face shape, and facial hair. Wang et al. showed that nose height and chin length notably influenced the leak site in loose-fitting masks [
19]. In a similar manner, Oestenstad et al. showed that the leak site changed with the face size and shape [
20]. Even gender and age matter—women often have leaks at the mask’s bottom [
21]. Overall, accurately measuring mask leakage is still challenging, and leakage testing should use anatomically accurate or even sub-specific face models [
22,
23,
24].
Previous studies have explored different methods to characterize mask flows, especially leakage flows and escaping particles. Using a Schlieren optical imaging system, Tang et al. found that while surgical masks block a cough’s forward jet, a poor fit can allow air leakage from the top and sides [
25]. Similarly, Su et al. reported comparable reductions in filtration efficiency among various loose-fitting masks (cloth, surgical, KN95) against ultrafine particles [
26]. Cappa et al. measured exhaled aerosols using a particle sizer and showed that imperfect sealing in surgical masks significantly reduced their ability to block expiratory particles during talking and coughing [
27]. Koh et al. simulated exhalation and coughing in a manikin with different masks. Interestingly, they demonstrated that poorly fitting N95 respirators may offer less protection than a simple surgical mask [
28]. Also using a manikin model, Brooks et al. demonstrated that knotting the mask band and tucking in the extra material of a surgical mask effectively improved mask fit and protection [
29]. Verma et al. utilized a manikin, foot pump, smoke generator, and a laser sheet to visualize a ‘cough’ through different masks [
30]. The team was able to identify sites of leakage in the masks and determine how far the ‘cough’ traveled after passing through the masks [
30]. For normal breathing, the foot pump delivers 500 mL of air to the model per breath, while for coughing, the foot pump delivers 1500 mL, mimicking the average amount of air expelled during a cough [
31].
Despite valuable insights obtained from previous visualization studies, they were limited to qualitative analysis and a single test participant wearing a mask. Moreover, the visualization results predominantly focused on exhalation flows. Due to ethical issues, the visualization methods with human subjects had to be safe, and many alternative optical approaches, such as laser-based methods, had to be excluded. The manikin models used in mask flow visualization were often for general purposes, with not-so-accurate facial morphologies. Even more significant, research on interpersonal droplet transmission is severely lacking, even though it is more relevant to evaluating the transmission risks of respiratory infections [
32]. One exception was Xu et al., who used a Schlieren system to study the effects of ventilation on airborne aerosol transmission between two persons and suggested that only a sufficiently high personal ventilation can effectively protect against infection [
33,
34].
The objective of this study is to visualize mask flows and the transmission of respiratory droplets between individuals. Specific aims include:
Explore different visualization methods for expiratory and inspiratory airflows due to mask-wearing, including systems based on Schlieren, laser, LED, smoke particles, and vapor droplets.
Compare facemask flow dynamics under different physical activities and between different mask types.
Measure the particle counts and leakage flow rates from different mask types, including cloth, surgical, and KN95.
Investigate interpersonal droplet transmission using a vapor-based visualization system between 3D-printed head models under various interaction scenarios.
2. Methods
2.1. Mask Flow Visualization Methods
2.1.1. Schlieren Optical Imaging System
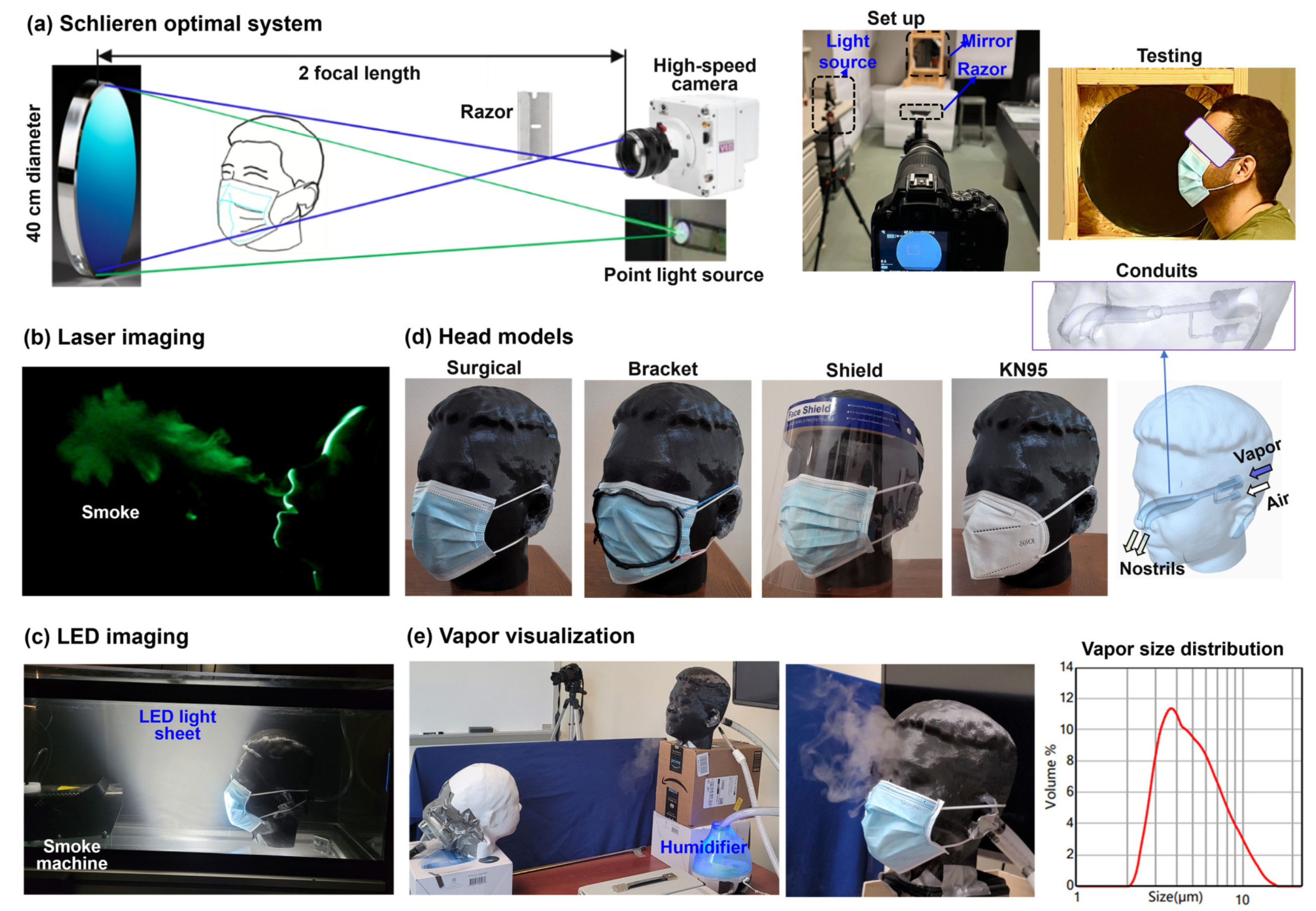
The Schlieren imaging system visualizes airflows by capturing light refraction changes between areas of differing pressure or temperature. This study used a single-mirror setup (
Figure 1a) with four parts: a concave mirror (AD015, 406 mm diameter, 1.8 m focal length), a pinhole LED light source 3.6 m away, a razor blade, and a Canon EOS Rebel T7 camera. The mirror reflects light through the test area to the razor, which blocks half the light. The remainder reaches the camera, producing an image. Dimmed lighting enhances airflow visibility. Image sensitivity depends on the mirror’s focal length to unobstructed object length ratio [
35]. During the test, the participant sat 20 cm from the mirror, with or without face coverings, as illustrated in the rightmost panel in
Figure 1a.
2.1.2. Laser-Particle Imaging System for Exhalation
The laser system was created using a laser sheet, a head model, a foot pump, and a fog machine. A laser sheet (100 mW, 488 nm) was used to illuminate expiratory flows that contain fog particles (
Figure 1b). The foot pump was used to pump a puff of air through the head model, simulating the way air is quickly released from the nose/mouth during coughing or breathing. A fog machine (CHAUVET DJ Hurricane 1000, Antwerpen, Belgium) was used to create tracer particles. The laser sheet was pointed toward the respiratory model to create a midsagittal cross-section of the expelled smoke. All testing was done in a dark room so that the illuminated particle-laden flows could be clearly visible. All testing was recorded using the same camera that was used for the Schlieren system images.
2.1.3. LED-Particle Imaging System for Inhalation and Exhalation
Considering that the foot pump could only generate expiratory flows, another visualization system was designed that allowed visualization of both inhalations and exhalations. To visualize the inspiratory flows that flow inwardly into the nose/mouth, seed particles need to be present outside the head model before inhalation. Ideally, these seed particles should be evenly suspended and as still as possible so that when inhalation starts, the particle trajectories converging toward the mouth/nose or mask can be sufficiently different from the surroundings. To achieve this, a 50-gallon fish tank was used, which was pre-filled with fog smoke five minutes before the test to allow the fog particles to stabilize (
Figure 1c). An LED sheet light was used to illuminate the mid-sagittal plane of the head model. A breathing machine was used to ventilate a sinusoidal pressure waveform to the head model, and the same camera that was used for the Schlieren imaging was used to record the flow dynamics around the mask (
Figure 1c). Note that, even for exhalation, this LED-fish tank system is different from the laser-foot-pump system described in 2.1.2 in that it visualizes how the ambient air is disturbed by expiratory flows, while the laser system visualizes how the exhaled particles are dispersed in the environment.
2.2. Quantitative Measurements of Flows, Temperature, and Particles
2.2.1. Leakage Flow Velocity and Mask Temperature
The velocity of leakage flows was measured using a TSI 9565 VelociCalc ventilation meter (Shoreview, MN). Considering that the flow would decay as it moved away from the gap, the probe was positioned 2 cm from the gap sites for all tests. The sites selected for velocity measurement included two nose top ridges, two lateral sides, and the chin, based on prior observations of leakage flows of different masks. When conducting the tests, the participant wore a mask with a good fit and breathed naturally. Each test was repeated five times. Meanwhile, the mask temperature was recorded with a FLIR ONE Pro iOS thermal camera (Wilsonville, OR, USA).
2.2.2. Particle Counter Testing
A Temtop PMD 331 Particle Counter was used to measure the number of droplets exhaled across the mask during normal breathing, as well as the size of each of the droplets. This particle counter can detect aerosol droplets from 0.3 µm to 10 µm in size and has seven channels. SARS CoV-2 virus is around 0.1 µm, which can escape mask filtration [
36]. However, viruses typically attach to larger droplets or particles to survive and spread, ranging from 0.3 µm to large drops of several millimeters [
37]. In addition to the control case (no mask), six types of masks were tested: surgical, KN95, cloth, cloth with HEPA filter, neck gaiter, and surgical with bracket. The tests were conducted in a controlled environment with 24
oC and 30% relative humidity. An air purifier operated for one hour prior to testing to ensure that the majority of droplet particles detected by the particle counter came from breathing.
2.3. Head Models
Several head models were used in this study. One was the respiratory model (Michigan Instruments, Grand Rapids, MI) that allowed air to be expelled through the mouth only. The other two head models were developed from scans of two volunteers using the iPhone app Bellus3D (Lilburn, GA). The use of human scans in this study has been approved by the Institutional Review Board (IRB) of the University of Massachusetts Lowell. The head model was further processed using SolidWorks (Dassault Systèmes, Waltham, MA) to add two conduits, with one connecting to a breathing simulator (Michigan Instruments, Grand Rapids, MI) and the other to a vapor source (rightmost panel,
Figure 1d). The head models were manufactured using a Dimension 1200es 3D printer and ABS printing material (Stratasys, Eden Prairie, MN). Due to its life-size and accurate facial topology, the head model can adequately simulate the mask fit to the face, as demonstrated in
Figure 1d.
2.4. Interpersonal Transmission Visualization System
To investigate interpersonal droplet transmission, two head models were placed at a specified distance and relative height (
Figure 1e). The head orientation of the two head models could also be adjusted as needed. Each head model was ventilated to a breathing simulator with a sinusoidal waveform (Michigan Instruments, Grand Rapids, MI). The source head model (black color,
Figure 1e) was also connected to the vapor source to simulate exhaled droplets, while the recipient model (white color,
Figure 1e) was only ventilated with tidal air flows. An ultrasonic humidifier (Pure Enrichment, Huntington Beach, CA, USA) was used to generate soft mist. Using a SprayLink laser diffraction spray particle size analyzer (Dickinson, TX, USA), the soft mist size distribution was measured to be 2.95, 4.62, and 8.55 µm for D10, D50, and D90, respectively [
38]. This size range is close to that of expiratory droplets from deep lungs [
39,
40,
41].
2.5. Numerical and Statistical Methods
2.5.1. Computational Fluid Dynamics (CFD) Simulations
Complementary CFD modeling and simulations were performed. An integrated mask-wearing model was developed that included the ambient air, facemask, face geometry, and airway. Gaps at the mask-face interface were modeled using individual volumes of the mask filter medium that had the properties of air rather than a porous medium. More details of the computational model, along with the mesh generation and grid-independent study, were provided in [
42,
43]. The low-Reynolds number (LRN) k-ω turbulent model was utilized to simulate the multi-regime flow dynamics. ANSYS Fluent 23 (Canonsburg, PA) was used to solve conservation equations for mass, momentum, and energy.
2.5.2. Statistical Analysis
Minitab 21.4 (State College, PA, USA) was applied to analyze the leakage flow velocities and cross-mask particle counts. A one-way analysis of variance (ANOVA) was used to evaluate the variability of the measurements. A p-value < 0.05 indicated statistical significance difference.
3. Results
3.1. Control Cases
3.1.1. Schlieren Optical Imaging System
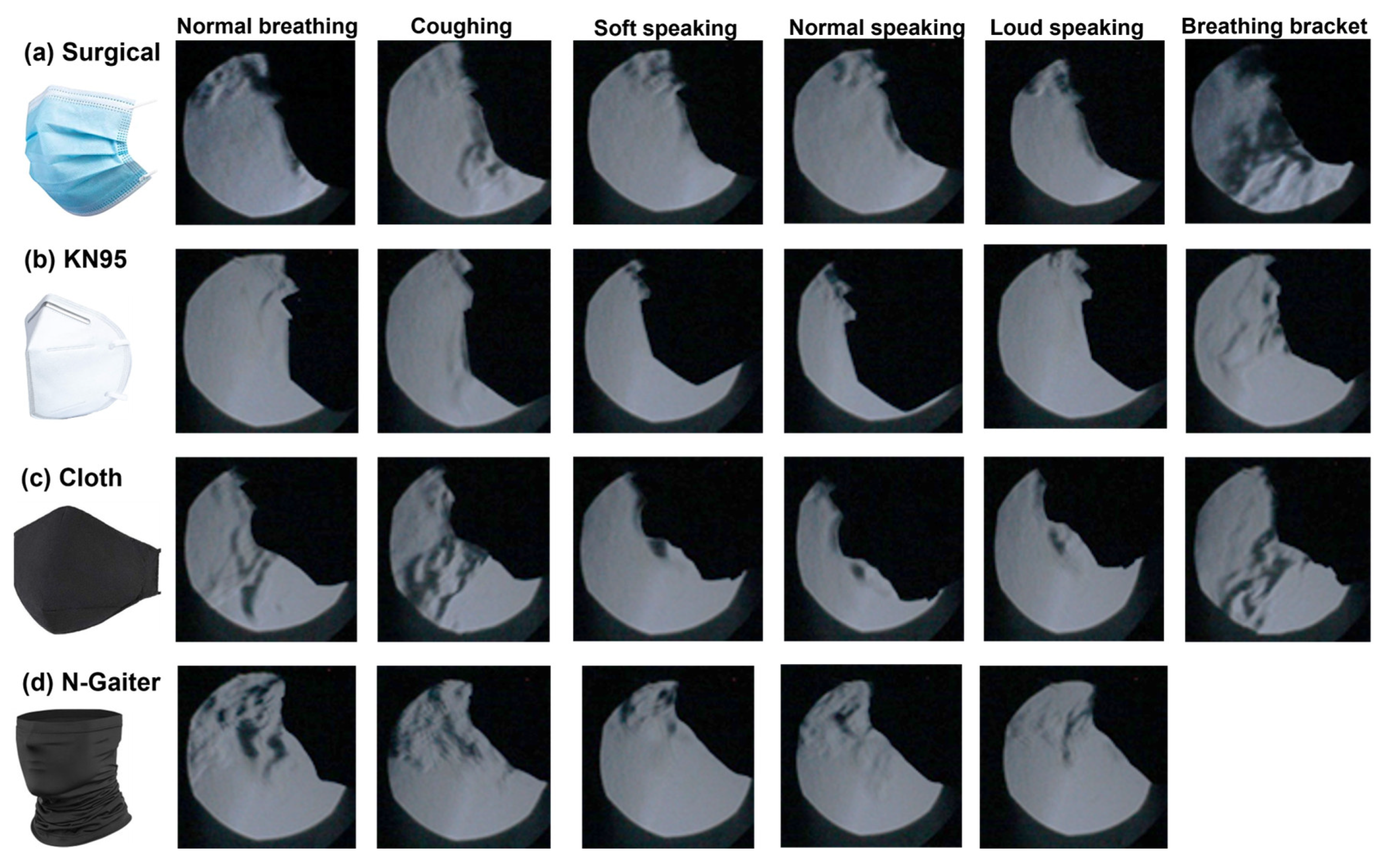
Figure 2 shows the Schlieren visualization results of the exhaled flows with different masks, i.e., surgical, KN95, cloth, and neck gaiter (N-Gaiter)
. For each mask, five activities were tested: normal breathing, coughing, soft speaking, normal speaking, and loud speaking. The effects of wearing a mask bracket were also evaluated for all masks except the neck gaiter. Considering that a good mask should distribute airflow over the mask and seal well, the mask performance was evaluated qualitatively based on how much air is seen escaping the mask and where leakages are occurring. For all masks tested, the KN95 mask was found to have the least amount of air escaping from the mask for all activities. By comparison, the cloth, surgical, and neck gaiter exhibited large amounts of air escaping from the tops/sides of the mask, as well as through the mask filter material itself. The jet-like flows through the cloth mask and neck gaiter are due to the larger fiber pores that allow air to pass through them more freely, while the KN95 mask has smaller filter pores. This makes it more difficult for airflows and particles to pass through and helps distribute the airflow all over the mask, thus lowering the exhaled flow speed crossing the mask and extending the effective filtration area.
Considering the three speaking scenarios (i.e., soft, normal, loud,
Figure 2), no apparent jet flows were observed through the masks, primarily because of the intermittent flow pulses during speaking, regardless of the speaking volume. When a mask bracket was used (last column,
Figure 2), intensified jet flows were observed crossing the KN95 and surgical masks, as the bracket effectively confined the exhaled flows within the boundary of the bracket. The purpose of a mask bracket is to fit the mask to the face better, specifically with surgical masks that can create gaps at the sides of an individual’s face.
For a given mask, we observed similar flow patterns among different activities, whether the test subject was breathing, coughing, or speaking at different volumes. This demonstrated the mask’s consistency in effectively containing exhaled airflows under different physical activities. Note that the considered activities have very different dynamics, where breathing releases air constantly from the nostrils, coughing releases air from the mouth quickly at high pressure, and speaking releases air intermittently from the mouth. Moreover, the latter two activities also involve the motion of the mouth, particularly the lips, which may affect the mask fit.
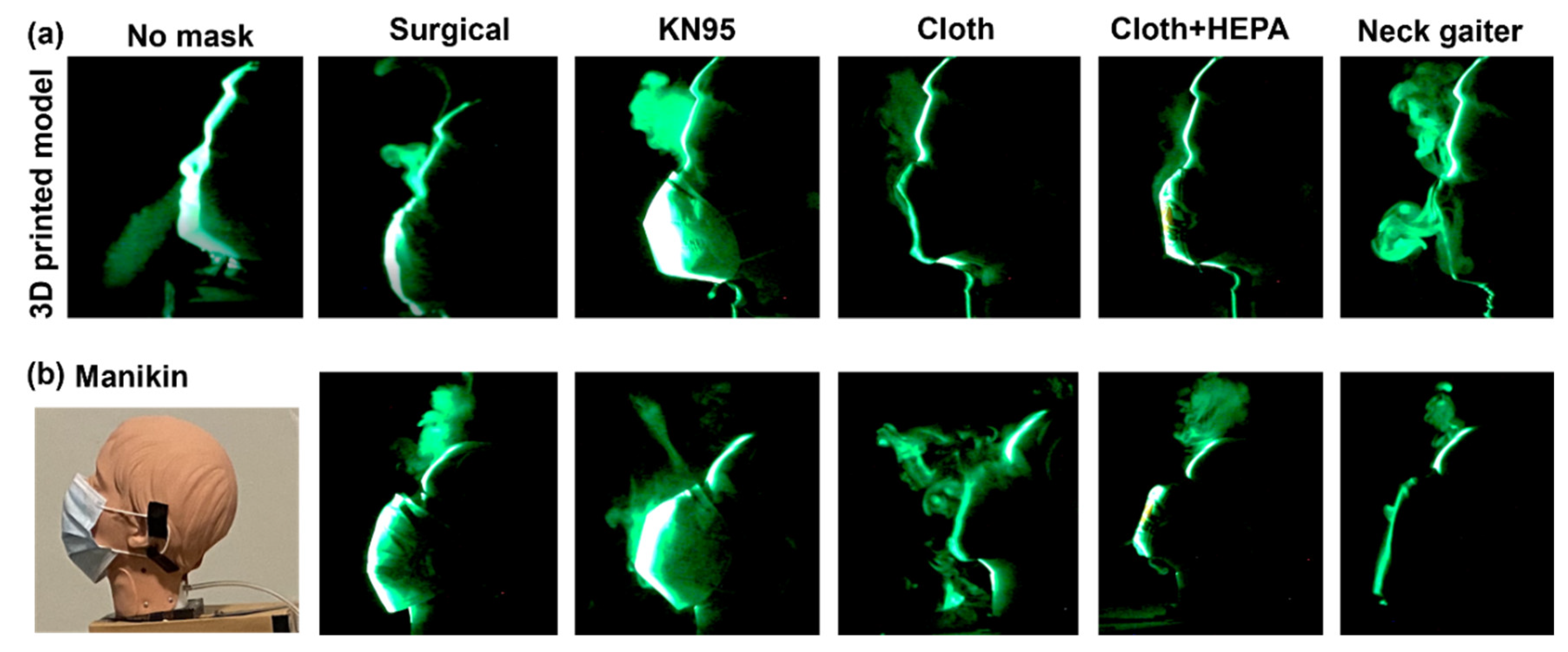
3.1.2. Laser Sheet System
Figure 3 shows the laser visualization of expiratory mask flows in two head models. It is observed that a breath exhaled from the head model traveled directly forward and dispersed in the air. A commonality among all the masks was leakages out of the top of the mask. In addition, fog particulates were able to pass directly through the cloth mask without a HEPA filter and through the neck gaiter. When the HEPA filter was placed in the cloth mask, there was no passage of fog directly through the mask. This finding highlights the effectiveness of the HEPA filter in improving the effectiveness of cloth masks.
Considering flows with the 3D-printed model (
Figure 3a), leakages occurred at the nose top with the surgical mask, KN95, and the neck gaiter. When comparing the results between the 3D-printed head and the manikin (Figs. 3a vs. 3b), one can see that the cloth masks (both with and without the HEPA filter) and surgical mask all performed better with the 3D head model. This is likely due to the 3D head model being larger in size and fitting the masks better than the manikin. These findings demonstrate the importance of proper mask designs for different population groups, such as adults and children.
Based on the findings from the laser sheet experiments, the neck gaiter and the cloth mask without the HEPA filter were less effective in preventing the spread of exhaled air due to their sites of leakage and their inability to distribute the airflow evenly across the filter. The surgical mask, the cloth mask with the HEPA filter, and the KN95 mask performed better in blocking and spreading the exhaled air despite having leakage through the top of the masks. These leakages highlight the important fact that masks are not 100% effective, necessitating social distancing and hand/face cleaning to mitigate viral transmission.
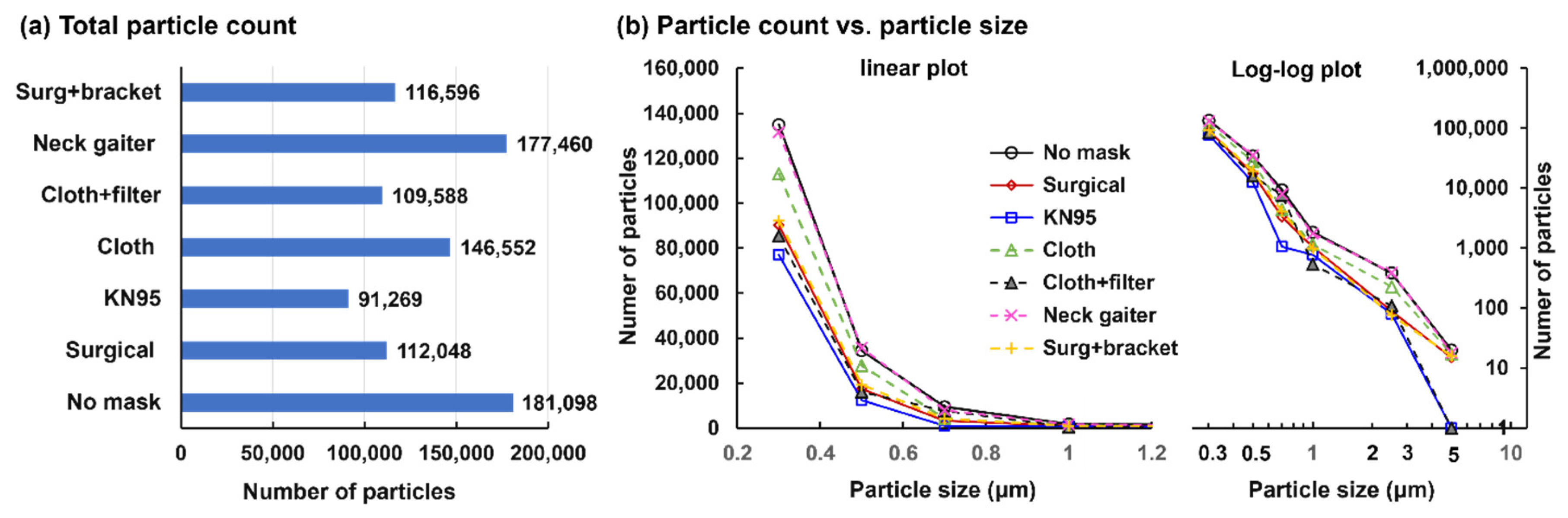
3.1.3. Particle Count across Various Masks
Figure 4 shows the exhaled particle counts across the masks. As expected, the largest number of exhaled droplets were detected with no mask-wearing, followed by the neck gaiter, cloth, surgical with bracket, surgical, cloth with HEPA filter and KN95. The slightly higher number of droplets from ‘surgical + bracket’ than from ‘surgical’ is because the bracket confined the exhaled flow-droplets within the bracket and reduced leakages from the mask-face interface.
3.2. Leakage Flow Visualization and Quantification
3.2.1. Leakage Flow Velocity Measurement
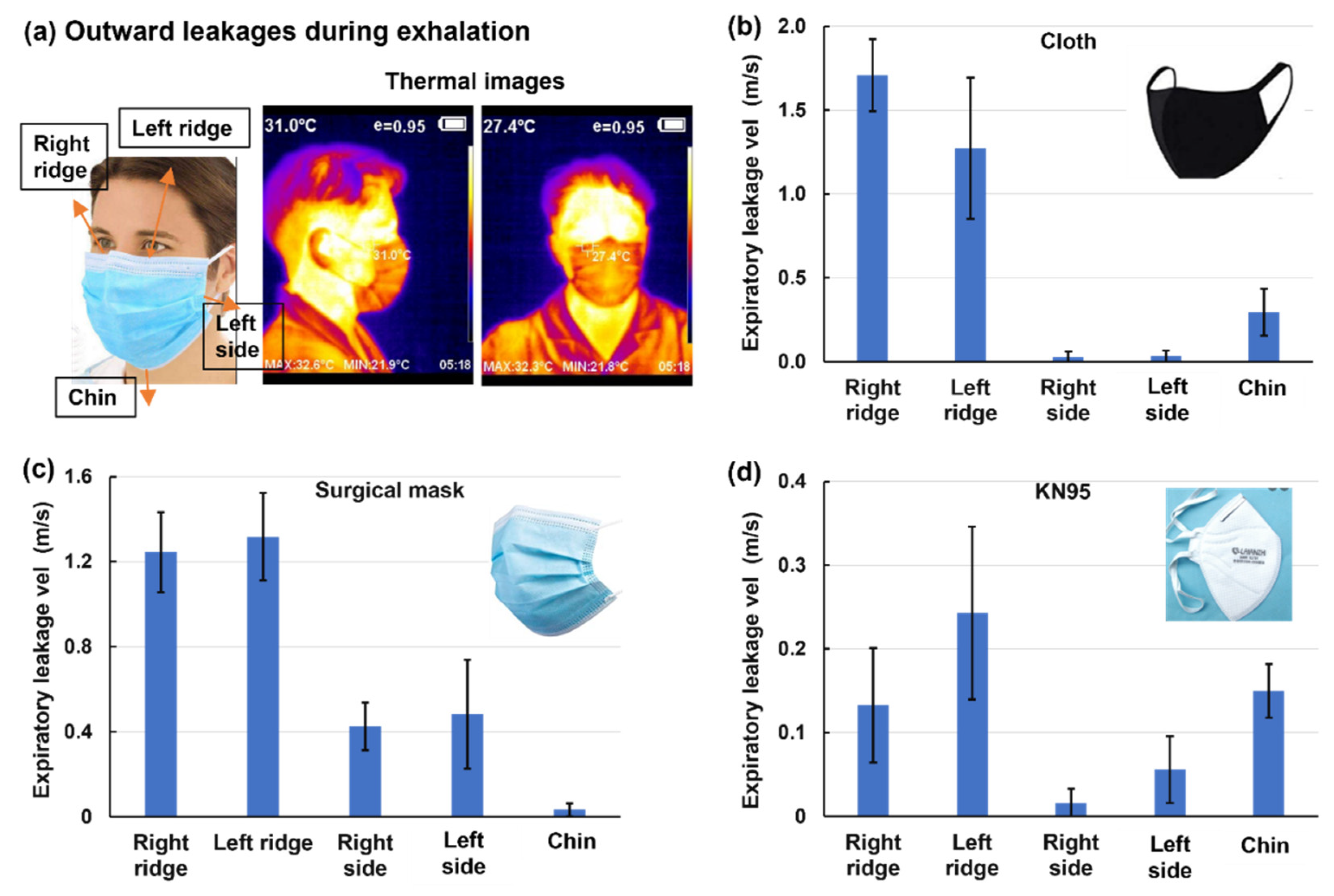
Considering that leakages are mostly observed at the nose top and two sides during exhalation, the speeds of the leakage flows were measured using a hotwire anemometer at the right and left ridges of the nose top, two sides, and the chin (
Figure 5a). For the three masks considered (cloth, surgical, and KN95), the leakage flow speeds are much higher from the two ridges than the two lateral sides and chin. Mask-specific differences are also observed, with more leakages from the chin for the cloth and KN95 masks and more lateral leakages from the surgical mask. These observations are consistent with the peculiar mask geometry and its fitting to the facial topology. Both the cloth and KN95 masks have a flat region covering the cheeks, while the surgical mask is tucked up on both sides, forming an arch that allows leakage flows. Among the three masks, the cloth mask had the highest leakage flow speed (1.7±0.5 m/s,
Figure 5b), followed by the surgical mask (1.3±0.5 m/s,
Figure 5c) and KN95 (0.24±0.1 m/s.
Figure 5d). This difference is also evident by the different ranges in the y-axis among Figs. 5b–d. By comparison, the jet flow velocity exhaled from the nostrils without a mask was measured to be 2~4 m/s, while the flow velocity across the mask was 0.1~0.2 m/s.
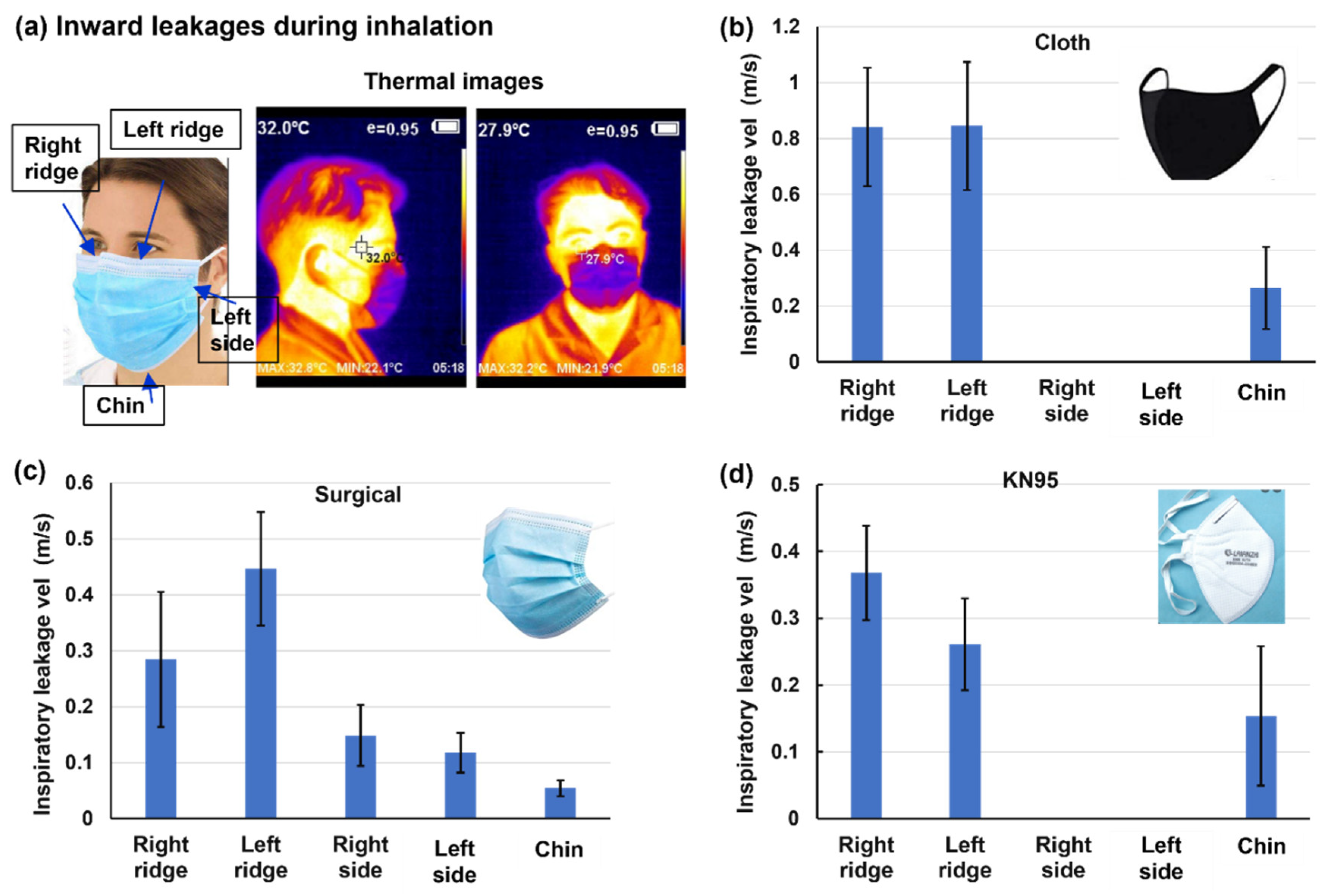
Figure 6 shows the speeds of the inward leakage flows at different sites during inhalation for three mask types. Note that for both inhalation and exhalation, the mask was carefully pressed to conform to the participant’s facial topology as much as possible, thus representing the best scenario of mask fit. The thermal images of the mask are blue in color, indicating the cooling effects from inspiratory ambient air, which contrasts with the warming effect (brown color) from the expiratory air at body temperature (Figs. 6a vs. 5a). The overall leakage speeds during inhalation are lower than those during exhalation (
Figure 6 vs.
Figure 5). This is due to the jet flow features of the expiratory flows in contrast to the converging flow features of inspiratory flows. Considering the side fitting, inward leakage is not observed in the cloth and KN95 masks (
Figure 6b&d) but is observed in the surgical masks (
Figure 6c), corroborating the finding that a tucked-up fold is more prone to mask-face gaps and leakage flows.
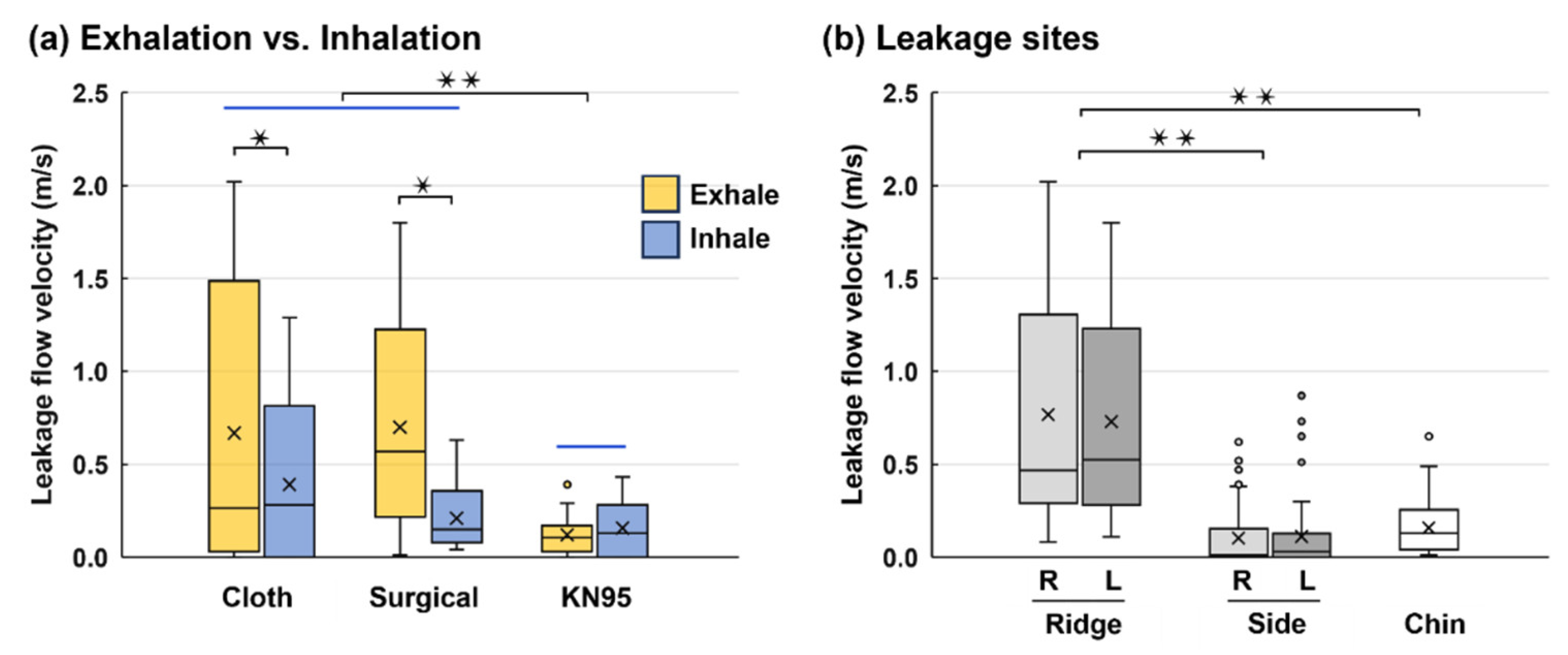
Figure 7 compares the leakage flow velocities between exhalation and inhalation for different mask types, considering all leakage sites. Significantly higher leakage flow velocities between exhalation and inhalation are observed for the two less-resistive masks (cloth: p-value = 0.043 and surgical: p = 0.031,
Figure 7a), while they are similar for the KN95 mask, indicating a better fit to the face. Considering different sites, significantly higher leakage flows occurred at the nose top than at the two sides and chin, reflecting the importance of using the nasal strip to anchor the mask to the ridge-shaped nose top. Insignificant differences are observed between the right and left ridges, as well as between the right and left sides, signifying the symmetry in both the mask shape and the human face.
3.2.2. In Vitro Visualization vs. CFD
Visualization of mask flows, especially underneath the mask, can provide detailed information on flow, pressure, and temperature to enhance our understanding of facemask dynamics and thermoregulation.
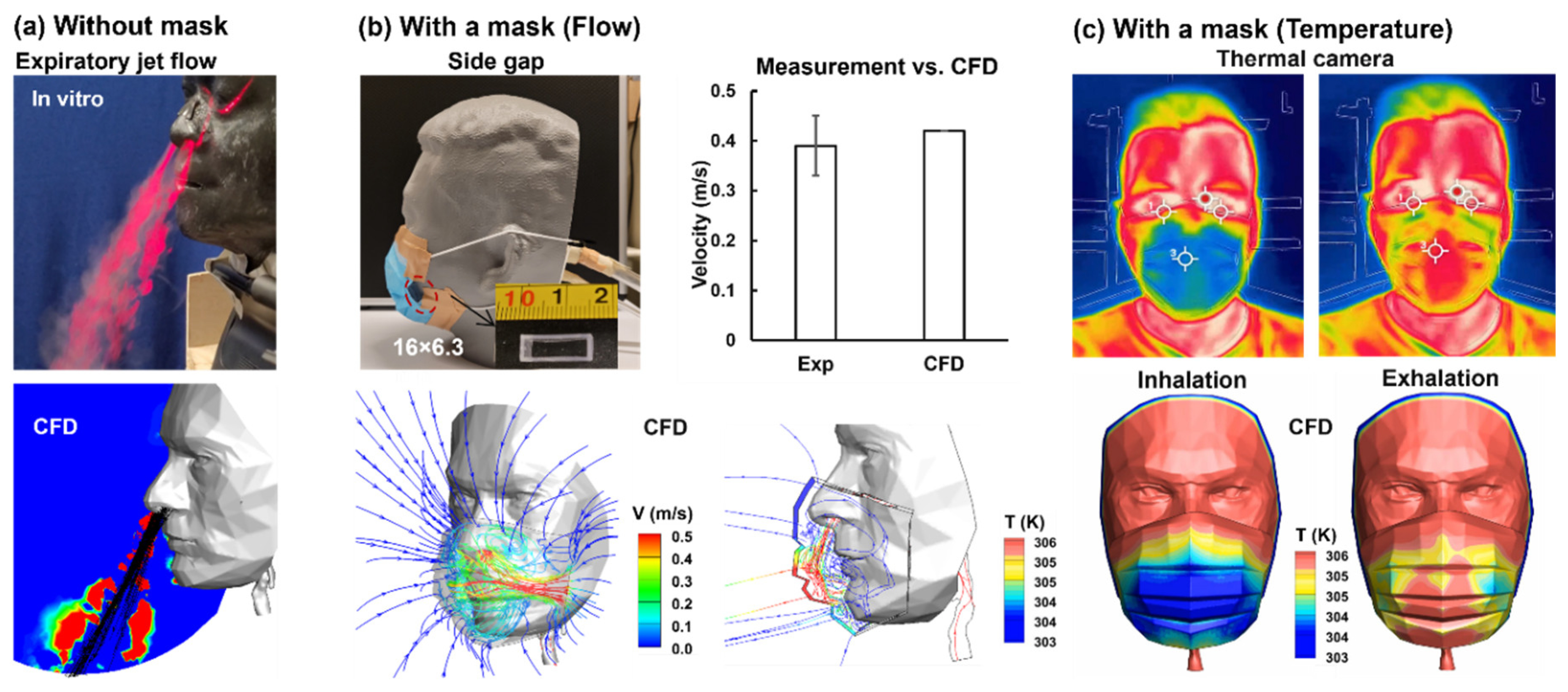
Figure 8 compares the experimental (upper panels) and computational (lower panels) results without mask-wearing (
Figure 8a), with a surgical mask and side gap (
Figure 8b), and with a well-fitted surgical mask (
Figure 8c). As shown in
Figure 8a, the expiratory jet flows from nostrils resembled each other between the laser-fog imaging and the predictions from computational fluid dynamics (CFD). The lower panel of
Figure 8b displays the inspiratory flow streamlines, which converge from all directions but intensify considerably through the side gap. To validate the CFD model, measurement of the flow velocities at the gap was conducted, which agreed favorably with the CFD simulations (
Figure 8b).
The lower panel of the third column (
Figure 8b) shows the CFD-predicted temperature distribution during exhalation, where the warm jet flows from the nostrils are cooled down and well mixed within the mask-face space. Due to the mask resistance, strong recirculation forms under the mask, eliciting quick mixing. Note the higher temperatures of the mask where the exhaled jet flow impinges compared to other regions of the mask.
Figure 8c compares the facial temperature obtained using a thermal camera and complementary CFD simulations. Again, high levels of resemblance are observed between the experiments and CFD for both inhalations and exhalations, further validating the computational model in capturing mask-wearing-associated thermoregulation, which in turn provides thermo-fluid details under the mask that are not easy to measure accurately or non-disruptively. Higher levels of similarity are also observed in comparison to the thermal images in Figs. 5&6.
3.3. LED-Fog System to Visualize Inhalation-Exhalation Flows
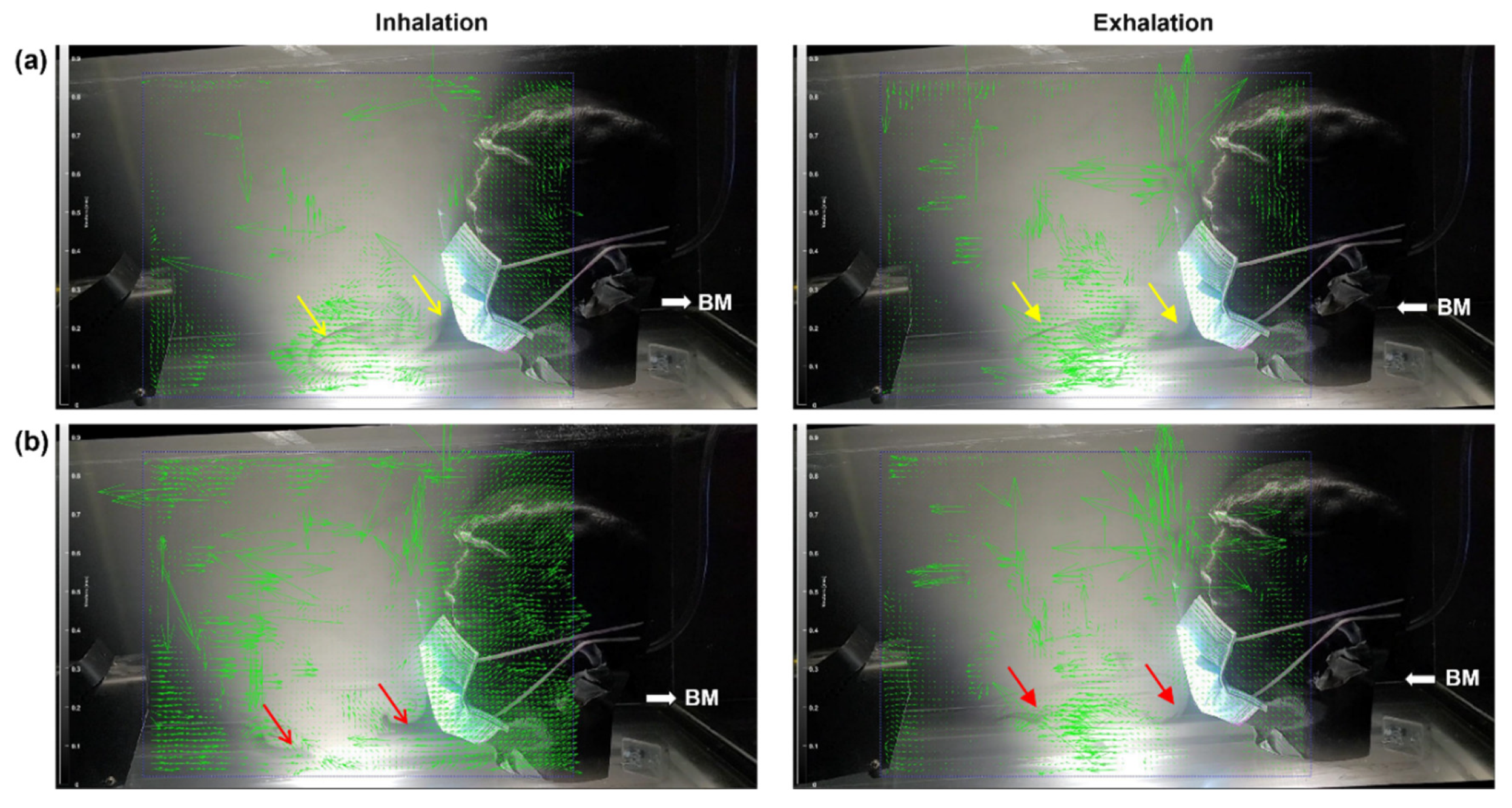
Figure 9 shows the inspiratory and expiratory vector fields in two breathing cycles in a head model wearing a surgical mask. Due to leakages at the nose top, high-speed flows are observed for both inhalation and exhalation cycles. The vectors at the nose top are much longer than the ones in front of the mask. Smoke streaks are observed moving away from the mask, as indicated by the yellow arrows in
Figure 9a and red arrows in
Figure 9b. These streams advance forward slowly and, at the same time, oscillate due to cyclic inhalation and exhalation, forming complex flow patterns. By contrast, the flows in the immediate proximity of the mask change quickly with the breathing cycle. The slow advancement of the smoke streaks away from the mask suggests that the exhaled droplets are likely to behave similarly. Thus, the overall advancement speed can indicate whether or when virus-laden respiratory droplets from an infected person reach the recipient at a specific distance.
3.4. Interpersonal Droplet Transmission
3.4.1. Without Face Covering (1.2 m)
The interpersonal transmission of exhaled droplets is shown in
Figure 10 after 5 minutes of exposure at a distance of 1.2 m between the source and recipient. The droplet deposition is visualized using SarGel, which turns from white to pink upon contacting water, with more deposited water mass correlating with a deeper pink color [
44]. The right three panels of Figs. 10a and 10b display different views of the droplet deposition on the head model that is ventilated with a tidal breathing machine. Heterogeneous deposition distributions are observed on the face in both test cases. However, much-enhanced deposition occurs at the lower cheek (or jaw) and neck (
Figure 10b), presumably because the droplet trajectories in the second case are more aligned with the recipient’s neck than in the first case.
3.4.2. Without Face Covering (1.5 m)
Figure 11 shows the effects of various types of face coverings on interpersonal droplet transmission at a distance of 1.5 m. In both cases, the source wore a surgical mask and a face shield. Thus, the exhaled droplets mainly escaped from the bottom, and a smaller fraction escaped from the two lateral sides of the face shield. Note that none of these three sites pointed to the recipient. The exposure lasted for twenty minutes, and photos of the droplet deposition were taken every five minutes with the mask on and removed. The deposition after 5 minutes of exposure was negligible in both cases and is thus not presented here.
Two observations are noteworthy regarding the droplet deposition from 10–20 minutes. First, the progression of deposition is nonlinear with time, which is noticeable during 0–5 minutes, becomes discernable during 5–10 minutes, but increases quickly between 10–20 minutes (
Figure 11a). This suggests that the exhaled droplet plume needs time to propagate from the source to the recipient. It appears that it takes about 15 minutes for the droplet plume to travel (by dispersion) a distance of 1.5 m from the source to the recipient. However, once the plume reaches the recipient, the deposition will be continuous and become noticeable after a short time, as illustrated by the quick color change from 10 to 15 minutes. Second, more deposition was observed in the mouth-nose region and the jaw-neck region. The deposition on the hairs of the frontal head is also notable.
Figure 11b illustrates the effects of double protection with the recipient adding a face shield on top of a surgical mask. Compared to wearing a surgical mask only, the face deposition is significantly lower after 10 minutes of exposure, indicating the effectiveness of extra face-shied protection, which indeed reduced the number of droplets reaching the face underneath the surgical mask. In this case, the droplets need to avoid impacting the face shield by moving downward in front of the shield and subsequently moving upward to reach the mask. This would decrease the leakage flows from the nose top and make more airflow cross the mask filter medium. Wearing a face shield might also have pressed down the tucked-up arch at two sides, reducing side leakages. Slight deposition in the philtrum region is observed after a 10-minute exposure, with no discernable deposition in other regions of the head model (
Figure 11b). An additional 10 minutes of exposure remarkably increased the deposition in the philtrum region, as well as in the nasofacial sulcus, and to a lesser degree, on the nasal ridge and neck (
Figure 11b).
4. Discussion
Even though mask-wearing has become ubiquitous, knowledge to quantify its performance or improve its performance is surprisingly limited and empirical at best. This study explored multiple methods to visualize mask-related flows and droplet transmission to better understand mask performance, including the Schlieren optical system, laser imaging system, thermal camera, and vapor-SarGel system. Interesting observations and further thoughts on mask-wearing are discussed below.
4.1. Leakage Flow Characterization
In this study, for all masks considered, leakages from the nose top were observed to be notably higher than those from any other sites (i.e., two lateral sides and chin). Therefore, efforts to improve the mask fit at the nose top are both compelling and rewarding. For instance, using a wider and stronger nasal strap in the surgical mask will not only improve the mask fit to the nose ridge but also help the mask maintain its good fit during various physical activities [
45]. A ‘Knot-and-tuck’ method also promises to achieve a better fit [
46]. By knotting the ear loops of a surgical mask and tucking excess material under the edges, a 3D cup-shaped mask forms that better conforms to the facial topology, thus improving the mask-face fit and reducing sideway leakages. It is noted that standardized methods or devices to quantify leakages or fit for disposable masks are lacking, even though commercial devices for tight-fitting respirators are available. One example is the PortaCount Respirator Fit Tester 8038 (TSI, Shoreview, MN, USA); a fit score of no less than 200 under all tested activities is considered a pass. However, when used for loose-fitting disposable masks, such as surgical masks, the fit scores based on the PortaCount Respirator Fit Tester are very low and exhibit large variability (e.g., 0–40). These erroneous readings are mainly due to the device’s working principle, which estimates the fit score based on the aerosol concentrations (generated from NaCl-solution) inside and outside the respirator.
For a loosely fit disposable mask, the aerosols will be easily inhaled across the filter medium or through the inward leakage, equalizing the aerosol concentrations inside and outside the mask, losing the good foundation for fit estimation [
47]. A more practical method is to leverage the temperature variations that are dependent on the leakage flow volume [
48,
49]. Moreover, the periodic temperature variation within one breathing cycle is expected to provide further information on leakage flows, including the leakage volume (gap size) and site, because the cooling effect during inhalation and warming effect during exhalation are highly sensitive to the leakage flow patterns [
50,
51,
52].
4.2. Droplet Deposition Visualization and Implications
Interpersonal transmission testing with vapor and SarGel provides a practical approach to visualize the transport and deposition of respiratory droplets that are often invisible in life conditions. It is observed that the relative orientation and distance can notably affect the transport and deposition of respiratory droplets (Figs. 10 and 11), making it necessary to systematically study these factors to gain a comprehensive understanding of viral transmission and mask protection. Zhang et al. reviewed the close-contact (or short-range) transmission of aerosols between two individuals and highlighted influencing physical parameters, such as their distance/orientation, body/head motion, exposure duration, and breathing intensity, among others [
53]. Zhang et al. also suggested that an exhaled droplet would need at least 0.6 s to reach the receiver at a distance of 1.5 m, based on an expiratory flow velocity of 2.4 m/s (i.e., 1.5 m over 2.4 m/s equals 0.6 s). Note that it can take much longer than 0.6 seconds for an exhaled droplet to travel from the source to the recipient because the exhaled droplets will quickly slow down within the ambient air. In this study, we observed that it took even longer (i.e., five minutes,
Figure 10) for the SarGel on the head model to turn pink under no-mask conditions and ten minutes and more when both the source and recipient were wearing masks (
Figure 11). It is reminded that the color change of SarGel with vapor deposition is a gradual process and becomes darker pink with accumulating deposition. To reach the color depth on the face in
Figure 11a, a deposition intensity of 0.24 mg/cm
2 of vapor is needed [
54].
One salient feature in vapor droplet deposition with no mask-wearing is the elevated deposition in the neck, chin, and jaw, as displayed in
Figure 11b, where the source is standing and the recipient is sitting. This preferential deposition can cause secondary self-inoculation of the recipient’s mucous membrane because people spontaneously touch their eyes, cheeks, chin, and mouth, unknowingly [
55,
56,
57]. According to a study involving 26 students in 2015, a person touched his or her own face 23 times per hour, with around 44% touching the mucous membranes (mouth, nose, and eyes) and 56% touching the non-mucosal areas (chin, cheek, neck, ears, forehead, and hair), with insignificant differences between right and left hands [
58]. In a more recent systematic review that considered ten observational studies, facial self-touching per hour was counted as 50.06 (±47) times [
59]. Considering the high deposition in the neck, chin, jaw, and mouth-nose region, hand/face/neck washing is highly recommended to reduce the chance of self-inoculation via spontaneous face-touching.
4.3. Effect of Double Masking
Double masking has been recommended as an extra measure during the COVID-19 pandemic to further curb viral transmission more effectively than wearing a single mask [
60]. It can significantly enhance protection against respiratory droplets that may contain viruses, including COVID-19 [
61]. This practice involves wearing one mask over another—typically a cloth mask over a surgical mask—to improve the overall fit and filtration capability of the masks [
62]. The CDC has noted that this method reduces exposure by 95% when both masks are worn properly [
63]. In this research, we evaluated the effect of double masking on interpersonal droplet transmission by comparing the recipient wearing a surgical mask vs. a surgical mask plus a face shield. Adding a face shield led to improvements: (a) prolonged the time when the droplet deposition became noticeable and (b) reduced the overall deposition on the face and neck of the recipient. Adding a face shield resulted in significant improvements in reducing deposition on the recipient, especially on the cheek and neck, even though deposition in the nose-mouth region was still observed (Figs. 11b vs. 11a). The time for deposition onset was also prolonged.
There might be two reasons behind these improvements: modified flow-particle dynamics and enhanced surgical mask fit. The presence of the face shield causes airflow and aerosols that approach the wearer to either collide with the shield or divert upwards from beneath the shield. This not only filters out a fraction of aerosols but also lowers their speeds, making it more difficult for these aerosols to be inhaled across the surgical mask. Furthermore, the face shield can also enhance the fit of the surgical mask to the face by pressing down the tucked-up folds, thus minimizing gaps around the edges of the mask. The seal of the face shield at the forehead also reduces the influx of unfiltered air through the nose top. Thus, adding a face shield to a surgical mask can effectively increase the number of barriers against potentially infectious aerosols while introducing relatively minimal extra resistance due to its side opening to the environment. It can be particularly useful in scenarios where higher protection is needed, such as crowded indoor spaces or when interacting closely with others. Also note that double masking is not recommended with two disposable masks or with two N95 masks, as these combinations do not enhance fit or filtration effectively and can make breathing more difficult [
64].
4.4. Limitations and Future Studies
Limitations of this study included an unbalanced focus on exhalation flows, the qualitative nature of results, and a limited number of interpersonal and mask-wearing scenarios. Overall, it is easier to visualize the expiratory flows than inspiratory flows both with and without a mask, due to the jet-like exhalation flows vs. the converging inhalation flows (Figs. 8a vs. 8b). The latter often have extremely low velocities (i.e., proportional to 1/r
3), except in the proximity of the nostrils and face-mask gaps (Figs. 8b and 9). By contrast, the exhalation core flow has a much higher velocity, penetrates a longer distance, and maintains a large temperature difference relative to the ambient air (
Figure 8b). It is unclear, for a mask with given gaps, whether the outward and inward leakage flow rates are the same or not. An accurate answer to this question will affect the estimation of the mask-fit effect on viral source control or receiver protection.
The major limitation of the visualization methods in this study is that they are qualitative only. The Schlieren optical system can also be used to visualize interpersonal droplet transport [
25,
33]. This, however, requires a large concave mirror, whose cost increases drastically with the mirror diameter [
65]. By comparison, the vapor-SarGel system developed in this study is low-cost and directly relevant to interpersonal viral transmission. The vapor droplets are measured to be 3.8 µm on average, which is consistent with the respiratory droplets generated in the alveolar region from liquid film ruptures [
39,
40,
41]. Note that the alveolar region is the major site of SARS-Cov-2 viral infections [
66].
Only four scenarios of interpersonal short-range airborne transmission were considered in this study. However, there are countless scenarios that can markedly influence the transmission risks. Edmunds et al. summarized such behaviors into four categories, with conversation accounting for 95% of all possible close contact events, i.e., conversation without physical contact (61%) and with physical contact (34%) [
67]. Zhang et al. [
53] proposed quantitative physical parameters to describe these close contact transmissions, including the two persons’ locations and head orientations (1), head/body movement (2), close contact frequency and duration (3), and breathing patterns/intensities (4). So far, the parameters most frequently studied are the interpersonal distance and exposure duration, which have been demonstrated to play an important role in viral transmission [
53]. In this study, in addition to the distance and duration, we also observed that the head relative height (i.e., recipient standing and recipient sitting) and face covering type can notably alter the aerosol transmission, including the exposure time for SarGel color change, the variation of deposition with time, and the deposition distribution (Figs. 10 and 11). Future studies are needed to examine how other close-contact parameters influence the transmission risk. Such datasets are vital for creating models of viral spread and planning future outbreak responses.
5. Conclusions
In summary, various visualization methods were attempted to better understand mask flows and interpersonal droplet transmission. Leakage flow velocities at the nose top, two sides, and chin were measured for the cloth, surgical, and KN95 masks, with significant differences among the three masks and between exhalation and inhalation for each mask. Particle counts were measured for five mask types (including neck gaiter and cloth + filter) with a good fit, which were consistent with their filtration efficiency in comparison to that with no mask. The vapor-SarGel visualization system for interpersonal droplet transmission provided a practical method to simulate the temporospatial transport and deposition of respiratory droplets under varying scenarios or with different face coverings. Significant deposition occurred in the mouth-nose region, as well as in the neck, chin, and jaw, increasing the risk of self-inoculation due to spontaneous face-touching, urging post-exposure hand/face washing. Adding a face shield to a surgical mask notably reduced total droplet deposition on the head and regional deposition in the neck and face, supporting that double face covering can be highly effective when one face covering is deemed inadequate.
Author Contributions
Conceptualization, J.H.P., R.N., X.S. and J.X.; methodology, J.S.X., M.T. and X.S.; software, M.T. and J.X.; validation, J.S.X., J.H.P., R.N., X.S., J.X., and M.R.; formal analysis, J.S.X., M.T. X.S.; investigation, J.S.X., M.T., and X.S.; Resources: X.S., J.H.P., R.N., J.X. data curation, M.T.; writing—original draft preparation, J.X.; writing—review and editing, X.S.; visualization, X.S.; supervision, J.X. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially sponsored by the Advanced Functional Fabrics of America and U.S. Army ARDEC under Agreement number W15QKN-16-3-0001.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We thank Amr Seifelnasr at UMass Lowell Biomedical Engineering for editing and proofreading this manuscript. Ms. Claire Lepont is gratefully acknowledged for the project management.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Kelly, E.; Arora, A.; Pirog, S.; Ward, J.; Clarkson, P.J. Comparing the fit of N95, KN95, surgical, and cloth face masks and assessing the accuracy of fit checking. PLOS One 2021, 16, e0245688. [Google Scholar] [CrossRef] [PubMed]
- Kähler, C.J.; Hain, R. Fundamental protective mechanisms of face masks against droplet infections. J. Aerosol Sci. 2020, 148, 105617. [Google Scholar] [CrossRef]
- Essa, W.K.; Yasin, S.A.; Saeed, I.A.; Ali, G.A.M. Nanofiber-based face masks and respirators as COVID-19 protection: a review. Membranes 2021, 11, 250. [Google Scholar] [CrossRef] [PubMed]
- BoyangYu; JianChen; DanerChen; RouxiChen; YuenanWang; XiujuanTang; Hsing-LinWang; Lian-PingWang; WeiweiDeng. Visualization of the interaction of water aerosol and nanofiber mesh. Phys. Fluids 2021, 33, 092106.
- Shen, H.; Zhou, Z.; Wang, H.; Zhang, M.; Han, M.; Durkin, D.P.; Shuai, D.; Shen, Y. Development of electrospun nanofibrous filters for controlling coronavirus aerosols. Environ. Sci. Technol. Lett. 2021, 8, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Passos de Oliveira Santos, R.; Rutledge, G.C. Examination of nanoparticle filtration by filtering facepiece respirators during the COVID-19 pandemic. ACS Appl. Nano Mater. 2021, 4, 3675–3685. [Google Scholar] [CrossRef]
- Ruckdashel, R.R.; Venkataraman, D.; Park, J.H. Smart textiles: A toolkit to fashion the future. Phys. Fluids 2021, 129, 130903. [Google Scholar] [CrossRef]
- Bradford Smith, P.; Agostini, G.; Mitchell, J.C. A scoping review of surgical masks and N95 filtering facepiece respirators: Learning from the past to guide the future of dentistry. Saf. Sci. 2020, 131, 104920–104920. [Google Scholar] [CrossRef]
- Smith, J.D.; MacDougall, C.C.; Johnstone, J.; Copes, R.A.; Schwartz, B.; Garber, G.E. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. Can. Med. Assoc. J. 2016, 188, 567–574. [Google Scholar] [CrossRef]
- Lai, A.C.K.; Poon, C.K.M.; Cheung, A.C.T. Effectiveness of facemasks to reduce exposure hazards for airborne infections among general populations. J. R. Soc. Interface 2012, 9, 938–948. [Google Scholar] [CrossRef]
- Lee, L.Y.-K.; Lam, E.P.-W.; Chan, C.-K.; Chan, S.-Y.; Chiu, M.-K.; Chong, W.-H.; Chu, K.-W.; Hon, M.-S.; Kwan, L.-K.; Tsang, K.-L.; et al. Practice and technique of using face mask amongst adults in the community: a cross-sectional descriptive study. BMC Public Health 2020, 20, 948–948. [Google Scholar] [CrossRef] [PubMed]
- Barnawi, G.M.; Barnawi, A.M.; Samarkandy, S. The association of the prolonged use of personal protective equipment and face mask during COVID-19 pandemic with various dermatologic disease manifestations: a systematic review. Cureus 2021, 13, e16544. [Google Scholar] [CrossRef] [PubMed]
- Ruhle, K.H.; Randerath, W. Measurement of mask leakage during CPAP in patients with obstructive sleep apnea. Pneumologie 2000, 54, 422–424. [Google Scholar] [PubMed]
- Persson, B.N.J. Side-leakage of face mask. Eur. Phys. J. E. Soft Matter. 2021, 44, 75. [Google Scholar] [CrossRef] [PubMed]
- Leidag, M.; Hader, C.; Keller, T.; Meyer, Y.; Rasche, K. Mask leakage in continuous positive airway pressure and C-Flex. J. Physiol. Pharmacol. 2008, 59 Suppl 6, 401–406. [Google Scholar]
- Mueller, J.T.; Karimi, S.; Poterack, K.A.; Seville, M.T.A.; Tipton, S.M. Surgical mask covering of N95 filtering facepiece respirators: The risk of increased leakage. Infect. Control Hosp. Epidemiol. 2021, 42, 627–628. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Grinshpun, S.A.; Reponen, T.; McKay, R.; Bergman, M.S.; Zhuang, Z. Effects of breathing frequency and flow rate on the total inward leakage of an elastomeric half-mask donned on an advanced manikin headform. Ann. Occup. Hyg. 2014, 58, 182–194. [Google Scholar] [PubMed]
- Crutchfield, C.D.; Park, D.L. Effect of leak location on measured respirator fit. Am. Ind. Hyg. Assoc. J. 1997, 58, 413–417. [Google Scholar] [CrossRef]
- Wang, T.K.; Solano, T.; Shoele, K. Bridge the gap: correlate face mask leakage and facial features with 3D morphable face models. J. Expo. Sci. Environ. Epidemiol. 2021. [Google Scholar] [CrossRef]
- Oestenstad, R.K.; Bartolucci, A.A. Factors affecting the location and shape of face seal leak sites on half-mask respirators. J. Occup. Environ. Hyg. 2010, 7, 332–341. [Google Scholar] [CrossRef]
- Solano, T.; Mittal, R.; Shoele, K. One size fits all?: A simulation framework for face-mask fit on population-based faces. PLoS One 2021, 16, e0252143. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Wang, J. A critical review on the role of leakages in the facemask protection against SARS-CoV-2 infection with consideration of vaccination and virus variants. Indoor Air 2022, 32, e13127. [Google Scholar] [CrossRef] [PubMed]
- Pushpawela, B.; Chea, P.; Ward, R.; Flagan, R.C. Quantification of face seal leakage using parallel resistance model. Phys. Fluids 2023, 35, 127127. [Google Scholar] [CrossRef]
- Wang, T.-K.; Solano, T.; Shoele, K. Bridge the gap: correlate face mask leakage and facial features with 3D morphable face models. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Liebner, T.J.; Craven, B.A.; Settles, G.S. A schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J. R. Soc. Interface 2009, 6 (Suppl. 6), S727–S736. [Google Scholar] [CrossRef] [PubMed]
- Su, W.C.; Lee, J.; Xi, J.; Zhang, K. Investigation of mask efficiency for loose-fitting masks against ultrafine particles and effect on airway deposition efficiency. Aerosol Air Qual. Res. 2022, 22, 210228. [Google Scholar] [CrossRef]
- Cappa, C.D.; Asadi, S.; Barreda, S.; Wexler, A.S.; Bouvier, N.M.; Ristenpart, W.D. Expiratory aerosol particle escape from surgical masks due to imperfect sealing. Sci. Rep. 2021, 11, 12110. [Google Scholar] [CrossRef] [PubMed]
- Koh, X.Q.; Sng, A.; Chee, J.Y.; Sadovoy, A.; Luo, P.; Daniel, D. Outward and inward protection efficiencies of different mask designs for different respiratory activities. J. Aerosol. Sci. 2022, 160, 105905. [Google Scholar] [CrossRef]
- Brooks, J.T.; Beezhold, D.H.; Noti, J.D.; Coyle, J.P.; Derk, R.C.; Blachere, F.M.; Lindsley, W.G. Maximizing fit for cloth and medical procedure masks to improve performance and reduce SARS-CoV-2 transmission and exposure, 2021. MMWR Morb. Mortal Wkly Rep. 2021, 70, 254–257. [Google Scholar] [CrossRef]
- Verma, S.; Dhanak, M.; Frankenfield, J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys. Fluids 2020, 32, 061708. [Google Scholar] [CrossRef]
- Eni, M.; Mordoh, V.; Zigel, Y. Cough detection using a non-contact microphone: A nocturnal cough study. PLoS One 2022, 17, e0262240. [Google Scholar] [CrossRef] [PubMed]
- Crawford, F.W.; Jones, S.A.; Cartter, M.; Dean, S.G.; Warren, J.L.; Li, Z.R.; Barbieri, J.; Campbell, J.; Kenney, P.; Valleau, T.; et al. Impact of close interpersonal contact on COVID-19 incidence: Evidence from 1 year of mobile device data. Sci. Adv. 2022, 8, eabi5499. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wei, X.; Liu, L.; Su, L.; Liu, W.; Wang, Y.; Nielsen, P.V. Effects of personalized ventilation interventions on airborne infection risk and transmission between occupants. Build. Environ. 2020, 180, 107008. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Nielsen, P.V.; Liu, L.; Jensen, R.L.; Gong, G. Human exhalation characterization with the aid of Schlieren imaging technique. Build. Environ. 2017, 112, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Talaat, M.; Barari, K.; Si, X.A.; Xi, J. Schlieren imaging and video classification of alphabet pronunciations: exploiting phonetic flows for speech recognition and speech therapy. Vis. Comput. Ind. Biomed. Art 2024, 7, 12. [Google Scholar] [CrossRef]
- Forouzandeh, P.; O’Dowd, K.; Pillai, S.C. Face masks and respirators in the fight against the COVID-19 pandemic: An overview of the standards and testing methods. Saf. Sci. 2021, 133, 104995. [Google Scholar] [CrossRef]
- Ranga, U. SARS-CoV-2 aerosol and droplets: an overview. Virusdisease 2021, 32, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Talaat, M.; Si, X.; Xi, J. Breathe out the secret of the lung: Video classification of exhaled flows from normal and asthmatic lung models using CNN-long short-term memory networks. J. Respir. 2023, 3, 237–257. [Google Scholar] [CrossRef]
- April Si, X.; Talaat, M.; Xi, J. SARS COV-2 virus-laden droplets coughed from deep lungs: Numerical quantification in a single-path whole respiratory tract geometry. Phys. Fluids 2021, 33, 023306. [Google Scholar] [CrossRef]
- Li, H.; Leong, F.Y.; Xu, G.; Kang, C.W.; Lim, K.H.; Tan, B.H.; Loo, C.M. Airborne dispersion of droplets during coughing: a physical model of viral transmission. Sci. Rep. 2021, 11, 4617. [Google Scholar] [CrossRef]
- Bake, B.; Larsson, P.; Ljungkvist, G.; Ljungström, E.; Olin, A.C. Exhaled particles and small airways. Respir. Res. 2019, 20, 8. [Google Scholar] [CrossRef]
- Xi, J.; Kim, J.; Si, X.A.; Mckee, E.; Corley, R.A.; Kabilan, S.; Wang, S. CFD modeling and image analysis of exhaled aerosols due to a growing bronchial tumor: towards non-invasive diagnosis and treatment of respiratory obstructive diseases. Theranostics 2015, 5, 443–455. [Google Scholar] [CrossRef]
- Xi, J.; Barari, K.; Si, X.A.; Abdollahzadeh Jamalabadi, M.Y.; Park, J.H.; Rein, M. Inspiratory leakage flow fraction for surgical masks with varying gaps and filter materials. Phys. Fluids 2022, 34, 041908. [Google Scholar] [CrossRef]
- Xi, J.; Lei, L.R.; Zouzas, W.; April Si, X. Nasally inhaled therapeutics and vaccination for COVID-19: Developments and challenges. MedComm 2021, 2, 569–586. [Google Scholar] [CrossRef]
- Roberge, R.J.; Palmiero, A.J.; Liu, Y.; Kim, J.H.; Zhuang, Z. Effect of upper strap downward displacement on n95 filtering facepiece respirator fit factors: a pilot study. J. Occup. Environ. Hyg. 2014, 11, 338–341. [Google Scholar] [CrossRef]
- South-Shore_Health. How the ‘Knot-and-Tuck’ Method Achieves a Better Fitting Face Mask. Available online: https://www.southshorehealth.org/wellness/blog/how-knot-and-tuck-method-achieves-better-fitting-face-mask (accessed on May 27).
- Su, W.-C.; Lee, J.; Xi, J.; Zhang, K. Investigation of mask efficiency for loose-fitting masks against ultrafine particles and effect on airway deposition efficiency. Aerosol Air Qual. Res. 2022, 22, 210228. [Google Scholar] [CrossRef]
- Barari, K.; Si, X.; Xi, J. Impacts of mask wearing and leakages on cyclic respiratory flows and facial thermoregulation. Fluids 2024, 9, 9. [Google Scholar] [CrossRef]
- Cherrie, J.W.; Wang, S.; Mueller, W.; Wendelboe-Nelson, C.; Loh, M. In-mask temperature and humidity can validate respirator wear-time and indicate lung health status. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 578–583. [Google Scholar] [CrossRef]
- Zheng, Z.; Dai, K.; Zhou, X.; Liu, J.; Liu, W.; Lu, J.; Fang, Z. Field investigation of thermal comfort with face masks in outdoor spaces in South China: A case study. Urban Clim. 2023, 51, 101632. [Google Scholar] [CrossRef]
- Hu, R.; Liu, J.; Xie, Y.; Jiao, J.; Fang, Z.; Lin, B. Effects of mask wearing duration and relative humidity on thermal perception in the summer outdoor built environment. Build. Simul. 2023, 16, 1601–1616. [Google Scholar] [CrossRef]
- Oner, E.; Seçkin, A.Ç.; Egeli, D.; Seçkin, M. Investigation of the Thermal Comfort Properties of Masks Used during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 11275. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, W.; Chan, P.T.; Yen, H.L.; Tang, J.W.; Li, Y. Close contact behavior in indoor environment and transmission of respiratory infection. Indoor Air 2020, 30, 645–661. [Google Scholar] [CrossRef]
- Xi, J.; Yuan, J.E.; Zhang, Y.; Nevorski, D.; Wang, Z.; Zhou, Y. Visualization and quantification of nasal and olfactory deposition in a sectional adult nasal airway cast. Pharm. Res. 2016, 33, 1527–1541. [Google Scholar] [CrossRef]
- Kwok, Y.L.; Gralton, J.; McLaws, M.L. Face touching: a frequent habit that has implications for hand hygiene. Am. J. Infect. Control 2015, 43, 112–114. [Google Scholar] [CrossRef]
- Chen, Y.J.; Qin, G.; Chen, J.; Xu, J.L.; Feng, D.Y.; Wu, X.Y.; Li, X. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw. Open 2020, 3, e2016924. [Google Scholar] [CrossRef]
- Wiener, R.C.; Trickett Shockey, A.K.; Waters, C.; Bhandari, R. Face-touching behavior during the COVID-19 pandemic: Self-inoculation and transmission potentials. J. Dent. Hyg. 2021, 95, 41–46. [Google Scholar]
- Mueller, S.M.; Martin, S.; Grunwald, M. Self-touch: Contact durations and point of touch of spontaneous facial self-touches differ depending on cognitive and emotional load. PLoS One 2019, 14, e0213677. [Google Scholar] [CrossRef]
- Rahman, J.; Mumin, J.; Fakhruddin, B. How frequently do we touch facial T-zone: A systematic review. Ann. Glob. Health 2020, 86, 75. [Google Scholar] [CrossRef]
- Nalunkuma, R.; Abila, D.B.; Ssewante, N.; Kiyimba, B.; Kigozi, E.; Kisuza, R.K.; Kasekende, F.; Nkalubo, J.; Kalungi, S.; Muttamba, W.; et al. Double face mask use for COVID-19 infection prevention and control among medical students at Makerere University: A cross-section survey. Risk Manag. Healthc. Policy 2022, 15, 111–120. [Google Scholar] [CrossRef]
- Sickbert-Bennett, E.E.; Samet, J.M.; Prince, S.E.; Chen, H.; Zeman, K.L.; Tong, H.; Bennett, W.D. Fitted filtration efficiency of double masking during the COVID-19 pandemic. JAMA Intern. Med. 2021, 181, 1126–1128. [Google Scholar] [CrossRef]
- Blachere, F.M.; Lemons, A.R.; Coyle, J.P.; Derk, R.C.; Lindsley, W.G.; Beezhold, D.H.; Woodfork, K.; Duling, M.G.; Boutin, B.; Boots, T.; et al. Face mask fit modifications that improve source control performance. Am. J. Infect. Control 2022, 50, 133–140. [Google Scholar] [CrossRef] [PubMed]
- (CDC), C.f.D.C.a.P. Maximizing fit for cloth and medical procedure masks to improve performance and reduce SARS-CoV-2 transmission and exposure, 2021. Available online: https://www.cdc.gov/mmwr/volumes/70/wr/mm7007e1.htm?s_cid=mm7007e1_w (accessed on May 27).
- NewYork-Presbyterian. Should You Be Double Masking? Available online: https://healthmatters.nyp.org/should-you-be-double-masking/ (accessed on May 27).
- Gena, A.W.; Voelker, C.; Settles, G.S. Qualitative and quantitative schlieren optical measurement of the human thermal plume. Indoor Air 2020, 30, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Bridges, J.P.; Vladar, E.K.; Huang, H.; Mason, R.J. Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax 2022, 77, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, W.J.; Kafatos, G.; Wallinga, J.; Mossong, J.R. Mixing patterns and the spread of close-contact infectious diseases. Emerg. Themes Epidemiol. 2006, 3, 10. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Experimental methods: (a) Schlieren optical imaging, (b) Laser imaging, (c) head models with various face coverings, (d) LED imaging, and (e) vapor-based visualization.
Figure 1.
Experimental methods: (a) Schlieren optical imaging, (b) Laser imaging, (c) head models with various face coverings, (d) LED imaging, and (e) vapor-based visualization.
Figure 2.
Schlieren imaging of expiratory mask flows under various activities: (a) surgical mask, (b) KN95, (c) cloth mask, and (d) neck gaiter. Test activities include normal breathing, coughing, soft speaking, normal speaking, loud speaking, and breathing with a bracket.
Figure 2.
Schlieren imaging of expiratory mask flows under various activities: (a) surgical mask, (b) KN95, (c) cloth mask, and (d) neck gaiter. Test activities include normal breathing, coughing, soft speaking, normal speaking, loud speaking, and breathing with a bracket.
Figure 3.
Laser visualization with different masks: (a) 3D printed head model, and (b) manikin.
Figure 3.
Laser visualization with different masks: (a) 3D printed head model, and (b) manikin.
Figure 4.
Exhaled particle counts across various masks: (a) total particle count, (b) particle count vs. particle size. ‘Surg+bracket’: Surgical plus bracket.
Figure 4.
Exhaled particle counts across various masks: (a) total particle count, (b) particle count vs. particle size. ‘Surg+bracket’: Surgical plus bracket.
Figure 5.
Leakage flow speed measurement during exhalation: (a) outward leakage diagram and thermal imaging, (b) cloth mask, (c) surgical mask, and (d) KN95.
Figure 5.
Leakage flow speed measurement during exhalation: (a) outward leakage diagram and thermal imaging, (b) cloth mask, (c) surgical mask, and (d) KN95.
Figure 6.
Leakage flow speed measurement during inhalation: (a) inward leakage diagram and thermal imaging, (b) cloth mask, (c) surgical mask, and (d) KN95.
Figure 6.
Leakage flow speed measurement during inhalation: (a) inward leakage diagram and thermal imaging, (b) cloth mask, (c) surgical mask, and (d) KN95.
Figure 7.
Quantitative comparison of leakage flow velocities: (a) between exhalation and inhalation, and (b) among various leakage sites.
Figure 7.
Quantitative comparison of leakage flow velocities: (a) between exhalation and inhalation, and (b) among various leakage sites.
Figure 8.
Experiments vs. computational fluid dynamic (CFD) simulations: (a) without a mask, (b) with a mask for flow, and (c) with a mask for temperature.
Figure 8.
Experiments vs. computational fluid dynamic (CFD) simulations: (a) without a mask, (b) with a mask for flow, and (c) with a mask for temperature.
Figure 9.
PIVlab analyses of the LED-illuminated smoke distribution during inhalation and exhalation in a head model with a surgical mask over two breathing cycles: (a) cycle A and (b) cycle B.
Figure 9.
PIVlab analyses of the LED-illuminated smoke distribution during inhalation and exhalation in a head model with a surgical mask over two breathing cycles: (a) cycle A and (b) cycle B.
Figure 10.
Visualization of interpersonal transmission of respiratory droplets after 5-minute exposure when both the source and recipient do not wear a mask: (a) both standing or sitting, and (b) source standing and recipient sitting.
Figure 10.
Visualization of interpersonal transmission of respiratory droplets after 5-minute exposure when both the source and recipient do not wear a mask: (a) both standing or sitting, and (b) source standing and recipient sitting.
Figure 11.
Effects of a face shield on inter-person transmission for varying exposure durations with the source person wearing both a surgical mask and face shield: (a) the recipient wearing a surgical mask, and (b) the recipient wearing a surgical mask and face shield.
Figure 11.
Effects of a face shield on inter-person transmission for varying exposure durations with the source person wearing both a surgical mask and face shield: (a) the recipient wearing a surgical mask, and (b) the recipient wearing a surgical mask and face shield.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).