1. Introduction
Cognitive impairment is a central feature of schizophrenia, significantly impacting functional outcomes [1–3]. Most patients experience deficits in attention/vigilance, verbal learning and memory, executive functioning, verbal fluency, and processing speed [4,5]. The severity of these impairments is consistently linked to various functional aspects and long-term disability [6–8]. Treatment has primarily focused on pharmacological and non-pharmacological approaches. Psychosocial treatments for cognitive remediation (CR) have shown more promising results compared to pharmacological methods [9–14].
CR therapy methodologies vary and include bottom-up vs. top-down, computer-based vs. non-computer-based, group vs. individual, tailored vs. standardized, and combined vs. not combined with other treatments [14–17]. A meta-analysis by Twamley et al. [18] showed that the effect size of computer-assisted CR therapy on cognition was greater than that of non-computer-assisted therapy. While the benefits of CR therapy are recognized, more information is required on effective CR therapy methodologies and related factors to formulate evidence-based recommendations for enhancing cognition in patients with schizophrenia.
We devised a CR therapy program utilizing the Japanese version of the Higher Brain Function Training System RehaCom® (
http://www.hasomed.de). While RehaCom®-based CR therapy has been used for patients with schizophrenia in other countries [19–21], the Japanese version was introduced in September 2019, and its impact on patients with schizophrenia in Japan remains unexplored. This study sought to assess the feasibility and effectiveness of augmenting treatment as usual (TAU) with RehaCom®-based CR therapy compared to TAU alone for cognition in patients with schizophrenia.
2. Materials and Methods
2.1. Study Design and Procedures
This randomized, parallel-two-arm, single-blind, controlled trial evaluated the effect of adding RehaCom®-based CR therapy to TAU on cognition and other outcomes compared to TAU alone in patients with schizophrenia. This study was conducted between December 2021 and July 2022 at the Medical Corporation Seitaikai Mental Support Soyokaze Hospital in the Nagano Prefecture, Japan, a suburban community.
Prospective patients met with the study team, reviewed the study procedures, and if interested, signed informed consent and scheduled a baseline assessment. After completing the baseline assessment, eligible patients were randomized into the CR + TAU and TAU alone groups. Post-treatment assessment occurred 12 weeks after baseline.
2.2. Participants
Between January 2021 and June 2022, participants were recruited from among inpatients and outpatients at the Medical Corporation Seitaikai Mental Support Soyokaze Hospital. The inclusion criteria were patients 20–60 years of age and diagnosed with schizophrenia based on the Structured Clinical Interview for DSM-5 Disorders Research Version (SCID-5-RV) [23]. The exclusion criteria were patients with current primary DSM-5 diagnosis other than schizophrenia, mental retardation, history of neurological disorders, active substance abuse within six months before consent, history of psychosis accounted for by substance abuse, current risk of suicide, and comorbid serious physical disorders.
2.3. Randomization and Blinding
Eligible participants were randomized into the CR + TAU and TAU-alone groups in a 1:1 ratio using a minimization procedure and they were stratified according to age (20–29, 30–39, 40–49, and 50–60 years) and sex (male/female). Randomization was performed using a computer-generated program and was independent and concealed at the individual level. The treatments were open-label; however, the assessors were blinded to the treatment allocation.
2.4. Interventions
2.4.1. CR Therapy
The CR therapy included computer and verbal sessions. The modules of the computer-assisted RehaCom®-based CR therapy modules included 24 individual 60-min sessions, 2 sessions/week, over 12 weeks. CR therapy was conducted on a computer with a special input panel using the Japanese version of the RehaCom® software package (KISSEI COMTEC CO., LTD.). RehaCom® is a computer system for continuous cognitive training such as attention, memory, executive function, and visual perception. Each RehaCom® module was classified according to the categories established by the MATRICS Consensus Cognitive Battery (MCCB) [24,25]: speed of processing, attention/vigilance, working memory, verbal learning and memory, visual learning and memory, reasoning and problem-solving, and social cognition (see
Appendix A). The characteristics of RehaCom® include a highly game-like training menu and a function that automatically adjusts the difficulty level according to the patient’s performance so that patients always receive the appropriate stimulation and continue to work on the training modules with motivation. Occupational therapists certified as cognitive remediation specialists administered the CR therapy. Patients used a desktop computer and an ergonomic keyboard. Each training session was divided into modules selected by the therapists in various cognitive domains.
Appendix B provides the details of the treatment schedule for CR therapy. Further details of the RehaCom® software are described at
http://www.hasomed.de. In addition to the computer sessions using RehaCom®, one-on-one verbal sessions to bridge the gap between improvements in cognitive impairment and daily functioning [12,13,26] were conducted with the therapist. Bridging interventions aid patients in applying their cognition to their daily functioning and promote socialization. The topics in this intervention included community living, the role of cognition in social skills, and problem-solving concerning compensatory strategies for dealing with daily living challenges. CR therapy was considered complete when patients participated in a minimum of 80% of the 24 scheduled treatment sessions.
2.4.2. TAU
All patients received standard treatment throughout the study, including regular meetings with a psychiatrist, antipsychotic medication, individual case management, and rehabilitation programs like occupational therapy, at the same frequency as the CR + TAU group.
2.5. Outcomes
2.5.1. Feasibility and Acceptability Outcomes
The feasibility and acceptability outcomes were the number of eligible patients randomized among the referred and recruited patients, retention rate by the proportion of participants with outcome measures at post-intervention and data completion, acceptability of treatments by dropout from the allocated group, and treatment adherence rate by the proportion of participants receiving a threshold dose of the CR therapy intervention (80% or more) in the CR + TAU group. CR therapy implemented in > 80% of the patients indicated good adherence to CR therapy protocols.
2.5.2. Efficacy Outcomes
Efficacy outcomes were collected at baseline and 12 weeks. The primary efficacy outcome was the change in cognition examined from the baseline to 12 weeks using the Brief Assessment of Cognition in Schizophrenia (BACS). The secondary efficacy outcomes included changes in intrinsic motivation, psychopathology, and functional levels from baseline to 12 weeks.
Cognition was examined with the BACS [27,28] and the Schizophrenia Cognition Rating Scale (SCoRS) [29–31]. The BACS consists of six domains: verbal memory (list learning), working memory (digit sequencing task), motor speed (token motor task), verbal fluency (category instances and letter fluency), attention and processing speed (symbol coding), and executive function (Tower of London test). The Z-scores were created to standardize each of the six domains, wherein the mean of healthy controls was set to zero and the standard deviation was set to one. The composite score was calculated as the average Z-score for each of the six BACS measures. The SCoRS is a 20-item interview-based measure of cognitive impairment with questions aimed at the degree to which this impairment impacts daily functioning. Additionally, a global rating, which reflects the overall impression of the level of cognitive difficulty of patients in 20 cognition areas, was generated. The items were developed to examine the cognitive domains of memory, learning, attention, working memory, problem-solving, motor skills, social cognition, and language production. Each item was rated on a scale ranging from 1 to 4, and the global rating ranged from 1 to 10, with higher ratings reflecting greater impairment. The anchor points for each item focused on the impairment degree in that ability and the degree to which the deficit impaired daily functioning. The evaluators only considered cognitive deficits and attempted to rule out non-cognitive sources of deficits. The complete SCoRS administration included three various ratings: ratings based on an interview with the patient, interview with an informant (i.e., the person who had the most regular contact with the patient in everyday situations), and generated by the interviewer who administered the scale to the patient and informant. SCoRS total is the sum of the 20 SCoRS items.
Intrinsic motivation was examined using the sum of the following three items from the Quality of Life Scale (QLS): sense of purpose, motivation, and curiosity [32–34] and the Intrinsic Motivation Inventory (IMI) [35]. The QLS is rated on a scale ranging from 0 to 6, with higher scores indicating better functioning. The IMI is a 21-item self-reporting scale that measures interest/enjoyment, value/usefulness, and perceived choice. The items were answered on a 7-point Likert scale with responses ranging from ‘‘not at all true’’ to ‘‘very true,’’ with a higher total score reflecting greater intrinsic motivation for a specified task.
Psychiatric symptoms were examined using the Positive and Negative Syndrome Scale (PANSS) [36], which is a 30-item rating scale designed to assess the psychotic symptom severity consisting of three domains: positive, negative, and general psychopathology. Each item is rated from 1 to 7, with higher scores indicating more severe symptoms.
Negative symptoms were assessed with the Scale for the Assessment of Negative Symptoms (SANS) [37], which consists of 30 items that result in global ratings in five symptom complexes: affective flattening, alogia, avolition/apathy, anhedonia/asociality, and attention. Each item and its global ratings range from 0 to 5, with higher scores indicating more severe negative symptoms.
The functional levels were examined using the modified Global Assessment of Functioning (mGAF-original) scale [38]. Eguchi et al. [39] translated the original mGAF [38] into Japanese and developed the psychological symptom (mGAF-S) and social functioning (mGAF-F) subscales by splitting the items and anchor points of the original mGAF scale. We used the mGAF-F, a single-item rating scale for measuring the functioning of the patients. Scores on each scale are rated between 21 and 90, with higher scores indicating better functioning.
2.6. Statistical Analysis
We summarized the descriptive statistics for the feasibility outcomes. T-tests for continuous data and χ2 tests for categorical data were used to determine the group differences in sociodemographic and clinical characteristics. We performed an analysis of covariance (ANCOVA) with an intention-to-treat analysis of each outcome measure using all available data to evaluate the efficacy outcomes. We conducted an ANCOVA with the post-intervention assessment score as the dependent variable and the baseline score, age, sex, baseline IMI total, and baseline QLS total as covariates to examine group differences in the primary efficacy outcome. Moreover, we examined group differences in secondary efficacy outcomes using ANCOVA, with post-intervention scores as the dependent variable and baseline scores, age, and sex as the covariates. Treatment effects were estimated at the 12-week point, with an interaction between group and time. Furthermore, we calculated the effect sizes with ηp2 for intervention changes. Statistical significance was set at p < 0.05 for a two-sided test. Statistical analyses were performed using IBM SPSS Statistics version 28.0. (IBM Corp., Tokyo, Japan).
3. Results
3.1. Participants
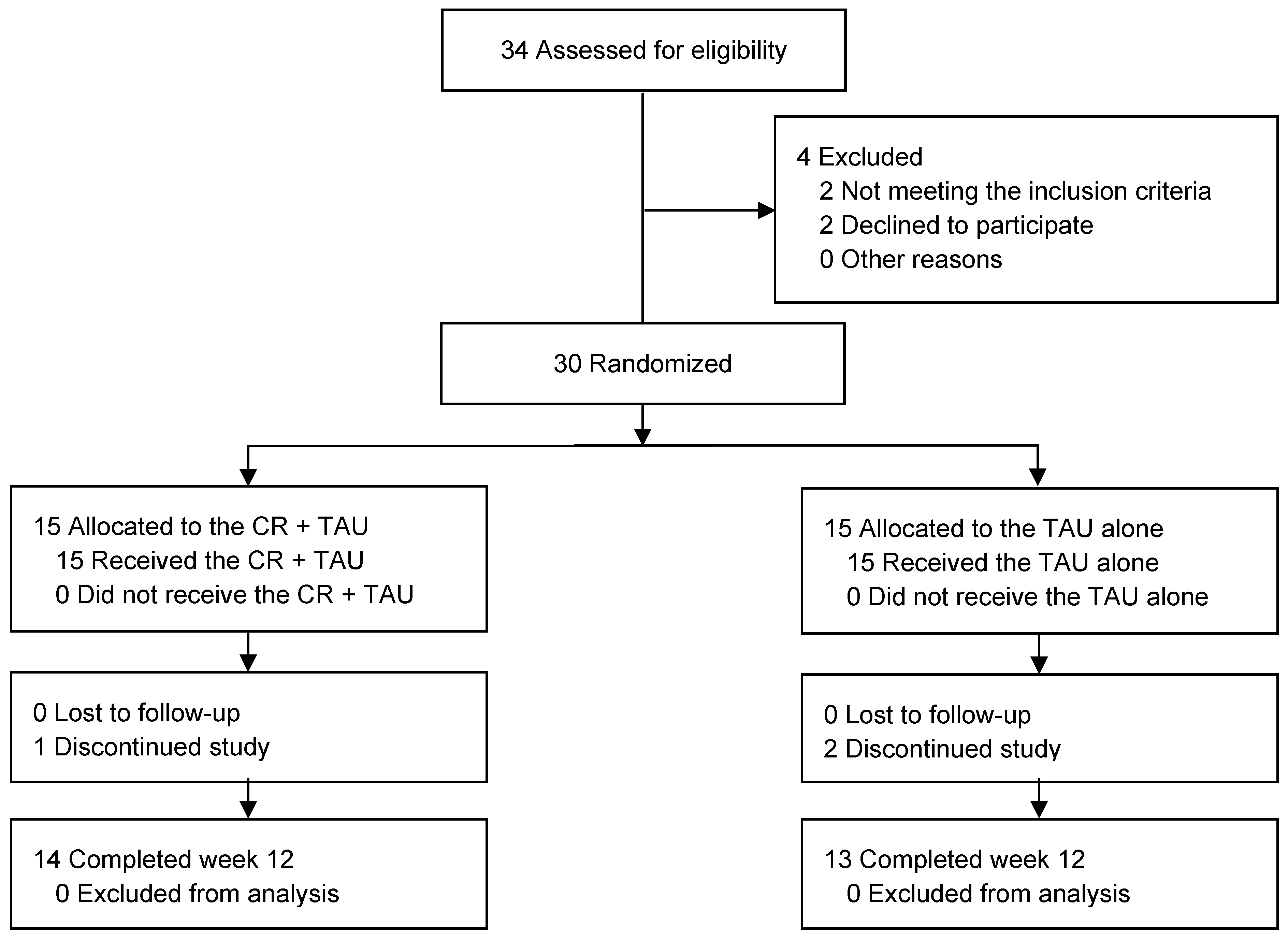
Figure 1 illustrates a flowchart of the study. Of the 34 patients enrolled in the trial, 30 met the inclusion criteria. Among the 30 patients who were randomized (mean age, 47.63 years [standard deviation (SD) = 9.44]; 19 [63%] males), 15 (53%) were in the CR + TAU group and 15 (73%) were in the TAU-alone group. Of these, 27(63%), 14 (50%), and 13 (71%) in the CR + TAU, CR + TAU, and TAU-alone groups, respectively, completed the trial. The demographic characteristics were not significantly different between the groups (
Table 1). Additionally, none of the groups exhibited considerable changes in medication use from baseline to 12 weeks. One patient in the CR + TAU and two in the TAU-alone groups dropped out. Reasons for dropping out of the study existed, including withdrawal of consent (n = 3).
3.2. Feasibility and Acceptability Outcomes
Overall, the results indicated retention rates of 90% and 93% in the CR + TAU and 87% in the TAU-alone groups, respectively. Furthermore, 14 patients (100%) in the CR + TAU group completed > 80% of the sessions. No accidents or injuries occurred during the CR therapy intervention.
3.3. Primary Efficacy Outcomes
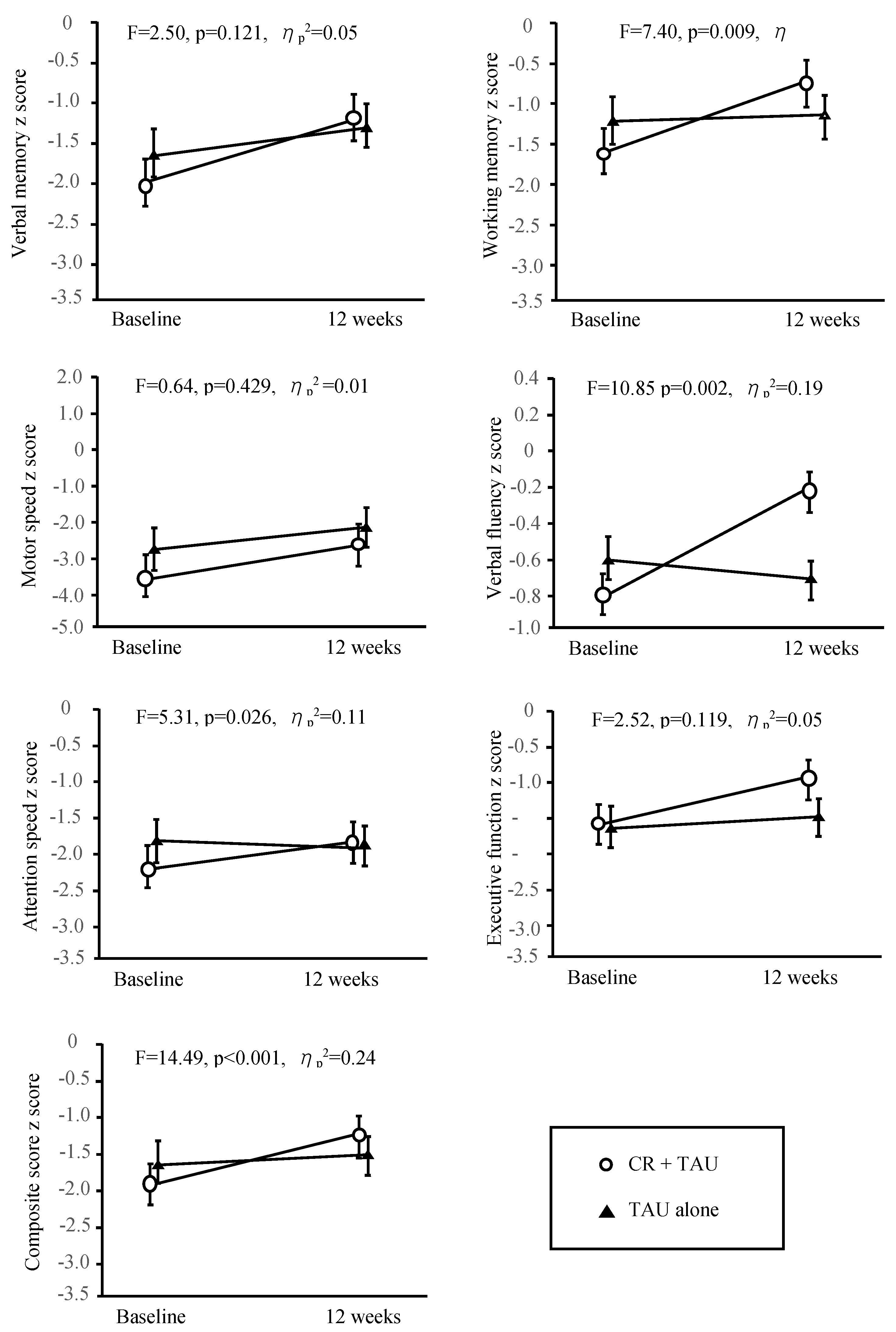
After 12 weeks of treatment, the mean absolute change in the BACS from baseline in verbal memory was 0.75 (95% confidence interval [CI], 0.289–0.823) for the CR + TAU and 0.26 (95% CI, 0.251–0.808) TAU-alone groups; working memory was 0.92 (95% CI, 0.351–0.872) for CR + TAU and 0.03 (95% CI, 0.091–0.614) for TAU-alone groups; motor speed was 0.55 (95% CI, 0.572–0.982) for CR + TAU and 0.29 (95% CI, 0.386–0.909) for TAU-alone groups; verbal fluency was 0.57 (95% CI, 0.419–0.916) for CR + TAU and −0.16 (95% CI, 0.139–0.684) for TAU-alone groups; attention and processing speed was 0.43 (95% CI, 0.177–0.711) for CR + TAU and −0.11 (95% CI, 0.462–0.684) for TAU-alone groups; executive function was 0.62 (95% CI, 0.351–0.872) for CR + TAU and 0.06 (95% CI, 0.462–0.950) for TAU-alone groups; and composite score was 0.65 (95% CI, 0.230–0.770) for CR + TAU and 0.06 (95% CI, 0.050–0.538) for TAU-alone groups.
The baseline assessment findings were comparable between the groups, indicating no significant differences. Table 2 and
Figure 2 show the differences in the changes from baseline to 12 weeks between the groups. The differences in change from baseline to 12 weeks for working memory (F = 7.40, p = 0.01, ηp2 = 0.14), verbal fluency (F = 10.85, p = 0.00, ηp2 = 0.19), attention (F = 5.31, p = 0.26, ηp2 = 0.11), and composite score (F = 14.49, p < 0.00, ηp2 = 0.24) were significant.
3.4. Secondary Efficacy Outcomes
The differences in the baseline assessment results were insignificant.
Table 2 shows the differences between the groups in terms of the changes from baseline to 12 weeks. The differences between groups in terms of the changes from baseline to 12 weeks were significant for SCoRS informant global rating (F = 9.26, p = 0.00, ηp2 = 0.17), SCoRS interviewer total (F = 4.76, p = 0.03, ηp2 = 0.09), and SCoRS interviewer global rating (F = 6.08, p = 0.02, ηp2 = 0.12); QLS score (F = 47.27, p < 0.00, ηp2 = 0.50); IMI Interest/enjoyment (F=6.35, p=0.02, ηp2 = 0.12), IMI total (F=6.51,p=0.01, ηp2 = 0.12); SANS anhedonia/asociality (F = 10.66, p = 0.00, ηp2 = 0.19), SANS total (F = 5.64, p = 0.02, ηp2 = 0.11); and mGAF-F score (F = 18.72, p < 0.00, ηp2 = 0.29).
The F-value in each panel is the test for the F-statistic of the interaction between time and treatment groups. Error bars indicate the standard error. BACS, Brief Assessment of Cognition in Schizophrenia; CR, Cognitive Remediation; TAU, Treatment As Usual
4. Discussion
4.1. Main Findings
This study assessed the feasibility and effectiveness of incorporating RehaCom®-based CR therapy into TAU on cognition and other outcomes in patients with schizophrenia at a Japanese psychiatric hospital. To the best of our knowledge, this is the first randomized trial indicating that utilizing the Japanese version of RehaCom® in CR therapy showed promising feasibility and efficacy in improving cognition in patients with schizophrenia.
This study offers encouraging evidence that adding RehaCom®-based CR therapy to TAU is feasible and well-received, as evidenced by high patient adherence and retention rates. Patients in the CR + TAU group successfully completed the program without worsening medical conditions and no adverse events, indicating good acceptability and tolerance. These findings suggest the feasibility f of employing RehaCom®-based CR therapy in a Japanese psychiatric hospital. The ability to adjust difficulty levels based on the patient's performance, a feature installed in RehaCom®, likely contributed to sustained motivation and engagement during CR therapy.
This study assessed the impact of adding RehaCom®-based CR therapy to TAU on cognition in patients with schizophrenia, finding significant improvements in several cognitive domains in the CR + TAU group compared to the TAU-alone group. These results align with that of a previous study on CR therapy using RehaCom® [19–21] and offer robust evidence for the efficacy of CR in enhancing cognition in schizophrenia. Although the TAU group also demonstrated a trend toward improved cognitive performance, the magnitude of these improvements in the CR + TAU group was greater than that in the TAU group. To improve outcomes, implementing CR therapy alone and CR with TAU may be crucial. As McGurk et al. and Wykes et al. reported, combining CR with other rehabilitation programs often enhances cognitive improvement compared with CR alone. This may have had a synergistic impact on the findings of cognitive performance in the CR therapy program in this study. Targeting patients with schizophrenia may maximize cognitive benefits and improve functional outcomes. Further validation is required to assess the effects of CR-induced cognitive gains in patients with schizophrenia.
Several factors may explain the cognitive improvement found in this study. Compared to the TAU alone group, the CR + TAU group demonstrated substantial improvement in cognition, intrinsic motivation [41–43], and negative symptoms [44–47], which are factors that enhance cognitive outcomes. Thus, improvements in intrinsic motivation and negative symptoms might contribute to greater cognitive gains with CR therapy [17,43]. However, the mechanisms through which RehaCom®-based CR therapy enhances cognitive performance in schizophrenia are not completely understood, necessitating further validation.
The primary goal of schizophrenia treatment involves improving functional outcomes [47,48]. Notably, our results revealed that CR + TAU led to superior outcomes in improving the mGAF-F functional level compared to TAU alone. This can be attributed to significant improvements in cognition, intrinsic motivation, and negative symptoms. The combined benefits of CR therapy on these areas alongside TAU, may enhance overall functional levels in patients with schizophrenia. The role of intrinsic motivation has been established [17,44,49]. Negative symptoms [44,50–52] are key elements of schizophrenia, affecting functional outcomes and mediating the link between cognition and functional outcomes. Therefore, proactive patients undergoing cumulative CR intervention can potentially improve cognition, intrinsic motivation, and negative symptoms, leading to better functional outcomes. Furthermore, training for cognitive task sessions and verbal sessions for bridging improved cognition and real-world functioning is crucial in CR therapy [12,13,26]. The results of this study revealed that the active participation of participants in CR resulted in improvements in cognition, intrinsic motivation, and negative symptoms. One-on-one verbal sessions with an occupational therapist and psychosocial treatments included in TAU may be effective in translating these improvements into real-world functioning.
4.2. Strengths and Limitations
This study’s primary strength lies in the fact that we verified CR’s feasibility and efficacy using RehaCom® for patients with schizophrenia for the first time in Japan. These findings valuable insights into the methodology of CR with RehaCom®, potentially guiding clinicians in implementing an evidence-based intervention to improve cognition in patients with schizophrenia.
However, this study has several limitations. Firstly, the study results were limited by a small sample size and were conducted at a single site; therefore, our findings should be considered preliminary until replicated by future studies. Secondly, the inclusion criteria may restrict the applicability of the findings to broader populations. Thirdly, since the study participants comprised inpatients and outpatients, caution is advised in interpreting functional-level assessments. Fourthly, the study did not explore the optimal dose (duration, intensity, and frequency) to maximize cognitive performance and other outcomes, necessitating further investigation. Fifthly, while an active control group is crucial for discerning essential treatment elements, comparing with TAU is an initial step necessary in evaluating the efficacy of novel therapies, such as CR. However, the TAU intervention received may have varied among patients, as the TAU content was not controlled. Finally, the absence of follow-up assessments to gauge lasting effects is a limitation. Additional follow-up studies are required to determine the impact of CR interventions on cognition.
5. Conclusions
This study demonstrates that integrating CR therapy into standard care effectively enhances cognition in patients with schizophrenia. Combining RehaCom®-based CR therapy with other psychosocial treatments may further elevate functional levels. These results advocate for the inclusion of this approach in schizophrenia treatment protocols. Additional research is needed to determine the prognostic significance of CR therapy.
Author Contributions
Conceptualization, A.Y. and M.K.; Methodology, A.Y. and T.S; Validation, A.Y. and T.S.; Formal analysis, A.Y., T.S., A.K. and M.K.; Investigation A.Y. and A.K.; Resources, A.Y. and T.S.; Writing—original draft preparation, A.Y. and T.S.; Supervision, T.S. and M.K.; Writing—review and editing, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Japanese Association of Occupational Therapists (2022-01).
Institutional Review Board Statement
The Ethics Committees of the School of Medicine, Shinshu University (No. 4643), and the Medical Corporation Seitaikai approved this study.
Informed Consent Statement
All participants provided written informed consent.
Data Availability Statement
This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) (UMIN000039521).
Acknowledgments
The authors express their gratitude to all participants. Additionally, we are grateful for the support received from Shoko Ito, Aya Makabe, Sonoko Miyasaka, and Sayaka Komatsu.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Classification of the RehaCom
® subprograms based on the MATRICS Consensus Cognitive Battery (MCCB) classification
| Cognitive domain |
RehaCom® subprogram |
| Attention / Vigilance |
a) Alertness, b) Saccadic Training, c) Divided Attention,d) Divided Attention 2,e) Sustained Attention |
| Speed of Processing |
f) Reaction Behavior, g) Responsiveness, h) Vigilance 2, i) Two-dimensional Operations, j) Attention and Concentration, k) Exploration |
| Reasoning and Problem-Solving |
l) Topological Memory, m) Logical Reasoning, n) Restoration Training |
| Working Memory |
o) Working Memory |
| Visual Learning and Memory |
p) Figural Memory |
| Verbal Learning and Memory |
q) Memory for Words, r) Verbal Memory |
| Social Cognition |
s) Physiognomic Memory |
| This classification was based on the categories established by the MCCB |
Appendix B
Details of the treatment schedule for cognitive remediation therapy using RehaCom®
| |
a |
b |
c |
d |
e |
f |
g |
h |
i |
j |
k |
l |
m |
n |
o |
p |
q |
r |
s |
| Session 1 |
○ |
○ |
|
|
|
○ |
○ |
|
|
|
|
○ |
|
|
○ |
○ |
○ |
|
○ |
| Session 2 |
|
|
○ |
○ |
|
|
|
○ |
○ |
|
|
|
○ |
|
○ |
○ |
|
○ |
○ |
| Session 3 |
|
|
|
|
○ |
|
|
|
|
○ |
○ |
|
|
○ |
○ |
○ |
○ |
|
○ |
| Session 4 |
○ |
○ |
|
|
|
○ |
○ |
|
|
|
|
○ |
|
|
○ |
○ |
|
○ |
○ |
| Session 5 |
|
|
○ |
○ |
|
|
|
○ |
○ |
|
|
|
○ |
|
○ |
○ |
○ |
|
○ |
| Session 6 |
|
|
|
|
○ |
|
|
|
|
○ |
○ |
|
|
○ |
○ |
○ |
|
○ |
○ |
| Session 7 |
○ |
○ |
|
|
|
○ |
○ |
|
|
|
|
○ |
|
|
○ |
○ |
○ |
|
○ |
| Session 8 |
|
|
○ |
○ |
|
|
|
○ |
○ |
|
|
|
○ |
|
○ |
○ |
|
○ |
○ |
| Session 9 |
|
|
|
|
○ |
|
|
|
|
○ |
○ |
|
|
○ |
○ |
○ |
○ |
|
○ |
| Session 10 |
○ |
○ |
|
|
|
○ |
○ |
|
|
|
|
○ |
|
|
○ |
○ |
|
○ |
○ |
| Session 11 |
|
|
○ |
○ |
|
|
|
○ |
○ |
|
|
|
○ |
|
○ |
○ |
○ |
|
○ |
| Session 12 |
|
|
|
|
○ |
|
|
|
|
○ |
○ |
|
|
○ |
○ |
○ |
|
○ |
○ |
| Session 13 |
○ |
○ |
|
|
|
○ |
○ |
|
|
|
|
○ |
|
|
○ |
○ |
○ |
|
○ |
| Session 14 |
|
|
○ |
○ |
|
|
|
○ |
○ |
|
|
|
○ |
|
○ |
○ |
|
○ |
○ |
| Session 15 |
|
|
|
|
○ |
|
|
|
|
○ |
○ |
|
|
○ |
○ |
○ |
○ |
|
○ |
| Session 16 |
○ |
○ |
|
|
|
○ |
○ |
|
|
|
|
○ |
|
|
○ |
○ |
|
○ |
○ |
| Session 17 |
|
|
○ |
○ |
|
|
|
○ |
○ |
|
|
|
○ |
|
○ |
○ |
○ |
|
○ |
| Session 18 |
|
|
|
|
○ |
|
|
|
|
○ |
○ |
|
|
○ |
○ |
○ |
|
○ |
○ |
| Session 19 |
○ |
○ |
|
|
|
○ |
○ |
|
|
|
|
○ |
|
|
○ |
○ |
○ |
|
○ |
| Session 20 |
|
|
○ |
○ |
|
|
|
○ |
○ |
|
|
|
○ |
|
○ |
○ |
|
○ |
○ |
| Session 21 |
|
|
|
|
○ |
|
|
|
|
○ |
○ |
|
|
○ |
○ |
○ |
○ |
|
○ |
| Session 22 |
○ |
○ |
|
|
|
○ |
○ |
|
|
|
|
○ |
|
|
○ |
○ |
|
○ |
○ |
| Session 23 |
|
|
○ |
○ |
|
|
|
○ |
○ |
|
|
|
○ |
|
○ |
○ |
○ |
|
○ |
| Session 24 |
|
|
|
|
○ |
|
|
|
|
○ |
○ |
|
|
○ |
○ |
○ |
|
○ |
○ |
| a) Alertness, b) Saccadic Training, c) Divided Attention, d) Divided Attention 2, e) Sustained Attention, f) Reaction Behavior, g) Responsiveness, h) Vigilance 2, i) Two-dimensional Operations, j) Attention and Concentration, k) Exploration, l) Topological Memory, m) Logical Reasoning, n) Restoration Training, o) Working Memory, p) Figural Memory, q) Memory for Words, r) Verbal Memory, s) Physiognomic Memory |
References
- Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012; 213: 11-37.
- Green MF, Harvey PD. Cognition in schizophrenia: past, present, and future. Schizophr Res Cogn. 2014; 1: e1-e9. [CrossRef]
- Galderisi S, Rossi A, Rocca P, et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry. 2014; 13: 275-287. [CrossRef]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998; 12: 426-445.
- Nuechterlein KH, Robbins TW, Einat H. Distinguishing separable domains of cognition in human and animal studies: what separations are optimal for targeting interventions? A summary of recommendations from breakout group 2 at the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr Bull. 2005; 31: 870-874. [CrossRef]
- Mohamed S, Rosenheck R, Swartz M, Stroup S, Lieberman JA, Keefe RS. Relationship of cognition and psychopathology to functional impairment in schizophrenia. Am J Psychiatry. 2008; 165: 978-987. [CrossRef]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004; 72: 41-51. [CrossRef]
- Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013; 70: 1107-1112.
- Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. 2005; 8: 457-472. [CrossRef]
- Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007; 64: 633-647. [CrossRef]
- Choi KH, Wykes T, Kurtz MM. Adjunctive pharmacotherapy for cognitive deficits in schizophrenia: meta-analytical investigation of efficacy. Br J Psychiatry. 2013; 203: 172-178. [CrossRef]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007; 164: 1791-1802.
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011; 168: 472-485. [CrossRef]
- Prikken M, Konings MJ, Lei WU, Begemann MJH, Sommer IEC. The efficacy of computerized cognitive drill and practice training for patients with a schizophrenia-spectrum disorder: A meta-analysis. Schizophr Res. 2019; 204: 368-374. [CrossRef]
- Best MW, Bowie CR. A review of cognitive remediation approaches for schizophrenia: from top-down to bottom-up, brain training to psychotherapy. Expert Rev Neurother. 2017; 17: 713-723. [CrossRef]
- Jahshan C, Vinogradov S, Wynn JK, Hellemann G, Green MF. A randomized controlled trial comparing a "bottom-up" and "top-down" approach to cognitive training in schizophrenia. J Psychiatr Res. 2019; 109: 118-125.
- Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. 2019; 18: 146-161. [CrossRef]
- Twamley EW, Jeste DV, Bellack AS. A review of cognitive training in schizophrenia. Schizophr Bull. 2003; 29: 359-382. [CrossRef]
- d'Amato T, Bation R, Cochet A, Jalenques I, Galland F, Giraud-Baro E, Pacaud-Troncin M, Augier-Astolfi F, Llorca PM, Saoud M, Brunelin J. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Schizophr Res. 2011; 125: 284-90. [CrossRef]
- Mak M, Tyburski E, Starkowska A, Karabanowicz E, Samochowiec A, Samochowiec J. The efficacy of computer-based cognitive training for executive dysfunction in schizophrenia. Psychiatry Res. 2019; 279: 62-70. [CrossRef]
- García-Fernández L, Cabot-Ivorra N, Rodríguez-García V, et al. Computerized cognitive remediation therapy, REHACOM, in first episode of schizophrenia: A randomized controlled trial. Psychiatry Res. 2019; 281: 112563. [CrossRef]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, fifth ed. American Psychiatric Association, Washington DC.
- First MB, Williams JBW, Karg RS, Spitzer RL. Structured clinical interview for DSM-5 disorders SCID-5-RV. Washington D.C: American Psychiatry Association ,2015. (translated by Takahashi, S. and Kitamura, T. (2020). Tokyo: Igaku-shoin).
- Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008; 165:203-13.
- Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008; 165: 214-20.
- Medalia A, Revheim N, Herlands T. Remediation of cognitive deficits in psychiatric patients: a clinician’s manual. New York: Montefiore Medical Center Press; 2002.
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004; 68: 283-297. [CrossRef]
- Kaneda Y, Sumiyoshi T, Keefe R, Ishimoto Y, Numata S, Ohmori T. Brief assessment of cognition in schizophrenia: validation of the Japanese version. Psychiatry Clin Neurosci. 2007; 61: 602-609. [CrossRef]
- Keefe RS, Poe M, Walker TM, Kang JW, Harvey PD. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am J Psychiatry. 2006; 163: 426-432. [CrossRef]
- Kaneda Y, Ueoka Y, Sumiyoshi T, et al. The Schizophrenia Cognition Rating Scale Japanese Version (SCoRS-J). Clin Psychiatry. 2010; 52: 1027-1030. Japanese.
- Keefe RS, Davis VG, Spagnola NB, et al. Reliability, validity and treatment sensitivity of the Schizophrenia Cognition Rating Scale. Eur Neuropsychopharmacol. 2015; 25: 176-184. [CrossRef]
- Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984; 10: 388-398. [CrossRef]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000; 55: 68-78.
- Fervaha G, Zakzanis KK, Foussias G, Graff-Guerrero A, Agid O, Remington G. Motivational deficits and cognitive test performance in schizophrenia. JAMA Psychiatry. 2014; 71:1058-1065. [CrossRef]
- Choi J, Mogami T, Medalia A. Intrinsic motivation inventory: an adapted measure for schizophrenia research. Schizophr Bull. 2010; 36: 966-976. [CrossRef]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987; 13: 261-276.
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989; 155: 49-58.
- Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995; 36: 267-275.
- Eguchi S, Koike S, Suga M, Takizawa R, Kasai K. Psychological symptom and social functioning subscales of the Modified Global Assessment of Functioning Scale: reliability and validity of the Japanese version. Psychiatry Clin Neurosci. 2015; 69: 126-127. [CrossRef]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G Power 3: a flexible statistical power analysis program for social, behavioral, and biomedical sciences. Behav. Res. Methods 2007; 39: 175-191.
- Medalia A, Saperstein A. The role of motivation for treatment success. Schizophr Bull. 2011; 37 :S122-128. [CrossRef]
- Fervaha G, Zakzanis KK, Foussias G, Graff-Guerrero A, Agid O, Remington G. Motivational deficits and cognitive test performance in schizophrenia. JAMA Psychiatry. 2014; 71: 1058-1065. [CrossRef]
- Saperstein AM, Medalia A. The role of motivation in cognitive remediation for people with schizophrenia. Curr Top Behav Neurosci. 2016; 27: 533-546.
- Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012; 69: 1216-1224.
- Mäkinen J, Miettunen J, Isohanni M, Koponen H. Negative symptoms in schizophrenia: a review. Nord J Psychiatry. 2008; 62: 334-341. [CrossRef]
- Fervaha G, Foussias G, Agid O, Remington G. Impact of primary negative symptoms on functional outcomes in schizophrenia. Eur Psychiatry. 2014; 29 (7): 449-455. [CrossRef]
- Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019; 381: 1753-1761.
- Fleischhacker WW, Arango C, Arteel P, et al. Schizophrenia--time to commit to policy change. Schizophr Bull. 2014; 40: S165-194. [CrossRef]
- Fervaha G, Foussias G, Agid O, Remington G. Motivational deficits in early schizophrenia: prevalent, persistent, and key determinants of functional outcome. Schizophr Res. 2015; 166: 9-16. [CrossRef]
- Mäkinen J, Miettunen J, Isohanni M, Koponen H. Negative symptoms in schizophrenia: a review. Nord J Psychiatry. 2008; 62: 334-341. [CrossRef]
- Fervaha G, Foussias G, Agid O, Remington G. Impact of primary negative symptoms on functional outcomes in schizophrenia. Eur Psychiatry. 2014; 29: 449-455. [CrossRef]
- Harvey PD, Strassnig M. Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry. 2012; 11: 73-79.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).