Submitted:
05 June 2024
Posted:
10 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Pole Test
2.3. Beam Walking Test
2.4. Rotarod Test
2.5. Buried Food Test
2.6. Y Maze
2.7. Immunohistochemical Assessment of TH Positive Dopaminergic Neurons in the SN and OB
2.8. Statistical Analysis
3. Results
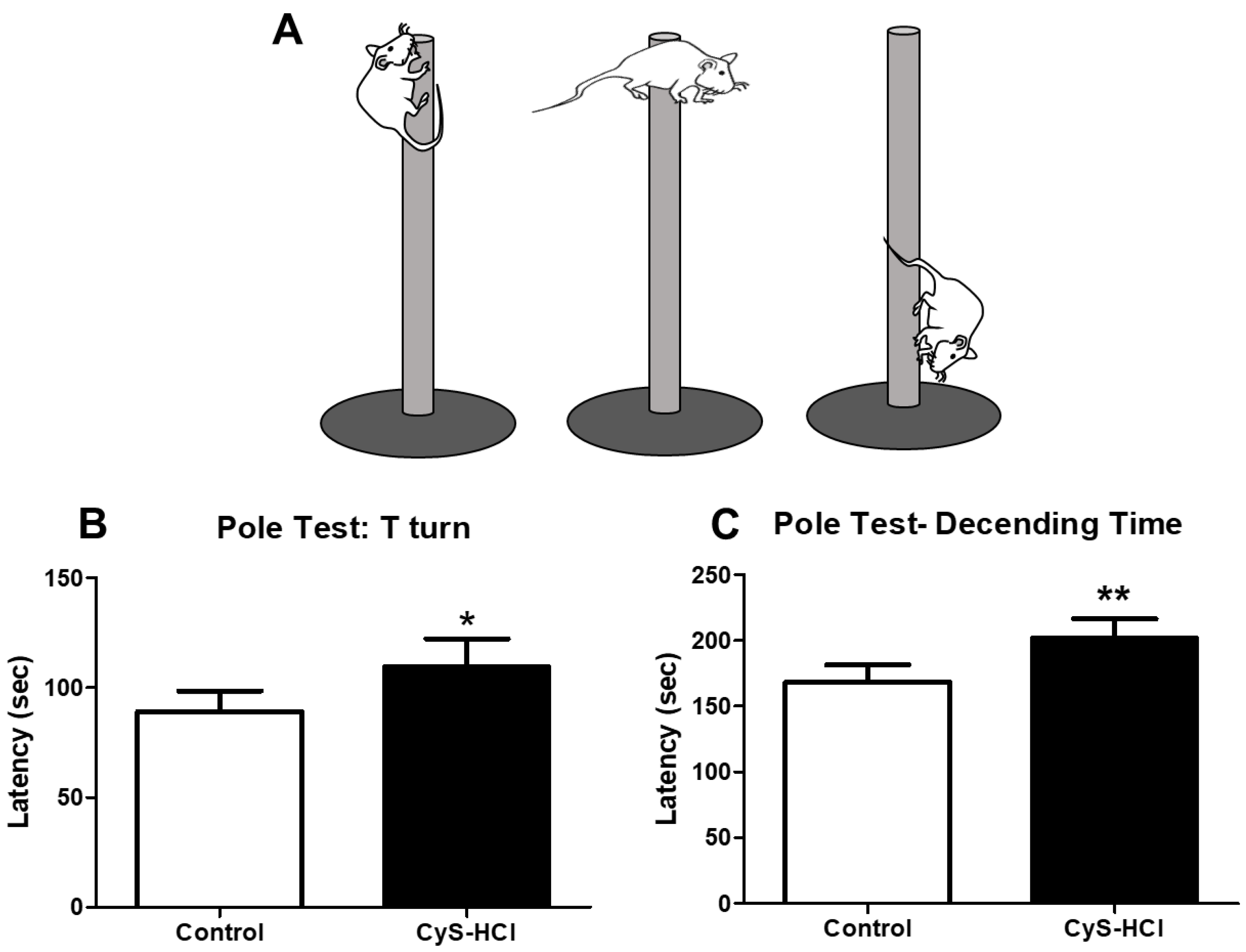
3.1. Cysteamine HCl Treated Mice Exhibited Delayed Motor Functions during the Pole Test
3.2. Cysteamine HCl Treated Mice Showed Impaired the Motor Coordination and Balance in Beam Walking Test

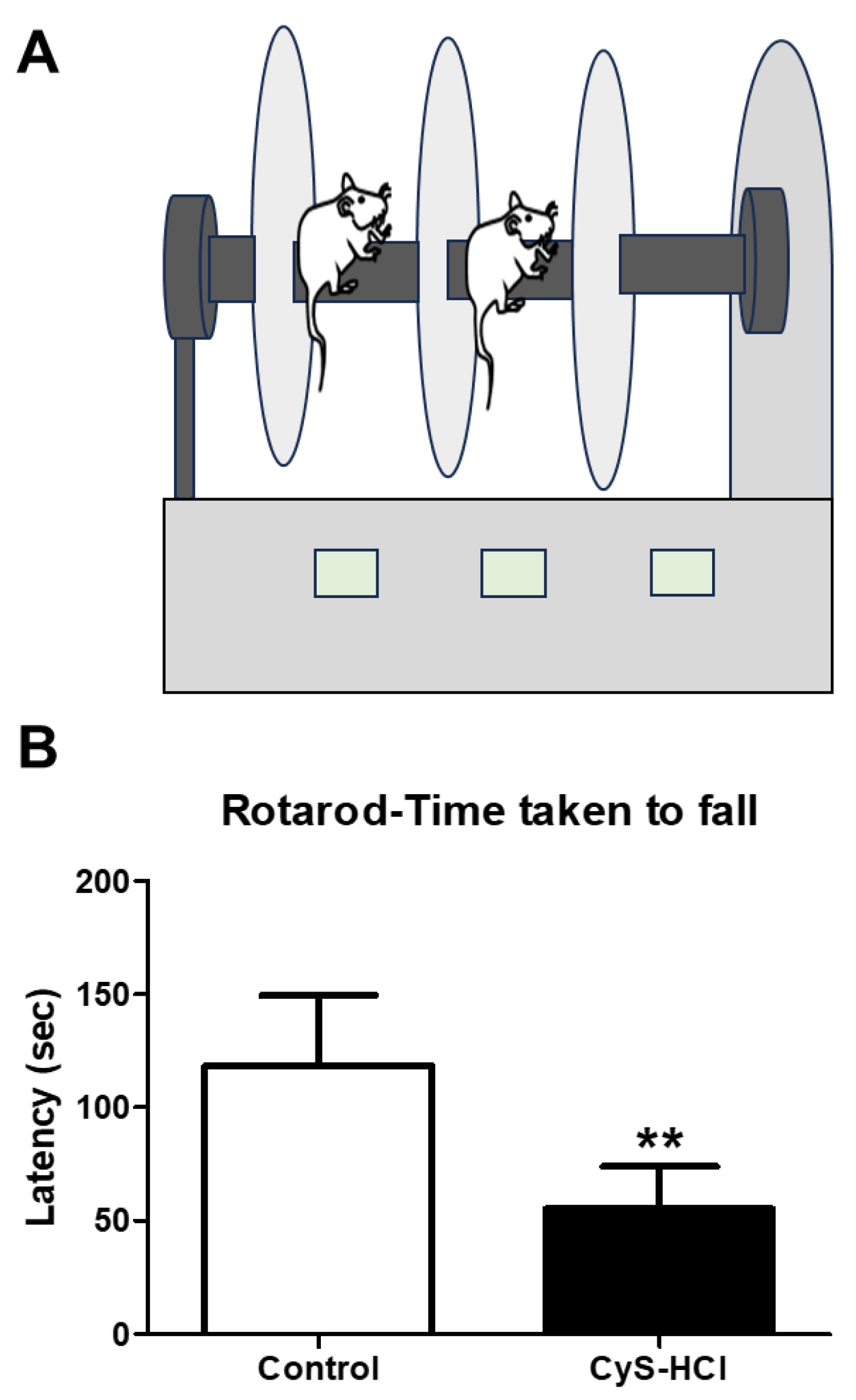
3.3. Cysteamine HCl Treatment Diminished the Locomotor Behavior in the Rotarod Test
3.4. Cysteamine HCl-Treated Animals Showed Deteriorated Olfactory Behavior

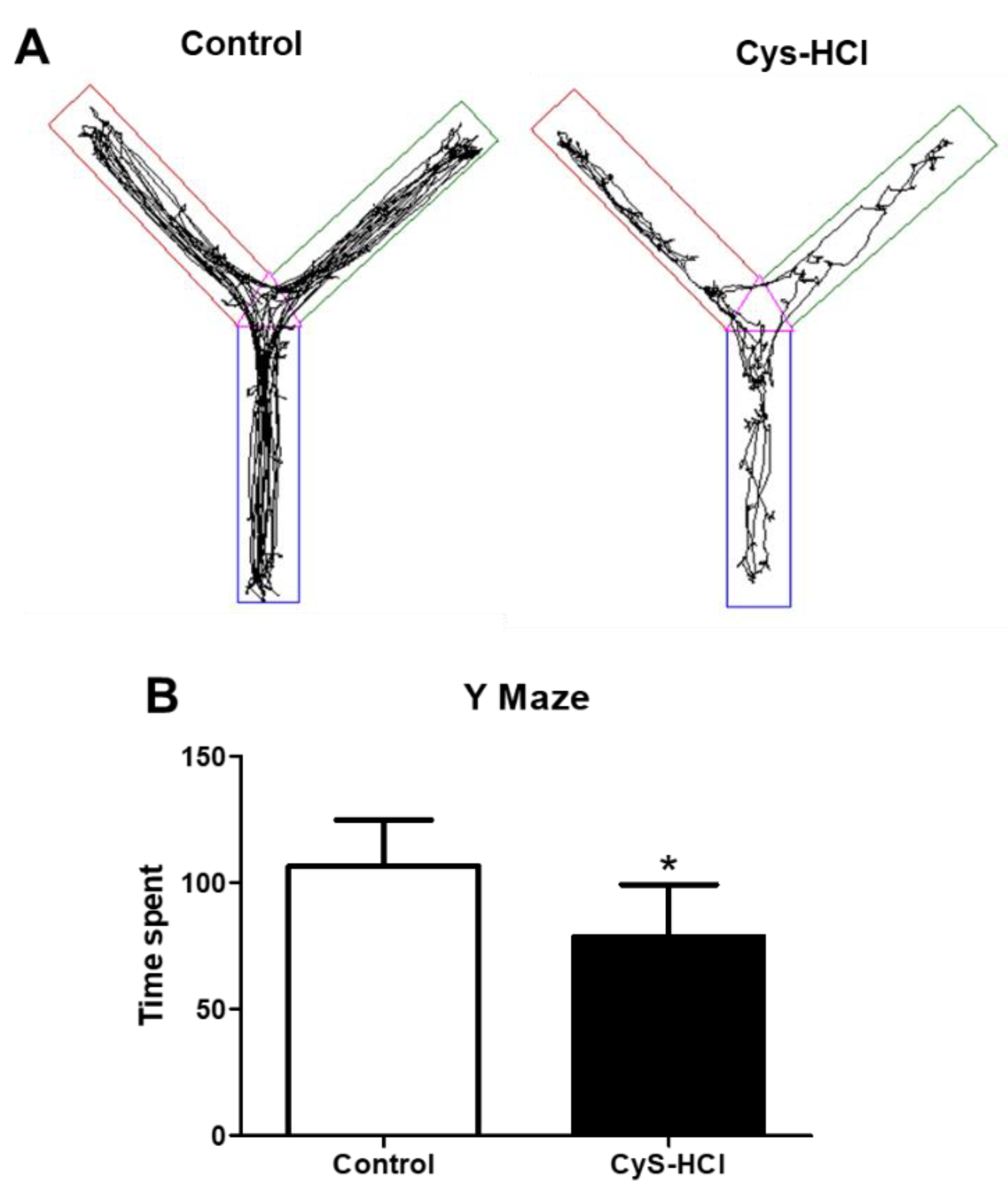
3.5. Impaired Spatial Memory in Cysteamine HCl Treatment during the Y Maze Test
3.6. Cysteamine HCl Treatment Decreased the Number of TH-Positive Cells in the Substantia Nigra and Olfactory Bulb


4. Discussion
5. Conclusion
Authors Contributions
Funding Information
Institutional Review Board Statement
Data Availability Statement
Acknowledgement
Informed Consent Statement
Conflicts of Interest
References
- Selye, H.; Szabo, S. Experimental Model for Production of Perforating Duodenal Ulcers by Cysteamine in the Rat. Nature 1973, 244, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.M.; Ismael, S.; Morais, J.; Araújo, J.R.; Faria, A.; Calhau, C.; Marques, C. Intestinal Alkaline Phosphatase: A Review of This Enzyme Role in the Intestinal Barrier Function. Microorganisms 2022, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Zahradnik-Bilska, J.; Brzozowski, B.; Magierowski, M.; Mach, T.; Magierowska, K.; Brzozowski, T. The Role of Intestinal Alkaline Phosphatase in Inflammatory Disorders of Gastrointestinal Tract. Mediators Inflamm. 2017, 2017, 9074601. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Gombert, M. Circadian Rhythms in the Pathogenesis of Gastrointestinal Diseases. World J. Gastroenterol. 2018, 24, 4297–4303. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.; Pihan, G. Development and Significance of Cysteamine and Propionitrile Models of Duodenal Ulcer. Chronobiol. Int. 1987, 4, 31–42. [Google Scholar] [CrossRef]
- Sikiric, P.; Boban Blagaic, A.; Krezic, I.; Zizek, H.; Kalogjera, L.; Smoday, I.M.; Vukovic, V.; Oroz, K.; Chiddenton, H.M.; Buric, S.; et al. From Selye’s and Szabo’s Cysteamine-Duodenal Ulcer in Rats to Dopamine in the Stomach: Therapy Significance and Possibilities. Pharmaceuticals 2023, 16, 1699. [Google Scholar] [CrossRef] [PubMed]
- Sri Rethinavel, H.; Selvaraj, D.B.; Balakrishnan, S.J.; Vergil Andrews, J.F.; Joseph, J.H.M.; Kandasamy, M. Omeprazole Treatment Manifests Anxiolytic Effects in a Cysteamine Hydrochloride Induced Mouse Model of Gastrointestinal Disorder. Heliyon 2022, 8, e09787. [Google Scholar] [CrossRef]
- Justino, L.; Welner, S.A.; Tannenbaum, G.S.; Schipper, H.M. Long-Term Effects of Cysteamine on Cognitive and Locomotor Behavior in Rats: Relationship to Hippocampal Glial Pathology and Somatostatin Levels. Brain Res. 1997, 761, 127–134. [Google Scholar] [CrossRef]
- Szabo, S.; Cho, C.H. From Cysteamine to MPTP: Structure-Activity Studies with Duodenal Ulcerogens. Toxicol. Pathol. 1988, 16, 205–212. [Google Scholar] [CrossRef]
- Terry, L.C.; Craig, R. Cysteamine Effects on Monoamines, Dopamine-Beta-Hydroxylase and the Hypothalamic-Pituitary Axis. Neuroendocrinology 1985, 41, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Martin-Iverson, M.T.; Radke, J.M.; Vincent, S.R. The Effects of Cysteamine on Dopamine-Mediated Behaviors: Evidence for Dopamine-Somatostatin Interactions in the Striatum. Pharmacol. Biochem. Behav. 1986, 24, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. Therapeutic Applications of Cysteamine and Cystamine in Neurodegenerative and Neuropsychiatric Diseases. Front. Neurol. 2019, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Yi, L.X.; Wang, D.Q.; Lim, T.M.; Tan, E.K. Role of Dopamine in the Pathophysiology of Parkinson’s Disease. Transl. Neurodegener. 2023, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Rethinavel, H.S.; Ravichandran, S.; Radhakrishnan, R.K.; Kandasamy, M. COVID-19 and Parkinson’s Disease: Defects in Neurogenesis as the Potential Cause of Olfactory System Impairments and Anosmia. J. Chem. Neuroanat. 2021, 115, 101965. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Horie, Y. Association Between Olfactory Impairment and Disease Severity and Duration in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2020, 7, 820–826. [Google Scholar] [CrossRef]
- Bhatia-Dey, N.; Heinbockel, T. The Olfactory System as Marker of Neurodegeneration in Aging, Neurological and Neuropsychiatric Disorders. Int. J. Environ. Res. Public. Health 2021, 18, 6976. [Google Scholar] [CrossRef]
- Best, J.A.; Nijhout, H.F.; Reed, M.C. Homeostatic Mechanisms in Dopamine Synthesis and Release: A Mathematical Model. Theor. Biol. Med. Model. 2009, 6, 21. [Google Scholar] [CrossRef]
- Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Brown, M.R.; Fisher, L.A.; Sawchenko, P.E.; Swanson, L.W.; Vale, W.W. Biological Effects of Cysteamine: Relationship to Somatostatin Depletion. Regul. Pept. 1983, 5, 163–179. [Google Scholar] [CrossRef]
- Widmann, R.; Sperk, G. Cysteamine-Induced Decrease of Somatostatin in Rat Brain Synaptosomes in Vitro. Endocrinology 1987, 121, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Haroutunian, V.; Mantin, R.; Campbell, G.A.; Tsuboyama, G.K.; Davis, K.L. Cysteamine-Induced Depletion of Central Somatostatin-like Immunoactivity: Effects on Behavior, Learning, Memory and Brain Neurochemistry. Brain Res. 1987, 403, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Singh, S. Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity. Int. J. Mol. Sci. 2020, 21, 2568. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, L.W.; Dokla, C.P. Morris Water Task Impairment and Hypoactivity Following Cysteamine-Induced Reductions of Somatostatin-like Immunoreactivity. Brain Res. 1989, 505, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, J.; Ruberg, M.; Moyse, E.; Javoy-Agid, F.; Dubois, B.; Agid, Y. Somatostatin and Dementia in Parkinson’s Disease. Brain Res. 1983, 278, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, X.; Li, X.; Liu, N.; Lou, F.; Ma, H.; Luo, X.; Ren, Y. Somatostatin Prevents Lipopolysaccharide-Induced Neurodegeneration in the Rat Substantia Nigra by Inhibiting the Activation of Microglia. Mol. Med. Rep. 2015, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, D.B.; Vergil Andrews, J.F.; Anusuyadevi, M.; Kandasamy, M. Ranitidine Alleviates Anxiety-like Behaviors and Improves the Density of Pyramidal Neurons upon Deactivation of Microglia in the CA3 Region of the Hippocampus in a Cysteamine HCl-Induced Mouse Model of Gastrointestinal Disorder. Brain Sci. 2023, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Sanchez, D.; Ubeda-Bañon, I.; Flores-Cuadrado, A.; Gonzalez-Rodriguez, M.; Villar-Conde, S.; Astillero-Lopez, V.; Martinez-Marcos, A. Somatostatin, Olfaction, and Neurodegeneration. Front. Neurosci. 2020, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Koh, S.-B. Many Faces of Parkinson’s Disease: Non-Motor Symptoms of Parkinson’s Disease. J. Mov. Disord. 2015, 8, 92–97. [Google Scholar] [CrossRef]
- Lin, L.-C.; Sibille, E. Reduced Brain Somatostatin in Mood Disorders: A Common Pathophysiological Substrate and Drug Target? Front. Pharmacol. 2013, 4, 110. [Google Scholar] [CrossRef]
- Goodwin, R.D.; Keyes, K.M.; Stein, M.B.; Talley, N.J. Peptic Ulcer and Mental Disorders among Adults in the Community: The Role of Nicotine and Alcohol Use Disorders. Psychosom. Med. 2009, 71, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Çamcı, G.; Oğuz, S. Association between Parkinson’s Disease and Helicobacter Pylori. J. Clin. Neurol. Seoul Korea 2016, 12, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Higinbotham, A.S.; Kilbane, C.W. The Gastrointestinal Tract and Parkinson’s Disease. Front. Cell. Infect. Microbiol. 2024, 13, 1158986. [Google Scholar] [CrossRef] [PubMed]
- Vernier, P.; Moret, F.; Callier, S.; Snapyan, M.; Wersinger, C.; Sidhu, A. The Degeneration of Dopamine Neurons in Parkinson’s Disease: Insights from Embryology and Evolution of the Mesostriatocortical System. Ann. N. Y. Acad. Sci. 2004, 1035, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current Understanding of the Molecular Mechanisms in Parkinson’s Disease: Targets for Potential Treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Arachchige, A.S.P.M. Depletion of Dopamine in Parkinson’s Disease and Relevant Therapeutic Options: A Review of the Literature. AIMS Neurosci. 2023, 10, 200–231. [Google Scholar] [CrossRef] [PubMed]
- Meredith, G.E.; Rademacher, D.J. MPTP Mouse Models of Parkinson’s Disease: An Update. J. Park. Dis. 2011, 1, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Demarco, C.; Coletta, M.; Bombardieri, G. INHIBITION OF PLASMA MONOAMINE OXIDASE BY CYSTEAMINE. Nature 1965, 205, 176. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Farooqui, R.; Kesler, N. Parkinson Disease: A New Link between Monoamine Oxidase and Mitochondrial Electron Flow. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 4890–4894. [Google Scholar] [CrossRef]
- Fujisawa, T.; Rubin, B.; Suzuki, A.; Patel, P.S.; Gahl, W.A.; Joshi, B.H.; Puri, R.K. Cysteamine Suppresses Invasion, Metastasis and Prolongs Survival by Inhibiting Matrix Metalloproteinases in a Mouse Model of Human Pancreatic Cancer. PLoS ONE 2012, 7, e34437. [Google Scholar] [CrossRef]
- Lee, C.-M. A Review on the Antimutagenic and Anticancer Effects of Cysteamine. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 2419444. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cui, Y.; Chang, Y.-Z.; Yu, P. Ferroptosis-Related Factors in the Substantia Nigra Are Associated with Parkinson’s Disease. Sci. Rep. 2023, 13, 15365. [Google Scholar] [CrossRef] [PubMed]
- Atallah, C.; Charcosset, C.; Greige-Gerges, H. Challenges for Cysteamine Stabilization, Quantification, and Biological Effects Improvement. J. Pharm. Anal. 2020, 10, 499–516. [Google Scholar] [CrossRef]
- Zecca, L.; Tampellini, D.; Gerlach, M.; Riederer, P.; Fariello, R.G.; Sulzer, D. Substantia Nigra Neuromelanin: Structure, Synthesis, and Molecular Behaviour. Mol. Pathol. 2001, 54, 414–418. [Google Scholar] [PubMed]
- D’Amato, R.J.; Alexander, G.M.; Schwartzman, R.J.; Kitt, C.A.; Price, D.L.; Snyder, S.H. Evidence for Neuromelanin Involvement in MPTP-Induced Neurotoxicity. Nature 1987, 327, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Siddu, A.; David, L.S.; Lauinger, N.; Chen, X.; Saint-Pierre, M.; Alpaugh, M.; Durcan, T.; Cicchetti, F. Beneficial Effects of Cysteamine in Thy1-α-Syn Mice and Induced Pluripotent Stem Cells with a SNCA Gene Triplication. Neurobiol. Dis. 2020, 145, 105042. [Google Scholar] [CrossRef] [PubMed]
- Chesselet, M.-F.; Richter, F.; Zhu, C.; Magen, I.; Watson, M.B.; Subramaniam, S.R. A Progressive Mouse Model of Parkinson’s Disease: The Thy1-aSyn (“Line 61”) Mice. Neurotherapeutics 2012, 9, 297–314. [Google Scholar] [CrossRef]
- Richter, F.; Stanojlovic, M.; Käufer, C.; Gericke, B.; Feja, M. A Mouse Model to Test Novel Therapeutics for Parkinson’s Disease: An Update on the Thy1-aSyn (“Line 61”) Mice. Neurotherapeutics 2023, 20, 97. [Google Scholar] [CrossRef]
- Rathke-Hartlieb, S.; Kahle, P.J.; Neumann, M.; Ozmen, L.; Haid, S.; Okochi, M.; Haass, C.; Schulz, J.B. Sensitivity to MPTP Is Not Increased in Parkinson’s Disease-Associated Mutant Alpha-Synuclein Transgenic Mice. J. Neurochem. 2001, 77, 1181–1184. [Google Scholar] [CrossRef]
- Sun, L.; Xu, S.; Zhou, M.; Wang, C.; Wu, Y.; Chan, P. Effects of Cysteamine on MPTP-Induced Dopaminergic Neurodegeneration in Mice. Brain Res. 2010, 1335, 74–82. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



