1. Introduction
Among pediatric skeletal injuries, forearm fractures represent a prevalent and clinically significant challenge, accounting for approximately 17.8% of all childhood fractures in the United States, out of an annual incidence rate of 9.47 per 1000 children [
1]. The later this damage is adequately addressed, the resultant loss of forearm motion and subsequent functional and social limitations in performing the activities of daily living, along with the psychological and esthetic impact will be more severe [
2]. Anatomy of the pediatric forearm provides essential guidance for fracture management. Relatively, the ulna is straight and static, while the radius is curved and rotates over the ulna during pronation and supination [
3]. These bones are connected by the interosseous membrane in the middle, and at the joints—both at the wrist and elbow through the proximal and distal radioulnar joints. Each bone has a proximal and distal physis, with the distal physis contributing significantly to longitudinal growth, accounting for 75% in the radius and 81% in the ulna [
4]. Growth polarization correlates with observations that fractures nearer to the distal ends have a higher remodeling potential than those closer to the elbow.
Location and nature of the fracture change with age; complete, displaced fractures are more common in adolescence, while younger children more frequently experience plastic deformation or greenstick fractures [
5]. Bilateral forearm fractures often result from indirect trauma, most commonly from falling onto an outstretched hand (FOOSH) [
2]. During a fall, a child typically extends the arm to protect against the impact, stiffening the wrist, which leads to fracture due to the axial load, that can injure the hand, wrist, forearm, elbow, and shoulder.
Deformities of the limb can indicate a FOOSH injury, but the diagnosis is primarily confirmed through anterioposterior (AP) and lateral orthogonal forearm radiographs, occasionally supplemented by CT or MR scans [
6]. Alternatively, a recent trial found the cost-effective, rapid, and radiation-free ultrasound as a viable diagnostic option [
7]. Distal pulses and capillary refill must be assessed too. Direct impacts can also cause isolated ulnar mid-shaft fractures (“nightstick fractures”) or less commonly of the radius. Abuse should be considered in children younger than three years old [
8].
Classification of pediatric diaphyseal fractures can be done using the internationally employed “AO Pediatric Comprehensive Classification of Long-Bone Fractures” (PCCF), which provides detailed guidelines for categorizing fractures based on the location and morphology of the break and covering different segments and subsegments of long bones [
9]. Fracture location is designated by numbers: the forearm bones are labeled with ‘2’, the proximal segment with ‘1’, the diaphyseal with ‘2’, and the distal with ‘3’. The letters ‘r’ and ‘u’ denote the radius and ulna, respectively. The specific subsegment of the bone (epiphysis, metaphysis, diaphysis) is indicated by “E”, “M”, and “D”. The second part of the code describes fracture morphology, including patterns specific to children. Severity is classified into two levels: simple (.1) and comminuted (broken into multiple fragments) (.2). Displacement is indicated by Roman numerals aiding in a clear and concise description of fractures. In case the fracture involves the growth plate, the Salter and Harris classification is utilized [
10].
Conservative therapy, which primarily involves immobilization with a cast, is a widely accepted and effective treatment for pediatric forearm fractures, especially when displacement is minimal [
8]. Typically, conservative treatment includes closed reduction followed by casting to restore alignment and immobilize the fracture. Casting type and duration depend on the characteristics of the fracture and the age of the child; for example, children under ten with angulations less than ten degrees often achieve complete remodeling and good function with casting alone. Intact periosteum in many of these fractures enhances stability and facilitates natural splinting, making conservative treatment particularly effective. For greenstick fractures, this is especially true, where the bone bends and cracks without breaking completely [
11]. Younger children benefit significantly from this method due to the higher remodeling potential of their bones, allowing for healing with minimal intervention and good functional outcomes. However, careful monitoring is essential to prevent complications such as repeated displacement, inadequate healing, or joint stiffness, with follow-up X-rays ensuring proper alignment. Despite these challenges, conservative therapy remains a cornerstone of pediatric fracture management, offering a less invasive option with excellent outcomes in many cases.
Operative therapy serves as a critical intervention for pediatric forearm fractures that are unstable, significantly displaced, involving both the radius and ulna, or unresponsive to conservative treatment [
12]. Warranting proper alignment and stability is essential for optimal healing and function preventing long-term complications.
Elastic Stable Intramedullary Nailing (ESIN) is a widely employed minimally invasive procedure for pediatric forearm fractures, which involves inserting flexible, strong metal rods into the medullary cavity of the bone, providing internal stabilization [
13]. Habitually, the nails are left in place for 6-12 months before removal. Casting is often used postoperatively for 3-4 weeks to ensure stability, with the elbow at 90 degrees and the forearm in neutral rotation. Advantages of ESIN include small incisions, reduced postoperative pain, and fast recovery times while allowing early mobilization beneficial for maintaining muscle strength and joint function compared to metal plating [
12]. Due to the drawbacks of metal implants, new resorbable intramedullary implants have been developed for the surgical treatment of pediatric forearm diaphyseal fractures, composed of biodegradable materials such as poly-L-lactide-co-glycolide (PLGA), offering temporary support during the healing process while gradually dissolving within the body, eliminating the need for a secondary procedure to remove the hardware [
14].
For more complex fractures, plate and screw fixation is often employed, applying a metal plate secured with screws to ensure precise alignment of the fracture. Plate and screw fixation proves particularly effective for fractures near joints or those that are comminuted, providing robust stability and promoting proper healing. Plates are habitually left in place for at least six months, and removal is considered if they cause discomfort or impede function. Postoperative immobilization usually involves casting or splinting for 4-6 weeks.
External fixation (fixateur externe) is another valuable option involving the stabilization of the bones from outside the body using a frame attached with pins or wires, allowing for adjustment, and is particularly useful in managing complex fractures with extensive soft tissue injuries [
15]. Typically, the fixator remains in place for 6-8 weeks, followed by a transition to a cast or splint for an additional 4-6 weeks, depending on the healing progress of the fracture. Hybrid fixation combines different methods to achieve optimal results [
16]. Surgical intervention carries inherent risks, such as infection or hardware-related irritation, but the benefits often outweigh these concerns in appropriately selected cases. Ensuring accurate bone healing, restoring full function, and preventing long-term sequelae such as deformity or chronic pain remains the primary objective of operative therapy.
Despite the apparent advantages of resorbable implants research on their use in pediatric populations has been limited. Only one study has investigated the outcomes of intramedullary PLGA implants for pediatric diaphyseal forearm fractures, with follow-ups at two [
17] and four [
18] years post-surgery. Another trial focused on distal fracture management [
19]. Therefore, our study aims to assess the mid-term functional and cosmetic results of bioabsorbable intramedullary implants in treating this common condition, providing a deeper understanding of their capabilities in pediatric diaphyseal forearm fractures.
2. Materials and Methods
2.1. Study Design and Patient Selection
A single-center, single-arm, descriptive cohort follow-up study was conducted at the Pediatric Surgical Division of the University of Pécs in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [
20]. Data were retrospectively collected from our hospital’s recordings of pediatric patients who underwent surgery for diaphyseal forearm fractures using PLGA intramedullary implants between May 2021 and March 2023. Then, they were prospectively recalled to evaluate their functional, esthetic, and psychological recovery one year post-surgery.
A total of 38 pediatric patients met the inclusion criteria, which were: (1) pediatric patients under the age of 18 years at the time of follow-up with (2) forearm fractures treated with absorbable intramedullary PLGA implants and (3) had at least one year of postoperative recovery. Eight patients were excluded due to loss of follow-up attributable to (1) missing contact information (2) not appearing on control examination (3) or because of bilateral fractures.
2.2. Intervention
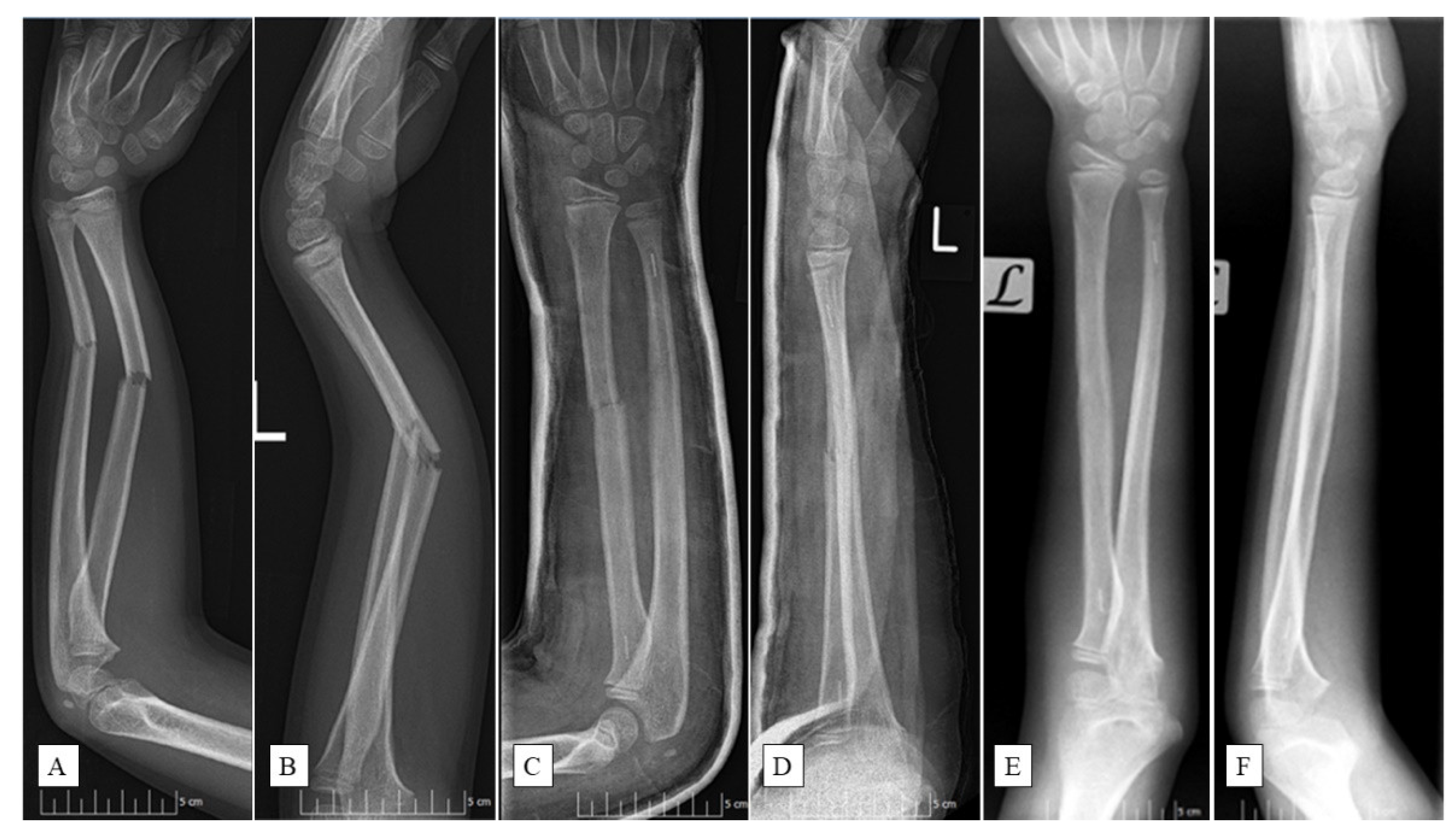
PLGA implants (Activa IM-Nail™, Bioretec Ltd., Tampere, Finland) function by maintaining their mechanical strength throughout the critical period of bone healing (
Figure 1).
These polymers degrade through hydrolysis, breaking down into lactic acid and glycolic acid monomers. Subsequently, the monomers are metabolized via the citric acid cycle, producing water and carbon dioxide as end products. This process lowers the local pH, creating an acidic environment around the implant which facilitates its gradual resorption. Complete degradation of PLGA typically occurs within 9-12 months, making it a reliable material for temporary internal fixation in pediatric patients. Additionally, the implants feature a tricalcium phosphate (β-TCP) marker for precise fluoroscopic placement (
Figure 1/C-F).
2.3. Surgical Protocol
PLGA implants are particularly advantageous for fractures of the radius, ulna, or both, provided proper immobilization is ensured. However, they are not suitable for oblique spiral, comminuted, or epiphyseal fractures, and are contraindicated in the presence of local infection or poor patient compliance.
Several key steps are involved in the surgical procedure for inserting PLGA implants in a minimally invasive manner. After cleaning and disinfecting the operative area and administering general anesthesia, the patient was positioned supine with the affected arm placed on a radiolucent table. Additional management, such as analgesia with 0.1–0.2 mg/kg nalbuphine injections (Nubain, ALTAMEDICS GmbH, Cologne, Germany), and sedation with midazolam (Dormicum, Egis Gyógyszergyár Zrt, Budapest, Hungary) were administered perioperatively based on clinical indications and parental consent. Small incisions were made dorsally on the distal radius and laterally on the proximal ulna. Entry points into the cortical bone had been created using an awl or drill. For the radius, an entry hole was made by positioning the drill perpendicular to the cortex and gradually angling it to form the smallest possible angle with the diaphyseal axis. Then, the medullary canal was reamed with a dilator that matched the implant size - and this process was repeated for the ulna.
Once the canals were prepared, the PLGA implants were introduced using an inserter, guaranteeing no rotational movements to prevent misalignment. Implant diameter choice is critical and should match the smallest diameter of the medullary canal, with available options being 2.7, and 3.2 mm in diameter, and lengths of 200, 300, and 400 mm. Fluoroscopy was used to verify implant positions, with the β-TCP tips aiding in visualization. Protruding ends of the implants were trimmed and smoothed to avoid soft tissue irritation, and the incisions were closed with absorbable sutures. Postoperative care includes immobilizing the limb in a cast above the elbow at a 90-degree angle for 4-6 weeks, with sports and strenuous activities avoided for 2-6 months.
2.5. Evaluated Metrics
Endpoints included patient demographics (such as age, sex, dominant hand) and fracture characteristics (time of injury, and affected side and bone), collected in Microsoft Excel 2021 (Microsoft Corporation, Redmond, WA, USA). Primary outcomes included the joint function evaluation via their range of motion (ROM) using a goniometer. ROM was calculated by adding the absolute values of opposing motions. Measurements from the operated extremity were compared to those of the unharmed limb to determine any discrepancies in ROM for the following movements:
Elbow Flexion and Extension: normal range is -10 to 150 degrees (°). Patients were instructed to fully extend and flex their elbows while standing with arms at their sides.
Forearm Pronation and Supination: standard interval is 80 to 90° in both directions from the neutral position. Children held a pen in a fist with elbows at 90°, rotating their forearms to achieve maximum pronation and supination.
Wrist Palmar Flexion and Dorsiflexion: typical ROM is 80° dorsiflexion and 70° palmar flexion. Patients placed their forearms on a horizontal surface, moving their wrists to the maximum palmar and dorsiflexion positions.
Surgical scars were evaluated using the Vancouver Scar Scale (VSS) [
21], assessing the composite score of four criteria:
Pigmentation: scored from 0 (normal) to 2 (severe hyperpigmentation).
Vascularity: counted from 0 (normal) to 3 (severe vascularity).
Pliability: graded from 0 (normal) to 5 (severe contracture).
Height: recorded from 0 (flat) to 3 (more than 5 mm).
Satisfaction was assessed through a questionnaire asking if the patient or their guardian would choose the same surgical method again under similar circumstances. This survey aimed to capture subjective satisfaction with the functional and aesthetic outcomes of the surgery.
2.6. Data Analysis and Visualization
Descriptive statistics were calculated until two decimals for all continuous outcomes utilizing means, standard deviations (SD), medians, interquartile ranges (IQR), 25th percentiles (IQR25), 75th percentiles (IQR75), counts, and ranges, while discrete endpoints were analyzed via count and percentage distributions. This study employed Python 3.12.3 (Python Software Foundation, Wilmington, DE, USA) for data visualization and statistical analysis, a versatile and open-source language, that facilitated data handling and testing operating several specialized libraries. Statistical analysis was conducted using scipy and numpy libraries. Shapiro-Wilk test was utilized to determine normality, suitable for smaller sample sizes. Two-sample t-tests were used to compare means from two independent groups when both samples were normally distributed, while non-parametric Mann-Whitney U tests differentiated not normally distributed samples. Additionally, a Chi-square (χ²) test determined the association between categorical variables. Differences were deemed significant p ≤ 0.05. Matplotlib was utilized for fundamental plot creation and customization while seaborn provided advanced plotting functions and aesthetic enhancements.
3. Results
This study included 38 pediatric patients with diaphyseal forearm fractures treated using PLGA implants. Patients age ranged from 5 to 15 years, with a mean age of 9.71 (SD = 2.69) years. Regarding sex distribution, the majority of the patients (76.32% of all cases) were female, and the affected side was more often the right forearm (55.26%,
n = 21) (
Table 1). Dominant hand analysis revealed that 85.71% (
n = 18) of the patients were right-handed, moreover, the non-dominant hand was involved in slightly more (52.12%,
n = 12) injuries, which was not statistically significant (
p = 0.513). In terms of fracture type, most of the fractures involved both the radius and ulna (84.21%,
n = 32), with only five children (13.16%) having fractures of the radius alone and one patient (2.63%) having a fracture of the ulna alone. Restricted elbow flexion (< 137°) was linked significantly (
p = 0.017) with radius-only fractures (80% of patients had limited mobility), while wide ROM (≥ 137°) was marginally associated (
p = 0.052) with fractures of both bones (64.52% of children had high mobility).
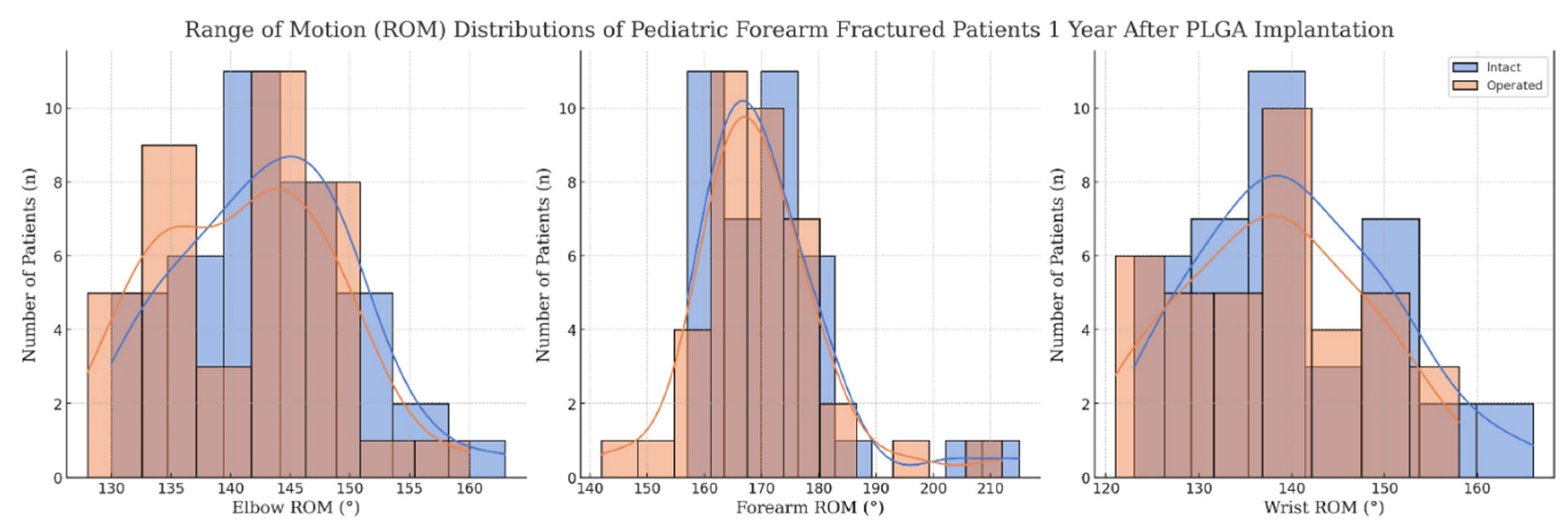
Functional outcomes were assessed via ROM for various movements of the forearm, including the elbow and wrist performance as well, and are summarized in
Table 2 and visualized in
Figure 2.
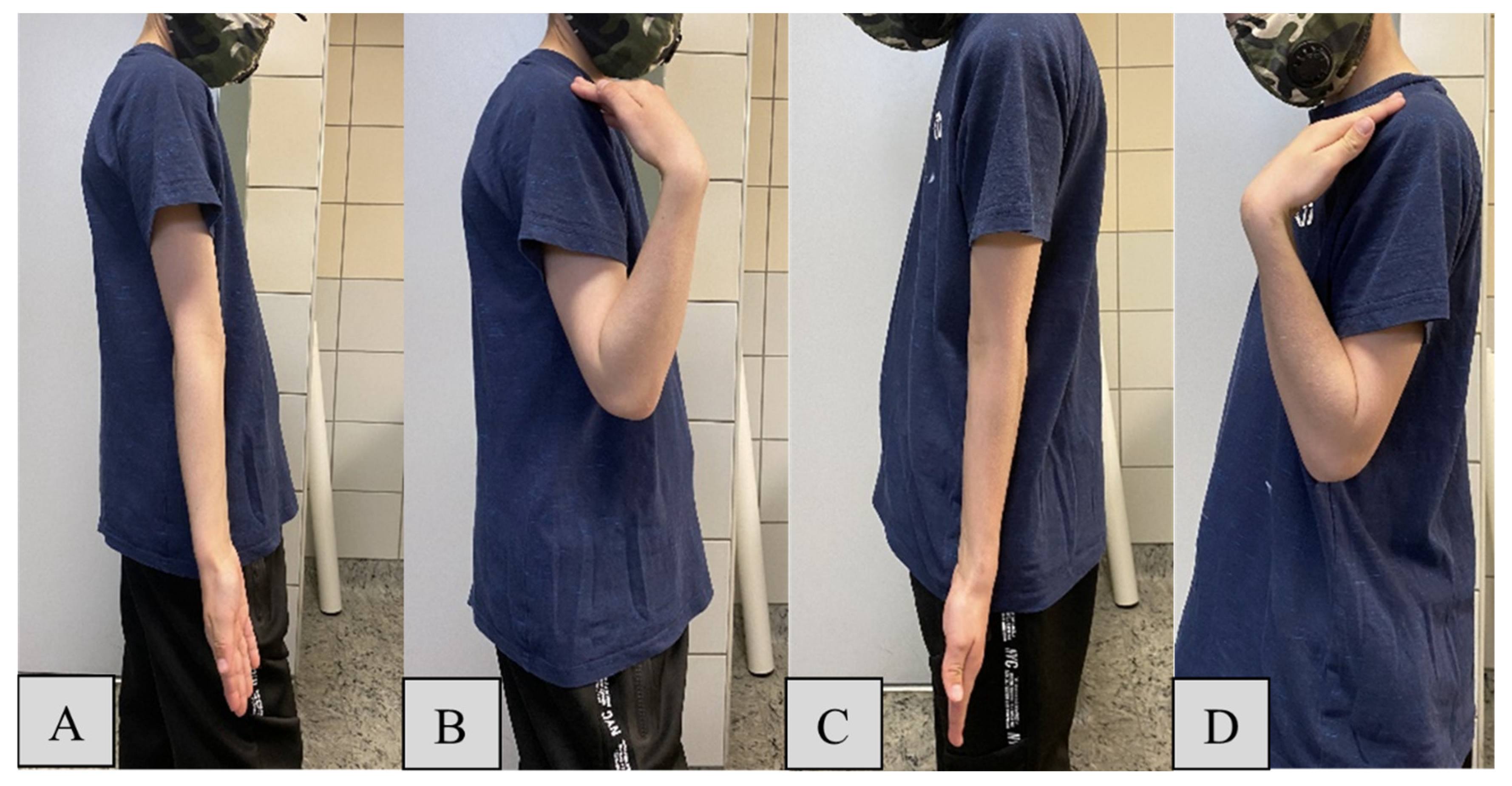
For elbow flexion, the mean maximum angle for the operated side (
Figure 3/D) was 139.3° (SD = 6.2) compared to 140.8° (SD = 6.2) on the intact side (
Figure 3/B). The minor reduction of -1.45° on the operated side suggests effective restoration of function by the PLGA implants, despite a statistically significant difference (
p = 0.282). Elbow extension showed mean ROM values of -1.1° (SD = 2.9) for the PLGA-treated (
Figure 3/C) and -1.3° (SD = 2.9) for the intact side (
Figure 3/A), with a negligible mean difference of 0.05° (
p = 0.098), indicating that the surgical intervention had no significant impact on extension capability.
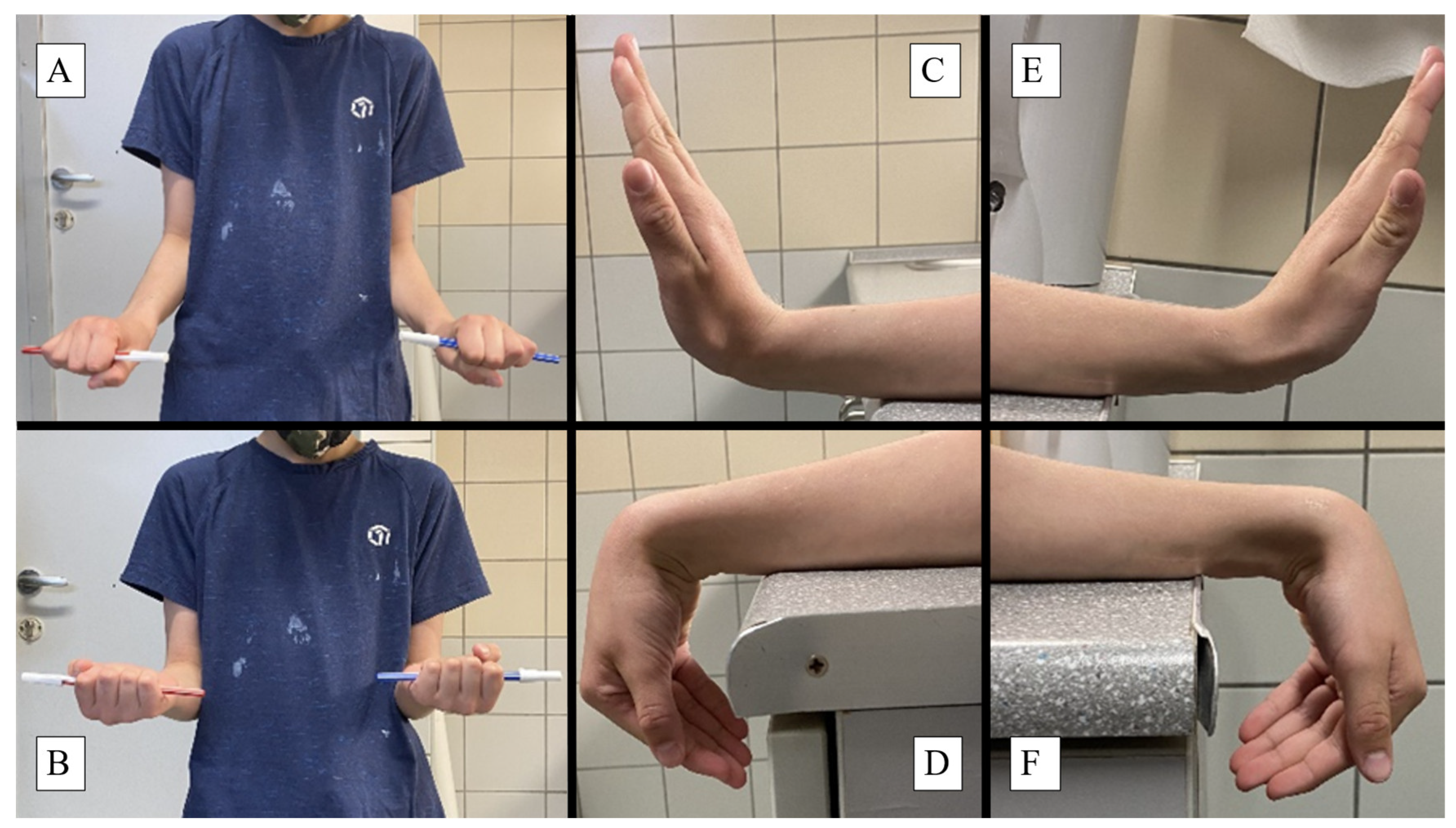
Forearm pronation (
Figure 4/A) exhibited a mean ROM of 80.8° (SD = 6.6) on the operated side and 82.4° (SD = 6.6) on the intact side. The slight reduction of -1.61° in pronation on the operated side (
p = 0.166), suggests a clinically minimal impact on rotational movement. Supination of the forearm (
Figure 4/B) demonstrated a mean ROM loss of 0.24° (
p = 0.141) for the operated side, representing no significant difference and thus supporting the efficacy of the PLGA implants in maintaining rotational movement.
Wrist dorsiflexion showed a mean difference of -0.34° (
p = 0.070) between the operated (
Figure 4/F) and intact sides (
Figure 4/C), representing no significant impact on wrist extension and underscoring the implant’s capacity to maintain wrist flexibility. Palmar flexion measurements indicated a mean difference of -0.89° (
p = 0.563) between the operated (
Figure 4/E) and intact (
Figure 4/D) sides, showing no statistically significant variation and highlighting the implant’s ability to preserve a wide range of wrist movements.
For the total scores, guardians rated the forearm scars with a mean score of 1.13 (SD = 1.14), while medical professionals provided a significantly (
p = 0.020) lower mean VSS score of 0.55 (SD = 0.80) (
Figure 5/C-D). Patient satisfaction was universally high, with all 38 patients reported as satisfied with the treatment outcomes. Comparisons are presented in brief in
Table 3.
4. Discussion
Restoring function while ensuring proper bone healing is the primary objective of pediatric fracture management [
8]. Our results indicate that PLGA intramedullary implants are effective in achieving this balance. PLGA is a biodegradable polymer that has garnered attention for its biocompatibility, controlled degradation properties, and minimal toxicity. Due to these characteristics, it is also one of the most promising drug delivery systems in nanoparticle formulation [
22]. It is possible to add further active ingredients (such as IGF1) into the implant with timed-release properties, for osteostimulation or to prevent infections [
23]. In pediatric traumatology, its features are particularly advantageous, as the implants gradually degrade, eliminating the need for a second surgery to remove hardware [
14]. Fewer interventions reduce the overall healthcare burden and mitigate the psychological impact of additional surgical interventions on young patients and their families. Eliminating a second surgical procedure also translates into fewer anesthesia-related risks and a reduced likelihood of postoperative complications, such as bleeding, infections, or scar tissue formation, which are particularly pertinent in pediatric populations [
24]. Furthermore, the shorter hospital stays and reduced need for follow-up visits significantly decrease the disruption to a child’s education and social life, which are vital for their overall development. Despite PLGA materials being generally well-tolerated, the potential for allergic reactions or adverse responses in certain patients should be investigated. Additionally, PLGA implants are nearly invisible on X-ray imaging, while they are compatible with MRI. To decrease the cost of control examinations and better compliance, β-TCP bits were incorporated into the tips of the implants, which show up as hyper-opacities on X-ray.
Expanding on our previous investigations [
25,
26], the current cohort included 38, predominantly female (76.32%) pediatric patients, generally with both of their diaphysis (84.21%) fractured on a single forearm with a mean age of 9.71. A novel observation was that originally, slightly limited elbow flexion (< 137°) correlated significantly (
p = 0.017) with radius-only fractures (80% of patients had restricted mobility), while a broad original ROM (≥ 137°) was marginally associated (
p = 0.052) with fractures of both diaphyseal bones (64.52% of children had high mobility) - which might also reveal to be significant within a larger analyzed population.
A minor reduction in mean elbow (absolute flexion difference: -1.45°,
p = 0.282; extension: 0.05°,
p = 0.098) forearm (pronation: -1.61°,
p = 0.166; supination: 0.24°,
p = 0.141) and wrist (palmar flexion: -0.89°,
p = 0.563; dorsiflexion: -1.55°,
p = 0.070) mobility highlights the precision of these implants in preserving near-normal ROM. A randomized controlled trial (RCT) found similar patterns regarding preserved ROM using PLGA. Moreover, they showed that the currently gold standard ESIN utilization slightly reduced forearm rotational ROM on the injured side, and increased postoperative pain compared to PLGA [
17]. These findings are significant given the crucial role of elbow and forearm movements in daily activities and play, which are essential for children’s development and quality of life. Consistent functional outcomes across ages, genders, and different fracture types underscore the versatility and reliability of PLGA implants. Another study found that they were also applicable in osteochondral fractures of the lateral condyle of the femur, patella, and radial head [
27]. Therefore, the observed uniformity suggests that surgeons can confidently employ these implants across a wide range of fractures, ensuring optimal outcomes irrespective of specific characteristics. This adaptability could be further enhanced by individualizing management to patient needs, considering factors such as the dominant hand and specific activity requirements. Advances in 3D printing and bioengineering could enable the creation of such patient-specific PLGA implants. Tailoring the size and shape of the implants to fit the unique anatomical and physiological needs of each child may further enhance the effectiveness and comfort of the treatment.
Our results also indicate a generally positive outcome for pediatric forearm fractures treated with PLGA implants, with high satisfaction rates and minor VSS scores, suggesting minimal scarring as assessed by both guardians and doctors. Although both groups gave low total VSS scores, it must be underscored, that guardians rated the scars 104.76% worse than healthcare professionals, which correlates with recent observations [
28]. Educating patients and their families about the benefits and care associated with PLGA implants can enhance compliance and satisfaction. Clear communication regarding the implant’s degradation process and expected recovery timeline is essential for setting realistic expectations.
Traditional methods of managing pediatric forearm fractures often involve metallic implants, which, while effective, necessitate removal surgeries and pose risks of long-term complications such as hardware irritation or migration [
14]. A rigid, non-degradable material may increase postoperative pain and distress, which can lead to a less comfortable recovery period for pediatric patients. Corrosion or mechanical wear can exacerbate cellular toxicity and tissue reactions [
29]. Chronic inflammation due to metal debris may also play a role in carcinogenesis [
30]. Using PLGA implants circumvents these issues, offering a more patient-friendly approach with fewer long-term risks. Given that PLGA degrades over time, it might also affect the growth plate less. A disadvantage of the degradation process is that it is theorized to lead to intermediary byproducts, which are acidic in nature and halt osteoblast activity thereby hindering recanalization. This has been studied in maxillo-facial surgeries and pediatric pelvic osteotomies with over 90% bone recanalization within two years and another study regarding the bone regrowth of the implant canal is underway by Hermann et al. [
31,
32]. On the other hand, one of its byproducts, lactate, has a crucial part in biochemical pathways and could exert therapeutic effects such as angiogenesis [
33]. Another important consideration is the environmental impact of materials. Metal implants contribute to medical waste and require energy-intensive production processes [
34]. In contrast, PLGA implants degrade naturally within the body, reducing the ecological footprint of surgical interventions, and aligning with the broader global efforts toward sustainability in healthcare practices.
Future perspectives and limitations of this research must also be discussed. Expanding the study to include larger and more diverse populations whose data was collected prospectively will provide more comprehensive insights into the generalizability of these findings and reduce possible bias. While the current study corroborates the efficacy of PLGA implants in maintaining functional outcomes, future research should explore the long-term effects of these implants on bone health and growth. Given the dynamic nature of pediatric bone development, it is essential to monitor how the gradual degradation of PLGA implants influences bone remodeling over more extended periods. Longitudinal studies tracking patients into adolescence and adulthood would provide valuable insights into any delayed effects and further solidify the implants’ safety profile. Additionally, investigating the economic impact of PLGA implants, including cost-effectiveness analyses directly compared to traditional methods, could further substantiate their adoption in clinical practice. Potential research could explore their usefulness in other pediatric orthopedic conditions, such as fractures of the femur, tibia, or even more complex multi-fragmentary fractures. Expanding the use of PLGA implants could standardize treatment protocols and streamline surgical training for pediatric surgeons. Lastly, the scar scales and questionnaires are subjective measurements, which could be further objectivized by specialized instruments, such as Laser Doppler Imaging (LDI) for quantifying scar blood flow, Cutometers for measuring elasticity, or ColorMeters for calculating the melanin index [
35]. Using advanced imaging techniques, for example, MRI would also reveal implant resorption rates [
18].
Management of pediatric forearm fractures using PLGA intramedullary implants has demonstrated promising outcomes, reflecting advancements in biomaterial technology and surgical techniques. Our study presents compelling evidence supporting the capabilities of PLGA implants in maintaining functional ROM while minimizing complications, thereby enhancing the overall recovery experience for young patients.
5. Conclusions
Our study demonstrates that PLGA intramedullary implants effectively restore function and ensure proper bone healing in pediatric diaphyseal forearm fractures. Children showed only a minor reduction in mobility, indicating the precision of these implants in preserving a near-normal range of motion. Consistent functional outcomes across different ages, genders, and fracture types highlight the versatility of PLGA implants. They offer a patient-friendly alternative to traditional metallic implants, which often require removal surgeries and pose long-term risks such as hardware irritation or migration. The biodegradable nature of PLGA eliminates the need for a second surgery, reducing healthcare burdens and psychological impacts on young patients and their families while decreasing anesthesia-related risks and postoperative complications such as bleeding, infections, or scar tissue formation. Additionally, fewer hospital stays and follow-up visits minimize disruptions to a child’s education and social life, crucial for their overall development. While PLGA’s degradation process may introduce some intermediary byproducts, the overall benefits seem to outweigh these concerns.
Future research should focus on the long-term effects of PLGA implants on bone health and growth, expanding to larger, diverse populations to validate findings. Additionally, investigating the economic impact and exploring their application in other pediatric orthopedic conditions could further substantiate PLGA implants’ adoption in clinical practice. Overall, PLGA implants represent a significant advancement in pediatric fracture management, enhancing recovery experiences for young patients.
Author Contributions
Conceptualization, A.L., Á.M.L. and G.J.; methodology, A.K., A.L, G.J. and H.N.; software, A.K., A.L. and H.N.; validation, A.K., A.L., Á.M.L, H.N. and G.J.; formal analysis., A.K., A.L., Á.M.L. and H.N.; investigation, Á.M.L., H.N. and G.J.; resources, G.J.; data curation, A.K., A.L., Á.M.L., and H.N. .; writing—original draft preparation, A.L.; writing—review and editing, A.K., Á.M.L., A.L. G.J., and H.N.; visualization, A.K., A.L. and H.N.; supervision, A.K., A.L. and G.J.; project administration, A.K., A.L., and G.J.; funding acquisition, G.J. All authors have read and agreed to the published version of the manuscript.